Insights into the Activities and Usefulness of Deoxynojirimycin and Morus alba: A Comprehensive Review
Abstract
1. Introduction
2. Materials and Methods
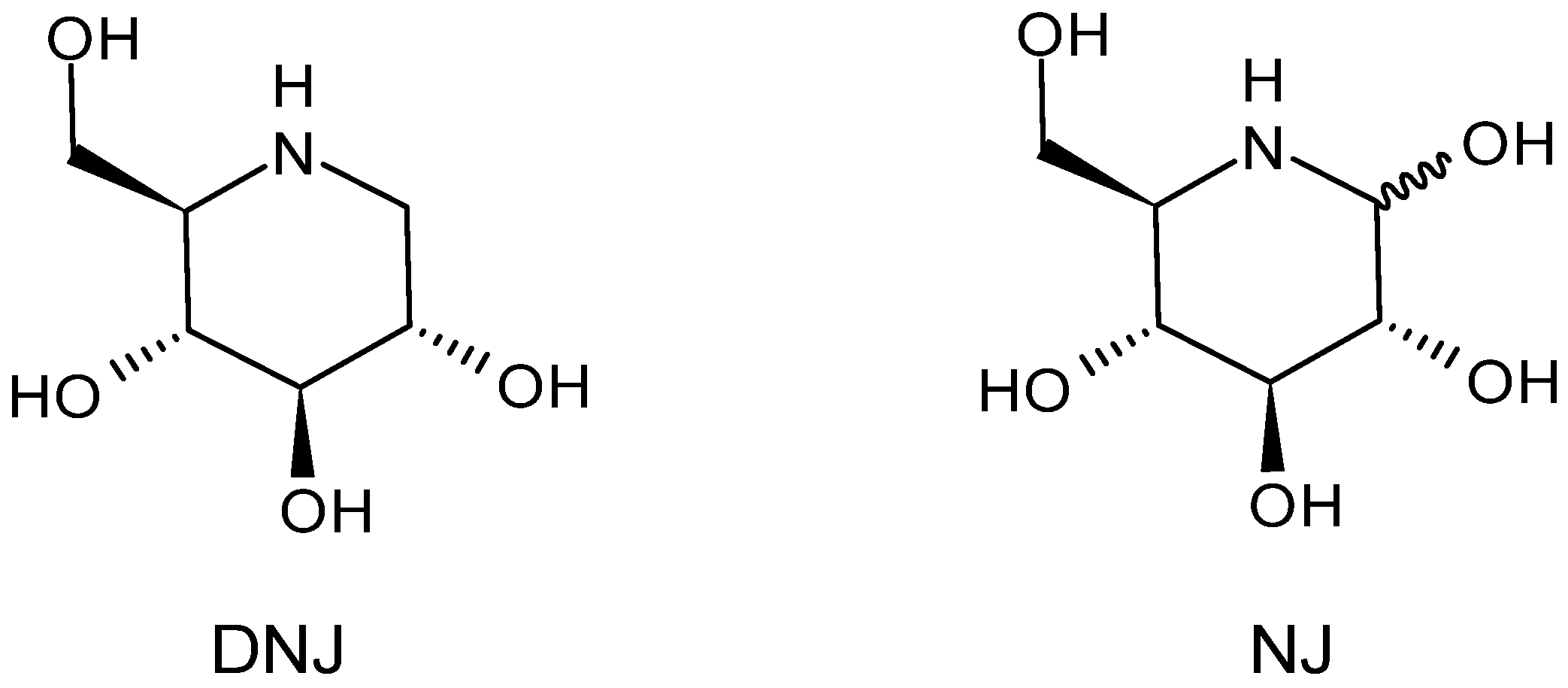
3. History and Chemistry of Deoxynojirimycin
4. Methods for Deoxynojirimycin Production
4.1. Production of Deoxynojirimycin from Plants, Microbes, and Insects
4.2. Methods for Enhancing DNJ Production
4.3. Improvements in the Preparation of DNJ and Quantitative Determination of DNJ
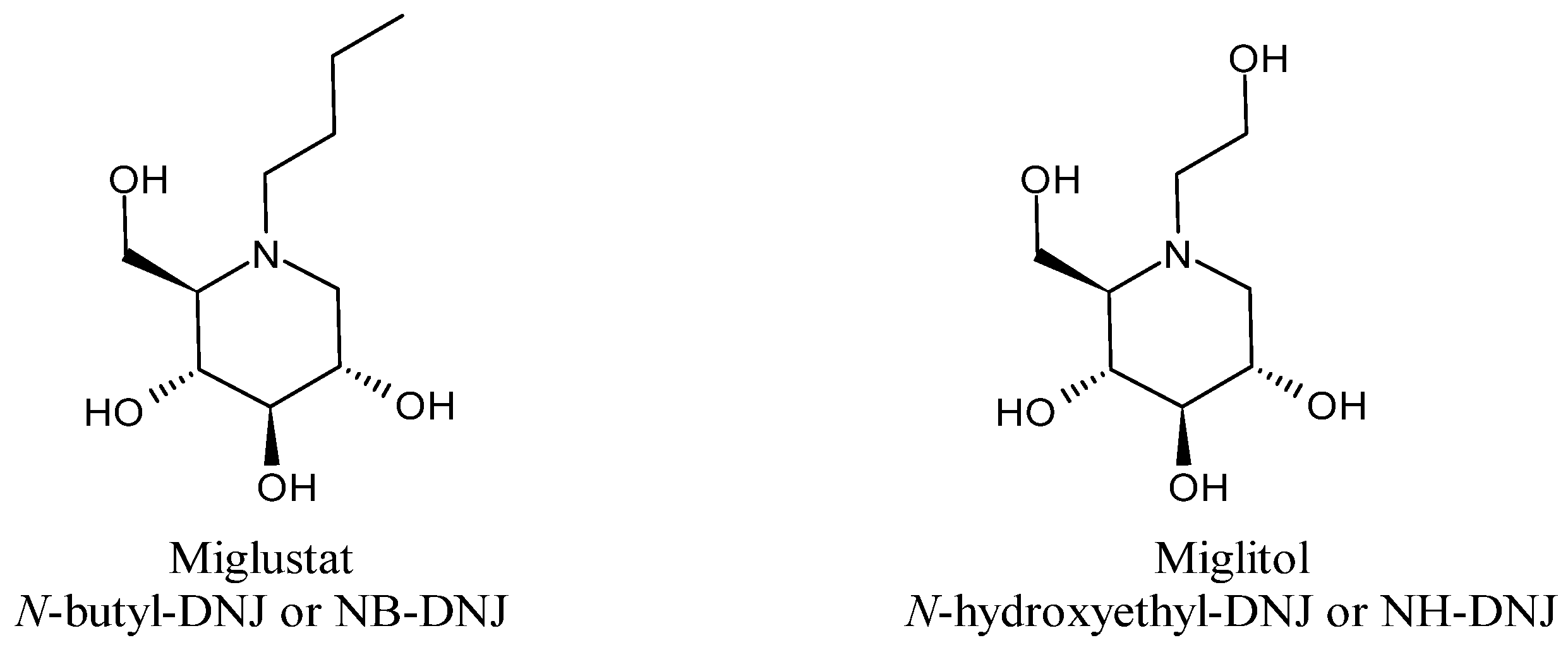
5. N-Alkylated Derivatives and Congeners of Deoxynojirimycin
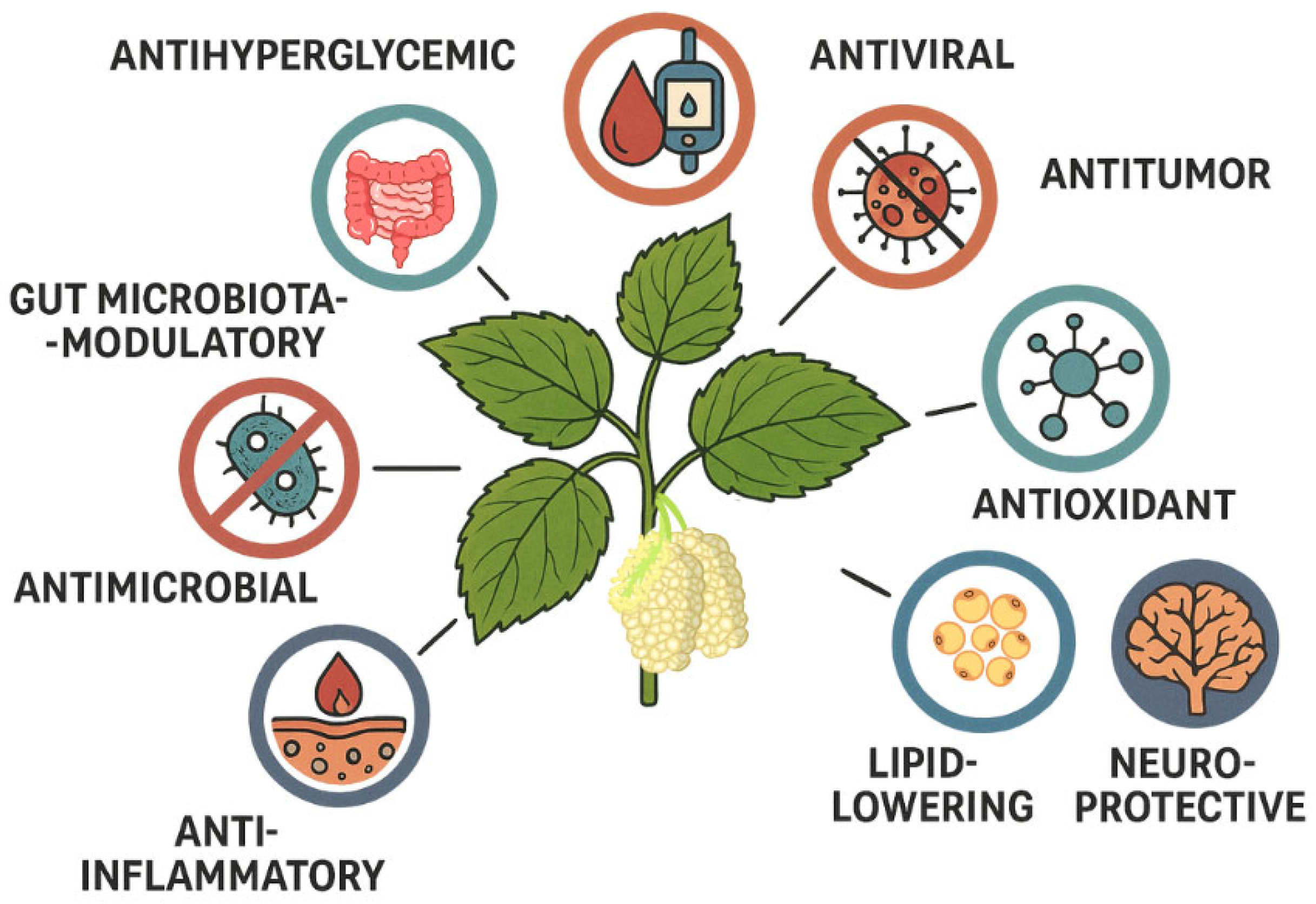
6. Biological Activities of Deoxynojirimycin
6.1. Antioxidant Activity
6.2. Antiviral Activity
6.3. Activity in Diabetes and Cardiovascular Diseases
6.4. Antiobesity Activity
6.5. Neuroprotective Effect
6.6. Anti-Inflammatory Activity
6.7. Anti-Hyperlipidemic, Liver Diseases, and Gut Microbiota-Modulatory Activities
6.8. Antimicrobial Activity
6.9. Anticancer Activity
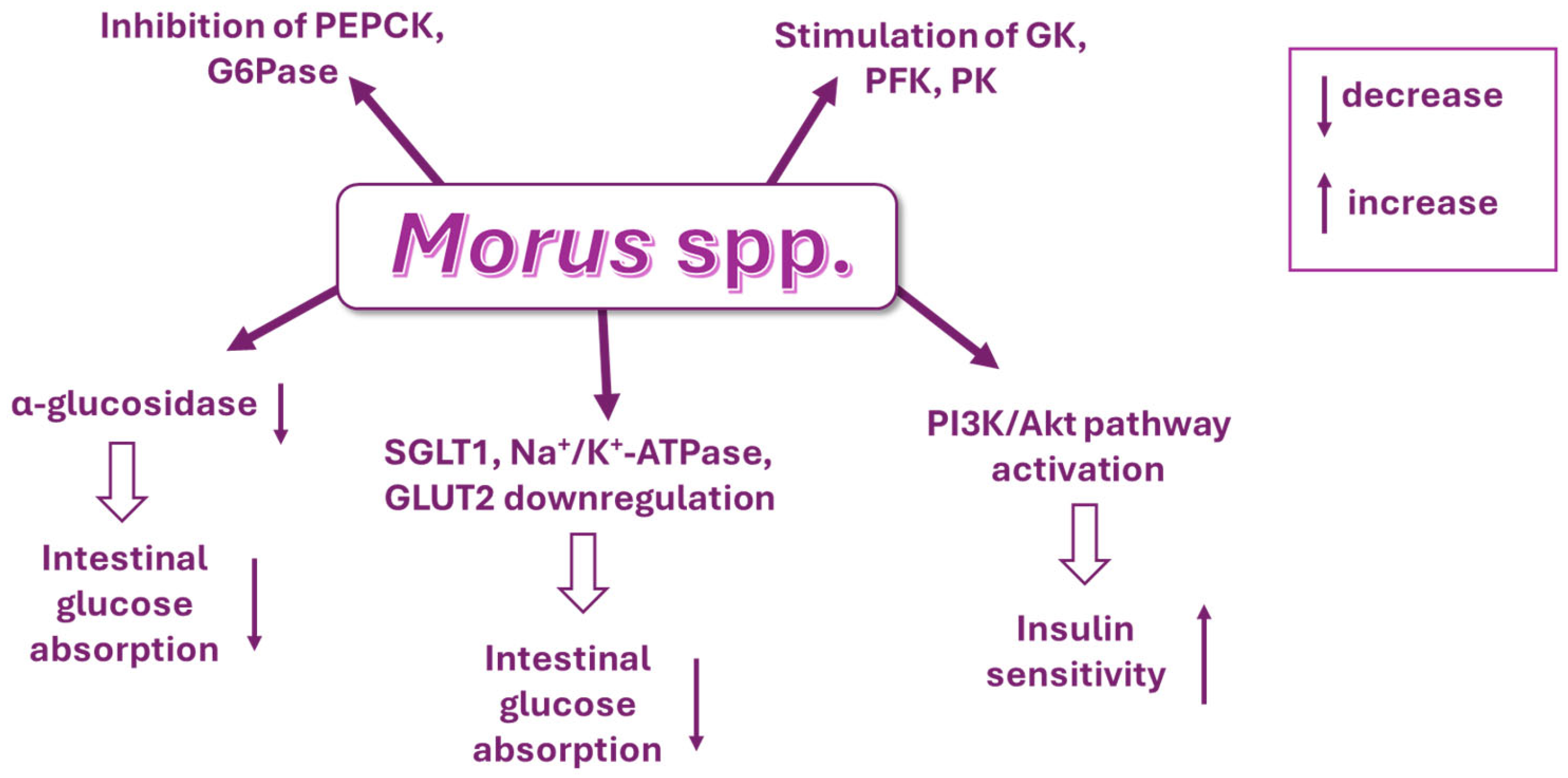
7. Mechanisms of Action of Deoxynojirimycin
8. Studies on Mulberry
8.1. Reducose®
Clinical Studies on Reducose®
8.2. Glubloc
Clinical Studies on GlublocTM
8.3. Studies on Mulberry spp.
8.3.1. Studies on Herbal Compositions and Plant-Based Supplements Containing Mulberry
8.3.2. Studies on Mulberry as a Functional Food
8.3.3. Clinical Studies on Mulberry
9. Toxicity and Allergies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | adenosine 5′-monophosphate-activated protein kinase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | ferric reducing antioxidant power |
| GLUT | glucose transporter |
| HUVEC | human umbilical vein endothelial cells |
| iAUC | incremental area under the curve |
| ICR | Institute of Cancer Research |
| IL | interleukin |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| LDL-C | low-density lipoprotein cholesterol |
| IRS1 | insulin receptor substrate-1 |
| MLE | mulberry leaf extract |
| MLP | mulberry leaf powder |
| NRF2 | nuclear factor (erythroid-derived 2)-like 2 |
| NQO-1 | NAD(P)H:quinone oxidoreductase-1 |
| OGG1 | 8-oxoguanine DNA glycosylase |
| PI3K | phosphatidylinositol 3-kinase |
| PPG | postprandial plasma/blood glucose |
| PPI | postprandial plasma insulin |
| SOD | superoxide dismutase |
| SIRT1 | sirtuin 1 |
| TC | total cholesterol |
| TG | triglycerides |
| TNF-α | tissue necrosis factor a |
References
- Kishnani, P.; Tarnopolsky, M.; Roberts, M.; Sivakumar, K.; Dasouki, M.; Dimachkie, M.M.; Finanger, E.; Goker-Alpan, O.; Guter, K.A.; Mozaffar, T.; et al. Duvoglustat HCl Increases Systemic and Tissue Exposure of Active Acid α-Glucosidase in Pompe Patients Co-Administered with Alglucosidase α. Mol. Ther. 2017, 25, 1199–1208. [Google Scholar] [CrossRef]
- Beck, M. Treatment strategies for lysosomal storage disorders. Dev. Med. Child Neurol. 2018, 60, 13–18. [Google Scholar] [CrossRef]
- Bellotti, A.S.; Andreoli, L.; Ronchi, D.; Bresolin, N.; Comi, G.P.; Corti, S. Molecular Approaches for the Treatment of Pompe Disease. Mol. Neurobiol. 2020, 57, 1259–1280. [Google Scholar] [CrossRef]
- Eruygur, N.; Dural, E. Determination of 1-Deoxynojirimycin by a Developed and Validated HPLC-FLD Method and Assessment of In-Vitro Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activity in Mulberry Varieties from Turkey. Phytomedicine 2019, 53, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.G.; Wen, P.; Shen, W.Z.; Liu, F.; Li, Q.; Li, E.N.; Liao, S.T.; Wu, H.; Zou, Y.X. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model. J. Nat. Prod. 2019, 82, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ji, W.; Liu, Q.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; Liu, S.; et al. Morus alba L. (Sangzhi) alkaloids (SZ-A) Exert Anti-Inflammatory Effects via Regulation of MAPK Signaling in Macrophages. J. Ethnopharmacol. 2021, 280, 114483. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sakamoto, Y.; Mizowaki, Y.; Iwagaki, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. Intake of Mulberry 1-Deoxynojirimycin Prevents Colorectal Cancer in Mice. J. Clin. Biochem. Nutr. 2017, 61, 47–52. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Silva, A.A.N.; Silva, V.A. Phytotherapy in Reducing Glycemic Index and Testicular Oxidative Stress Resulting from Induced Diabetes: A Review. Braz. J. Biol. 2017, 77, 68–78. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Li, T.; Zhao, C.; Barcenas, A.; Serrano, B.; Qu, L.; Shen, M.; Zhao, W. The Effects of 1-Deoxynojirimycin from Mulberry on Oxidative Stress and Inflammation in Laying Hens and the Direct Effects on Intestine Epithelium Cells In Vitro. Animals 2023, 13, 2830. [Google Scholar] [CrossRef]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef]
- Misumi, I.; Li, Z.; Sun, L.; Das, A.; Shiota, T.; Cullen, J.; Zhang, Q.; Whitmire, J.K.; Lemon, S.M. Iminosugar Glucosidase Inhibitors Reduce Hepatic Inflammation in Hepatitis A Virus-Infected Ifnar1−/− Mice. J. Virol. 2021, 95, e0005821. [Google Scholar] [CrossRef]
- Hong, K.Q.; Fu, X.M.; Yin, H.; Li, S.T.; Chen, T.; Wang, Z.W. Advances in the Extraction, Purification and Detection of the Natural Product 1-Deoxynojirimycin. Crit. Rev. Anal. Chem. 2021, 51, 246–257. [Google Scholar] [CrossRef]
- Phimarn, W.; Wichaiyo, K.; Silpsavikul, K.; Sungthong, B.; Saramunee, K. A Meta-analysis of Efficacy of Morus alba Linn. to Improve Blood Glucose and Lipid Profile. Eur. J. Nutr. 2017, 56, 1509–1521. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry Leaves and their Potential Effects against Cardiometabolic Risks: A Review of Chemical Compositions, Biological Properties and Clinical Efficacy. Pharm. Biol. 2018, 56, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.; Khan, S.N.; Haque, I.; Alam, M.; Mushfiq, M.; Khan, A.U. Novel Anti-adherence Activity of Mulberry Leaves: Inhibition of Streptococcus mutans Biofilm by 1-Deoxynojirimycin Isolated from Morus alba. J. Antimicrob. Chemother. 2008, 62, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, J.; Zheng, S.; Liang, F.; Luo, Y.; Huang, K.; Xu, W.; He, X. Mulberry Leaves Ameliorate Obesity through Enhancing Brown Adipose Tissue Activity and Modulating Gut Microbiota. Food Funct. 2019, 10, 4771–4781. [Google Scholar] [CrossRef]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of High 1-Deoxynojirimycin (DNJ) Content Mulberry Tea and Use of Response Surface Methodology to Optimize Tea-Making Conditions for Highest DNJ Extraction. LWT Food Sci. Technol. 2012, 45, 226–232. [Google Scholar] [CrossRef]
- Kong, W.-H.; Oh, S.-H.; Ahn, Y.-R.; Kim, K.-W.; Kim, J.-H.; Seo, S.-W. Antiobesity Effects and Improvement of Insulin Sensitivity by 1-Deoxynojirimycin in Animal Models. J. Agric. Food Chem. 2008, 56, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, Y.; Zhao, L.; Ye, Y. 1-Deoxynojirimycin and its Derivatives: A Mini Review of the Literature. Curr. Med. Chem. 2021, 28, 628–643. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Thakur, K.; Zhang, Y.Y.; Mocan, A.; Zhang, F.; Zhang, J.G.; Wei, Z.J. 1-Deoxynojirimycin, its Potential for Management of Non-Communicable Metabolic Diseases. Trends Food Sci. Technol. 2019, 89, 88–99. [Google Scholar] [CrossRef]
- Yamagishi, K.; Onose, S.; Takasu, S.; Ito, J.; Ikeda, R.; Kimura, T.; Nakagawa, K.; Miyazawa, T. Lactose Increases the Production of 1-Deoxynojirimycin in Bacillus amyloliquefaciens. Food Sci. Technol. Res. 2017, 23, 349–353. [Google Scholar] [CrossRef]
- Shi, J.; Xu, J.; Liu, X.; Goda, A.; Salem, S.H.; Deabes, M.M.; Ibrahim, M.I.M.; Naguib, K.; Mohamed, S.R. Evaluation of Some Artificial Food Preservatives and Natural Plant Extracts as Antimicrobial Agents for Safety. Discov. Food 2024, 4, 89. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, Y.; Wang, W.; Yan, Y.; Ye, H.; Jabbar, S.; Zeng, X. Extraction Optimization, Characterization and Antioxidant Activity In Vitro of Polysaccharides from Mulberry (Morus alba L.) Leaves. Carbohydr. Polym. 2015, 128, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Glycosidase-inhibiting Alkaloids: Isolation, Structure, and Application. In Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Fattorusso, E., Taglialatela-Scafati, O., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 111–138. [Google Scholar]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional Constituents of Mulberry and Their Potential Applications in Food and Pharmaceuticals: A Review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- de Paiva Brandão, M.L.; Romano, C.A.; Paz, A.T.S.; de Paula, J.R. What Is the Impact of Research with Morus nigra?–A Scientometric Study. Res. Soc. Dev. 2021, 10, e49310212838. [Google Scholar] [CrossRef]
- Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15, 65. [Google Scholar] [CrossRef]
- Xin, X.; Jiang, X.; Thomas, A.; Niu, B.; Zhang, M.; Xu, X.; Zhang, R.; Li, H.; Gui, Z. Studies on 1-Deoxynojirimycin Biosynthesis in Mulberry (Morus alba L.) Seeds through Comparative Transcriptomics. Nat. Prod. Res. 2024, 38, 2585–2594. [Google Scholar] [CrossRef]
- Rayamajhi, V.; Dhakal, D.; Chaudhary, A.K.; Sohng, J.K. Improved Production of 1-Deoxynojirymicin in Escherichia coli through Metabolic Engineering. World J. Microbiol. Biotechnol. 2018, 34, 77. [Google Scholar] [CrossRef]
- Wrodnigg, T.M. From Lianas to Glycobiology Tools: Twenty-Five Years of 2,5-Dideoxy-2,5-imino-D-mannitol. Mon. Chem. 2002, 133, 393–426. [Google Scholar] [CrossRef]
- Mehmood, A.; Battino, M.; Chen, X. 1-Deoxynojirimycin: A Comprehensive Review of Sources, Biosynthesis Pathways, Strategies to Enhance Its Production, and Anti-Diabetic Activities. Food Funct. 2025, 16, 4673–4701. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, W.; Wu, H.; Liang, Z. An Overview of the Biological Production of 1-Deoxynojirimycin: Current Status and Future Perspective. Appl. Microbiol. Biotechnol. 2019, 103, 9335–9344. [Google Scholar] [CrossRef]
- Asano, N.; Kato, A.; Watson, A.A. Therapeutic Applications of Sugar-mimicking Glycosidase Inhibitors. Mini Rev. Med. Chem. 2001, 1, 145–154. [Google Scholar] [CrossRef]
- Conforti, I.; Marra, A. Iminosugars as Glycosyltransferase Inhibitors. Org. Biomol. Chem. 2021, 19, 5439–5475. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Chen, Y.; Wang, Y. Functional Benefits of Mulberry Leaf Tea or Extracts to Alleviate Metabolic Diseases: Current Opinion and Perspectives. Food Biosci. 2024, 59, 104218. [Google Scholar] [CrossRef]
- Trimarco, V.; Gallo, P.; Ghazihosseini, S.; Izzo, A.; Rozza, P.I.; Spinelli, A.; Cristiano, S.; De Rosa, C.; Rozza, F.; Morisco, C. White Mulberry Plant Extracts in Cardiovascular Prevention: An Update. Nutrients 2025, 17, 2262. [Google Scholar] [CrossRef] [PubMed]
- Morales Ramos, J.G.; Esteves Pairazamán, A.T.; Mocarro Willis, M.; Collantes Santisteban, S.; Caldas Herrera, E. Medicinal Properties of Morus alba for the Control of Type 2 Diabetes Mellitus: A Systematic Review. F1000Research 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Awolade, P.; Cele, N.; Kerru, N.; Gummidi, L.; Oluwakemi, E.; Singh, P. Therapeutic Significance of β-Glucuronidase Activity and its Inhibitors: A Review. Eur. J. Med. Chem. 2020, 187, 111921. [Google Scholar] [CrossRef] [PubMed]
- Dhara, D.; Dhara, A.; Bennett, J.; Murphy, P.V. Cyclisations and strategies for stereoselective synthesis of piperidine iminosugars. Chem. Rec. 2021, 21, 2958–2979. [Google Scholar] [CrossRef]
- Chang, J.; Block, T.M.; Guo, J.T. Antiviral Therapies Targeting Host ER Alpha-glucosidases: Current Status and Future Directions. Antivir. Res. 2013, 99, 251–260. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.E.; Hong, S.C.; Kim, S.Y.; Kwon, H.C.; Hyun, C.G.; Choi, J. Genome Analysis of Streptomyces nojiriensis JCM 3382 and Distribution of Gene Clusters for Three Antibiotics and an Azasugar Across the Genus Streptomyces. Microorganisms 2021, 9, 1802. [Google Scholar] [CrossRef] [PubMed]
- Mayasa, V.; Raghuveer, P.; Chintamaneni, P.K.; Gali, C.C.; Neerudu, U.K. Safety Assessment of a Standardized Polyherbal Formulation (Glubloc™): Acute Toxicity Study and 28-Day Repeated Dose Toxicological Studies. bioRxiv, 2023; in press. [Google Scholar] [CrossRef]
- Bennett, J.J.; Murphy, P.V. Flow Chemistry Based Catalytic Hydrogenation for Improving the Synthesis of 1-Deoxynojirimycin (DNJ) from an L-sorbose Derived Precursor. Carbohyd. Res. 2023, 529, 108845. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Seo, M.J.; Jang, S. 1-Deoxynojirimycin-Producing Bacteria: Production, Optimization, Biosynthesis, Biological Activities. Biotechnol. Bioproc. Eng. 2024, 29, 981–992. [Google Scholar] [CrossRef]
- Kato, A.; Kato, N.; Kano, E.; Adachi, I.; Ikeda, K.; Yu, L.; Okamoto, T.; Banba, Y.; Ouchi, H.; Takahata, H.; et al. Biological properties of D-and L-1-deoxyazasugars. J. Med. Chem. 2005, 48, 2036–2044. [Google Scholar] [CrossRef]
- van den Nieuwendijk, A.M.; van den Berg, R.J.; Ruben, M.; Witte, M.D.; Brussee, J.; Boot, R.G.; van der Marel, G.A.; Aerts, J.M.F.G.; Overkleeft, H.S. Synthesis of Eight 1-Deoxynojirimycin Isomers from a Single Chiral Cyanohydrin. Eur. J. Org. Chem. 2012, 2012, 3437–3446. [Google Scholar] [CrossRef]
- Kawamura, M.Y.; Talero, A.G.; Santiago, J.V.; Garambel-Vilca, E.; Rosset, I.G.; Burtoloso, A.C. Six-Step Syntheses of (−)-1-Deoxyaltronojirimycin and (+)-1-Deoxymannonojirimycin from N-Z-O-TBDPS-l-serinal. J. Org. Chem. 2016, 81, 10569–10575. [Google Scholar] [CrossRef]
- De Angelis, M.; Sappino, C.; Mandic, E.; D’Alessio, M.; De Dominicis, M.G.; Sannino, S.; Primitivo, L.; Mencarelli, P.; Ricelli, A.; Righi, G. Stereodivergent Synthesis of Piperidine Iminosugars 1-Deoxy-D-nojirimycin and 1-Deoxy-D-altronojirimycin. Tetrahedron 2021, 79, 131837. [Google Scholar] [CrossRef]
- Orena, M.; Rinaldi, S. Chiral Nonaromatic Nitrogen-Heterocycles by Asymmetric Intramolecular Haloamination and Haloamidation. Organics 2024, 5, 163–204. [Google Scholar] [CrossRef]
- Ren, F.; Ji, N.; Zhu, Y. Research Progress of α-Glucosidase Inhibitors Produced by Microorganisms and their Applications. Foods 2023, 12, 3344. [Google Scholar] [CrossRef]
- Dahiya, J.; Kumar, G.; Narula, A.K. The Multifaceted Potential of Azasugars: Synthetic Approaches, Molecular Interactions and Therapeutic Usage. J. Chem. Lett. 2025, 6, 79–92. [Google Scholar] [CrossRef]
- Lim, H.J.; Siziya, I.N.; Seo, M.J. Verification and Applications of 1-Deoxynojirimycin Biosynthesis: Recent Advances and Future Prospects. Proc. Biochem. 2024, 141, 118–131. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.U.; Lee, H.S.; Kim, I.; Ahn, M.Y.; Ryu, K.S. Determination of 1-Deoxynojirimycin in Morus alba L. Leaves by Derivatization with 9-Fluorenylmethyl Chloroformate Followed by Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. A 2003, 1002, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Takahashi, M.; Nishida, M.; Miyauchi, M.; Kizu, H.; Kameda, Y.; Arisawa, M.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J.; et al. Homonojirimycin analogues and their glucosides from Lobelia sessilifolia and Adenophora spp. (Campanulaceae). Carbohydr. Res. 2000, 323, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Shibano, M.; Kakutani, K.; Taniguchi, M.; Yasuda, M.; Baba, K. Antioxidant Constituents in the Dayflower (Commelina communis L.) and their α-Glucosidase-Inhibitory Activity. J. Nat. Med. 2008, 62, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Kato, N.; Adachi, I.; Hollinshead, J.; Fleet, G.W.; Kuriyama, C.; Ikeda, K.; Asano, N.; Nash, R.J. Isolation of glycosidase-inhibiting hyacinthacines and related alkaloids from Scilla socialis. J. Nat. Prod. 2007, 70, 993–997. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.J.; Bucheli, P.; Zhang, P.F.; Wei, D.Z.; Lu, Y.H. Phytochemical Profiles of Different Mulberry (Morus sp.) Species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef]
- Qiao, Y.; Ito, M.; Kimura, T.; Ikeuchi, T.; Takita, T.; Yasukawa, K. Inhibitory Effect of Morus australis Leaf Extract and its Component Iminosugars on Intestinal Carbohydrate-Digesting Enzymes. J. Biosci. Bioeng. 2021, 132, 226–233. [Google Scholar] [CrossRef]
- Yatsunami, K.; Ichida, M.; Onodera, S. The Relationship Between 1-Deoxynojirimycin Content and α-Glucosidase Inhibitory Activity in Leaves of 276 Mulberry Cultivars (Morus spp.) in Kyoto, Japan. J. Nat. Med. 2008, 62, 63–66. [Google Scholar] [CrossRef]
- Karim, M.B.; Kanaya, S.; Altaf-Ul-Amin, M. Prediction of Natural Products as Binding Molecules to COVID-19 Spike Proteins. Res. Sq. 2022; preprint. [Google Scholar] [CrossRef]
- da Fonseca, S.S.S.; Rodrigues, T.V.P.; de Souza Pinheiro, W.B.; Teixeira, E.B.; dos Santos, K.I.P.; da Silva, M.G.D.O.P.; de Sousa, A.M.; do Vale, D.M.C.; Pinho, J.D.; Araùjo, T.M.T.; et al. The Effect of 1-Deoxynojirimycin Isolated from Logging Residue of Bagassa guianensis on an In Vitro Cancer Model. Front. Chem. Eng. 2024, 6, 1342755. [Google Scholar] [CrossRef]
- Momeni, H.; Salehi, A.; Absalan, A.; Akbari, M. Hydro-Alcoholic Extract of Morus nigra Reduces Fasting Blood Glucose and HbA1c% in Diabetic Patients, Probably via Competitive and Allosteric Interaction with alpha-Glucosidase Enzyme; A Clinical Trial and In Silico Analysis. J. Complement. Integr. Med. 2022, 19, 763–769. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, L.C.; Chen, H.; Chen, G.H. Optimization of the Fermentation Conditions for 1-Deoxynojirimycin Production by Streptomyces lavendulae Applying the Response Surface Methodology. Int. J. Food Eng. 2011, 7, 1–10. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Y.; Chen, L.; Chen, G.G.; Liang, Z.Q. A Novel Strategy to Regulate 1-Deoxynojirimycin Production Based on Its Biosynthetic Pathway in Streptomyces lavendulae. Front. Microbiol. 2019, 10, 1968. [Google Scholar] [CrossRef]
- Lee, S.M. 1-Deoxynojirimycin Isolated from a Bacillus subtilis Stimulates Adiponectin and GLUT4 Expressions in 3T3-L1 Adipocytes. J. Microbiol. Biotechnol. 2013, 23, 637–643. [Google Scholar] [CrossRef]
- Lee, H.; Jung, D.H.; Seo, D.H.; Chung, W.H.; Seo, M.J. Genome Analysis of 1-Deoxynojirimycin (1-DNJ)-Producing Bacillus velezensis K26 and Distribution of Bacillus sp. Harboring a 1-DNJ Biosynthetic Gene Cluster. Genomics 2021, 113, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.N.; Kim, Y.; Maibunkaew, S.; Park, J.; Nguyen, M.T.; Oh, D.-B.; Kwon, O. Enhanced Production of 1-Deoxynojirimycin in Bacillus subtilis subsp. Inaquosorum by Random Mutagenesis and Culture Optimization. Biotechnol. Bioprocess. Eng. 2021, 26, 265–276. [Google Scholar] [CrossRef]
- Kang, K.D.; Cho, Y.S.; Song, J.H.; Park, Y.S.; Lee, J.Y.; Hwang, K.Y.; Rhee, S.K.; Chung, J.H.; Kwon, O.; Seong, S.I. Identification of the Genes Involved in 1-Deoxynojirimycin Synthesis in Bacillus subtilis MORI 3K-85. J. Microbiol. 2011, 49, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.P.; Singh, D.K.; Singh, S.N.; Singh, S.P.; Kumar, M.; Singh, S. A Review on Silkworm (Bombyx mori Linn.) an Economic Important Insect. Biol. Forum–Int. J. 2022, 14, 482–491. [Google Scholar]
- Rattana, S.; Katisart, T.; Butiman, C.; Sungthong, B. Total Flavonoids, Total Phenolics, 1-Deoxynojirimycin Content and alpha-Glucosidase Inhibitory Activity of Thai Silkworm Races (Bombyx mori Linn.). Pak. J. Pharm. Sci. 2019, 32, 2539–2544. [Google Scholar]
- Anusha, S.; Negi, P.S. Processing of Silkworm (Bombyx mori) Pupae Waste and mealworm (Tenebrio molitor) Larvae: Chemical Characterization of Extracts Rich in Anti-Oxidant, Anti-Diabetic, and Anti-Obesity Activity. Biomass Convers. Bioref. 2025; in press. [Google Scholar] [CrossRef]
- Park, J.W.; Kook, P.R.; Park, J.S.; Cho, Y.H.; Park, S.K.; Lee, J.H.; Kang, S.K.; Kim, S.W.; Kim, S.R.; Jeong, S.H. Comparison of the Nutritional Composition of Silkworms Reared on Artificial and Mulberry Leaf Diets. Int. J. Ind. Entomol. Biomater. 2025, 49, 18–25. [Google Scholar] [CrossRef]
- Liu, G.; Tang, J.; Tu, J.; Guo, X. Solvent Fractionation and LC-MS Profiling, Antioxidant Properties, and α-Glucosidase Inhibitory Activity of Bombyx batryticatus. Molecules 2025, 30, 1021. [Google Scholar] [CrossRef]
- Zhao, Q.; Jia, T.Z.; Cao, Q.C.; Tian, F.; Ying, W.T. A Crude 1-DNJ Extract from Home Made Bombyx batryticatus Inhibits Diabetic Cardiomyopathy-Associated Fibrosis in db/db Mice and Reduces Protein N-Glycosylation Levels. Int. J. Mol. Sci. 2018, 19, 1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Xu, H.; Ge, A.Q.; Yang, K.L.; He, Q.; Ge, J.W. Bombyx batryticatus Extract Activates Coagulation Factor XII to Promote Angiogenesis in Rats with Cerebral Ischemia/Reperfusion Injury. J. Ethnopharmacol. 2024, 319, 117081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Luo, Y.; Xia, X.; He, J.; Zhang, J.; Zeng, Q.; Li, D.; Ma, B.; Zhang, S.; Zhai, C. Dehydrogenase MnGutB1 Catalyzes 1-Deoxynojirimycin Biosynthesis in Mulberry. Plant Physiol. 2023, 192, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Straube, H. Am-aza-ing Antidiabetic: Mulberry Dehydrogenase MnGUTB1 Contributes to the Biosynthesis of 1-Deoxynojirimycin. Plant Physiol. 2023, 193, 700–702. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, S.; Li, Y.; Wei, Y.; Wang, Z.; Xia, X.; Li, D.; He, N. Unraveling the Mechanism of 1-Deoxynojirimycin (DNJ) Accumulation: The Role of SWEET3 in Mulberry Chloroplasts. bioRxiv, 2025; in press. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Wang, D.; Liu, J.; Yu, X.; Wei, Y.; Ouyang, Z. Transcriptome Analysis and Identification of Key Genes Involved in 1-Deoxynojirimycin Biosynthesis of Mulberry (Morus alba L.). PeerJ 2018, 6, e5443. [Google Scholar] [CrossRef]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis Impact on Plant Growth, Soil Health and Environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, J.; Deng, Z.; Wang, Y.; Jiang, X.; Wang, J. Soil Application of Bacillus subtilis Regulates Flavonoid and Alkaloids Biosynthesis in Mulberry Leaves. Metabolites 2024, 14, 180. [Google Scholar] [CrossRef]
- Liao, Y.; Du, W.; Wan, J.; Fan, J.; Pi, J.; Wu, M.; Wei, Y. Mining and Functional Characterization of NADPH-Cytochrome P450 Reductases of the DNJ Biosynthetic Pathway in Mulberry Leaves. BMC Plant Biol. 2024, 24, 133. [Google Scholar] [CrossRef]
- Fan, J.; Liao, Y.; Zhao, Y.; Wan, J.; Wei, Y.; Ouyang, Z. A CYP450 Monooxygenase MaCYP82C169 Discovered from Mulberry Leaves Catalyzes the Methyl Oxidation Reaction in 1-Deoxynojirimycin Biosynthesis. Food Biosci. 2025, 66, 106177. [Google Scholar] [CrossRef]
- Sonthisut, M.; Wongpanya, R.; Phonphoem, A.; Phonphoem, W.P. Enhancement of 1-Deoxynojirimycin Production in Mulberry (Morus spp.) using LED irradiation. Plant Cell Tissue Organ. Cult. 2022, 148, 167–176. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Maibunkaew, S.; Oh, D.B.; Kim, S.G.; Le Han, H.; Kwon, O. Enhanced 1-Deoxynojirimycin Production in Bacillus amyloliquefaciens TU11 Strain via Random Mutagenesis and Statistical Optimization. Biotechnol. Bioprocess Eng. 2025, 30, 386–400. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Lu, Y.; Wu, N.; Chen, J.; Ji, Z.; Zhan, Y.; Ma, X.; Chen, J.; Cai, D.; et al. Metabolic Engineering of Bacillus amyloliquefaciens for Efficient Production of α-Glucosidase Inhibitor 1-Deoxynojirimycin. Synth. Syst. Biotechnol. 2023, 8, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Siziya, I.N.; Lim, H.J.; Jung, D.H.; Baek, S.; Lee, S.; Seo, M.J. Enhancement of Iminosugar Production, 1-Deoxynojirimycin and 1-Deoxymannojirimycin, in Recombinant Corynebacterium glutamicum. Food Sci. Biotechnol. 2025, 34, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Acikara, Ö.B. Ion-exchange Chromatography and Its Applications. Col. Chromatog. 2013, 10, 55744. [Google Scholar] [CrossRef]
- Marchetti, L.; Saviane, A.; Montà, A.d.; Paglia, G.; Pellati, F.; Benvenuti, S.; Bertelli, D.; Cappellozza, S. Determination of 1-Deoxynojirimycin (1-DNJ) in Leaves of Italian or Italy-Adapted Cultivars of Mulberry (Morus sp.pl.) by HPLC-MS. Plants 2021, 10, 1553. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, F.; Tang, C.; Xiao, G.; Li, Z.; Luo, G. Quantitative Determination of 1-Deoxynojirimycin in 146 Varieties of Mulberry Fruit. Int. J. Food Prop. 2021, 24, 1214–1221. [Google Scholar] [CrossRef]
- Sharmila Banu, G.; Latha, R. Extraction and quantification of 1-Deoxynojirimycin in Silkworm during metamorphosis and moulting stages. Res. J. Biotechnol. 2023, 18, 15–21. [Google Scholar] [CrossRef]
- Maikhunthod, B.; Chaipayang, S.; Jittmittraphap, A.; Thippornchai, N.; Boonchuen, P.; Tittabutr, P.; Eumkeb, G.; Sabuakham, S.; Rungrotmongkol, T.; Mahalapbutr, P.; et al. Exploring the Therapeutic Potential of Thai Medicinal Plants: In Vitro Screening and In Silico Docking of Phytoconstituents for Novel AntiSARS-CoV-2 Agents. BMC Complement. Altern. Med. 2024, 24, 274. [Google Scholar] [CrossRef]
- Walkowiak-Bródka, A.; Piekuś-Słomka, N.; Wnuk, K.; Kupcewicz, B. Analysis of White Mulberry Leaves and Dietary Supplements, ATR-FTIR Combined with Chemometrics for the Rapid Determination of 1-Deoxynojirimycin. Nutrients 2022, 14, 5276. [Google Scholar] [CrossRef]
- Ma, J.; Ye, Y.; He, R.; Xiong, Y.; Xiao, R.; Wang, K.; Zhang, Y.; Wu, X. Enrichment of Deoxynojirimycin in Mulberry Using Cation Exchange Resin: Adsorption/Desorption Characteristics and Process Optimization. Food Chem. 2025, 463, 141281. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, Y.; He, J.; Huang, G.; Jiang, X.; Li, Y.; Li, H.; Zhang, R.; Gui, Z. 1-Deoxynojirimycin Derivative Containing Tegafur Induced HCT-116 Cell Apoptosis through Mitochondrial Dysfunction and Oxidative Stress Pathway. ACS Med. Chem. Lett. 2024, 15, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kubota, H.; Kimura, T.; Yamashita, S.; Tsuzuki, T.; Oikawa, S.; Miyazawa, T. Occurrence of orally administered mulberry 1-deoxynojirimycin in rat plasma. J. Agric. Food Chem. 2007, 55, 8928–8933. [Google Scholar] [CrossRef]
- Zervas, M.; Somers, K.L.; Thrall, M.A.; Walkley, S.U. Critical Role for Glycosphingolipids in Niemann-Pick Disease Type C. Curr. Biol. 2001, 11, 1283–1287. [Google Scholar] [CrossRef]
- Watson, A.A.; Fleet, G.W.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids—Natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265295. [Google Scholar] [CrossRef]
- Gu, X.; Gupta, V.; Yang, Y.; Zhu, J.-Y.; Carlson, E.J.; Kingsley, C.; Tash, J.S.; Schönbrunn, E.; Hawkinson, J.; Georg, G.I. Structure-Activity Studies of N-butyl-1-deoxynojirimycin (NB-DNJ) Analogues: Discovery of Potent and Selective Aminocyclopentitol Inhibitors of GBA1 and GBA2. ChemMedChem 2017, 12, 1977–1984. [Google Scholar] [CrossRef]
- Cox, T.; Lachmann, R.; Hollak, C.; Aerts, J.; Zimran, A. Novel Oral Treatment of Gaucher’s Disease with N-Butyldeoxynojirimycin (OGT 918) to Decrease Substrate Biosynthesis. Lancet 2000, 355, 1481–1485. [Google Scholar] [CrossRef]
- Scott, L.J.; Spencer, C.M. Miglitol. Drugs 2000, 59, 521–549. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Tuning Protein Folding in Lysosomal Storage Diseases: The Chemistry Behind Pharmacological Chaperones. Chem. Sci. 2018, 9, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Foucart, Q.; Shimadate, Y.; Marrot, J.; Kato, A.; Désiré, J.; Blériot, Y. Synthesis and Glycosidase Inhibition of Conformationally Locked DNJ and DMJ Derivatives Exploiting a 2-oxo-C-Allyl Iminosugar. Org. Biomol. Chem. 2019, 17, 7204–7214. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Shimadate, Y.; Cheng, B.; Kanekiyo, U.; Kato, A.; Wang, J.Z.; Li, Y.X.; Jia, Y.M.; Fleet, G.W.J.; Yu, C.Y. Synthesis and Glycosidase Inhibition of 5-C-Alkyl-DNJ and 5-C-Alkyl-l-ido-DNJ Derivatives. Eur. J. Med. Chem. 2021, 224, 113716. [Google Scholar] [CrossRef]
- Hatano, A.; Kanno, Y.; Kondo, Y.; Sunaga, Y.; Umezawa, H.; Okada, M.; Yamada, H.; Iwaki, R.; Kato, A.; Fukui, K. Synthesis and Characterization of Novel, Conjugated, Fluorescent DNJ Derivatives for α-Glucosidase Recognition. Bioorg. Med. Chem. 2017, 25, 773–778. [Google Scholar] [CrossRef]
- Iftikhar, M.; Lu, Y.; Zhou, M. An Overview of Therapeutic Potential of N-alkylated 1-Deoxynojirimycin Congeners. Carbohydr. Res. 2021, 504, 108317. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Chan, K.W.K.; Vasudevan, S.G.; Low, J.G. α-Glucosidase Inhibitors as Broad-Spectrum Antivirals: Current Knowledge and Future Prospects. Antivir. Res. 2025, 238, 106147. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Zhou, Y.; Sakamoto, Y.; Yamazaki, A.; Kurumiya, E.; Yamazaki, R.; Hayashi, K.; Kasuya, Y.; Watanabe, K.; Kasahara, J.; et al. N-Butyldeoxynojirimycin (Miglustat) Ameliorates Pulmonary Fibrosis through Inhibition of Nuclear Translocation of Smad2/3. Biomed. Pharmacother. 2023, 160, 114405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.; Jiao, R.; Liu, Z.; Zhang, T.; Chai, D.; Meng, L.; Yang, Z.; Liu, Y.; Wu, H.; et al. Miglustat Ameliorates Isoproterenol-Induced Cardiac Fibrosis via Targeting UGCG. Mol. Med. 2025, 31, 55. [Google Scholar] [CrossRef]
- Esposito, A.; D’Alonzo, D.; Stabile, M.; Firpo, V.; Migliaccio, A.; Artiano, R.; D’Errico, S.; De Gregorio, E.; Guaragna, A. Synthesis of a Di-O-acylated Deoxynojirimycin (DNJ) Derivative and Evaluation of its Antibacterial and Antibiofilm Activity against Staphylococcus aureus and Stenotrophomonas maltophilia. Carbohydr. Res. 2025, 550, 109379. [Google Scholar] [CrossRef]
- Esposito, A.; Rossi, A.; Stabile, M.; Pinto, G.; De Fino, I.; Melessike, M.; Tamanini, A.; Cabrini, G.; Lippi, G.; Aureli, M.; et al. Assessing the Potential of N-Butyl-l-deoxynojirimycin (l-NBDNJ) in Models of Cystic Fibrosis as a Promising Antibacterial Agent. ACS Pharmacol. Transl. Sci. 2024, 7, 1807–1822. [Google Scholar] [CrossRef]
- Callahan, M.; Treston, A.M.; Lin, G.; Smith, M.; Kaufman, B.; Khaliq, M.; Evans DeWald, L.; Spurgers, K.; Warfield, K.L.; Lowe, P.; et al. Randomized Single Oral Dose Phase 1 Study of Safety, Tolerability, and Pharmacokinetics of Iminosugar UV-4 Hydrochloride (UV-4B) in Healthy Subjects. PLoS Negl. Trop. Dis. 2022, 16, e0010636. [Google Scholar] [CrossRef]
- Verhaegen, M.; Vermeire, K.; Verhaegen, M.; Vermeire, K.; Verhaegen, M.; Vermeire, K. The Endoplasmic Reticulum (ER): A Crucial Cellular Hub in Flavivirus Infection and Potential Target Site for Antiviral Interventions. Npj Viruses 2024, 2, 24. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. 1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling. Appl. Sci. 2024, 14, 3186. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, M.; Li, T.; Li, D.; Feng, Y.; Wang, Y.; Qu, L.; Barcenas, A.R.; Serrano, B.R.; Shen, M.; et al. Effects of 1-Deoxynojirimycin Extracts of Mulberry Leaves on Oxidative Stress and the Function of the Intestinal Tract in Broilers Induced by H2O2. Animals 2024, 14, 3319. [Google Scholar] [CrossRef]
- Xing, W.; Li, M.; Wang, B.; Huo, L.; Tian, W.; Ge, F.; Shen, M.; Sun, L.; Liu, J.; Yu, S. Effect of 1-DNJ on Oxidative Stress-Induced Apoptosis in Porcine Ovarian GCs through Modulation of the PERK-ATF4/MFN2 Signaling Pathway. Antioxidants 2025, 14, 456. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Liu, T.; Fan, J.; Xia, Z.; Zhou, Y.; Deng, X. Expanding Horizons of Iminosugars as Broad-Spectrum Anti-Virals: Mechanism, Efficacy and Novel Developments. Nat. Prod. Bioprospect. 2024, 14, 55. [Google Scholar] [CrossRef]
- Ferjancic, Z.; Bihelovic, F.; Vulovic, B.; Matovic, R.; Trmcic, M.; Jankovic, A.; Pavlovic, M.; Djurkovic, F.; Prodanovic, R.; Djelmas, A.D.; et al. Development of Iminosugar-based Glycosidase Inhibitors as Drug Candidates for SARS-CoV-2 Virus Via Molecular Modelling and In Vitro Studies. J. Enzym. Inhib. Med. Chem. 2024, 39, 2289007. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Koyama, A.H.; Malfer, C.; Gee, S.L.; Schlesinger, M.J. The Effects of Inhibitors of Glucosidase I on the Formation of Sindbis Virus. Virus Res. 1985, 2, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, P.S.; Bowlin, T.L.; Liu, P.S.; Sjoerdsma, A. Antiretroviral Activity of Castanospermine and Deoxynojirimycin, Specific Inhibitors of Glycoprotein Processing. Biochem. Biophys. Res. Commun. 1987, 148, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C.; Robinson, W.E., Jr.; Mitchell, W.M. Role of Protein N-Glycosylation in Pathogenesis of Human Immunodeficiency Virus Type 1. Proc. Natl. Acad. Sci. USA 1988, 85, 9248–9252. [Google Scholar] [CrossRef]
- Lad, V.J.; Shende, V.R.; Gupta, A.K. Effect of Catanospermine, 1-Deoxynojirimycin or 1-Deoxymannojirimycin on Biological and Functional Activities of Japanese Encephalitis Virus in Porcine Stable Kidney Cells. Microbiol. Res. 2013, 4, e3. [Google Scholar] [CrossRef]
- Qu, X.; Pan, X.; Weidner, J.; Yu, W.; Alonzi, D.; Xu, X.; Butters, T.; Block, T.; Guo, J.T.; Chang, J. Inhibitors of Endoplasmic Reticulum α-Glucosidases Potently Suppress Hepatitis C Virus Virion Assembly and Release. Antimicrob. Agents Chemother. 2011, 55, 1036–1044. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Ma, K.; Shen, M.; Liu, J.; Zhang, Y.; Sun, L. Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus. Animals 2025, 15, 1207. [Google Scholar] [CrossRef]
- Durantel, D.; Carrouée-Durantel, S.; Branza-Nichita, N.; Dwek, R.A.; Zitzmann, N. Effects of Interferon, Ribavirin, And Iminosugar Derivatives on Cells Persistently Infected with Noncytopathic Bovine Viral Diarrhea Virus. Antimicrob. Agents Chemother. 2004, 48, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Karade, S.S.; Franco, E.J.; Rojas, A.C.; Hanrahan, K.C.; Kolesnikov, A.; Yu, W.; MacKerell, A.D., Jr.; Hill, D.C.; Weber, D.J.; Brown, A.N.; et al. Structure-Based Design of Potent Iminosugar Inhibitors of Endoplasmic Reticulum α-Glucosidase I with Anti-SARS-CoV-2 Activity. J. Med. Chem. 2023, 66, 2744–2760. [Google Scholar] [CrossRef] [PubMed]
- Dwek, R.A.; Bell, J.I.; Feldmann, M.; Zitzmann, N. Host-Targeting Oral Antiviral Drugs to Prevent Pandemics. Lancet 2022, 399, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Arman, B.Y.; Hill, M.L.; Kiappes, J.L.; Alonzi, D.S.; Makower, L.L.; Witt, K.D.; Gileadi, C.; Rangel, V.; Dwek, R.A.; et al. Assessment of Repurposed Compounds against Coronaviruses Highlights the Antiviral Broad-Spectrum Activity of Host-Targeting Iminosugars and Confirms the Activity of Potent Directly Acting Antivirals. Antivir. Res. 2025, 237, 106123. [Google Scholar] [CrossRef]
- Courageot, M.-P.; Frenkiel, M.-P.; Duarte Dos Santos, C.; Deubel, V.; Desprès, P. α-Glucosidase Inhibitors Reduce Dengue Virus Production by Affecting the Initial Steps of Virion Morphogenesis in the Endoplasmic Reticulum. J. Virol. 2000, 74, 564–572. [Google Scholar] [CrossRef]
- Perera, N.; Brun, J.; Alonzi, D.S.; Tyrrell, B.E.; Miller, J.L.; Zitzmann, N. Antiviral Effects of Deoxynojirimycin (DNJ)-Based Iminosugars in Dengue Virus-Infected Primary Dendritic Cells. Antivir. Res. 2022, 199, 105269. [Google Scholar] [CrossRef]
- Tyrrell, B.E.; Kumar, A.; Gangadharan, B.; Alonzi, D.; Brun, J.; Hill, M.; Bharucha, T.; Bosworth, A.; Graham, V.; Dowall, S.; et al. Exploring the Potential of Iminosugars as Antivirals for Crimean-Congo Haemorrhagic Fever Virus, Using the Surrogate Hazara Virus: Liquid-Chromatography-Based Mapping of Viral N-Glycosylation and in Vitro Antiviral assays. Pathogens 2023, 12, 399. [Google Scholar] [CrossRef]
- Cui, W.Y.; Luo, K.Y.; Xiao, Q.; Sun, Z.Y.; Wang, Y.F.; Cui, C.F.; Chen, F.C.; Xu, B.; Shen, W.J.; Wan, F.C.; et al. Effect of Mulberry Leaf or Mulberry Leaf Extract on Glycemic Traits: A Systematic Review and Meta-Analysis. Food Funct. 2023, 14, 1277–1289. [Google Scholar] [CrossRef]
- Cho, I.H.; Cheng, R.; Jung, C.W.; Won, T.H.; Wang, D.; Jang, H.H.; Hwang, I.G.; Kwon, S.W. Effects of 1-Deoxynojirimycin on Glycemic Control: A Systematic Review and Meta-Analysis. NFS J. 2024, 38, 100210. [Google Scholar] [CrossRef]
- Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients 2022, 14, 4538. [Google Scholar] [CrossRef] [PubMed]
- Elbakry, M.; Elremaly, W. Antidiabetic Activity of Some Common Medicinal Plants. Biol. Biomed. J. 2023, 1, 1–16. [Google Scholar] [CrossRef]
- Kang, C.W.; Park, M.; Lee, H.J. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life 2022, 12, 1630. [Google Scholar] [CrossRef]
- Zhuang, Q.; Guo, F.; Fu, L.; Dong, Y.; Xie, S.; Ding, X.; Hu, S.; Zhou, X.D.; Jiang, Y.; Zhou, H.; et al. 1-Deoxynojirimycin Promotes Cardiac Function and Rescues Mitochondrial Cristae in Mitochondrial Hypertrophic Cardiomyopathy. J. Clin. Investig. 2023, 133, e164660. [Google Scholar] [CrossRef] [PubMed]
- Ntalouka, F.; Tsirivakou, A. Morus alba: Natural and Valuable Effects in Weight Loss Management. Front. Clin. Diabetes Healthc. 2024, 5, 1395688. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G.; McPhee, D.J. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice. Molecules 2015, 20, 21700–21714. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Y.; Cui, W.; Lu, G.; Wang, Y.; Gao, H.; Huang, L.; Mu, Z. A Polysaccharide Extract of Mulberry Leaf Ameliorates Hepatic Glucose Metabolism and Insulin Signaling in Rats with Type 2 Diabetes Induced by High Fat-Diet and Streptozotocin. Int. J. Biol. Macromol. 2015, 72, 951–959. [Google Scholar] [CrossRef]
- Cai, S.; Sun, W.; Fan, Y.; Guo, X.; Xu, G.; Xu, T.; Hou, Y.; Zhao, B.; Feng, X.; Liu, T. Effect of Mulberry Leaf (Folium mori) on Insulin Resistance via IRS-1/PI3K/Glut-4 Signalling Pathway in Type 2 Diabetes Mellitus Rats. Pharm. Biol. 2016, 54, 2685–2691. [Google Scholar] [CrossRef]
- Chen, W.; Liang, T.; Zuo, W.; Wu, X.; Shen, Z.; Wang, F.; Li, C.; Zheng, Y.; Peng, G. Neuroprotective effect of 1-Deoxynojirimycin on Cognitive Impairment, β-Amyloid Deposition, and Neuroinflammation in the SAMP8 Mice. Biomed. Pharmacother. 2018, 106, 92–97. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Ito, J.; Eitsuka, T.; Nakagawa, K. 1-Deoxynojirimycin Attenuates Pathological Markers of Alzheimer’s Disease in the In Vitro Model of Neuronal Insulin Resistance. FASEB J. 2024, 38, e23800. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Cao, H.; Ji, W.; Li, C.; Huan, Y.; Lei, L.; Fu, Y.; Gao, X.; Liu, Y.; et al. Ramulus Mori (Sangzhi) Alkaloids (SZ-A) Ameliorate Glucose Metabolism Accompanied by the Modulation of Gut Microbiota and Ileal Inflammatory Damage in Type 2 Diabetic KKAy Mice. Front. Pharmacol. 2021, 12, 642400. [Google Scholar] [CrossRef]
- Peng, D.; Zhuge, F.; Wang, M.; Zhang, B.; Zhuang, Z.; Zhou, R.; Zhang, Y.; Li, J.; Yu, Z.; Shi, J. Morus alba L.(Sangzhi) Alkaloids Mitigate Atherosclerosis by Regulating M1/M2 Macrophage Polarization. Phytomedicine 2024, 128, 155526. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Shang, Y.; Wang, Y.; Chen, Y.; Mu, B.; Zheng, Q.; Liu, H. 1-DNJ Alleviates Obesity-Induced Testicular Inflammation in Mice Model by Inhibiting IKKβ/NF-kB Pathway. Reprod. Sci. 2024, 31, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, S.; Yu, J.; Sun, Y.; Zhu, J.; Ji, D.; Wu, C. The Mulberry-Derived 1-Deoxynojirimycin (DNJ) Inhibits High-Fat Diet (HFD)-Induced Hypercholesteremia and Modulates the Gut Microbiota in a Gender-Specific Manner. J. Funct. Foods 2019, 52, 63–72. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C. The Gut Microbiota-Produced Indole-3-Propionic Acid Confers the Antihyperlipidemic Effect of Mulberry-Derived 1-Deoxynojirimycin. Msystems 2020, 5, e00313-20. [Google Scholar] [CrossRef]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of NASH in Endocrine Diseases. Endocr. Connect. 2021, 10, R52–R65. [Google Scholar] [CrossRef]
- Le, M.H.; Le, D.M.; Baez, T.C.; Wu, Y.; Ito, T.; Lee, E.Y.; Lee, K.; Stave, C.D.; Henry, L.; Barnett, S.D.; et al. Global Incidence of Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis of 63 Studies and 1,201,807 Persons. J. Hepatol. 2023, 79, 287–295. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, L.; Hu, B.; Zou, X.; Hu, H.; Zhang, Z.; Jiang, N.; Ma, J.; Yang, H.; Liu, H. 1-Deoxynojirimycin Improves High Fat Diet-Induced Nonalcoholic Steatohepatitis by Restoring Gut Dysbiosis. J. Nutr. Biochem. 2019, 71, 16–26. [Google Scholar] [CrossRef]
- Do, H.J.; Chung, J.H.; Hwang, J.W.; Kim, O.Y.; Lee, J.Y.; Shin, M.J. 1-Deoxynojirimycin Isolated from Bacillus subtilis Improves Hepatic Lipid Metabolism and Mitochondrial Function in High-Fat-Fed Mice. Food Chem. Toxicol. 2015, 75, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Liu, J.X.; Wang, F.F.; Qu, L.P. Mulberry Leaf Extract and Deoxynojirimycin Modulates Glucose and Lipid Levels via the IRS1/PI3K/AKT Signaling Pathway in Cells. J. Food Biochem. 2025, 2025, 7345044. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Tian, Y.Y.; Chen, W.W.; Wang, J.; Zhang, M.M.; Wang, S.W.; Xie, Y.H. 1-Deoxynojirimycin Ameliorates Diabetic Liver Injury by Regulating AMPK/SIRT1 and Oxidative Stress in db/db Mice. Endocr. Metab. Immune Dis. Drug Targets, 2025; in press. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; D’Amato, A.; Lauria, G.; Saturnino, C.; Andreu, I.; Longo, P.; Sinicropi, M.S. Diarylureas: New Promising Small Molecules against Streptococcus mutans for the Treatment of Dental Caries. Antibiotics 2023, 12, 112. [Google Scholar] [CrossRef]
- Gaviappa, D.; Matthew, S.; Deveswaran, R.; Bharkhavy, K.V. Evaluation of 1-DNJ Isolated from Mulberry Plant for its Anticariogenic Efficacy. In Advancements in Science and Technology for Healthcare, Agriculture, and Environmental Sustainability; CRC Press: Boca Raton, FL, USA, 2024; pp. 89–93. ISBN 9781032708348. [Google Scholar]
- Yoo, Y.; Seo, D.H.; Lee, H.; Cho, E.S.; Song, N.E.; Nam, T.G.; Nam, Y.D.; Seo, M.J. Inhibitory Effect of Bacillus velezensis on Biofilm Formation by Streptococcus mutans. J. Biotechnol. 2019, 298, 57–63. [Google Scholar] [CrossRef]
- Wang, R.J.; Yang, C.H.; Hu, M.L. 1-Deoxynojirimycin Inhibits Metastasis of B16F10 Melanoma Cells by Attenuating the Activity and Expression of Matrix Metalloproteinases-2 and -9 and Altering Cell Surface Glycosylation. J. Agric. Food Chem. 2010, 58, 8988–8993. [Google Scholar] [CrossRef]
- Ramappa, V.K.; Srivastava, D.; Singh, P.; Kumar, U.; Singh, V. Mulberry 1-Deoxynojirimycin (DNJ): An Exemplary Compound for Therapeutics. J. Hortic. Sci. Biotechnol. 2020, 95, 679–686. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, S.; Ai, X.; Wei, L. 1-Deoxynojirimycin Affects High Glucose-Induced Pancreatic Beta-Cell Dysfunction through Regulating CEBPA Expression and AMPK Pathway. Biochem. Cell Biol. 2024, 103, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, M.; Zhang, Y.; Zhang, H. Improvement Activity of 1-Deoxynojirimycin in the Growth of Dairy Goat Primary Mammary Epithelial Cell through Upregulating LEF-1 Expression. Biomed Res. Int. 2018, 2018, 7809512. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Chen, J.J.; Li, Q.Q.; Zeng, G.Y.; Chen, Q.Y.; Chen, J.L.; Liao, Z.M.; Jin, P.; Wang, K.S.; Yang, Z.C. Alpha-Glucosidase Inhibitor 1-Deoxynojirimycin Promotes Beige Remodeling of 3T3-L1 Preadipocytes via Activating AMPK. Biochem. Biophys. Res. Commun. 2019, 509, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Goel, B.; Bhardwaj, N.; Vishwakarma, R.A.; Jain, S.K. Exploring the Potential of Chemical Inhibitors for Targeting Post-Translational Glycosylation of Coronavirus (SARS-CoV-2). ACS Omega 2022, 7, 27038–27051. [Google Scholar] [CrossRef]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars Past, Present and Future: Medicines for Tomorrow. Drug Discov. Today 2011, 16, 107–118. [Google Scholar] [CrossRef]
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef]
- Yu, C.; Hu, Q. Regulating the PI3K and AMPK pathway: The secret of 1-deoxynojirimycin’s success in alleviating chronic diseases. J. Future Foods 2025, 5, 551–560. [Google Scholar] [CrossRef]
- Porasar, P.; Gibo, R.; Gogoi, B.; Sharma, D.; Bharadwaj, A.; Gam, S.; Hazarika, D.; Dutta, K.N. A Systematic Review on the Phytochemistry, Isolated Compounds, Nutritional Benefits, Pharmacology and Toxicology of the Plant Species Morus alba L. Discover Plants 2025, 2, 7. [Google Scholar] [CrossRef]
- Maqsood, M.; Anam Saeed, R.; Sahar, A.; Khan, M.I. Mulberry Plant as a Source of Functional Food with Therapeutic and Nutritional Applications: A Review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef]
- Sarkhel, S. Nutrition Importance and Health Benefits of Mulberry Leaf Extract: A Review. J. Pharmacog. Phytochem. 2020, 9, 689–695. [Google Scholar] [CrossRef]
- Fatima, M.; Dar, M.A.; Dhanavade, M.J.; Abbas, S.Z.; Bukhari, M.N.; Arsalan, A.; Liao, Y.; Wan, J.; Shah Syed Bukhari, J.; Ouyang, Z. Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges. Biology 2024, 13, 506. [Google Scholar] [CrossRef]
- Chang, B.Y.; Koo, B.S.; Kim, S.Y. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Tong, Y.; Wang, J.; Zhang, J.; Wei, X.; Si, D.; Zhang, R. Potential Role and Mechanism of Mulberry Extract in Immune Modulation: Focus on Chemical Compositions, Mechanistic Insights, and Extraction Techniques. Int. J. Mol. Sci. 2024, 25, 5333. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.I.; Jang, S.; Kim, K.H. Morus alba L. for Blood Sugar Management: A Systematic Review and Meta-Analysis. Evid. Based Complement Alternat. Med. 2022, 2022, 9282154. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Zadeh, A.M.; Asemi, Z.; Farrokhnezhad, A.H.; Memarzadeh, M.R.; Banikazemi, Z.; Shariat, M.; Shafabakhsh, R. Morus alba Leaf Extract Affects Metabolic Profiles, Biomarkers Inflammation and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Double-Blind Clinical Trial. Clin. Nutr. ESPEN 2022, 49, 68–73. [Google Scholar] [CrossRef]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of Mulberry Leaves’ Hypoglycemic Properties and Hypoglycemic Mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, B.; Armas Diaz, Y.; Cassotta, M.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Xiao, J.B.; Zhang, D.; Xiaobo, Z.; et al. Therapeutic Potential of Mulberry (Morus alba L.) Byproducts for Cardiovascular Diseases. J. Berry Res. 2025, 15, 3–11. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Hussain, A.; Tebyaniyan, H. Green Alternatives as Antimicrobial Agents in Mitigating Periodontal Diseases: A Narrative Review. Microorganisms 2023, 11, 1269. [Google Scholar] [CrossRef]
- Gunjal, S.; Ankola, A.; Bhat, K. In Vitro Antibacterial Activity of Ethanolic Extract of Morus alba Leaf against Periodontal Pathogens. Indian J. Dent. Res. 2015, 26, 533–536. [Google Scholar] [CrossRef]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.d.C.A.; Freitas, K.d.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, Pharmacology, and Clinical Trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Su, Y.; Jia, J.; Bian, X.; Gu, Y.; Lv, G.; Chen, S.; Jiang, N. Mulberry–Nutritional Value, Health Benefits, and its Applications in Food, Biomaterials, and Medicine: A Systematic Review with Bibliometric Analysis. Nat. Prod. Comm. 2025, 20, 1934578X251314698. [Google Scholar] [CrossRef]
- Rohela, G.K.; Muttanna, P.S.; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An Ideal Plant for Sustainable Development. Trees For. People 2020, 2, 100011. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A Review of Bioactive Compounds and Advanced Processing Technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Shao, Y.; Lin, L.; Xu, W.; Gong, Z.; Li, J.; Zhang, J.; Yan, X.; Liu, Z.; Xiao, W. Investigation on Quality Characteristics and Antidiabetic Properties of Mulberry Leaf Fu Brick Tea. J. Food Biochem. 2024, 2024, 8826062. [Google Scholar] [CrossRef]
- Seneta, W.; Dolatowski, J. Dendrologia; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2007. [Google Scholar]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, Bioactivities and Future Prospects of Mulberry Leaves: A Review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Baciu, E.-D.; Baci, G.-M.; Moise, A.R.; Dezmirean, D.S.A.; Baciu, E.-D.; Baci, G.-M.; Moise, A.R.; Dezmirean, D.S. A Status Review on the Importance of Mulberry (Morus spp.) and Prospects towards Its Cultivation in a Controlled Environment. Horticulturae 2023, 9, 444. [Google Scholar] [CrossRef]
- Saini, P.; Rohela, G.K.; Kumar, J.S.; Shabnam, A.A.; Kumar, A. Cultivation, Utilization, and Economic Benefits of Mulberry. In The Mulberry Genome; Gnanesh, B.N., Vijayan, K., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 13–56. ISBN 978-3-031-28478-6. [Google Scholar]
- Wang, Y.; Ai, Q.; Gu, M.; Guan, H.; Yang, W.; Zhang, M.; Mao, J.; Lin, Z.; Liu, Q.; Liu, J. Comprehensive Overview of Different Medicinal Parts from Morus alba L.: Chemical compositions and Pharmacological Activities. Front. Pharmacol. 2024, 15, 1364948. [Google Scholar] [CrossRef]
- Marx, T.K.; Glávits, R.; Endres, J.R.; Palmer, P.A.; Clewell, A.E.; Murbach, T.S.; Hirka, G.; Pasics, I. A 28-Day Repeated Dose Toxicological Study of an Aqueous Extract of Morus alba L. Int. J. Toxicol. 2016, 35, 683–691. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Hirka, G.; Pasics Szakonyiné, I. A 90-Day Preclinical Toxicological Evaluation in Rats of a Highly Purified and Concentrated Mulberry Leaf Extract. J. Appl. Toxicol. 2024, 44, 1504–1517. [Google Scholar] [CrossRef]
- Lee, D.; Baek, J.Y.; Choi, Y.J.; Han, M.J.; Kim, S.H.; Kim, T.H.; Lee, S.; Kang, K.S. Glucose-Lowering Effect of Reducose® Enriched with 1-Leoxynojirimycin and l-Leucine: Studies on Insulin Secretion in INS-1 Cells and Reduction of Blood Glucose in Diabetic Rats. Heliyon 2024, 10, e25499. [Google Scholar] [CrossRef] [PubMed]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-Extract Improves Glucose Tolerance and Decreases Insulin Concentrations in Normoglycaemic Adults: Results of a Randomised Double-Blind Placebo-Controlled Study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef] [PubMed]
- Thondre, P.S.; Lightowler, H.; Ahlstrom, L.; Gallagher, A. Mulberry Leaf Extract Improves Glycaemic Response and Insulaemic Response to Sucrose in Healthy Subjects: Results of a Randomized, Double Blind, Placebo-Controlled Study. Nutr. Metab. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Thondre, P.S.; Butler, I.; Tammam, J.; Achebe, I.; Young, E.; Lane, M.; Gallagher, A. Understanding the Impact Of Different Doses of Reducose® Mulberry Leaf Extract on Blood Glucose and Insulin Responses after Eating a Complex Meal: Results from a Double-Blind, Randomised, Crossover Trial. Nutrients 2024, 16, 1670. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Dietary Bioactive Compounds and Human Health: The Role of Bioavailability. Nutrients 2024, 17, 48. [Google Scholar] [CrossRef]
- Gali, C.C.; Palle, L. Glubloc™ Reduces Postprandial Blood Glucose Surge in Healthy Individuals (A Placebo Controlled Pilot study). Prepr. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Dash, R.; Venugopal, G.; Chakravarthi Gali, C.; Palle, L.; Ramadass, B. Abstract No: ABS071. GLUBLOC™—The Effect on Post-High Carbohydrate Meal Intake in Healthy Adults and their Postprandial Glucose and Insulin Level Management—Randomized, Placebo-Controlled, Single-Blinded, Double Crossover Study. 2023. Available online: https://journals.lww.com/indjem/fulltext/2023/27001/oral.2.aspx?context=latestarticles (accessed on 15 May 2025).
- Venugopal, G.; Dash, R.; Agrawal, S.; Ray, S.; Kumar Sahoo, P.; Ramadass, B. A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study. Nutrients 2024, 16, 2237. [Google Scholar] [CrossRef]
- Konda, S.G.; Bhashyam, S.; Kasivelu, G.; Ramala, S.; Gali, C.C. A Ready-To-Mix Nutraceutical Supplement (GLUBLOC) Lowers Postprandial Blood Glucose Levels in Healthy Individuals—A Randomised, Placebo-Controlled, Crossover Study. Nutrire 2024, 49, 56. [Google Scholar] [CrossRef]
- Guntupalli, S.P.; Karpuram, M.; Samala, M.; Gali, C.C. A Novel Nutritional Supplement Reduces Postprandial Glucose Response in Healthy Individuals in a Randomised, Placebo-Controlled, Crossover Clinical Study. Nutrire 2024, 49, 33. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Nakagawa, K. A Comprehensive Review on the Production, Pharmacokinetics and Health Benefits of Mulberry Leaf Iminosugars: Main Focus on 1-Deoxynojirimycin, d-Fagomine, and 2-O-α-d-Galactopyranosyl-DNJ. Crit. Rev. Food Sci. Nutr. 2023, 63, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Angelini, F.; Belli, L.; Cicero, A.F.; Da Ros, R.; De Pergola, G.; Gaudio, G.V.; Lupi, A.; Sartore, G.; et al. Nutraceuticals and Supplements in Management of Prediabetes and Diabetes. Nutrients 2025, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sohouli, M.H.; Nateghi, M.; Melekoglu, E.; Fatahi, S. Impact of Mulberry Consumption on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. J. Clin. Pharm. Ther. 2022, 47, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Wu, Y.L.; Yu, M.H.; Hung, T.W.; Chan, K.C.; Wang, C.J. Mulberry Leaf and Neochlorogenic Acid Alleviates Glucolipotoxicity-Induced Oxidative Stress and Inhibits Proliferation/Migration via Downregulating Ras and FAK Signaling Pathway in Vascular Smooth Muscle Cell. Nutrients 2022, 14, 3006. [Google Scholar] [CrossRef]
- Boers, H.M.; van Dijk, T.H.; Duchateau, G.S.; Mela, D.J.; Hiemstra, H.; Hoogenraad, A.-R.; Priebe, M.G. Effect of Mulberry Fruit Extract on Glucose Fluxes after a Wheat Porridge Meal: A Dual Isotope Study in Healthy Human Subjects. Eur. J. Clin. Nutr. 2023, 77, 741–747. [Google Scholar] [CrossRef]
- Mela, D.J.; Boers, H.M.; Kadam, T.; Hiemstra, H.; Kalathil, R.; Seijen ten Hoorn, J.W. Effect of Mulberry Fruit Extract on Post-Prandial Glycaemic and Insulinemic Responses to Different Rice Types: A Randomised Trial in Healthy Adults. Br. J. Nutr. 2023, 130, 2088–2094. [Google Scholar] [CrossRef]
- Mela, D.J.; Cao, X.-Z.; Govindaiah, S.; Hiemstra, H.; Kalathil, R.; Lin, L.; Manoj, J.; Mi, T.; Verhoeven, C.H. Dose-Response Efficacy of Mulberry Fruit Extract for Reducing Post-Prandial Blood Glucose and Insulin Responses: Randomized Trial Evidence in Healthy Adults. Br. J. Nutr. 2023, 129, 771–778. [Google Scholar] [CrossRef]
- Mela, D.J.; Alssema, M.; Hiemstra, H.; Hoogenraad, A.R.; Kadam, T. Effect of Low-Dose Mulberry Fruit Extract on Postprandial Glucose and Insulin Responses: A Randomized Pilot Trial in Individuals with Type 2 Diabetes. Nutrients 2024, 16, 2177. [Google Scholar] [CrossRef]
- Józefczuk, J.; Malikowska, K.; Glapa, A.; Stawińska-Witoszyńska, B.; Nowak, J.K.; Bajerska, J.; Lisowska, A.; Walkowiak, J. Mulberry Leaf Extract Decreases Digestion and Absorption of Starch in Healthy Subjects—A Randomized, Placebo-Controlled, Crossover Study. Adv. Med. Sci. 2017, 62, 302–306. [Google Scholar] [CrossRef]
- Ann, J.Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) Ameliorate Obesity-Induced Hepatic Lipogenesis, Fibrosis, and Oxidative Stress in High-Fat Diet-Fed Mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-Deoxynojirimycin in Mulberry (Morus indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients with Coronary Heart Disease by Improving Antioxidant and Anti-Inflammatory Capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Ranjan, B.; Kumar, R.; Verma, N.; Mittal, S.; Pakrasi, P.L.; Venkatesh Kumar, R. Evaluation of the Antidiabetic Properties of S-1708 Mulberry Variety. Pharmacogn. Mag. 2017, 13, S280–S288. [Google Scholar] [CrossRef]
- Tond, S.B.; Fallah, S.; Salemi, Z.; Seifi, M. Influence of Mulberry Leaf Extract on Serum Adiponectin, Visfatin and Lipid Profile Levels in Type 2 Diabetic Rats. Brazilian Arch. Biol. Technol. 2016, 59, 16160297. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abd El-Twab, S.M.; Abdel-Reheim, E.S. Consumption of Polyphenol-rich Morus alba Leaves Extract Attenuates Early Diabetic Retinopathy: The Underlying Mechanism. Eur. J. Nutr. 2017, 56, 1671–1684. [Google Scholar] [CrossRef]
- Riche, D.M.; Riche, K.D.; East, H.E.; Barrett, E.K.; May, W.L. Impact of Mulberry Leaf Extract on Type 2 Diabetes (Mul-DM): A Randomized, Placebo-Controlled Pilot Study. Complement. Ther. Med. 2017, 32, 105–108. [Google Scholar] [CrossRef]
- Pan, Y.; Song, Y.; Zhao, M.; Yang, M.; Xiao, N.; Wang, J.; Feng, F. Mulberry Leaf Extract Ameliorates High-Fat Diet-Induced Obesity in Mice by Regulating the Gut Microbiota and Metabolites. Food Biosci. 2024, 62, 105359. [Google Scholar] [CrossRef]
- Li, D.X.; Xu, S.Q.; Jiang, H.; Li, Y.T.; Zhao, Y.L.; Jin, P.; Ji, S.; Du, Y.; Tang, D.Q. Gut Microbiota and Intestinal FXR Signalling Involved in the Alleviation of Mulberry (Morus alba L.) Leaf Ethanol Extract on Type 2 Diabetes Mellitus in db/db Mice. J. Funct. Foods 2024, 123, 106600. [Google Scholar] [CrossRef]
- Forsan, H.F.; Fayed, M.R.; Farahat, N.M.; Gabr, W.M.; Alswerky, E.M.; Abd El-Hak, A.E.; Fouda, M.A.; Safwat, M.A. Polyphenols of Mulberry White (Morus alba L.) Leaves as a Source of Functional Food: A Review. Al-Kitab J. Pure Sci. 2025, 9, 104–127. [Google Scholar] [CrossRef]
- Ai, J.; Bao, B.; Battino, M.; Giampieri, F.; Chen, C.; You, L.; Cespedes, C.; Ognyanov, M.; Tian, L.; Bai, W. Recent advances on bioactive polysaccharides from mulberry. Food Funct. 2021, 12, 5219–5523. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, X.; Deng, Q.; Yang, M.; Li, S.; Zhang, Q.; Sun, Y.; Chen, H. Extraction, Structural Characterization and Biological Activities of Polysaccharides from Mulberry Leaves: A Review. Int. J. Biol. Macromol. 2024, 257, 128669. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Chua An Wen, A.; Wang, S.; Zhang, L.; Yang, H.; Mao, Y.; Jia, J.; Wang, D.; Wang, J.; et al. Combined Mulberry Leaf Polysaccharide-Caged Liposomes for Effective Oral Drug Delivery in Rat Model. Int. J. Nanomed. 2025, 20, 5377–5391. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, B.; Kwon, E.-B.; Chung, H.-S.; Choi, J.-G. Mulberrofuran G, a Mulberry Component, Prevents SARS-CoV-2 Infection by Blocking the Interaction between SARS-CoV-2 Spike protein S1 Receptor-Binding Domain and Human Angiotensin-Converting Enzyme 2 Receptor. Nutrients 2022, 14, 4170. [Google Scholar] [CrossRef]
- Tian, L.L.; Bi, Y.X.; Zhang, H. Structurally Diverse Stilbenoids as Potent α-Glucosidase Inhibitors with Antidiabetic Effect from Morus alba. J. Agric. Food Chem. 2024, 72, 17938–17952. [Google Scholar] [CrossRef]
- Dalla Costa, V.; Piovan, A.; Brun, P.; Filippini, R. Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development. Molecules 2025, 30, 2073. [Google Scholar] [CrossRef]
- Sureshan, M.; Brintha, S.; Jothi, A. Identification of Mulberrofuran as a Potent Inhibitor of Hepatitis A Virus 3CPro and RdRP Enzymes Through Structure-Based Virtual Screening, Dynamics Simulation, and DFT Studies. Mol. Divers. 2024, 28, 1609–1628. [Google Scholar] [CrossRef]
- Soonthornsit, N.; Pitaksutheepong, C.; Hemstapat, W.; Utaisincharoen, P.; Pitaksuteepong, T. In Vitro Anti-Inflammatory Activity of Morus alba L. Stem Extract in LPS-Stimulated RAW 264.7 Cells. Evid.-Based Complement. Altern. Med. 2017, 2017, 3928956. [Google Scholar] [CrossRef]
- Gan, L.; Inamura, Y.; Shimizu, Y.; Yokoi, Y.; Ohnishi, Y.; Song, Z.; Kumaki, Y.; Kikukawa, T.; Demura, M.; Ito, M.; et al. A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites. Metabolites 2023, 13, 1003. [Google Scholar] [CrossRef]
- Parklak, W.; Chottidao, M.; Munkong, N.; Komindr, S.; Monkhai, S.; Wanikorn, B.; Makaje, N.; Kulprachakarn, K.; Chuljerm, H.; Somnuk, S. Nutraceutical Properties of Thai Mulberry (Morus alba L.) and Their Effects on Metabolic and Cardiovascular Risk Factors in Individuals with Obesity: A Randomized, Single-Blind Crossover Trial. Nutrients 2024, 16, 4336. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Liu, J.; Li, M.; Lu, J.; Zhou, J.; Zhang, M.; Ferri, N.; Chen, H. Mulberry Leaf and Its Effects Against Obesity: A Systematic Review of Phytochemistry, Molecular Mechanisms and Applications. Phytomedicine 2024, 128, 155528. [Google Scholar] [CrossRef]
- Mahboubi, M. Morus alba (Mulberry), a Natural Potent Compound in Management of Obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef]
- Aramwit, P.; Supasyndh, O.; Siritienthong, T.; Bang, N. Mulberry Leaf Reduces Oxidation and C-Reactive Protein Level in Patients with Mild Dyslipidemia. Biomed Res. Int. 2013, 2013, 787981. [Google Scholar] [CrossRef]
- Chatterji, S.; Fogel, D. Study of the Effect of the Herbal Composition SR2004 on Hemoglobin A1c, Fasting Blood Glucose, and Lipids in Patients with Type 2 Diabetes Mellitus. Integr. Med. Res. 2018, 7, 248–256. [Google Scholar] [CrossRef]
- Lange, E.; Kęszycka, P.K.; Pałkowska-Goździk, E.; Billing-Marczak, K. Comparison of Glycemic Response to Carbohydrate Meals without or with a Plant-Based Formula of Kidney Bean Extract, White Mulberry Leaf Extract, and Green Coffee Extract in Individuals with Abdominal Obesity. Int. J. Environ. Res. Public Health 2022, 19, 12117. [Google Scholar] [CrossRef]
- Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials. Fermentation 2025, 11, 258. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.; Feng, J.; Yin, J.; Zou, X.; Wang, J.; Wang, B. Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3. Fermentation 2023, 9, 829. [Google Scholar] [CrossRef]
- Chew, H.C.; Tang, P.L.; Tan, X.Y.; Tan, H.Y. Effect of Mulberry Leaf Extract Fortification and Probiotic Fermentation on the Bioactivities of Cottage Cheese. J. Food Measur. Charact. 2022, 16, 486–499. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Gramza-Michalowska, A.; Kmiecik, D.; Flaczyk, E.; Korczak, J. Mulberry Fruit as an Antioxidant Component in Muesli. Agric. Sci. 2013, 4, 130–135. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Dziedziński, M.; Szymanowska, D.; Szczepaniak, O.; Byczkiewicz, S.; Telichowska, A.; Szulc, P. The Effects of Morus alba L. Fortification on the Quality, Functional Properties and Sensory Attributes of Bread Stored under Refrigerated Conditions. Sustainability 2020, 12, 6691. [Google Scholar] [CrossRef]
- Ding, F.; Wang, Q.; Xie, C.; Wang, M.; Zhang, L.; Gao, M.; Yang, Z.; Ma, J.; Shi, X.; Chen, W.; et al. The Impact of Mulberry Leaf Extract at Three Different Levels on Reducing the Glycemic Index of White Bread. PLoS ONE 2023, 18, e0288911. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Niu, X.; Liu, Y.; Xu, L.; Wang, Y.; Zhang, Q.; He, J.; Liu, Y.; Zhang, K.; et al. Postprandial Glycemic Effects of Lactose-Hydrolyzed Milk Supplemented with Mulberry Leaf and Corn Silk Extracts in Adults with Type 2 Diabetes: A Randomized Crossover Trial. Clin. Nutrit. ESPEN 2025, 67, 549–554. [Google Scholar] [CrossRef]
- Midori, Y.; Kenichiro, Y.; Iori, Y.; Kanako, N.; Madoka, S.; Ai, T.M. Mulberry Leaves and Water Chestnut Tea Reduces Postprandial Blood Glucose in Borderline Diabetic Japanese: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Asia Pac. J. Clin. Nutr. 2025, 34, 174–182. [Google Scholar] [CrossRef]
- Lee, C.L.; Chee, W.S.S.; Lee, Y.Y.; Gilcharan Singh, H.K.; Misra, S. Postprandial Glycemic Effect of Mulberry Leaf Extract: A Randomized Crossover Pilot Study in Asians. Nutr. Food Sci. 2025, 55, 661–673. [Google Scholar] [CrossRef]
- Fauzi, A.; Kifli, N.; Noor, M.H.M.; Hamzah, H.; Azlan, A. Bioactivity, Phytochemistry Studies and Subacute In Vivo Toxicity of Ethanolic Leaf Extract of White Mulberry (Morus alba Linn.) in Female Mice. J. Ethnopharmacol. 2024, 325, 117914. [Google Scholar] [CrossRef]
- Velez, M.D. A White Mulberry Leaf Fatality: Re-Examining a Venerable Herb in Light of a High-Profile Event. J. Chin. Med. 2023, 132, 47–54. [Google Scholar]
- Papia, F.; Incorvaia, C.; Genovese, L.; Gangemi, S.; Minciullo, P.L. Allergic Reactions to Genus Morus Plants: A Review. Clin. Mol. Allergy 2020, 18, 1. [Google Scholar] [CrossRef]
| Virus | Ref. |
|---|---|
| Sindbis Virus | [121] |
| Moloney murine leukemia virus | [122] |
| Human Immunodeficiency Virus Type 1 | [123] |
| Japanese Encephalitis Virus | [124] |
| Hepatitis C Virus | [125] |
| Bovine Viral Diarrhea Virus | [126] |
| Porcine Epidemic Diarrhea Virus | [127] |
| SARS-CoV-2 | [128] |
| Dengue Virus | [131,132] |
| Crimean-Congo Hemorrhagic Fever Virus | [133] |
| Activity | Ref. |
|---|---|
| Inhibition of HFD-Induced Hypercholesteremia and Modulation of Gut Microbiota in Male and Female C57BL/6J Mice | [150] |
| Antihyperlipidemic Effect in Male and Female ICR mice | [151] |
| Improvement of HFD-Induced Nonalcoholic Steatohepatitis by Restoring Gut Dysbiosis. | [153] |
| Improvement of Hepatic Lipid Metabolism and Mitochondrial Function in High-Fat-Fed C57BL/6 Male Mice | [154] |
| Modulation of Glucose and Lipid Levels via the IRS1/PI3K/Akt Signaling Pathway in HepG2 Cells | [155] |
| Amelioration of Diabetic Liver Injury by Regulation of AMPK/SIRT1 and Oxidative Stress in db/db Mice | [156] |
| Title (Number) and Type of the Clinical Trial | Participants | Source of GlublocTM and Location of the Study | Conclusions of the Study | Ref. |
|---|---|---|---|---|
| Mulberry extract to modulate blood glucose in healthy adults (ISRCTN14597438) Double-blind, randomized, repeat measure, crossover design trial. | Out of forty randomized normoglycemic healthy adults aged 19–59 years, 37 subjects completed the study | Source: Reducose® extract was provided by Phynova Location: Functional Food Centre at Oxford Brookes University. | Reducose® co-administered with 50 g maltodextrin substantially reduces the increase in plasma glucose after ingestion of maltodextrin over 120 min. | [196] |
| Understanding the impact of different doses of Reducose® mulberry leaf extract on blood glucose and insulin responses after eating a complex meal (ISRCTN99601810) Randomized, double blind, placebo-controlled study. | Out of thirty-eight healthy men and women (aged between 18 and 60 years) thirty-seven participants completed the study. | Source: Reducose® aqueous extract was provided by Sponsor (Phynova Group Ltd., Long Hanborough, UK) by Purapharm Pharmaceuticals Co., Ltd. (Nanning, China). Location: England, United Kingdom | After an overnight fast, participants were given 75 g sucrose + white mulberry leaf extract, or 75 g sucrose alone. The addition of MLE to sucrose resulted in a significantly lower glycemic response and insulinemic response compared to a matched placebo (sucrose alone). | [197] |
| A clinical trial to investigate the effect of a proprietary mulberry leaf extract (Reducose®) on lowering blood glucose rises after consuming a drink containing sugar (sucrose) (ISRCTN18212231) Double-blind, randomized, placebo-controlled, repeat-measure, crossover design trial | Forty-three healthy participants were recruited (18 to 56 years) for the study. Thirty-seven healthy individuals completed the study | Source: Reducose® capsules (batch number 181102) were manufactured by Hunan Hill Pharmaceutical Co., Ltd., Hunan, China. Location: Oxford Brookes Centre for Nutrition and Health. | Participants consumed capsules containing 200 mg, 225 mg, 250 mg Reducose® or placebo before a test meal consisting of 150 g white bread and egg mayo filler. All three doses of Reducose® significantly lowered glucose iAUC 120 and plasma insulin iAUC 120. | [198] |
| Clinical Trial | Participants | Source of GlublocTM and Location of the Study | Conclusions of the Study | Ref. |
|---|---|---|---|---|
| Randomized, crossover, single-blind clinical trial CTRI/2023/05/052654 (Clinical Trial Registry of India (http://ctri.nic.in/) | 116 healthy participants, 85 subjects aged 18–60 years completed the day 1 and 5 crossover study | Source: GlublocTM was provided by My PuraVida Wellness Pvt Ltd., Hyderabad, Telangana, India. Location: Tertiary care hospital, AIIMS Bhubaneswar, Odisha, India. | Premeal supplementation with GlublocTM significantly reduced the postprandial surge in blood glucose and insulin levels after a carbohydrate-rich meal or sucrose drink intake over 120 min in healthy individuals. None of the participants reported any side effects, and no adverse events were recorded during this study. | [202] |
| Randomized, placebo-controlled, crossover study. CTRI/2024/01/061799 (Clinical Trial Registry of India (http://ctri.nic.in/) | 107 healthy subjects aged between 18 and 60 years were recruited, with 102 subjects successfully completing both the study assessments | Source: Standardized MLE + apple peel extract sachets (Glubloc™) were provided by INU Energy Pvt Ltd., Hyderabad, Telangana, India. Location: Department of Internal Medicine, Yashoda hospitals, Hitech City, Hyderabad, Telangana, India | Subjects were asked to eat 3 slices of bread toast with jam (75 g of bread with 45 g of jam, ~480 kilocalories), within 15 min or less. GlublocTM mixed with the high carbohydrate and sucrose meal, significantly reduced the postprandial blood glucose spike by reducing the rate of carbohydrate processing and by delaying its absorption. None of the subjects experienced major gastrointestinal side effects. | [203] |
| Randomized, placebo-controlled, crossover study CTRI/2023/08/056330 (Clinical Trial Registry of India (http://ctri.nic.in/) | 30 healthy south Indian subjects (both male and female), aged between 18 and 60 years | Standardized MLE + apple peel extract sachets (Glubloc™) were provided by My PuraVida Wellness Pvt Ltd., Hyderabad, Telangana, India. Location: department of Medicover hospital, Hitech City, Hyderabad, Telangana, India. | Glubloc™ tablets were administered before the meal, taken orally with a glass of water. Subjects were asked to eat 300 g of carbohydrate- and sugar-rich meal (250 g of Poha with 50 g of Gulab jamun mix, ~600 kcal), within 15 min or less. A single-dose supplementation of GlublocTM 10 min before the carbohydrate- and sugar-rich meal intake, significantly reduced the postprandial blood glucose spike and serum insulin levels. None of the subjects experienced any major gastrointestinal side effects. | [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tricase, A.F.; Cavalluzzi, M.M.; Catalano, A.; De Bellis, M.; De Palma, A.; Basile, G.; Sinicropi, M.S.; Lentini, G. Insights into the Activities and Usefulness of Deoxynojirimycin and Morus alba: A Comprehensive Review. Molecules 2025, 30, 3213. https://doi.org/10.3390/molecules30153213
Tricase AF, Cavalluzzi MM, Catalano A, De Bellis M, De Palma A, Basile G, Sinicropi MS, Lentini G. Insights into the Activities and Usefulness of Deoxynojirimycin and Morus alba: A Comprehensive Review. Molecules. 2025; 30(15):3213. https://doi.org/10.3390/molecules30153213
Chicago/Turabian StyleTricase, Angela Fulvia, Maria Maddalena Cavalluzzi, Alessia Catalano, Michela De Bellis, Annalisa De Palma, Giovanna Basile, Maria Stefania Sinicropi, and Giovanni Lentini. 2025. "Insights into the Activities and Usefulness of Deoxynojirimycin and Morus alba: A Comprehensive Review" Molecules 30, no. 15: 3213. https://doi.org/10.3390/molecules30153213
APA StyleTricase, A. F., Cavalluzzi, M. M., Catalano, A., De Bellis, M., De Palma, A., Basile, G., Sinicropi, M. S., & Lentini, G. (2025). Insights into the Activities and Usefulness of Deoxynojirimycin and Morus alba: A Comprehensive Review. Molecules, 30(15), 3213. https://doi.org/10.3390/molecules30153213









