Abstract
Ricinus communis (Euphorbiaceae), commonly known as the castor oil plant, is prized for its versatile applications in medicine, industry, and agriculture. It features large, deeply lobed leaves with vibrant colours, robust stems with anthocyanin pigments, and extensive root systems for nutrient absorption. Its terminal panicle-like inflorescences bear monoecious flowers, and its seeds are enclosed in prickly capsules. Throughout its various parts, R. communis harbours a diverse array of bioactive compounds. Leaves contain tannins, which exhibit astringent and antimicrobial properties, and alkaloids like ricinine, known for anti-inflammatory properties, as well as flavonoids like rutin, offering antioxidant and antibacterial properties. Roots contain ellagitannins, lupeol, and indole-3-acetic acid, known for anti-inflammatory and liver-protective effects. Seeds are renowned for ricin, ricinine, and phenolic compounds crucial for industrial applications such as biodegradable polymers. Pharmacologically, it demonstrates antioxidant effects from flavonoids and tannins, confirmed through minimum inhibitory concentration (MIC) assays for antibacterial activity. It shows potential in managing diabetes via insulin signalling pathways and exhibits anti-inflammatory properties by activating nuclear factor erythroid 2-related factor 2 (Nrf2). Additionally, it has anti-fertility effects and potential anticancer activity against cancer stem cells. This review aims to summarize Ricinus communis’s botanical properties, therapeutic uses, chemical composition, pharmacological effects, and industrial applications. Integrating the current knowledge offers insights into future research directions, emphasizing the plant’s diverse roles in agriculture, medicine, and industry.
1. Introduction
The castor bean plant, or Ricinus communis, is a significant member of the Euphorbiaceae family, native to tropical and subtropical regions [1]. It showcases remarkable phenotypic plasticity, with diverse morphological traits, such as lobed leaves and a deep taproot system [1]. The castor bean plant seeds are a vital source of essential oil seeds, primarily cultivated in South America, Africa, and India [2]. Extensive research has been conducted on this plant, underscoring its diverse chemical composition and wide-ranging applications [3]. Studies have revealed that the plant’s leaves contain phytochemicals that can combat various infections by acting as antibacterial and antifungal agents [4]. The plant’s potential for environmental remediation has also been explored, particularly its effectiveness in handling soils contaminated with heavy metals [5]. Ricinus communis is chemically constituted of biologically active molecules like ricinoleic acid, which is a principal fatty acid in castor oil possessing anti-inflammatory activity; ricinine, which is an alkaloid possessing insecticidal and putative anticonvulsant activities; rutin, which is a flavonoid with antioxidant activity; lupeol, which is a triterpenoid with anti-inflammatory and anticancer activities; and tannins, which are polyphenolic compounds possessing antimicrobial and astringent activities [6]. These molecules are referred to in this manuscript below with respect to their therapeutic and toxicological activities.
Moreover, research has delved into the plant’s toxic properties, evaluating its genotoxic effects and toxicity against insect vectors [7,8]. The plant has been harnessed to produce gold nanoparticles, showcasing its versatility in material science and nanotechnology [9]. Studies have also focused on using castor bean oil in biodiesel production, emphasising the optimisation of processing parameters [2]. Additionally, the plant’s pharmacological and toxicological effects on mammalian cells have been investigated, providing insights into its potential therapeutic uses [10]. The seeds contain various compounds, with ricinoleic acid being a prominent feature in the extracted castor oil; in contrast, the seed coat houses the well-known protein ricin, showcasing the plant’s multifaceted applications [11].
Comprehensive studies have revealed variations in chemical profiles across different cultivars and plant parts, presenting a rich landscape for exploration [3]. The castor bean plant’s chemical complexity has spurred research into its industrial applications and medicinal properties [12,13]. Historically, R. communis has left an indelible mark on traditional medicine, featuring prominently in medieval practices [14]. The plant’s applications have evolved, culminating in its recognition as an essential oil seed crop with vast agronomic importance [3]. The adaptability of different cultivars, with varying maturation cycles and stress tolerances, underscores its relevance to diverse agricultural practices [15]. The potential of R. communis as a bioresource has stimulated a broad spectrum of studies, delving into diverse facets, from seed maturation and plant development to responses to biotic and abiotic stresses [16,17].
The captivating blend of historical significance, botanical diversity, and chemical complexity converges in the medicinal realm, where the oil extracted from R. communis seeds has demonstrated pharmacological promise [5,18]. Scarpa and Guerci’s pioneering study [19] laid the foundation for understanding the medicinal uses of R. communis, revealing its global prevalence in traditional medicine. However, as the authors suggested, a deeper exploration into the phytochemical and pharmacological aspects was warranted to unravel the compounds responsible for the plant’s medicinal properties [19]. This call for further investigation has since spurred a plethora of research dedicated to unravelling the chemical composition and pharmacological activities of R. communis extracts and isolated compounds.
This literature review aims to unravel the complexities of R. communis, comprising its botanical features, medicinal significance, chemical composition, and pharmacological activities. By examining the diverse studies that span traditional medicinal uses, industrial applications, and innovative biotechnological research, the aim is to provide a holistic understanding of this enigmatic plant. Through synthesising the existing knowledge, this review seeks to illuminate the pathways for future research, fostering a deeperappreciation for the multifaceted nature of R. communis and its pivotal role in agriculture, medicine, and industry.
2. Methodology
This review was carried out by systematically searching the literature in scientific databases such as PubMed, Scopus, ScienceDirect, and Google Scholar. The keywords used for the search were “Ricinus communis”, “ricin toxicity”, “chemical composition”, “pharmacological activity”, “traditional uses”, “industrial applications,” and “botanical features.” Boolean operators “AND” and “OR” were used to restrict the searches. The papers were chosen based on relevance, recency (2010–2023), and scientific merit. Duplicate studies and articles that were not in the English language were excluded.
Following the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) standards, a systematic literature search was conducted to find papers relevant to R. communis. The search covered publications from 1980 to 2024, ensuring a comprehensive historical and contemporary overview of the scientific discourse on the species. The identification phase involved searching on four major electronic databases, namely, PubMed (n = 450), Scopus (n = 350), Web of Science (n = 300), and Google Scholar (n = 150), resulting in 1250 records. An initial screening process removed 200 duplicate entries and 180 studies that did not directly pertain to R. communis, leaving 620 unique records for title and abstract screening. The full texts of 620 articles were subsequently evaluated for eligibility. A total of 161 studies were excluded for the following reasons: irrelevance to the research question (n = 110), inappropriate study design (n = 45), and language limitations (articles not published in English; n = 6).
Ultimately, 129 studies met all inclusion criteria and were incorporated into the qualitative synthesis. These studies form the empirical and conceptual foundation of this literature review and are represented in the accompanying PRISMA flow diagram in Figure 1 below.
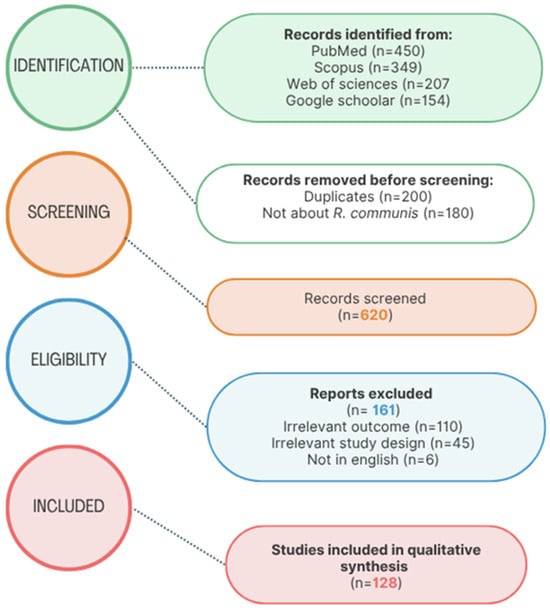
Figure 1.
PRISMA flow diagram illustrating the systematic process of identification, screening, eligibility assessment, and inclusion of studies in the literature review.
3. Taxonomy and Botanical Features
3.1. Euphorbiaceae Family
According to Rahman et al. [20], the Euphorbiaceae family, commonly called the spurge family, is a diversified collection of plants with a wide range of species that show exceptional adaptation across numerous ecological niches. The family’s morphological diversity, which ranges from tiny herbs to enormous trees, reflects this adaptability and illustrates the family’s evolutionary flexibility [20]. One of the most extensive and varied families of flowering plants, the Euphorbiaceae family, usually referred to as the spurge family, is widely distributed throughout the tropics and comprises more than 300 genera and 8000 species [21]. The Euphorbiaceae family is well known for its ethnomedicinal applications and for producing bioactive diterpenoids, which have been linked to the biological activity of numerous species [22]. A 5/7/6-tricyclic ring system with poly-hydroxyl groups characterises the naturally occurring daphnane diterpenoids, which are primarily found in plants belonging to the Thymelaeaceae and Euphorbiaceae families [22]. Certain unique types also possess a distinctive orthoester motif triaxially connected at carbon positions [23].
A few of the 6300 species and 219 genera in the Euphorbiaceae family are native to Brazil and are found in tropical and subtropical climates [24]. Moreover, with 6745 species and 218 genera, the Euphorbiaceae family of angiosperms is one of the most taxonomically complicated and varied [25]. The family is renowned for its abundant rubber, oil, timber, starch, medicines, and other plant items vital to the economy [26]. Naki et al. [27] further mentioned that the Euphorbiaceae family is widely distributed throughout Indonesia and has long been utilised experimentally in medicine.
The Euphorbiaceae family is recognised as the most prevalent in some forest regions, such as the Congo Basin and the Amazon Rainforest [28]. Rich and varied flora, including Manihot esculenta (cassava) and Jatropha curcas (Barbados nut), which are abundant in both small-scale mined and unmined areas, are further characteristics of the Euphorbiaceae family that attest to its adaptation and resilience [29]. Furthermore, a few of the Euphorbiaceae family members are well known for producing latex, a liquid that acts as a defence against pests and herbivores [30]. One prominent example is the rubber tree (Hevea brasiliensis), which yields economically valuable latex in natural rubber manufacturing [20]. The family also includes simple but highly variable leaves, and small, usually unisexual or bisexual flowers frequently gathered in inflorescences [31]. In contrast to many other plant families, the Euphorbiaceae have structures in genera such as Euphorbia called cyathia, depicted in Figure 2, which are composites of several flowers that resemble a single flower [32]. According to Lopes et al. [33], the fruits of Euphorbiaceae plants are often capsules that can rupture and release seeds over a long distance, providing a broader seed dispersion for the species’ survival and propagation.
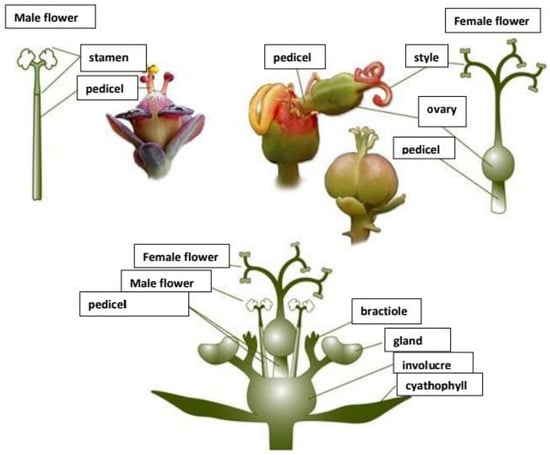
Figure 2.
Diagrammatic representation of Cyanthia of the genera Euphorbia [34].
3.1.1. Taxonomy of R. communis
According to Chouhan et al. [35], the castor oil plant is classified as follows: Kingdom: Plantae, Phylum: Spermatophyta, Subphylum: Angiospermae, Class: Dicotyledonae, Order: Euphorbiales, Family: Euphorbiaceae, Genus: Ricinus, Species: communis.
3.1.2. Botanical Features of Ricinus communis
Leaves
The leaves of R. communis have a firm texture and glossy green hue and are relatively large, as seen in Figure 3a [36]. These leaves can grow to a diameter of 60 centimetres and are deeply split, with palmately lobed surfaces [36]. The ornamental attractiveness of the leaves is attributed to their usual five to eleven-pointed lobes that radiate from a central point and have serrated borders [37]. According to Suurbaar et al. [4], the veins of the leaves are visible and run outward from the central midrib to the tips of the lobes, where they aid in the movement of nutrients and provide structural support. The leaves can range from vivid green to deep red, depending on the anthocyanin pigments present [38]. As the castor oil plant leaves develop, they change from a dark reddish-purple or bronze shade to a dark green one, as seen in Figure 3b [38]. The observed shades in the leaves are influenced by the different stages of their development and are caused by the variable levels of anthocyanin colouring present in the leaves [38].
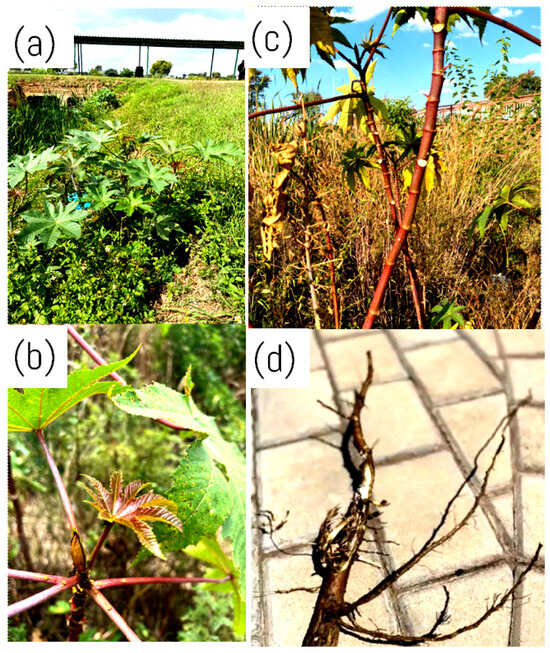
Figure 3.
Different Ricinus communis plant parts growing wild in its natural habitat at the Sefako Makgatho Health Sciences University campus (25.6192° S, 28.0161° E). (a) Shrub; (b) stem; (c) young leaf; (d) roots. Image captured by T.P. Ramothloa.
Stem
Ricinus communis has a strong stem and smooth surface, essential for maintaining the plant’s overall structure [31]. The stem grows vertically and branches into a distinct structure that supports the plant’s stability and resilience in various environmental circumstances [31]. According to the literature, R. communis has a robust, erect, and succulent stem that can grow up to 1–2 m in height [39]. The stem is typically smooth, hairless, and light green, but some cultivars may have reddish or purple stems due to anthocyanin pigments depicted in Figure 3c [40]. These anthocyanin pigments are water-soluble pigments belonging to the flavonoid class of compounds responsible for the red, purple, and blue hues in various fruits, flowers, and plant stems [41]. According to Li et al. [42], these plant pigments have several purposes, such as attracting pollinators, protecting plants from UV rays, and fending off herbivores. The stem has prominent leaf scars and is covered by a waxy cuticle with a white, milky sap inside, also known as latex [43]. It is hollow inside, with a central pith surrounded by a ring of vascular bundles composed of xylem and phloem tissues that transport water and nutrients between the roots and the rest of the plant [44].
Roots
Ricinus communis roots are thick and fleshy and can grow up to 2 m deep into the ground [35]. The roots primarily comprise principal and secondary roots, with some adventitious roots emerging from the stem [45]. The primary roots are thick and gradually taper, forming a taproot system that aids the plant’s anchoring and access to profound soil moisture [35]. Secondary roots are thin and fibrous, branching out laterally from the primary roots to form a dense network that absorbs moisture and nutrients from the soil [45]. The roots have a thin layer of root hairs that maximise the surface area for moisture and nutrient absorption, depicted in Figure 3d [35].
Flowers and Inflorescences
Ricinus communis has a terminal panicle-like inflorescence of closely spaced clusters of tiny, monoecious flowers [31]. On the same plant, R. communis yields both male and female flowers [46]. However, the female flowers are usually at the top of the inflorescence, and the male flowers are at the bottom [46]. The flowers are produced in terminal racemes or panicles, including male and female ones [47]. The male flowers are relatively large and greenish, with a three-lobed calyx, a three-lobed ovary, and a single style with three stigmas, depicted in Figure 4a [46]. The female flowers are small and reddish, with a five-lobed calyx, a five-petaled corolla, and numerous stamens depicted in Figure 4b [47]. Since the flowers are protandrous, the male flowers reach maturity before the female flowers, which assists in cross-pollination [46]. This unusual flower arrangement plays a vital role in developing recognisable castor oil seeds and aiding the plant’s reproductive strategy [46].
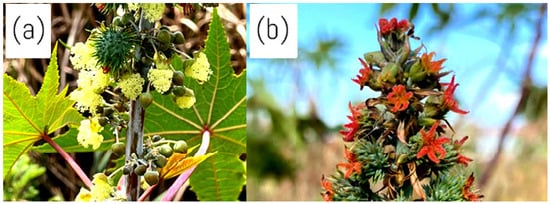
Figure 4.
Ricinus communis flowers from a plant in its natural habitat at Sefako Makgatho Health Sciences University. (a) The male flower. (b) female flower. Image captured by T.P. Ramothloa.
Seeds
Ricinus communis seeds have complex morphological characteristics that add to their uniqueness and ecological significance [48]. The seeds are firm and glossy, with colours ranging from light brown to dark reddish-brown, and they are enclosed in a spiky, spherical fruit capsule [49]. These seeds have an oval shape and a smooth, glossy surface; they are relatively large, usually ranging from one to two centimetres [1]. Their rigid and resilient exterior integument acts as a buffer, preventing outside forces and environmental stressors from wounding the developing plant inside [50]. When examined more closely, the seeds exhibit distinctive marks and carvings that enhance their visual attractiveness in addition to complex patterns and textures (Figure 5) [48]. According to Herawati et al. [51], the seeds’ surface may display detailed differences in texture that are indicative of both underlying genetic variety and environmental factors. Additionally, because of their sturdy construction and appealing look, which attract a variety of seed dispersers like birds and mammals, the seeds serve a crucial ecological function in seed dispersal mechanisms [52].

Figure 5.
Variations in size and colour of Ricinus communis seeds [53].
3.1.3. Geographical Distribution of Ricinus communis
Ricinus communis is native to the Indian subcontinent, Eastern Africa, and the Southeast Mediterranean Basin [54]. However, it has been widely cultivated and naturalized in many other regions, including tropical and subtropical areas such as the Americas and China, and throughout all the continents except Antarctica [31]. Its seeds were valued for their industrial and therapeutic qualities in ancient civilisations [14]. Rana et al. [14] shed light on the plant’s traditional usage and pharmaceutical potential, highlighting its complex value. Due to the plant’s adaptation to warm, sunny climates and well-drained soil, it has historically been cultivated in these areas, which has helped it become a profitable crop [31]. Through various factors, including human trade and accidental seed spread, R. communis’s global distribution surpassed its original range [31]. The distribution of R. communis in South Africa is influenced by various factors, including its historical origins, ecological adaptability, and human activities [55]. The plant’s distribution in South Africa is closely linked to its adaptability to diverse environmental conditions, including well-drained soils and warm temperatures [56]. The plant’s historical uses in traditional medicine and its economic significance have also contributed to its intentional cultivation and spread within the country [57]. Furthermore, R. communis in South Africa raises concerns about its potential invasiveness and ecological impacts on native vegetation and ecosystems [58].
South Africa’s provinces show a varied distribution of R. communis as it can survive in various environments, from dry inland regions to coastal locations [59]. It is found in the fynbos biome of the Western Cape, especially in places with well-drained soils and moderate rainfall [59]. It also spreads to the Eastern Cape, taking over scrublands and grasslands [60]. This species, adapted to extreme environmental circumstances, is also common in semi-desert parts of the Northern Cape [61]. Moreover, it is also found in disturbed places like roadside ditches and uninhabited fields in Gauteng [62]. Its frequent occurrence in savanna and forest habitats in the provinces of Limpopo and Mpumalanga demonstrates its capacity to flourish in various ecosystems [59,63]. Additionally, it exists in the KwaZulu-Natal (KZN) province in the riverbank and coastal regions where there is relatively more precipitation [59,63]. This distribution pattern highlights the ecological adaptability of R. communis, which enables it to endure and proliferate in various South African settings [64]. Table 1 summarises the distribution of R. communis globally, emphasising the climate and type of soil found in the area, while Table 2 summarises the distribution of R. communis in South Africa, also emphasising the climate and the soil type found in the area, as well as the habitat of the plant.

Table 1.
Geographical distribution of Ricinus communis globally.

Table 2.
Distribution of Ricinus Communis across provinces of South Africa based on habitat, weather/climate conditions, and soil type.
4. Traditional Applications
Ricinus communis has many uses that demonstrate the symbiotic interaction between humans and the natural environment and show its historical significance and ongoing relevance in modern contexts [20]. The customary applications of R. communis are profoundly embedded throughout a wide range of global civilisations [14]. The seeds have long been used mainly for castor oil production, and then the oil was used as a strong laxative that often assisted in relieving constipation and encouraging regular bowel movements [76]. According to Suurbaar et al. [4], castor oil has also been included in several topical skincare formulations due to its moisturising and antibacterial qualities, which make it useful in the treatment of dermatological diseases like dermatitis, eczema, and acne. Castor oil is highly valued for its skin-cleansing and rejuvenating properties in traditional medical systems such as Ayurveda and traditional Chinese medicine; hence, it is commonly used in herbal medicines and cosmetic goods [77].
Additionally, various parts of the plant offer specific therapeutic benefits. The leaves act as an insecticide against aphids, rust mites, mosquitoes, and whiteflies, and are used to increase milk production in cattle and nursing mothers [78]. They also serve medicinal purposes, such as relieving stomach aches and treating eye infections and narcotic poisoning [78]. Using hot water extracts, the roots are valued for their efficacy in treating toothaches and jaundice [78]. Ricinus communis is a multipurpose plant in agricultural settings, as its allelopathic properties help deter pests and promote biodiversity in agroecosystems [3]. Its rapid growth and resilience make it suitable for soil conservation efforts [10]. Additionally, R. communis is culturally significant in various societies’ folklore, mythology, and traditional rituals, as it is associated with purification and protection against evil spirits [11].
In certain regions of South America, castor oil is traditionally used as an antiparasitic drug, primarily for the expulsion of intestinal worms. Brazilian and Colombian ethnomedicinal applications involve oral intake of small amounts of warm castor oil to cure helminthic infections, with activity noted against Strongyloides, tapeworms, and roundworms [79]. This folk use is supported by in vitro pharmacological studies, which showed that castor oil is cysticidal against Entamoeba histolytica and other gastrointestinal parasites [80]. Table 3 summarises the traditional uses of R. communis for treating various diseases or ailments.

Table 3.
Traditional uses of Ricinus communis for the treatment of different ailments and diseases.
5. Chemical Composition
The chemical composition provides information about the molecular makeup of an item or organism by describing the types and arrangements of chemical elements and compounds that are present [3]. Conversely, active constituents are substances found in a natural product or organism with pharmacological or physiological effects [83]. R. communis is a chemically complex plant, with each anatomical part consisting of distinct sets of bioactive constituents that add to its overall pharmacological, therapeutic, and toxicological properties [3]. Phytochemical constituents found in the leaves, seeds, roots, stems, and flowers of the plant not only explain its medicinal importance but also identify its ecological standing [2].
The leaves of R. communis have drawn extensive studies on their phytochemical constituents, revealing the occurrence of prominent secondary metabolites such as tannins, saponins, alkaloids, and flavonoids [84]. These phytochemicals are commonly renowned for their medicinal significance, particularly due to their antioxidant, antimicrobial, and anti-inflammatory activities [85]. Phytochemical analysis of the leaf extracts was consistent with the presence of selected phenolic acids, such as gallic acid, ellagic acid, and quercetin, and flavonoids, such as rutin and epicatechin [86]. The co-presence of these bioactive compounds validates the therapeutic significance of the leaves to traditional and modern phytotherapeutic practices.
Similarly, the seeds of R. Communis possess a stable and intricate chemical makeup that serves as the basis for most of the plant’s pharmaceutical and industrial uses. Alkaloids like ricinine, flavonoids, saponins, and phenolic acids are found in the seeds [11,86]. These phytochemicals are not just implicated in the well-known anti-inflammatory and anticancer activities of the plant but also its toxicity, as exemplified by the ricin content, a highly toxic protein toxin that has also been extracted from the seeds [2]. This two-sidedness of the compounds makes the seed extracts a subject of considerable interest in both pharmacological and toxicological research. Besides the leaves and seeds, the roots of R. communis contain numerous bioactive components. Among them is lupeol, a triterpenoid with well-established anti-inflammatory and anticancer activities [5]. The roots are also discovered to contain indole-3-acetic acid, a plant growth regulator that participates in the biosynthesis of other secondary metabolites, which functions to enhance the plant’s versatility and provide potential drug applications [5]. While not as well-documented as the seeds or leaves, these compounds in the root contribute to the plant’s overall medicinal value.
The R. communis chemical profile is also found in stems and flowers, though the studies are less extensive. Available data report the presence of tannins, which have antioxidant activity, and β-caryophyllene, a sesquiterpenoid with anti-inflammatory activity and plant defence properties [11]. Also, ricinine, present in seeds, was found to be present in stems, playing a role in plant defence chemicals [11].
The R. communis flowers, although less studied, are also associated with their pharmacological activity. Floral tissues were found to possess flavonoids such as rutin, which are both recognised for their vascular protective and antioxidant activity [3]. This suggests that even the lesser studied components of the plant may be of therapeutic significance that should be investigated. Collectively, R. communis shows a chemically intricate and pharmacologically diverse profile for all its components. Figure 6 depicts the chemical structures of some of the active constituents found in the various parts of the plant. Table 4 summarises the chemical compounds in different plant parts and their most abundant active constituents.
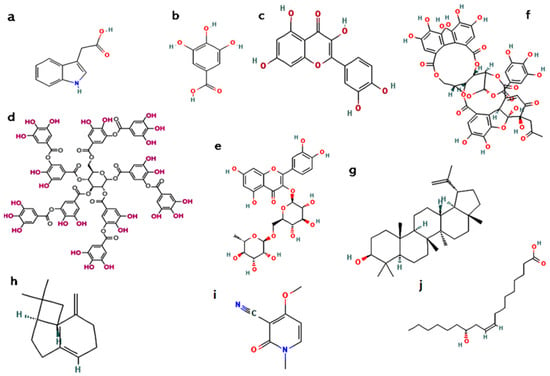
Figure 6.
Chemical structures of active constituents found in various parts of the Ricinus communis plant: (a) indole-3-acetic acid; (b) gallic acid; (c) quercetin; (d) tannic acid, (e) rutin, (f) ellagitannins; (g) lupeol; (h) β-caryophyllene; (i) ricinine; (j) ricinoleic acid.

Table 4.
Chemical compounds and active constituents found in different plant parts of Ricinus communis.
6. Pharmacological Activity
Pharmacological activities include a variety of actions that substances, especially medications or other medical compounds, take on biological systems [88]. These activities may have therapeutic benefits, such as reducing symptoms, preventing illness, or enhancing well-being, in addition to possible negative consequences or reactions [88]. When discussing medicinal plants, the term “pharmacological activities” refers to the range of biological effects that these plants’ bioactive chemicals display [76]. Ricinus communis is well known for its pharmacological properties, mostly linked to its seeds [89]. Ricinus communis seeds yield castor oil, traditionally used for its therapeutic benefits, and ricin, a poisonous protein [89].
6.1. Antioxidant Activity
The discovery of the antioxidant activity of R. communis has been intensively improved by several investigations [90,91]. To evaluate the antioxidant potential of R. communis roots, various solvent extracts (methanol, ethanol, ethyl acetate, n-hexane, chloroform, n-butanol, and aqueous) were prepared and analysed using standard antioxidant assays. Ahmed and Iqbal [90] investigated these extracts for their ability to scavenge DPPH radicals and measured their total phenolic content (TPC) and total flavonoid content (TFC). The results indicated significant antioxidant activity across all extracts, with ethyl acetate and chloroform showing the highest DPPH scavenging activity at 41%. Additionally, the TPC analysis revealed notable activity in aqueous, n-butanol, and ethyl acetate extracts, with values of 131 mg/mL, 127 mg/mL, and 117 mg/mL gallic acid equivalent, respectively. Regarding TFC, methanol and aqueous extracts demonstrated the highest activity at 32 µg/mL each, compared to 38 µg/mL in the control. These findings highlight R. communis roots as a powerful antioxidant source, implying that they could be useful in medicinal applications.
In a related study, Ihekuna et al. [91] investigated the antioxidant activity of ethanolic extracts from R. communis seeds and leaves. In both in vivo and in vitro assessments, R. communis extracts displayed potent antioxidant activity, with IC50 values of 77.17 ± 0.36 and 69.33 ± 0.26 μg/mL for seed and leaf extracts in DPPH assays and 62.86 ± 0.54 and 62.39 ± 0.18 μg/mL in nitric oxide radical inhibition assays, respectively. Treatment with these extracts boosted catalase and superoxide dismutase (SOD) levels in the liver and kidneys, suggesting a protective effect against oxidative stress. According to their findings, the ethanolic extracts had a substantial amount of antioxidant activity, suggesting the presence of bioactive substances that could reduce oxidative damage and neutralise free radicals.
Tuyen et al. [85] investigated the antioxidant qualities of R. communis leaves. Using spectrophotometry and chromatography, the researchers found many phenolic chemicals in the leaves, such as flavanol glycosides, aromatic acids, and coumarinolignan. The compounds identified included cleomiscosin A, kaempferol-3-O-β-D-glucopyranoside, kaempferol-3-O-β-D-xylopyranoside, gallic acid, and vanillic acid. Cleomiscosin A was discovered for the first time in the Ricinus genus and had low DPPH radical scavenging activity (SC50 = 403.23 μg/mL). The presence of these compounds in R. communis leaves emphasises the plant’s ability to scavenge free radicals and guard against oxidative stress, as these compounds are known for their antioxidant properties.
The studies mentioned above used solvents of varying polarity, such as ethyl acetate, chloroform, methanol, and ethanol, which likely influenced the antioxidant activities observed by selectively extracting different phytochemicals. This methodological variation suggests a lack of standardization that complicates direct comparison between studies, though it also highlights the importance of solvent choice in maximizing bioactive compound yield. This variation in solvent polarity is crucial, as more polar solvents (like methanol and aqueous) are more effective at extracting hydrophilic compounds such as phenolics and flavonoids, while less polar solvents (like chloroform and hexane) may extract non-polar antioxidant constituents. By linking solvent polarity to the type of compounds extracted, it becomes clearer why different extracts demonstrated varying levels of antioxidant activity. While Ahmed and Iqbal [90] emphasised the correlation between total phenolic and flavonoid content and antioxidant activity, Tuyen et al. [85] identified specific phenolic compounds like cleomiscosin A with low activity, indicating that not all phenolics contribute equally to antioxidant potential. Nonetheless, all three studies consistently demonstrate that R. communis exhibits noteworthy antioxidant activity, supporting its potential application in oxidative stress-related therapies.
6.2. Antifungal/Antimicrobial Activity
Ricinus communis, known for its diverse pharmacological properties, has strong antifungal activity since its effectiveness against a range of fungal species has been demonstrated by research on its antifungal properties [92]. The antifungal potential of R. communis was investigated by Dikhoba et al. [92], who showed that the plant’s leaf extract was effective against fungi, with the minimum inhibitory concentration (MIC) ranging from 0.08 to 2.5 mg/mL, with R. communis leaf extract, among others, exhibiting promising antifungal activity. Additionally, R. communis had a MIC value of 0.08 mg/mL against Fusarium verticilloides, comparable to the antibiotic Amphoteracin B. Furthermore, the overall efficacy of R. communis against Aspergillus ochraceous was notable, indicating its potency in preventing fungal growth. To ascertain the MIC values or the concentration at which the growth of the fungal pathogen is stopped, the investigators used standard microbiological techniques.
Moreover, Suurbaar et al. [4] used assays such as disc diffusion, MIC, minimal lethal concentration (MLC), and minimum fungicidal concentration (MFC) methods to study the antibacterial and antifungal properties of R. communis leaf extracts. The solvent extracts from R. communis’s leaves displayed bacteriostatic and bactericidal effects against the tested organisms, with MIC values ranging from 3.13 to 25.0 mg/mL and MBCs from 200 to 400 mg/mL. For Candida albicans, MFCs fell between 200 and 400 mg/L, indicating potential antifungal activity. These findings affirm the antimicrobial properties of R. communis’s leaves and highlight the extractability of biologically relevant phytochemicals using aqueous, methanol, and ethanol solvents from its leaves.
Additionally, bimetallic nanoparticles made using R. communis leaf extracts have been shown to have antibacterial and antifungal activities by López-Ubaldo et al. [93]. The antibacterial activity of the nanoparticles was assessed using microbiological assays. The synthesised nanoparticles showed promising antibacterial activity against Staphylococcus aureus, Escherichia coli, and Aspergillus niger, with MIC concentrations ranging from 1.25 to 2.45 μg and MLC allowances from 2.45 to 9.81 μg.
Additionally, R. communis leaf extracts and fractions were studied by Istaufa et al. [94] for their antibacterial properties against various diseases, including tuberculosis (TB), which is caused by the bacteria Mycobacterium tuberculosis. The ethanolic extract showed the most promising results, completely inhibiting bacterial growth at 200 μg/mL, with an average colony count reduction from 20.33 at 100 μg/mL to 0 at 200 μg/mL. Other solvents, such as methanol and acetone, also exhibited inhibitory effects, with the methanol extract inhibiting growth at 100 μg/mL and reducing colonies from 17.33 at 100 μg/mL to 0 at 150 μg/mL. The acetone extracts reduced colony counts from 55.66 at 100 μg/mL to 0 at 200 μg/mL. The minimum inhibitory levels of various extracts were assessed, with n-hexane showing activity at 10,000 μg/mL, ethyl acetate at 40,000 μg/mL, and chloroform at 5000 μg/mL. These findings suggest that Ricinus communis L. leaf extracts have significant potential as a supplementary treatment for tuberculosis, effectively inhibiting Mycobacterium tuberculosis growth at various concentrations and solvents.
The different studies mentioned above used a variety of solvents, ranging from ethanol and methanol to chloroform and n-hexane, and techniques, like MIC, MLC, and disc diffusion, to assess the antifungal and antimicrobial properties of R. communis, with each method and solvent choice influencing the observed potency. While Suurbaar et al. [4] demonstrated strong antifungal activity using plant extracts, López-Ubaldo et al. [93] showed that the synthesis of bimetallic nanoparticles from R. communis could further enhance antimicrobial efficacy, suggesting a promising nanotechnology-based approach. Across all studies, R. communis consistently exhibited notable antifungal activity against pathogens like Fusarium verticilloides, Aspergillus niger, and Candida albicans, reinforcing its potential as a natural antifungal agent.
6.3. Antidiabetic Activity
Ricinus communis may have antidiabetic properties; recent research has explored this possibility, providing insight into the plant’s possible benefits [10]. In a study conducted by Benariba et al. [95], the objective was to ascertain the levels of total phenolic and flavonoid compounds in hydro-acetone extracts obtained from the roots of R. communis and the aerial parts of Teucrium polium. Additionally, the study aimed to evaluate these extracts’ antioxidant and antidiabetic properties using DPPH, FRAP, and α-amylase activity assays. Moreover, T. polium exhibited greater efficiency in inhibiting the α-amylase enzyme (EC50 = 924 ± 0.19 µg/mL) compared to R. communis extract (EC50 = 1300 ± 0.03 µg/mL) in the α-amylase activity assay. The study highlights the potential antidiabetic activity of R. communis extract, particularly its ability to inhibit α-amylase activity. The findings suggest that the hydro-acetone extract from the roots of R. communis may offer promising therapeutic benefits for managing diabetes or related metabolic disorders.
Additionally, Hajrah et al. [10] evaluated the antidiabetic effect of R. communis leaf extract using gene expression profiling in MCF7 cells. The results of the study showed that treatment with the extract at concentrations of 10 μg/mL and 50 μg/mL significantly increased the expression of PPAR-γ, a key regulator of glucose metabolism and fatty acid storage, by 2.5 and 3.2-fold, respectively, and PPARGC1A (PGC-1α), which interacts with PPAR-γ to regulate genes related to energy metabolism, by 1.8 and 2.4-fold, respectively. Furthermore, DPP4 expression was downregulated by 2.1 and 2.7-fold, respectively, in the extract. DPP4 is implicated in the breakdown of incretins, including GLP-1, which increases insulin production and lowers blood glucose levels. These results imply that phytochemicals included in R. communis leaf extract may be able to better manage diabetes by enhancing insulin resistance, lowering serum glucose levels, and enhancing glucose uptake in muscle and adipose tissue.
Moreover, the study conducted by Dayyih et al. [96] and colleagues investigated the influence of castor oil (CAO) on glycated haemoglobin (HbA1c) levels in rats with induced type 2 diabetes mellitus (T2D), particularly in comparison to the Sodium–Glucose Cotransporter 2 (SGLT2) inhibitor empagliflozin. Their research aimed to shed light on CAO’s potential as a therapeutic agent for diabetes management. HbA1c was utilised as a long-term glycaemic regulation predictor, with 6.5% or above indicating diabetes, 5.7–6.4% suggesting pre-diabetes, and less than 5.7% indicating health. Rats were divided into eight groups, including healthy and diabetic, and treated with CAO for various durations. Significant decreases in HbA1c were observed from the ninth week onwards in healthy and diabetic rats treated with CAO compared to non-treated groups (p < 0.05), indicating a reduction in blood sugar levels. Specifically, a decrease in HbA1c values was noted in healthy rats given CAO daily, whereas HbA1c levels increased in healthy rats without drug treatment. However, CAO alone did not achieve the same efficacy in lowering HbA1c levels as empagliflozin, a diabetes medication. Empagliflozin-treated rats showed a more significant decrease in HbA1c levels than CAO-treated rats, with an earlier onset of effect observed in the empagliflozin group (p < 0.05). The results of their study revealed a noteworthy finding regarding the impact of CAO on glycaemic control. This disparity suggests a differential effect of CAO in diabetic conditions, possibly due to the complex pathophysiology of diabetes. Despite its traditional use as a laxative, the study indicates that CAO may possess some antidiabetic activity, albeit less potent than conventional medications like empagliflozin.
The studies mentioned above investigating the antidiabetic potential of R. communis used a range of methodologies, including biochemical assays, gene expression profiling, and animal models with differing extract types and target mechanisms, which may account for variations in observed efficacy [10,95,96]. While Benariba et al. [95] demonstrated moderate α-amylase inhibition through hydro-acetone root extracts, Hajrah et al. [9] highlighted the molecular effects of leaf extract, showing the upregulation of genes like PPAR-γ and the downregulation of DPP4 involved in glucose metabolism. Dayyih et al. [96], using in vivo models, showed that castor oil led to modest reductions in HbA1c levels. However, it was less effective than standard SGLT2 inhibitors, suggesting that R. communis may contribute to glycaemic control but likely serves best as a complementary rather than primary therapy. Overall, the studies collectively indicate that R. communis possesses antidiabetic properties through enzymatic inhibition and gene-regulatory pathways, with potential for use in supportive diabetes management.
6.4. Anti-Inflammatory Activity
The anti-inflammatory qualities of R. communis have been highlighted by a recent study, suggesting that it may be helpful as a treatment for inflammatory ailments. To clarify the anti-inflammatory properties of R. communis leaves, Lee et al. [97] investigated how they might effectively activate Nrf2 to mitigate the effects of dexamethasone-induced muscular atrophy. The study evaluated muscle atrophy and Nrf2 activation using in vivo and in vitro models, offering mechanistic insights into the anti-inflammatory qualities of R. communis leaves. Rutin, identified as the predominant compound in R. communis with a content of 159.5 mg/100 g dry weight of extract, effectively prevented DEX-induced myotube atrophy in vitro, reducing muscle atrophy-related gene expression, proteasome activity, and intracellular reactive oxygen species (ROS) accumulation while improving mitochondrial function.
Additionally, R. communis leaf extract’s anti-inflammatory properties were also investigated by Hajrah et al. [10] using gene expression profiling in MCF7 cells. The results of the investigation showed that the TNFAIP6 gene (tumour necrosis factor-inducible gene 6) was upregulated by 2.3- and 3.1-fold, respectively, after treatment with the extract at doses of 10 μg/mL and 50 μg/mL. By reducing neutrophil infiltration in illness models, TNFAIP6, often referred to as TNF-stimulated gene 6 (TSG-6), plays a critical role in mediating anti-inflammatory and protective effects. The gene’s product aids in the anti-inflammatory response by binding to and interacting with several chemokines. These results suggest that the leaf extract of R. communis possesses the capacity to produce notable anti-inflammatory effects, which could potentially augment its medicinal advantages.
Furthermore, R. communis and Withania somnifera hydroalcoholic extracts were evaluated by Hussain et al. [98] for their anti-inflammatory properties. They showed potent antioxidant and anti-inflammatory properties both in vitro and in vivo. The study assessed the antioxidant and anti-inflammatory activities of R. communis leaf extract and W. somnifera extract. Both extracts demonstrated the dose-dependent inhibition of oedema in inflammation models. Ricinus communis extracts showed the maximum inhibition of ear oedema (51.49 ± 2.54%) and paw oedema (46.62 ± 8.98%) in comparison to W. somnifera, with R. communis also significantly inhibiting carrageenan-induced paw oedema (72.88 ± 13.79%). These effects were attributed to the extracts’ high phenolic and flavonoid contents. In comparison, R. communis displayed more promising antioxidant and anti-inflammatory effects than W. somnifera.
The studies mentioned above explored the anti-inflammatory activity of R. communis employing diverse models and analytical approaches, such as gene expression profiling, muscle atrophy assays, and inflammation-induced oedema models, leading to a broad understanding of its therapeutic potential [10,98]. Lee et al. [97] demonstrated that rutin-rich leaf extracts activated Nrf2 and countered dexamethasone-induced muscle atrophy by reducing oxidative stress and proteasome activity, while Hajrah et al. [10] reported the upregulation of the TNFAIP6 gene, which plays a central role in suppressing inflammation. Hussain et al. [98] further supported these findings by showing that R. communis extracts significantly reduced oedema in animal models, outperforming W. somnifera in anti-inflammatory and antioxidant effects. Overall, these studies confirm that R. communis exhibits strong anti-inflammatory activity through both gene-regulatory mechanisms and the phenolic compound-mediated inhibition of inflammation.
6.5. Anti-Fertility Activity
A study by McNeil et al. [99] investigated the short-term and long-term spermatogenic effects of a small R. communis seed variety. The n-hexane extract of the seeds was given intraperitoneally to male rats, and semen analysis was performed regularly. Spermatogenesis and sperm motility were shown to be suppressed for up to six weeks following therapy, according to the data. Microscopic analysis revealed normal appearance, liquefaction, and consistency of semen samples in all groups. However, within 72 h of treatment with the extract from R. communis seeds, semen parameters were significantly suppressed. The percentage of normal sperm forms decreased by 50% (from 80% in controls to 40% in treated groups), and motility decreased by 56% (compared to 88% in controls). The semen count was also markedly reduced to 49 × 106 (compared to 99.5 × 106 in controls). Over the following weeks, there was a gradual improvement in mean normal forms and motility, reaching 60% and 65%, respectively, by the sixth week, while semen count began recovering by the eighth week. These findings indicated that Ricinus communis seed extract had a reversible impact on semen parameters, suggesting its potential as a male contraceptive agent that requires further investigation. Thus, R. communis is shown to be a potent yet reversible anti-spermatogenic agent with notable anti-motility qualities, showing potential as a readily available and reasonably priced male contraceptive drug because of the plant’s origin.
A study by Desouky et al. [100] evaluated the impact of Eucalyptus oil and R. communis seed extract on the reproductive biology of Theba pisana snails using assays including egg laying capacity, hatching rates, histopathological analysis of hermaphrodite glands, antioxidant enzyme activities, and hormonal analysis. Results showed significant reductions in egg-laying capacity (p < 0.05) and hatching rates (p < 0.01) with both extracts, particularly with the Ricinus extract displaying a more pronounced effect. Additionally, histopathological analysis revealed disruption in the hermaphrodite glands of treated snails, indicating interference with spermatogenesis and oogenesis. Hormonal analysis demonstrated disturbances in reproductive hormones, including increased levels of luteinising hormone (p < 0.05), follicle-stimulating hormone (p < 0.01), and testosterone (p < 0.001). These findings suggest that both Eucalyptus oil and Ricinus extract adversely affect the reproductive physiology of T. pisana, with Ricinus extract exerting more substantial anti-fertility effects.
Another study by Iornmube et al. [101] investigated the contraceptive efficacy of R. communis minor (RICOM-1013-J) through oral administration in women volunteers. The results showed that a dosage range of 2.5–2.7 gm of RICOM-1013-J conferred protection against conception for 12 months. Sexual activity frequency was 2–3 times per week, and menstrual flow remained within typical limits throughout the study period. Examination of hormone profiles revealed notable differences between the treatment and control groups. Specifically, individuals in the treatment group exhibited significantly elevated levels of prolactin compared to those in the control group. Women administered RICOM-1013-J showed significantly elevated prolactin levels compared to the control group (mean: 1154.4 mIU/L vs. 171.5 mIU/L, p < 0.01). In contrast, luteinising hormone (LH) and follicle-stimulating hormone (FSH) levels varied across the groups (mean LH: 2.25 IU/L vs. 1.47 IU/L, mean FSH: 3.14 IU/L vs. 3.67 IU/L, p > 0.05). The findings suggest that RICOM-1013-J may induce alterations in hormone levels associated with contraceptive effects, warranting further investigation into its mechanism of action and long-term effects on hormonal regulation.
The above studies explored the anti-fertility effects of R. communis utilising diverse models ranging from mammalian systems to invertebrate systems and human trials demonstrating both physiological and hormonal mechanisms underlying its contraceptive potential [99,100,101]. McNeil et al. [99] showed that n-hexane seed extracts significantly suppressed spermatogenesis and motility in male rats, with partial recovery observed over time, indicating reversible male contraceptive effects. Desouky et al. [100] found that R. communis extract impaired reproductive capacity in T. pisana snails through hormonal disruption and glandular damage, while Iornmube et al. [101] reported effective contraceptive outcomes in women using RICOM-1013-J, associated with significantly elevated prolactin levels. Overall, these findings indicate that R. communis exhibits potent and multi-pathway anti-fertility activity, making it a promising candidate for both male and female contraceptive development.
6.6. Anti-Hepatotoxic Activity
The hepatoprotective activity of R. communis has generated interest due to its possible therapeutic benefits for liver health. The study by Shati [102] investigated the hepatoprotective effects of R. communis against cyanide-induced liver damage. The results showed that cyanide administration led to a significant decrease in enzymatic activities: catalase (CAT) decreased from 68.6 ± 2.3 to 24.1 ± 1.8 U/mg protein, superoxide dismutase (SOD) from 12.4 ± 0.9 to 5.2 ± 0.4 U/mg protein, glutathione reductase (GSH-Red) from 3.5 ± 0.3 to 1.1 ± 0.1 U/mg protein, and glutathione peroxidase (GSH-Px) from 15.2 ± 1.2 to 5.8 ± 0.6 U/mg protein. The non-enzymatic glutathione (GSH) level decreased from 5.8 ± 0.4 to 2.3 ± 0.2 μmol/mg protein. Additionally, lipid peroxidation increased, indicated by Thiobarbituric Acid Reactive Substance (TBARS) levels rising from 1.8 ± 0.1 to 5.9 ± 0.5 nmol/mg protein. Liver function markers showed significant increases: aspartic transaminase (AST) from 45.2 ± 3.5 to 136.4 ± 10.2 U/L, alanine transferase (ALT) from 42.3 ± 3.1 to 124.7 ± 8.9 U/L, and total bilirubin from 0.9 ± 0.1 to 3.5 ± 0.3 mg/dL. Serum levels of total cholesterol increased from 120.5 ± 8.2 to 198.3 ± 12.4 mg/dL, total lipids from 5.4 ± 0.3 to 11.6 ± 0.7 g/L, and total protein from 6.2 ± 0.5 to 9.8 ± 0.6 g/dL. However, treatment with R. communis extract significantly alleviated these adverse effects, restoring enzymatic activities, reducing lipid peroxidation, and normalizing liver function markers and serum parameters. Moreover, the over-expression of genes related to oxidative stress and inflammation (P53, Bcl-2, IL-4, and IL-12) induced by cyanide toxicity was managed effectively by R. communis extract. This study concluded that R. communis extract has a promising role in treating cyanide-induced hepatotoxicity.
Additionally, Naveen et al. [103] assessed R. communis’s hepatoprotective effect in rats, showing the plant’s possible advantages for liver health. The study demonstrated the hepatoprotective qualities of R. communis by evaluating its ability to prevent liver damage using animal models and biochemical testing. The effects of aqueous extract of R. communis leaves on hepatotoxicity induced by carbon tetrachloride (CCl4) in rats were investigated. The results revealed that the administration of CCl4 led to elevated levels of aspartate aminotransferase (AST) and alanine transaminase (ALT), indicative of liver damage. However, treatment with the aqueous extract of R. communis leaves resulted in a significant decrease in AST and ALT levels compared to the standard group treated with CCl4 alone. For instance, AST levels decreased from 266.7 ± 25.68 (standard group) to 144.5 ± 12.92 (Group III) and 161.7 ± 11.92 (Group V) with treatment with 100 mg/kg and 500 mg/kg of the extract, respectively. Similarly, ALT levels decreased from 86.67 ± 4.47 (Standard group) to 30.67 ± 3.565 (Group III) and 42 ± 2.828 (Group V) with treatment with 100 mg/kg and 500 mg/kg of the extract, respectively. These findings underscore the potential hepatoprotective properties of R. communis extract against CCl4-induced liver damage.
The above-mentioned studies investigated the anti-hepatotoxic activity of R. communis employing different hepatotoxic models, cyanide-induced and carbon tetrachloride (CCl4)-induced, to assess its therapeutic potential in mitigating liver damage [102,103]. Shati [102] demonstrated that R. communis extract significantly restored antioxidant enzyme levels, reduced lipid peroxidation, and normalised liver function markers in cyanide-exposed rats, while also modulating stress-related gene expression. Similarly, Naveen et al. [103] reported that aqueous extracts of R. communis leaves markedly reduced elevated AST and ALT levels caused by CCl4, indicating protective effects on liver function. Overall, these studies affirm that R. communis exhibits strong hepatoprotective activity by counteracting oxidative stress and restoring normal liver enzyme levels.
6.7. Anticancer Activity
Ricinus communis has received considerable attention for its potential anticancer effects. Herawati et al. [104] investigated the cytotoxic effects of crude ricin (CR), derived from R. communis, on A549 lung cancer cells compared to cisplatin, a standard cancer drug. CR showed cytotoxicity against A549 cells with an IC50 value of 40.94 ppm, higher than that of cisplatin (10.98 ppm). Flow cytometry analysis revealed that CR induced apoptosis in A549 cells in a concentration-dependent manner, with a higher proportion of cells undergoing early apoptosis than necrosis. Western blot analysis confirmed that CR activated caspase-9 and caspase-3, indicating apoptotic cell death. Additionally, CR and cisplatin inhibited A549 cell migration in a concentration- and time-dependent manner. Furthermore, CR inhibited autophagy in A549 cells, as evidenced by decreased expression of LC3-II and Beclin-1 and increased expression of p62 and Atg5, suggesting a potential mechanism for its anticancer activity. These findings suggest that CR has potential as an anticancer agent against lung cancer cells, with apoptotic induction and autophagy inhibition as critical mechanisms.
Furthermore, in a study by Hajrah et al. [10], the gene expression profiling of R. communis leaf extract was investigated, shedding light on its effects on mammalian cells. MCF7 cells were subjected to sublethal doses of 10 µg/mL and 50 µg/mL of the extract, followed by RNA-Seq analysis to assess changes in gene expression. Significant alterations in gene expression were observed, with 245 upregulated genes and 134 downregulated genes identified at 10 µg/mL and 168 upregulated genes and 73 downregulated genes at 50 µg/mL, compared to control cells treated with DMSO. The study underscored dose-dependent responses to the extract, revealing a subset of genes consistently modulated across both doses. Cluster analysis further corroborated these findings, highlighting the impact of Ricinus extract on the gene expression profile of MCF7 cells. These results contribute to understanding the potential anticancer activity of Ricinus extract, suggesting its relevance as a therapeutic agent in cancer treatment.
Additionally, R. communis fruit extract could potentially possess antimetastatic potential since it has been reported by Mabasa et al. [58] to decrease breast cancer cell migration, adhesion, and invasiveness. The study investigated the impact of the fruit extract on cancer cell behaviour using cell culture and molecular biology techniques, offering insights into the fruit extract’s antimetastatic processes. The fraction exhibited significant reductions in cell viability for both BUD-8 and MCF-7 cells at concentrations between 300 and 500 µg/mL for BUD-8 and 400 to 500 µg/mL for MCF-7. Furthermore, it inhibited various metastasis-related processes, including cell migration, adhesion, invasiveness, and MMP-2 activity. Additionally, the fraction modulated the expression of essential proteins involved in metastasis and angiogenesis, suggesting its potential as an antimetastatic agent for treating malignant cancers.
Moreover, a study by Majumder et al. [11] investigated the cytotoxic effects of R. communis fruit extract (RCFE) on breast cancer cells MCF-7 and MDA-MB-231. Treatment with RCFE significantly increased cytotoxicity in both cell lines in a dose- and time-dependent manner. Specifically, 1 µg/mL of RCFE treatment induced considerable cell death in both MCF-7 and MDA-MB-231 cells. Furthermore, RCFE exhibited cytotoxic specificity against various cancer cell lines, including HER2-positive MDA-MB-453, triple-positive ZR-75-1 breast cancer cells, colon cancer cell line HT-29, and adenocarcinoma cell line A549, while showing minimal effects on regular cell lines HEK293 and mouse embryonic fibroblast (MEF) cells. Additionally, RCFE demonstrated inhibitory effects on the migration, adhesion, and invasion of MCF-7 and MDA-MB-231 cells. The extract significantly inhibited cell migration and adhesion in a dose-dependent manner, with higher concentrations exhibiting more pronounced effects; at 10 µg/mL and 50 µg/mL, RCFE significantly reduced adhesion and migration in MCF-7 cells, with 50 µg/mL almost completely inhibiting migration. RCFE treatment also led to a substantial reduction in invasion capability in both cell lines, with 50 µg/mL reducing invasion by up to 85% in MDA-MB-231. Further analysis revealed that RCFE treatment reduced the expression of metastasis-associated matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9) in MCF-7 and MDA-MB-231 cells in a concentration-dependent manner, with reductions of 48% and 64% at 50 µg/mL and 100 µg/mL, respectively. These findings suggest the potential of RCFE as a promising therapeutic agent for breast cancer treatment due to its cytotoxic effects and inhibition of metastatic properties in breast cancer cells.
Moreover, a study by Loan et al. [105] aimed to enhance the specificity of ricin for cancer treatment by conjugating/encapsulating it with DOTAP/DOPE liposomes, forming ricin–liposome complexes. These complexes were then analysed for their characteristics and evaluated for their effects on SKMEL-28 melanoma cells. The results indicated that the ricin–liposome complexes exhibited even size distribution, with an average size of approximately 340 nm. These complexes demonstrated the ability to penetrate cells via endocytosis, with ricin-liposome3 showing the highest efficacy. Importantly, ricin–liposome3 exhibited significant toxicity against SKMEL-28 cells, with the lowest IC50 (62.4 ng/mL) among the tested complexes. Furthermore, at the IC10 concentration, ricin–liposome3 induced necrosis and apoptosis in SKMEL-28 cells. Additionally, ricin–liposome3 displayed potent anticancer properties, as evidenced by its ability to significantly reduce the migration, invasion, and tumour formation abilities of SKMEL-28 cells compared to control cells. In conclusion, the study suggests that, despite ricin’s reputation as one of nature’s most poisonous substances, its specificity can be enhanced for treating melanoma and potentially other cancers when utilised in complex forms with liposomes. This research offers promising insights into developing targeted therapies using ricin–liposome complexes.
The studies mentioned above evaluated the anticancer potential of R. communis utilising various plant parts (seeds, fruits, and leaves) as well as different types of extracts, with a variety of methodologies like gene expression analysis, cytotoxicity assays, flow cytometry, and nanocarrier systems [10,11,58,104,105]. One of the areas where there is a variation is in the solvents and types of extracts utilised, for example, crude ricin protein, methanolic fruit extracts, and aqueous leaf extracts, each with different polarity, hence selectively extracting different types of bioactive compounds [9,10,11,104]. Polar solvents like methanol are better at extracting hydrophilic compounds like flavonoids and phenolic acids, which are characterised as antioxidants with cytotoxic effects, whereas protein-based extracts like ricin exert different apoptotic mechanisms.
The diversity in solvent polarity and target compounds would be one way, then, of accounting for the disparity in potency as well as the mechanism of action between studies. Ricin, for instance, activates caspases and inhibits autophagy, whereas methanolic fruit extracts downregulate metastasis-associated targets such as MMP-2 as well as MMP-9 [11,104]. Equally, the application of liposome encapsulation in Loan et al. [105] reflects increasing interest in maximising specificity as well as cellular internalisation through drug delivery systems, a more focused as well as efficient therapeutic profile than crude or conventional extracts. Overall, the diversity in solvents of extraction, plant components, and delivery forms not only highlights R. communis’s rich anticancer potential but also highlights how necessary it is to associate specific bioactivities observed with extraction methods and chemical properties for more judicious therapeutic development.
6.8. Antiparasitic Activity
There are recent studies that affirm that R. communis, particularly its seed oil and ricinoleic acid, exhibits profound antiparasitic activity. Muñoz-Sánchez et al. [80] tested three commercial-grade castor oils in their in vitro research on the cysticidal activity against the protozoan parasite Entamoeba histolytica, the causative agent of amoebiasis. The experiments treated cultures of cysts with each oil at 10–100 µg/mL concentration for 10 h. Morphological examination showed profound membrane disruption, cytoplasm leakage, and dissolution of cyst wall integrity in a dose-dependent fashion. EC50 values reported were 35–50 µg/mL, which fall within the calculated intestinal concentrations with frequent oral administration. The authors credited this activity to the high level of ricinoleic acid, which is cytolytic to the membrane of protozoa by fatty acid-induced micellisation and osmotic imbalance [80].
Moreover, Berhanu et al. [106] analysed the isolation and characterisation of ricinoleic acid and methyl ester anthelmintic activity against R. communis seed. Petroleum ether extraction, base hydrolysis, and esterification were carried out to obtain pure fatty acid derivatives, which were screened against Caenorhabditis elegans as a nematode model and activity was analysed by worm motility and viability using light microscopy. The agents at 1 mg/mL dose level killed more than 97% in 8 h, with no recovery of surviving worms within 24 h. Molecular docking study revealed strong binding energies of ricinoleic acid to succinate dehydrogenase (−5.41 kcal/mol) and glucose-6-phosphate dehydrogenase (−3.76 kcal/mol), enzymes essential to helminth metabolism. Furthermore, in silico ADME screening also revealed satisfactory oral bioavailability, drug-likeness, and low toxicity risk. The study confirmed that ricinoleic acid has great potential for translation as a plant anthelmintic drug [106].
These studies evaluated R. communis antiparasitic activity according to various experimental models for determining the efficacy against protozoan and helminth infections [80,106]. Muñoz-Sánchez et al. [80] demonstrated that pharmaceutical-grade castor oil strongly disrupted. E. histolytica cysts in vitro with EC50 values of 35–50 µg/mL and induced severe morphological damage after 10 h of incubation. This was attributed to the membrane-disrupting activity of ricinoleic acid, which is the principal fatty acid of castor oil. Similarly, Berhanu et al. [106] isolated ricinoleic acid and methyl ester of ricinoleic acid from petroleum ether seed extract of R. communis and reported >97% mortality of C. elegans at 1 mg/mL. These findings were supported by molecular docking tests, which proved to have good binding to helminthic enzymes such as succinate dehydrogenase and glucose-6-phosphate dehydrogenase. All these tests establish that R. communis is significantly potent against parasites through highly active antiparasitic activity, supplemented by the ability of ricinoleic acid to disrupt parasite metabolism and membrane stability. Table 5 summarises the pharmacological activities and mechanisms of R. communis extracts.

Table 5.
Pharmacological activities and mechanisms of Ricinus communis extracts.
7. Industrial Application
Castor oil is produced from the seeds of the R. communis plant and has a wide range of industrial uses in several industries [12]. Castor oil’s several industrial uses highlight its adaptability, durability, and ongoing significance in contemporary manufacturing processes and environmental stewardship programs [107]. In the industrial realm, castor oil made from seeds is a valuable raw material in many industries, as it is an essential component in producing biodegradable plastics, lubricants, and pharmaceuticals [107]. Sen et al. [108] stated that, due to its exceptional viscosity and lubricating qualities, it is an essential ingredient in the creation of high-performance lubricants and greases that guarantee the seamless functioning of industrial machinery and automotive components, even in the face of harsh temperature and pressure fluctuations.
To further address the growing demand for sustainable materials in packaging, automotive parts, and consumer goods, castor oil derivatives, particularly ricinoleic acid, plays a crucial role in the production of biodegradable plastics and polymers [109,110,111]. The castor oil derivatives are also helpful emulsifiers and surfactants, and they are essential for stabilising solutions in various sectors, from paints and coatings to textiles [110,111]. Furthermore, there is growing momentum behind castor oil’s potential as a sustainable biofuel feedstock, providing a greener substitute for traditional fossil fuels and aiding in the fight against climate change [107].
Castor oil has become a leading bio-renewable feedstock in the chemical industry due to its unique profile of hydroxylated fatty acids [112]. It can be used directly as a polyol in the production of polyurethane and polyester, thus enabling the production of green adhesives, coatings, foams, plastics, and composite resins [113]. An extensive study by Mutlu and Meier [112] investigated castor oil as a chemical platform, demonstrating that its major constituent, ricinoleic acid, can undergo esterification and polymerisation without the use of severe catalysts, hence fulfilling the requirements for green chemistry. Their study emphasised the capability of castor oil to be functionalized at the hydroxyl group as well as unsaturation positions, making it extremely versatile for designed chemical synthesis.
Additionally, Lim et al. [113] prepared waterborne polyurethane dispersions based on modified castor oil, showing that the products exhibited improved elongation-at-break, tensile strength, and thermal stability. This work further demonstrates the capacity of castor oil to perform in challenging applications like flexible coatings and biodegradable films with performance metrics that are comparable to, or even better than, petrochemical counterparts. The prevalence of ricinoleic acid in castor oil enables a broad range of functional group modifications, such as epoxidation, amidation, and sulfation, that are critical for the development of high-performance specialty polymers [111]. Additionally, its non-edible status removes competition with food sources, rendering it an economically and ethically acceptable option for chemical feedstocks [114]. This work also highlights the potential for local castor oil production and processing in developing countries, and hence decentralizing bio-industrial development.
In addition to industrial uses, castor oil derivatives are now found to be widely used in the military sector, especially in the preparation of composite solid rocket propellants [115]. The hydroxyl groups of ricinoleic acid make it easy to achieve reactive plasticizers for enhancing the flexibility, burning rate, and energy efficiency of rocket grains [115]. Ahmad et al. [115] compared thermochemical and mechanical behaviour of plasticizers based on castor oil and found that not only did they improve mechanical flexibility of HTPB-based propellants, but also allowed better control over the decomposition kinetics, a desirable safety feature in an explosive compound.
Kovačević et al. [116] presented recent work on composite propellants which established the fact that castor-based polyols improved mechanical and viscoelastic stability upon being added to HTPB-based propellant formulations. Specifically, the research measured storage modulus, tan delta, and cross-link density and concluded that castor-based additives had raised the structural bonding and reduced thermal cracking risks in combustion. Jia et al. [110] demonstrated the polyol esters made from castor oil were effective in stabilizing PVC structures and retaining elasticity against thermal and oxidative environments [110]. Such stabilizing action is similarly in great demand in high-energy military composites with materials exposed to fluctuating environmental and mechanical environments.
These attributes are due to the chemical cross-linking potential of castor oil derivatives, which enable control over combustion performance and support safe storage [115]. In addition, the renewable and low-toxicity nature of castor oil is in line with current defence-agency goals for greener, safer, and more sustainable materials, as defence agencies increasingly look toward replacements for non-renewable or toxic inputs to ammunition, propulsion, and structural systems [111]. Table 6 summarises the industrial applications of R. communis in many industries such as pharmaceutical, agriculture, and cosmetics, thus highlighting the purpose and application of the plant.

Table 6.
Industrial application of Ricinus communis in various industrial sectors.
8. Toxicology
Ricinus communis has been extensively researched for its toxicological qualities, i.e., ricin, a poisonous protein found in varied amounts throughout the plant, particularly in the seeds, leaves, and pericarp [118]. Ricin is renowned for its high toxicity and has been linked to poisoning in both humans and animals, with symptoms ranging from gastrointestinal distress to organ failure and, in severe cases, death [118]. The assessment of R. communis toxicity, as detailed in the study by Joshua et al. [119], provides crucial insights into the safety profile of extracts derived from its seeds. The study’s findings reveal distinct outcomes between the unfermented and fermented methanol extracts of R. communis seeds. Notably, the unfermented extract exhibited no toxicity at doses as high as 5000 mg/kg body weight, confirming its relatively benign nature under controlled conditions. In contrast, the fermented extract demonstrated toxicity at the same dosage level, resulting in mortality among test subjects, likely attributable to increased organic acid content from the fermentation process. These findings underscore the critical role of extraction methods in determining the safety of R. communis extracts and highlight the necessity for rigorous toxicity assessments in both medicinal and agricultural contexts.
Furthermore, research evaluating its impact on model species such as Drosophila melanogaste by Ai et al. [77] emphasised the potential toxicity of R. communis, with the plant’s ethanol extract exhibiting toxic effects. The results showed that the ethanol extract of R. communis expressed its high toxicity against the 2nd instar larvae of Drosophila melanogaste with the LD50 value of 64.63 mg/mL, underlining the need for caution in its usage and handling. Additionally, Bernstein et al. [120] conducted a review of the toxicological effects of R. communis in traditional medicine. The review’s findings highlight that castor oil, derived from R. communis seeds, has historically been significant in pharmacological applications, notably for inducing labour in over 57.7% of treated women within 24 h at doses such as 59 mL. However, the use resulted in adverse effects such as irregular uterine contractions, gastrointestinal distress (vomiting, diarrhoea), and dehydration, underscoring concerns about its potential toxicity for maternal and foetal health. These findings emphasise the critical need for comprehensive toxicological evaluations to ensure the safety of castor oil ingestion, particularly during pregnancy.
The possible effects of R. communis on cattle and animal feeding systems were investigated concerning the toxicological consequences of this plant [121]. Research findings on the nutrient profile and toxicity of the R. communis seeds in common livestock species revealed that the defatted seed meal contained 32–48% crude protein and approximately 3200 kcal/kg of gross energy. The seeds had a ricin content of 9.3 mg/g and an RCA (Ricinus communis Agglutinin) content of 9.9 mg/g. The meal showed high lectin activity, with agglutination occurring at about 4.70 mg/mL. Various detoxification methods showed differing levels of success, with high pH, moist heating, and microbial techniques being most effective in deactivating ricin, though strategies for allergen detoxification were inconclusive. The study concluded that castor seed could be a valuable feedstuff if detoxified safely, cost-effectively, and efficiently for commercial use.
These results demonstrate the wide range of toxicological consequences associated with R. communis and the necessity of thorough evaluations to guarantee the safety of its use in different contexts and to reduce any potential negative impacts on the environment and animal health [18,122].
Given the known toxicity of ricin and the other constituents of R. communis, traditional and semi-purified plant extracts are widely utilised in indigenous medicine without reported acute toxicity, suggesting the presence of effective detoxification protocols. Disregarding the extreme toxicity of ricin, heat or aqueous traditional preparation techniques have been found to inactivate or reduce the toxic activity of the protein. Ricin is a heat-labile protein that denatures under moist heat, thereby destabilising its two-chain form, making it unable to bind and inactivate ribosomes [91]. Boiling or roasting of seeds and leaves of R. communis used extensively in ethnomedicine depletes ricin bioactivity through disruption of disulfide bonds between A and B chains required for its internalisation in host cells [123]. Aqueous preparations by the traditional decoctions also facilitate detoxification by flushing out water-soluble toxins like ricin and ricinine from plant material, particularly if coupled with the drainage of the liquid [81]. Scientific experiments confirm that ricin content in such preparations is far lower or below detectable levels, and toxicity experiments establish reduced effects in animal models and in vitro systems [124].
Pingale et al. [124], in a study of acute toxicity, gave Swiss mice a freshly prepared aqueous leaf juice of R. communis at a single dose of 2000 mg/kg body weight and monitored the mice for 14 days. No death, behavioural alteration, or sign of systemic toxicity occurred, and significant parameters such as food/water consumption, body weight, and cage-side behaviour were within the normal range. This high-dose test was negative for toxicity and suggested that water-soluble toxins such as ricin were either inactivated during preparation or available at subtoxic concentrations due to the thermal and aqueous lability of the protein. This parallels ancient means through which aqueous extraction and heat are used to prepare Ricinus drugs, also mirroring their relative safety with proper processing.
Such outcomes support the empirical safety of traditional preparation methods and underscore that not all Ricinus products are equally risky, especially when processed through traditionally guided detoxification processes. Table 7 represents the LD50 phytochemical values components of R. communis in animal models across various exposure pathways.

Table 7.
LD50 values for components of Ricinus communis in animal models across various exposure pathways.
9. Future Directions and Research Gaps
Research on R. communis is still developing, and several new areas call for investigation and creativity. One promising direction is to clarify the plant’s epigenetic regulatory mechanisms and how they affect its biochemical profile and therapeutic potential. Gaining knowledge about how environmental stimuli alter R. communis gene expression patterns might help improve the quantity and calibre of bioactive chemicals produced. Studying the interactions between the plant and symbiotic and soil microbiota species may also reveal new ways to optimise cultivation methods and reduce environmental stressors, increasing the plant’s resilience and productivity in various agroecosystems.
Notable gaps in knowledge still exist in R. communis study, even with significant achievements. The thorough characterisation of its secondary metabolites and their cooperative relationships is one crucial area that merits consideration. Although the pharmacological characteristics of some bioactive chemicals have been thoroughly investigated, the entire range of metabolites found in R. communis is still poorly understood. Furthermore, additional research is necessary to determine the precise mechanisms regulating these chemicals’ production, storage, and secretion in various plant tissues. Closing these gaps will help us better understand the biochemical complexity of R. communis and make it easier to manipulate metabolic pathways specifically for improved industrial and medicinal uses.
10. Conclusions
The culmination of this literature review presents a comprehensive overview of its multifaceted attributes and potential applications. Through a synthesis of diverse studies, key findings reveal the plant’s rich composition of bioactive compounds, including ricin and ricinoleic acid, which underpin its pharmacological significance. Across traditional medicinal practices and modern pharmaceutical research, R. communis emerges as a promising source of therapeutic agents with diverse biological activities. From its anti-inflammatory and antimicrobial properties to its potential in cancer treatment, the plant’s bioactive constituents hold promise for addressing various health challenges.
Looking forward, the implications for future research underscore several critical avenues for exploration. Firstly, there is a pressing need to deepen our understanding of the underlying mechanisms driving the pharmacological effects of R. communis compounds. Unravelling these mechanisms at the molecular level can not only enhance our knowledge of plant-based therapeutics but also inform the development of targeted pharmaceutical interventions. Moreover, exploring synergistic interactions among the plant’s bioactive components may unveil novel therapeutic combinations with enhanced efficacy and reduced side effects.
Beyond its medicinal applications, the literature review also sheds light on the industrial potential of R. communis. The plant’s oil-rich seeds offer a sustainable alternative to conventional resources, from biodiesel production to synthesising biopolymers and surfactants. However, realising the full potential of R. communis in industrial applications necessitates further research into optimising cultivation practices, enhancing seed yield, and exploring innovative processing techniques. By addressing these challenges, R. communis can contribute to diverse sectors, fostering innovation, sustainability, and economic growth.
Author Contributions
Conceptualization, T.P.R. and C.M.N.; methodology, T.P.R. and C.M.N.; investigation, T.P.R.; data curation, T.P.R. and C.M.N.; writing—original draft preparation, T.P.R. and C.M.N.; writing—review and editing, T.P.R., C.M.N., N.M.M., M.C.M., M.A.M. and M.M.M.; visualization, N.M.M., M.C.M. and M.M.M.; supervision, C.M.N.; project administration, C.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to extend our sincere appreciation to the Department of Biology and Environmental Sciences, School of Science and Technology, at Sefako Makgatho Health Sciences University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alsubeie, M.S. Morphological, Genetic Characterisation, and Chemical Analysis of Castor Bean (Ricinus communis) Growing in Riyadh Saudi Arabia. Entomol. Appl. Sci. Lett. 2023, 10, 38–44. [Google Scholar] [CrossRef]
- Patel, V.; Dumancas, G.; Viswanath, L.; Maples, R.; Subong, B. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, LPI.S40233. [Google Scholar] [CrossRef]
- Yeboah, A.; Ying, S.; Lu, J.; Xie, Y.; Amoanimaa-Dede, H.; Boateng, K.G.A.; Chen, M.; Yin, X. Castor Oil (Ricinus communis): A Review on the Chemical Composition and Physicochemical Properties. Food Sci. Technol. 2021, 41 (Suppl. S2), 399–413. [Google Scholar] [CrossRef]
- Suurbaar, J.; Mosobil, R.; Donkor, A.M. Antibacterial and Antifungal Activities and Phytochemical Profile of Leaf Extract from Different Extractants of Ricinus communis Against Selected Pathogens. BMC Res. Notes 2017, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, A.; Lu, J.; Gu, S.; Shi, Y.; Amoanimaa-Dede, H.; Agyenim-Boateng, K.; Yin, X. The Utilisation of Ricinus communis in the Phytomanagement of Heavy Metal-Contaminated Soils. Environ. Rev. 2020, 28, 466–477. [Google Scholar] [CrossRef]
- Ekoja, A.I.; Audu-Peter, J.D. Medicinal properties of Ricinus communis and the need for novel formulation of the extracts: A review. Univers. J. Pharm. Res. 2023, 8, 45–51. [Google Scholar] [CrossRef]
- Wachira, S.W.; Omar, S.; Jacob, J.W.; Wahome, M.; Alborn, H.T.; Spring, D.R.; Masiga, D.K.; Torto, B. Toxicity of Six Plant Extracts and Two Pyridone Alkaloids from Ricinus communis Against the Malaria Vector Anopheles gambiae. Parasites Vectors 2014, 7, 312. [Google Scholar] [CrossRef]
- Sabitha, Y.; Fatima, S. Evaluation of Genotoxicity of Ricinus communis (Leaves and Seeds) by Their Silver Nanoparticles Using Comet Assay. J. Adv. Sci. Res. 2022, 13, 146–150. [Google Scholar]
- Ghramh, H.A.; Khan, K.; Ibrahim, E.A.; Setzer, W.N. Synthesis of Gold Nanoparticles (AuNPs) Using Ricinus communis Leaf Ethanol Extract, Their Characterization, and Biological Applications. Nanomaterials 2019, 9, 765. [Google Scholar] [CrossRef]
- Hajrah, N.M.; Abdul, W.; Al-Garni, S.; Sheikh, A.; Ahmed, M.; Hall, N.; Bora, R. Gene Expression Profiling to Elucidate the Pharmacological and Toxicological Effects of Ricinus communis L. Leaf Extract in Mammalian Cells. Biotechnol. Biotechnol. Equip. 2019, 33, 397–407. [Google Scholar] [CrossRef]
- Majumder, M.; Debnath, S.; Gajbhiye, R.L.; Saikia, R.; Gogoi, B.; Samanta, S.K.; Das, D.K.; Biswas, K.; Jaisankar, P.; Mukhopadhyay, R. Ricinus communis L. Fruit Extract Inhibits Migration/Invasion, Induces Apoptosis in Breast Cancer Cells and Arrests Tumor Progression In Vivo. Sci. Rep. 2019, 9, 14493. [Google Scholar] [CrossRef]
- Obanla, O.R.; Mohammed, F.; Alebiosu, O.S.; Ojewumi, M.E.; Oladimeji, T.E.; Babatunde, D.E. Study on the Lubricating Properties of Castor (Ricinus communis) and Hydroxylated Rubber (Hevea brasiliensis) Seed Oil. ACS Omega 2021, 6, 28471–28476. [Google Scholar] [CrossRef]
- Jena, J.; Gupta, A. Ricinus communis Linn: A Phytopharmacological Review. Int. J. Pharm. Pharm. Sci. 2012, 4, 25–29. [Google Scholar]
- Rana, M.; Dhamija, H.; Prashar, B.; Sharma, S. Ricinus communis L.—A Review. Int. J. PharmTech Res. 2012, 4, 1706–1711. [Google Scholar]
- Severino, L.S. Studies on Yield Components and Seed Physiology of Castor (Ricinus communis L.); Texas Tech University: Lubbock, TX, USA, 2012. [Google Scholar]
- Das, R.; Pal, A.; Paul, A.K. Production of Biopolyester Poly (3-Hydroxybutyrate) by Bacillus cereus RCL 02, a Leaf Endophyte of Ricinus communis L. J. Microbiol. Biotechnol. Res. 2017, 7, 32–41. [Google Scholar] [CrossRef][Green Version]
- Severino, L.S.; Auld, D.L. Study on the Effect of Air Temperature on Seed Development and Determination of the Base Temperature for Seed Growth in Castor (‘Ricinus communis’ L.). Aust. J. Crop Sci. 2014, 8, 290–295. [Google Scholar]
- Utami, P.; Nurdiana. Disinfection Effect of 10% Ricinus communis Oil on Candida albicans Counts of Heat Polymerized Acrylic Resin. Dentika Dent. J. 2021, 24, 6–10. [Google Scholar] [CrossRef]
- Scarpa, A.; Guerci, A. Various Uses of the Castor Oil Plant (Ricinus communis L.): A Review. J. Ethnopharmacol. 1982, 5, 117–137. [Google Scholar] [CrossRef]
- Rahman, A.Y.A.; Usharraj, A.O.; Misra, B.B.; Thottathil, G.P.; Jayasekaran, K.; Feng, Y.; Hou, S.; Ong, S.Y.; Ng, F.L.; Lee, L.S.; et al. Draft Genome Sequence of the Rubber Tree Hevea brasiliensis. BMC Genom. 2013, 14, 75. [Google Scholar] [CrossRef]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A Review of the Ethnomedicinal Uses, Biological Activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef]
- King, A.; Brown, G.; Gilday, A.; Larson, T.; Graham, I. Production of Bioactive Diterpenoids in the Euphorbiaceae Depends on Evolutionarily Conserved Gene Clusters. Plant Cell 2014, 26, 3286–3298. [Google Scholar] [CrossRef]
- Jin, Y.; Shi, L.; Zhang, D.; Wei, H.; Si, Y.; Ma, G.; Zhang, J. A Review on Daphnane-Type Diterpenoids and Their Bioactive Studies. Molecules 2019, 24, 1842. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.A.G.d.; Pinto, L.d.S.; Chaves, C.F.; Silva, A.J.R.d.; Silva, M.F.d.G.F.d.; Cotinguiba, F. The Chemophenetic Significance of Anomalously Cleanups Metabolites Are Revealed by Dereplication Using Molecular Networking Tools. Molecules 2021, 26, 925. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.C.R.; Bigio, N.C.; Külkamp, J.; Pereira-Silva, R.A. Synoptic Treatment of Dalechampia (Euphorbiaceae) from Espírito Santo, Brazil: Distribution, Morphology, Illustration and New Occurrences. Rodriguésia 2022, 73, e00412021. [Google Scholar] [CrossRef]
- Duan, Q.; Li, G.; Qu, Y.; Yin, D.; Zhang, C.; Chen, Y. Genome-Wide Identification, Evolution and Expression Analysis of the Glutathione S-Transferase Supergene Family in Euphorbiaceae. Front. Plant Sci. 2022, 13, 808279. [Google Scholar] [CrossRef]
- Naki, M.I.; Abdullah, A.; Susilawati, E. Literature Review of the Pharmacological Activities of the Euphorbiaceae Family Plants. J. Farm. Sains Prakt. 2023, 9, 62–71. [Google Scholar] [CrossRef]
- Ruziman, H.H.; Ismail, A.P.; Radzun, K.A.; Ishak, N.S.; Zohari, A.F.; Kusin, M.; Pardi, F. Tree Species Diversity and Conservation Status of Keniam Forest, Taman Negara, Pahang. IOP Conf. Ser. Earth Environ. Sci. 2022, 1019, 012014. [Google Scholar] [CrossRef]
- Essandoh, P.K.; Ampofo, E.A.; Okae-Anti, D.; Bryant, I.M. Comparison of Flora of Small-Scale Mined and Unmined-Sites in Dunkwa-East Municipality, Ghana. Environ. Nat. Resour. Res. 2019, 9, 86. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Salihu, B.; Gana, A.K.; Apuyor, B. Castor Oil Plant (Ricinus communis L.): Botany, Ecology and Uses. Int. J. Sci. Res. 2014, 3, 1333–1341. [Google Scholar]
- Parveen, M.; Ahmad, F.; Malla, A.M.; Azaz, S.; Alam, M.; Basudan, O.A.; Silva, M.R.; Silva, P.S.P. Acetylcholinesterase and Cytotoxic Activity of Chemical Constituents of Clusia lanceolata Leaves and Its Molecular Docking Study. Nat. Prod. Bioprospect. 2016, 6, 267–278. [Google Scholar] [CrossRef]
- Lopes, M.P.B.; Gomes, M.E.; Celin, G.d.S.; Bello, H.N.; Henrique, R.L.P.; Magalhães, I.P.; Santos, L.V.; Tropaldi, L.; Pascholate, S.F.; Furtado, E.L.; et al. Initial Studies of the Response of Rubber Tree Seedlings Treated with Saprobic Fungi from the Semiarid Region of Northeast Brazil to Anthracnose. Plants 2022, 11, 2477. [Google Scholar] [CrossRef]
- Flower Tales. Cyathium: An Astounding Performer; Flower Tales: Singapore, 2015; Available online: https://flower-tales.blogspot.com/2015/12/cyathium-astounding-performer.html (accessed on 25 May 2024).
- Chouhan, H.S.; Swarnakar, G.; Jogpal, B. Medicinal Properties of Ricinus communis: A Review. Int. J. Pharm. Sci. Res. 2021, 12, 3632–3642. [Google Scholar]
- Wang, S.; Zhao, Y.; Guo, J.; Liu, Y. Antioxidative Response in Leaves and Allelochemical Changes in Root Exudates of Ricinus communis under Cu, Zn, and Cd Stress. Environ. Sci. Pollut. Res. 2018, 25, 32747–32755. [Google Scholar] [CrossRef] [PubMed]
- Nour, I.H.; Alhadead, K.; Ellmouni, F.Y.; Badr, R.; Saad, T.I.; El-Banhawy, A.; Abdel Rahman, S.M. Morphological, Anatomical and Chemical Characterization of Ricinus communis L. (Euphorbiaceae). Agronomy 2023, 13, 985. [Google Scholar] [CrossRef]
- Pina, F.; Alejo-Armijo, A.; Clemente, A.; Mendoza, J.; Seco, A.; Basílio, N.; Parola, A. Evolution of Flavylium-Based Color Systems in Plants: What Physical Chemistry Can Tell Us. Int. J. Mol. Sci. 2021, 22, 3833. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, M.K.; Singh, A.K.; Kumar, S. Pharmacognostical Investigation of Ricinus communis Stem. Int. J. Pharm. Sci. Res. 2010, 1, 89–94. [Google Scholar]
- Liu, Y.; Tikunov, Y.; Schouten, R.; Marcelis, L.; Visser, R.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Li, J.; Wu, K.; Li, L.; Ma, G.; Fang, L.; Zeng, S. Transcriptomic Analysis Reveals Biosynthesis Genes and Transcription Factors Related to Leaf Anthocyanin Biosynthesis in Aglaonema commutatum. BMC Genom. 2023, 24, 28. [Google Scholar] [CrossRef]
- Alves de Siqueira, J.I.; Rodrigues Vieira, I.; Ferreira Chaves, E.M.; Sanabria-Diago, O.L.; Rodrigues Lemos, J. Biocultural Behavior and Traditional Practices on the Use of Species of Euphorbiaceae in Rural Home Gardens of the Semiarid Region of Piauí State (NE, Brazil). Caldasia 2020, 42, 70–84. [Google Scholar] [CrossRef]
- Khan, A.S. Flowering Plants: Structure and Industrial Products; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zheng, J.; Suhono, G.B.; Li, Y.; Jiang, M.Y.; Chen, Y.; Siddique, K.H. Salt-Tolerance in Castor Bean (Ricinus communis L.) Is Associated with Thicker Roots and Better Tissue K+/Na+ Distribution. Agriculture 2021, 11, 821. [Google Scholar] [CrossRef]
- Landoni, M.; Bertagnon, G.; Ghidoli, M.; Cassani, E.; Adani, F.; Pilu, R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy 2023, 13, 2076. [Google Scholar] [CrossRef]
- Claßen-Bockhoff, R.; Frankenhäuser, H. The ‘Male Flower’ of Ricinus communis (Euphorbiaceae) Interpreted as a Multi-Flowered Unit. Front. Cell Dev. Biol. 2020, 8, 313. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.S.M.; Soliman, A.S. Diversity Assessment by Molecular Barcoding and Seed Morphology in Ricinus communis L. Baghdad Sci. J. 2021, 18, 41. [Google Scholar]
- Drumond, A.A.L.; Sales, J.D.F.; Zuchi, J.; Camelo, G.N.; Souza, M.M.V. Physiological Quality of Castor Seeds (Ricinus communis L.) after Processing. J. Seed Sci. 2019, 41, 224–232. [Google Scholar] [CrossRef]
- Brandon, D.L.; McKeon, T.A.; Patfield, S.A.; Kong, Q.; He, X. Analysis of Castor by ELISAs that Distinguish Ricin and Ricinus communis Agglutinin (RCA). J. Am. Oil Chem. Soc. 2016, 93, 359–363. [Google Scholar] [CrossRef]
- Herawati, I.E.; Lesmana, R.; Levita, J.; Subarnas, A. Determination and Purification of Ricin Protein from Ricinus communis L. Seeds Using CLC (Column Liquid Chromatography) and FPLC (Fast Protein Liquid Chromatography). Int. J. Pharm. Sci. Technol. 2021, 8, 118–124. [Google Scholar]
- Worbs, S.; Skiba, M.; Söderström, M.; Rapinoja, M.L.; Zeleny, R.; Russmann, H.; Dorner, M.B. An International Proficiency Test to Detect, Identify and Quantify Ricin in Complex Matrices. Toxins 2015, 7, 4987–5010. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Fathi-Achachlouei, B.; Alirezalu, K.; Alirezalu, A.; Hesari, J.; Emami, S. Physiological and Medicinal Properties of Castor Oil. In Recent Progress in Medicinal Plants; Studium Press (India) Pvt. Ltd.: Delhi, India, 2011. [Google Scholar]
- Foster, J.T.; Allan, G.J.; Chan, A.P.; Rabinowicz, P.D.; Ravel, J.; Jackson, P.J.; Keim, P. Single Nucleotide Polymorphisms for Assessing Genetic Diversity in Castor Bean (Ricinus communis). BMC Plant Biol. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Mashela, P.W.; Nthangeni, M.E. Efficacy of Ricinus communis Fruit Meal with and Without Bacillus Species on Suppression of Meloidogyne incognita and Growth of Tomato. J. Phytopathol. 2002, 150, 399–402. [Google Scholar] [CrossRef]
- Akbar, S. Ricinus communis L. (Euphorbiaceae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1539–1550. [Google Scholar]
- Nemudzivhadi, V.; Masoko, P. In Vitro Assessment of Cytotoxicity, Antioxidant, and Anti-Inflammatory Activities of Ricinus communis (Euphorbiaceae) Leaf Extracts. Evid. Based Complement. Alternat. Med. 2014, 2014, 625961. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, R.; Malemela, K.; Serala, K.; Kgakishe, M.; Matsebatlela, T.; Mokgotho, M.; Mbazima, V. Ricinus communis Butanol Fraction Inhibits MCF-7 Breast Cancer Cell Migration, Adhesion, and Invasiveness. Integr. Cancer Ther. 2021, 20, 1534735420977684. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Pendota, S.C. Medicinal plants for mitigating pain and inflammatory-related conditions: An appraisal of ethnobotanical uses and patterns in South Africa. Front. Pharmacol. 2021, 12, 758583. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.A.; Otang, W.M.; Afolayan, A.J. The prevalence and perceived efficacy of medicinal plants used for stomach ailments in the Amathole District Municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2017, 108, 144–148. [Google Scholar] [CrossRef]
- De Beer, J.J.; Van Wyk, B.E. An ethnobotanical survey of the Agter–Hantam, Northern Cape Province, South Africa. S. Afr. J. Bot. 2011, 77, 741–754. [Google Scholar] [CrossRef]
- Engelbrecht, A.; Cilliers, A.M. More about… emergency medicine: Poisonous plants. Cont. Med. Educ. 2012, 30, 420–423. [Google Scholar]
- Mboyazi, S.N.; Nqotheni, M.I.; Maliehe, T.S.; Shandu, J.S. In vitro antibacterial and in silico toxicity properties of phytocompounds from Ricinus communis leaf extract. Pharmacogn. J. 2020, 12, 5. [Google Scholar] [CrossRef]
- Qin, L.; Han, J.; Wang, C.; Xu, B.; Tan, D.; He, S.; Guo, L.; Bo, X.; Xie, J. Key defatting tissue pretreatment protocol for enhanced maldi ms imaging of peptide biomarkers visualization in the castor beans and their attribution applications. Front. Plant Sci. 2022, 13, 1083901. [Google Scholar] [CrossRef]
- Gonzalez, S.L.; Ghermandi, L. Overgrazing causes a reduction in the vegetation cover and seed bank of Patagonian grasslands. Plant Soil. 2021, 464, 75–87. [Google Scholar] [CrossRef]
- Delgado Sánchez, L.; Rojas Andrés, B.M.; López González, N.; Padilla-García, N.; Martínez Ortega, M.M. IAPT chromosome data 28. Taxon 2018, 67, 1235–1245. [Google Scholar] [CrossRef]
- Chen, S.; Lin, B.; Li, Y.; Zhou, S. Spatial and Temporal Changes of Soil Properties and Soil Fertility Evaluation in a Large Grain-Production Area of Subtropical Plain, China. Geoderma 2020, 357, 113937. [Google Scholar] [CrossRef]
- Abd El-Ghani, M.M.; Huerta-Martínez, F.M.; Hongyan, L.; Qureshi, R.; El-Ghani, M.M.A.; Huerta-Martínez, F.M.; Qureshi, R. The Inland Western Desert of Egypt. In Plant Responses to Hyperarid Desert Environments; Springer International Publishing: Cham, Switzerland, 2017; pp. 179–256. [Google Scholar]
- Gebre, G.D.; Abera, G.B.; Shiferaw, S.; Adaramola, S. Soil Physicochemical and Macrobiota Diversity Dynamics Under Enclosed Areas of Central Rift Valley of Ethiopia. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Lokpal, C.P. Genesis and Quality of Black and Associated Red Soils Under Teak and Sandalwood in Seoni District of Madhya Pradesh. Ph.D. Thesis, Panjabrao Deshmukh Krishi Vidyapeeth, Akola, India, 2017. [Google Scholar]
- Bautista-Cruz, A.; De León-González, F.; Carrillo-González, R.; Robles, C. Identification of soil quality indicators for maguey mezcalero (Agave angustifolia Haw.) plantations in Southern Mexico. Afr. J. Agric. Res. 2011, 6, 4795–4799. [Google Scholar]
- Onyekwelu, C.C.; Onwubuariri, C.N.; Mgbeojedo, T.I.; Al-Naimi, L.S.; Ijeh, B.I.; Agoha, C.C. Geo-electrical investigation of the groundwater potential of Ogidi and environs, Anambra State, South-eastern Nigeria. J. Pet. Explor. Prod. Technol. 2021, 11, 1053–1067. [Google Scholar] [CrossRef]
- Javed, A.; Iqbal, M.; Shehzadi, R. Effect of Plastic Film and Straw Mulch on Wheat Yield, Water Use Efficiency and Soil Properties in Punjab, Pakistan. J. Bioresour. Manag. 2020, 7, 5. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Natural Resources Conservation Service. Soil Health; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2023. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/health/ (accessed on 25 April 2023).
- Luseba, D.; Elgorashi, E.E.; Ntloedibe, D.T.; Van Staden, J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. S. Afr. J. Bot. 2007, 73, 378–383. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An ancient story for a timeless plant toxin. Toxins 2019, 11, 324. [Google Scholar] [CrossRef]
- Ai, T.T.T.; Phien, H.H.; Men, T.T. Phytochemical constituents and toxicity of the ethanol extract of Ricinus communis (L.) in drosophila melanogaster. Asian J. Biol. 2021, 12, 21. [Google Scholar]
- Abomughaid, M.M.; Teibo, J.O.; Akinfe, O.A.; Adewolu, A.M.; Teibo, T.K.A.; Afifi, M.; Al-Farga, A.M.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; et al. A phytochemical and pharmacological review of Ricinus communis L. Discov. Appl. Sci. 2024, 6, 315. [Google Scholar]
- Badria, F.; El-Naggar, M.; Bar, F. Chemistry, economic, and biological aspects of Ricinus communis plant. Asian J. Res. Pharm. Sci. Biotechnol. 2020, 8, 9–24. [Google Scholar] [CrossRef]
- Muñoz-Sánchez, D.; Pinto, Y.; Valencia-Hernández, J.; Lora-Suarez, F.; Sanchez, J.; Gómez-Marín, J. In vitro activity of Ricinus communis (castor oil) on cysts of Entamoeba histolytica. Phytomed. Plus 2024, 4, 100404. [Google Scholar] [CrossRef]
- Marwat, S.K.; Rehman, F.; Khan, E.A.; Baloch, M.S.; Sadiq, M.; Ullah, I.; Javaria, S.; Shaheen, S. Review—Ricinus communis: Ethnomedicinal Uses and Pharmacological Activities. Pak. J. Pharm. Sci. 2017, 30, 1815–1827. [Google Scholar] [PubMed]
- Krunal, D.; Rabinarayan, A. Therapeutic Importance of Eranda (Ricinus communis Linn.) in Ayurveda—A Review. Ayurpharm Int. J. Ayur. Alli. Sci. 2013, 2, 281–295. [Google Scholar]
- Ahl, H.; Hikal, W. Antileishmanial Derivates of Natural Products from Ricinus communis. Aswan Univ. J. Environ. Stud. 2022, 3, 41–75. [Google Scholar]
- Mamoucha, S.; Tsafantakis, N.; Fokialakis, N.; Christodoulakis, N. Structural and Phytochemical Investigation of the Leaves of Ricinus communis. Aust. J. Bot. 2017, 65, 58–66. [Google Scholar] [CrossRef]
- Tuyến, P.N.K.; Linh, T.T.T.; Son, D.V.; Thang, N.V.; Dang, V.; Trang, N.T.Q.; Duong, H.T. Phenolic Compounds from the Leaves of Ricinus communis Linn. Sci. Technol. Dev. J. 2020, 23, 1069–1072. [Google Scholar] [CrossRef]
- Thi, V. Contribution to Results of the Chemical Constituents of Ricinus communis. Vietnam J. Sci. Technol. 2018, 54, 523. [Google Scholar]
- Purushothaman, D.; Chandra, M. Evaluation of phytochemical constituents and antioxidant activities of Ricinus communis L. Res. J. Pharm. Technol. 2022, 15, 5619–5624. [Google Scholar]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Franke, H.; Scholl, R.; Aigner, A. Ricin and Ricinus communis in Pharmacology and Toxicology—From Ancient Use and “Papyrus Ebers” to Modern Perspectives and “Poisonous Plant of the Year 2018”. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1181–1208. [Google Scholar] [CrossRef]
- Ahmed, F.; Iqbal, M. Antioxidant Activity of Ricinus communis. Org. Med. Chem. Int. J. 2018, 5, 107–112. [Google Scholar] [CrossRef]
- Ihekuna, O.; Imaga, N.; Adewusi, B. Antioxidant and Haematological Activities of Ethanolic Extract of Ricinus communis. FASEB J. 2019, 33, 491–499. [Google Scholar] [CrossRef]
- Dikhoba, P.; Mongalo, N.; Elgorashi, E.; Makhafola, T. Antifungal and Anti-Mycotoxigenic Activity of Selected South African Medicinal Plants Species. Heliyon 2019, 5, e02668. [Google Scholar] [CrossRef] [PubMed]
- López-Ubaldo, F.; Sánchez-Mendieta, V.; Olea-Mejía, O.F.; María, S.G.P.; Luckie, R.A.M. Biosynthesis of Ag/Cu Bimetallic Nanoparticles Using Ricinus communis and Their Antibacterial and Antifungal Activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025018. [Google Scholar] [CrossRef]
- Istaufa, F.S.; Subagio, Y.; Suswati, I.; Kartikasari, I.; Rachmaniah, D.; Andiyan, A. Castor Plant (Ricinus communis L.) Leaf Extract as Potential Antibacterial against the Growth of Mycobacterium tuberculosis. Folia Medica Indones. 2022, 58, 371–376. [Google Scholar] [CrossRef]
- Benariba, N.; Fekhikher, Z.; Brixi-Gormat, R.; Benramdane, H.; Youbi, H.; Kouar, I.; Rachid, A.Z.Z. In Vitro Antioxidant and Antidiabetic Activities of the Hydro-Acetone Extracts of Ricinus communis and Teucrium polium from Tlemcen Area. J. Nat. Prod. Res. Appl. 2023, 3, 34–46. [Google Scholar]
- Dayyih, W.A.; Manaysa, M.; Hailat, M.; Zakareia, Z.; El Hajji, F. Influence of Castor Oil on Glycated Hemoglobin (HbA1c) in Induced Type 2 Diabetes Mellitus in Rats. Jordan J. Pharm. Sci. 2021, 14, 341–349. [Google Scholar]
- Lee, H.; Kim, Y.I.; Kim, M.J.; Hahm, J.H.; Seo, H.D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Castor Oil Plant (Ricinus communis L.) Leaves Improve Dexamethasone-Induced Muscle Atrophy via Nrf2 Activation. Front. Pharmacol. 2022, 13, 891762. [Google Scholar] [CrossRef]
- Hussain, A.; Aslam, B.; Muhammad, F.; Faisal, M.N. In Vitro Antioxidant Activity and In Vivo Anti-Inflammatory Effect of Ricinus communis (L.) and Withania somnifera (L.) Hydroalcoholic Extracts in Rats. Braz. Arch. Biol. Technol. 2021, 64, e21200783. [Google Scholar] [CrossRef]
- McNeil, R.T.; Adebesin, A.; Ekwere, E. Ricinus communis-Linn (Castor Plant), Male Contraceptives and Reproductive Health of Women. Afr. J. Reprod. Health 2021, 25, 135–141. [Google Scholar]
- Desouky, M.M.; Abd El-Atti, M.S.; Elsheakh, A.A.; Elgohary, W.S. Effect of Eucalyptus globulus Oil and Ricinus communis Methanolic Extract as Potential Natural Molluscicides on the Reproductive Biology and Some Antioxidant Enzymes of the Land Snail, Theba pisana. Heliyon 2022, 8, e11497. [Google Scholar] [CrossRef]
- Iornmube, U.J.; Ekwere, E.O.; McNeil, R.T.; Francis, O.K. The Mechanism of Action and Effect on the Cervix of the Seed of Ricinus communis Var. Minor (RICOM-1013-J) in Women Volunteers in Nigeria. J. Health Sci. 2016, 8, 211–220. [Google Scholar]
- Shati, A.A. Protective Effects of Allium kurrat and Ricinus communis Against Cyanide-Induced Hepatotoxicity in Balb/C Mice. Toxicol. Rep. 2011, 5, 1046–1052. [Google Scholar]
- Naveen, A.; Shankar, J.; John, P.; Venkatanarayana, N. Evaluation of Hepatoprotective Activity of Aqueous Extract of Ricinus communis in Wistar Rats. Int. J. Basic Clin. Pharmacol. 2016, 5, 358–361. [Google Scholar] [CrossRef][Green Version]
- Herawati, I.E.; Lesmana, R.; Levita, J.; Subarnas, A. Cytotoxicity, Apoptosis, Migration Inhibition, and Autophagy-Induced by Crude Ricin from Ricinus communis Seeds in A549 Lung Cancer Cell Lines. Med. Sci. Monit. Basic Res. 2022, 28, e936683. [Google Scholar] [CrossRef] [PubMed]
- Loan, N.T.B.; Trung, N.N.; Le Na, N.T.; Thang, N.D. Anticancer Activities of Ricin-Liposome Complexes on SKMEL-28 Cells. Asian Pac. J. Cancer Prev. 2019, 20, 2117–2122. [Google Scholar]
- Berhanu, T.; Tewelde, E.; Yeshak, M.; Bisrat, D.; Asres, K. Anthelmintic potential and in silico studies of ricinoleic acid from the seed oil of Ricinus communis L. Int. J. Mol. Sci. 2025, 26, 1636. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. Elastomers 2017, 1, 1–12. [Google Scholar]
- Sen, B.; Gupta, M.; Mia, M.; Pimenov, D.; Mikolajczyk, T. Performance Assessment of Minimum Quantity Castor-Palm Oil Mixtures in Hard-Milling Operation. Materials 2021, 14, 198. [Google Scholar] [CrossRef]
- Kunduru, K.R.; Basu, A.; Zada, M.H.; Domb, A.J. Castor Oil-Based Biodegradable Polyesters. Biomacromolecules 2015, 16, 2572–2587. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Feng, G.; Bo, C.; Zhou, Y. Synthesis and Application of Environmental Castor Oil-Based Polyol Ester Plasticizers for Poly(vinyl chloride). ACS Sustain. Chem. Eng. 2015, 3, 2187–2193. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Sarma, S.J.; Brar, S.K. A Comprehensive Review of Castor Oil-Derived Renewable and Sustainable Industrial Products. Environ. Prog. Sustain. Energy 2022, 42, e1377. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Lim, W.B.; Min, J.G.; Seo, M.J.; Lee, J.H.; Bae, J.H.; Huh, P. Synthesis and properties of biodegradable waterborne polyurethane modified as castor oil. Results Mater. 2023, 19, 100433. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Ahmad, M.; Ibrahim, W.H.W.; Sazali, J.; Izhab, I.; Hassan, Z. Thermal process of castor and plant-based oil. Indones. J. Chem. 2019, 20, 237–247. [Google Scholar] [CrossRef]
- Kovačević, T.; Mijatov, S.; Gržetić, J.; Cakić, S.; Fidanovski, B.; Brzić, S. The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin. Def. Technol. 2024, 33, 66–77. [Google Scholar] [CrossRef]
- Qian, S.; Wang, H.; Huang, C.; Zhao, Y. Experimental Investigation on the Tribological Properties of Modified Carbon Nanotubes as the Additive in Castor Oil. Ind. Lubr. Tribol. 2018, 70, 499–505. [Google Scholar] [CrossRef]
- Bianchi, M.; Vargas, T.; Filho, R.; Guimarães, L.; Heck, L.; Pavarini, S.; Driemeier, D. Spontaneous Poisoning by Ricinus communis in Sheep. Acta Sci. Vet. 2018, 46, 4. [Google Scholar] [CrossRef]
- Joshua, P.E.; Chukwuka, R.S.; Arazu, A.V.; Ngoga, G.; Ogwuegbu, M.C.; Cosmas, S. Phytochemical and Toxicity Analysis of Ricinus communis. Asian J. Biol. 2020, 10, 34–41. [Google Scholar] [CrossRef]
- Bernstein, N.; Akram, M.; Yaniv-Bachrach, Z.; Daniyal, M. Is It Safe to Consume Traditional Medicinal Plants During Pregnancy? Phytother. Res. 2020, 35, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Akande, T.; Odunsi, A.; Akinfala, E. A Review of Nutritional and Toxicological Implications of Castor Bean (Ricinus communis L.) Meal in Animal Feeding Systems. J. Anim. Physiol. Anim. Nutr. 2015, 100, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Das, S.K. Comparative Evaluation of Mahua (Bassia latifolia) Oil Cake and Castor Bean (Ricinus communis) Seed as Fish Toxicants for Tilapia (Oreochromis mossambicus) and Panchax (Aplocheilus panchax) with Residual Toxicity Assessment on Labeo bata. Aquac. Res. 2019, 50, 2341–2349. [Google Scholar] [CrossRef]
- Herawati, I.; Lesmana, R.; Levita, J.; Subarnas, A. Analysis of crude ricin from Ricinus communis originated from Nganjuk, East Java, Indonesia, using liquid chromatography, column liquid chromatography, and fast protein liquid chromatography (FPLC). Rasayan J. Chem. 2022, 15, 850–857. [Google Scholar] [CrossRef]
- Pingale, S. Toxicity study of Ricinus communis. J. Curr. Pharm. Res. 2019, 9, 28–34. [Google Scholar]
- Whitfield, S.; Padgen, D.; Knight, S.; Gwyther, R.; Holley, J.; Clark, G.; Green, A. Establishment of a novel oral murine model of ricin intoxication and efficacy assessment of ovine ricin antitoxins. Toxins 2020, 12, 784. [Google Scholar] [CrossRef]
- Benson, J.; Gomez, A.; Wolf, M.; Tibbetts, B.; March, T. The acute toxicity, tissue distribution, and histopathology of inhaled ricin in Sprague Dawley rats and BALB/c mice. Inhal. Toxicol. 2011, 23, 247–256. [Google Scholar] [CrossRef]
- Falach, R.; Sapoznikov, A.; Evgy, Y.; Aftalion, M.; Makovitzki, A.; Agami, A.; Mimran, A.; Lerer, E.; Ben David, A.; Zichel, R.; et al. Post-exposure anti-ricin treatment protects swine against lethal systemic and pulmonary exposures. Toxins 2020, 12, 354. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Pereira, L.F.; Ribeiro, R.L.; Wolfman, C.; Medina, J.H.; Scorza, F.A.; Da Cunha, C. Ricinine-elicited seizures: A novel chemical model of convulsive seizures. Pharmacol. Biochem. Behav. 2000, 65, 577–583. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).