Stretchability and Melt Strength Enhancement of Biodegradable Polymer Blends for Packaging Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In-Situ Blend Preparation
2.2.1. Determination of Optimal DCP Content
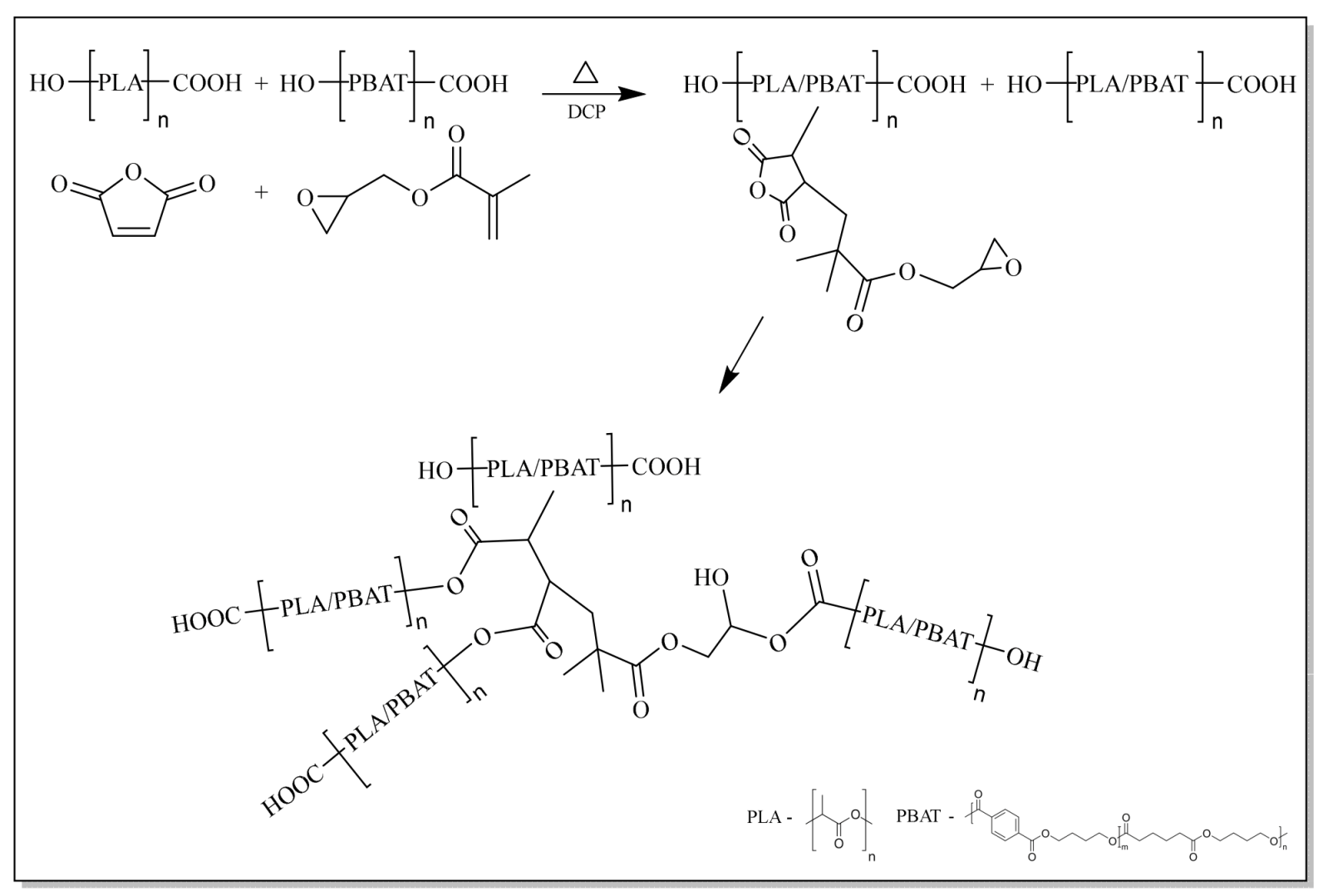
2.2.2. Incorporation of Chain Extenders
2.3. Methods
2.3.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3.2. Rheological Behavior
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Dynamical Mechanical Analysis (DMA)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Tensile Testing
3. Results and Discussion
3.1. Determination of Optimal DCP Content
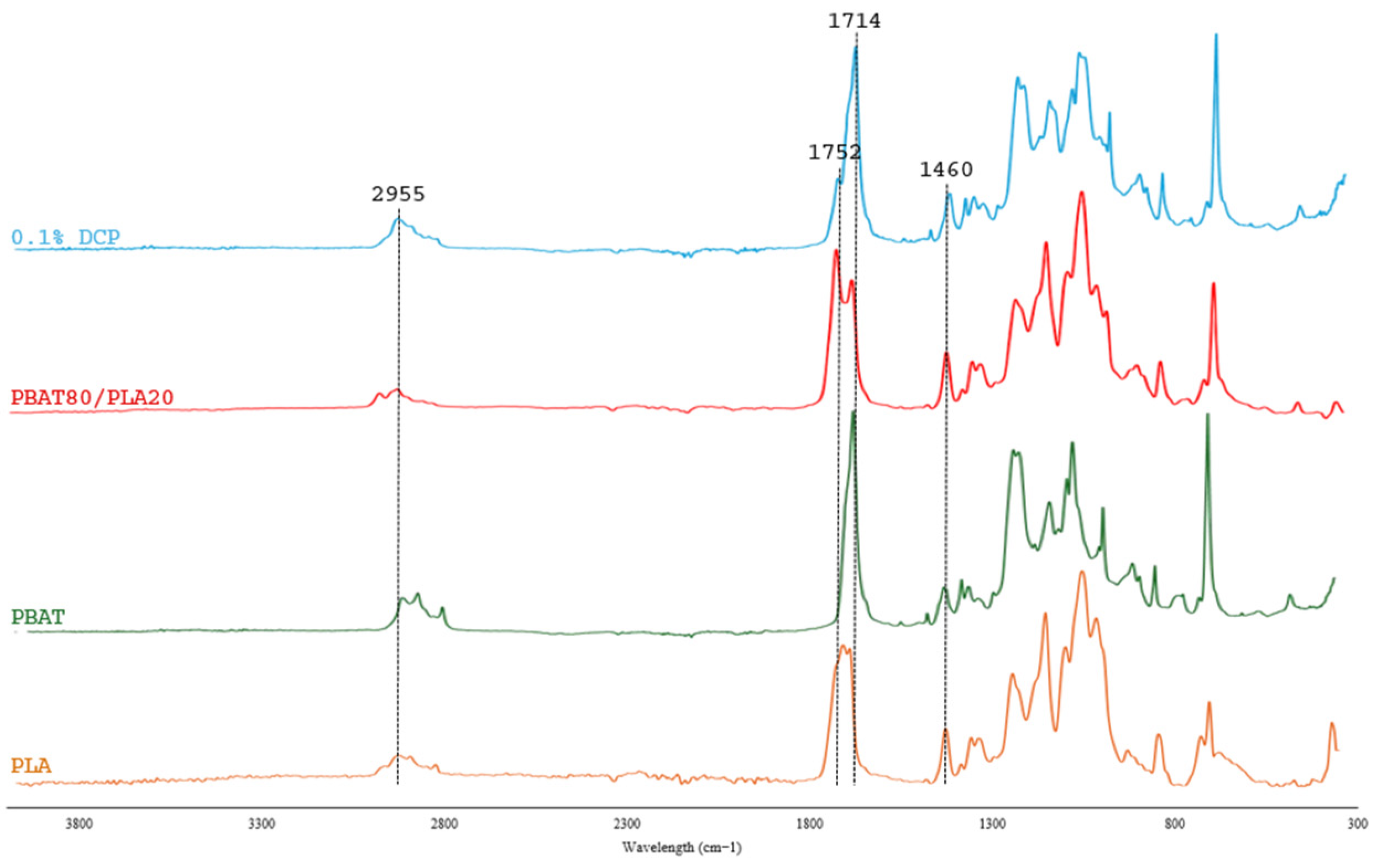
3.1.1. FT-IR Analysis
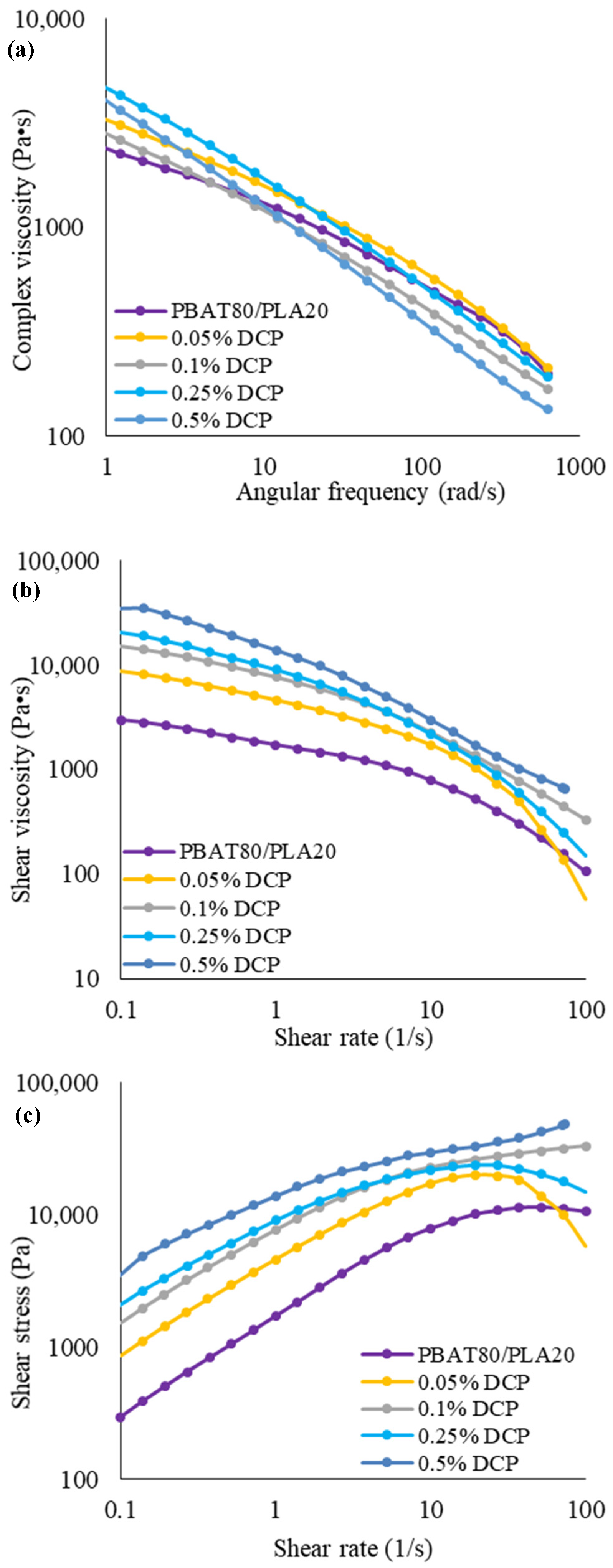
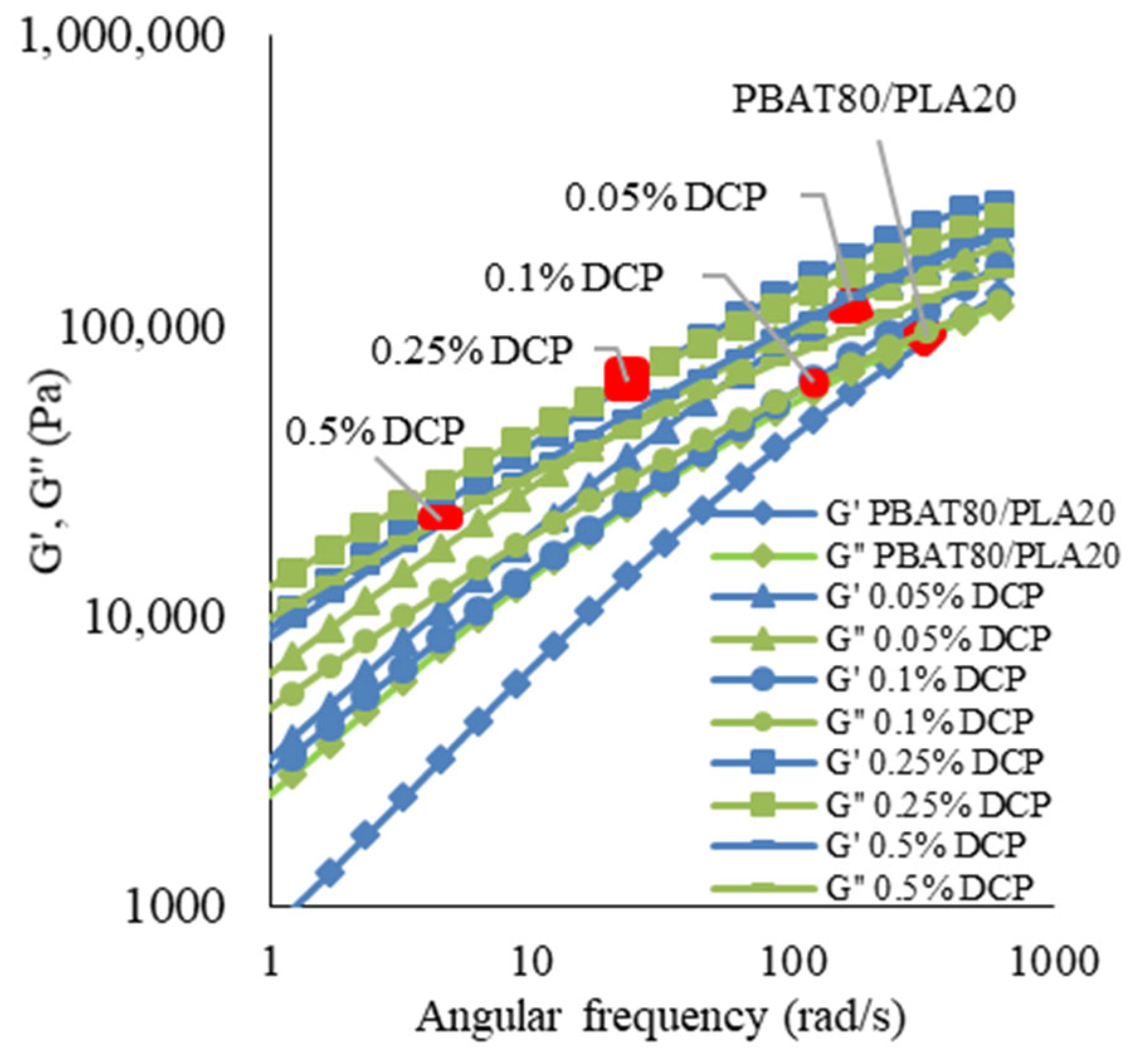
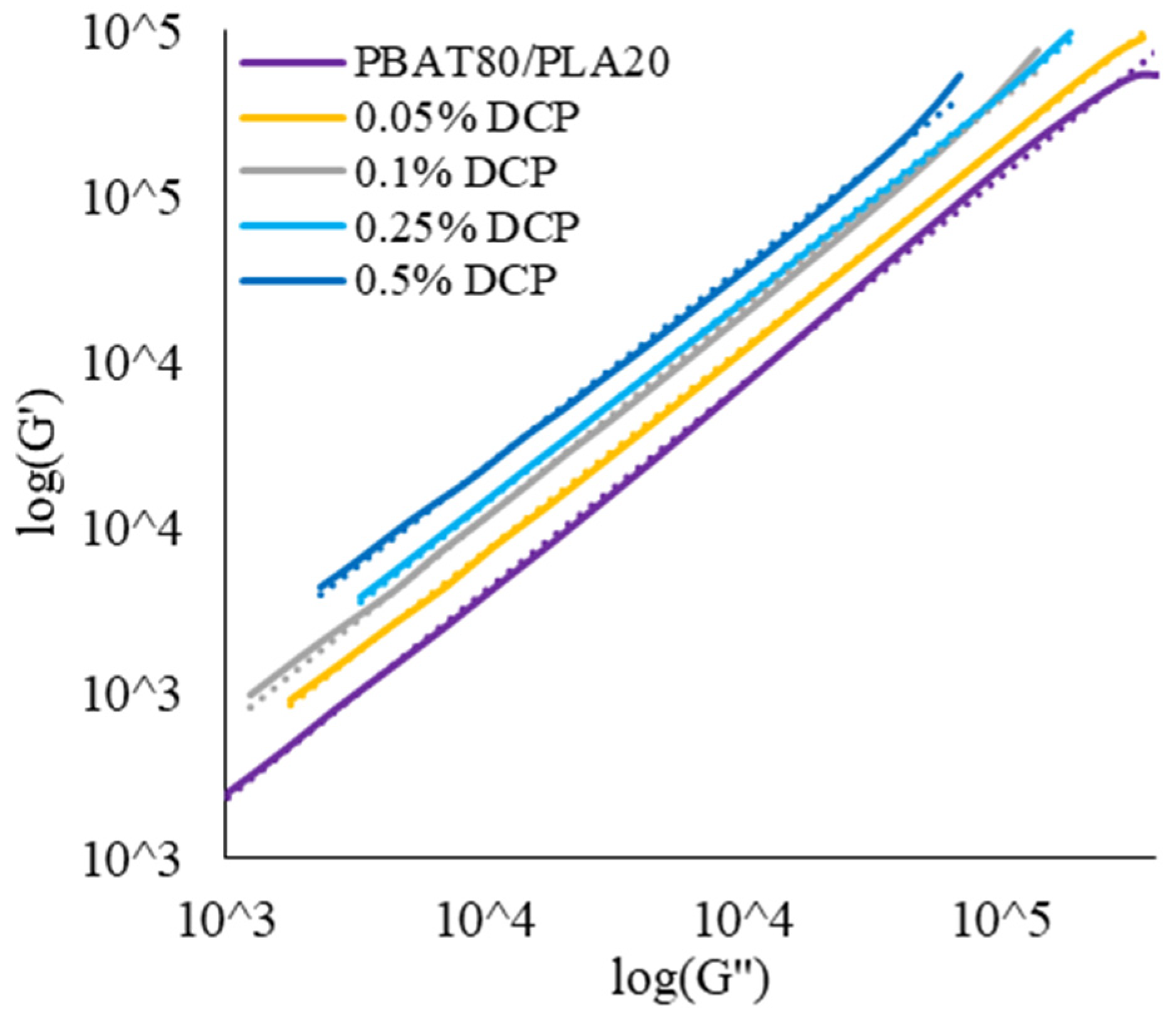
3.1.2. Rheological Properties
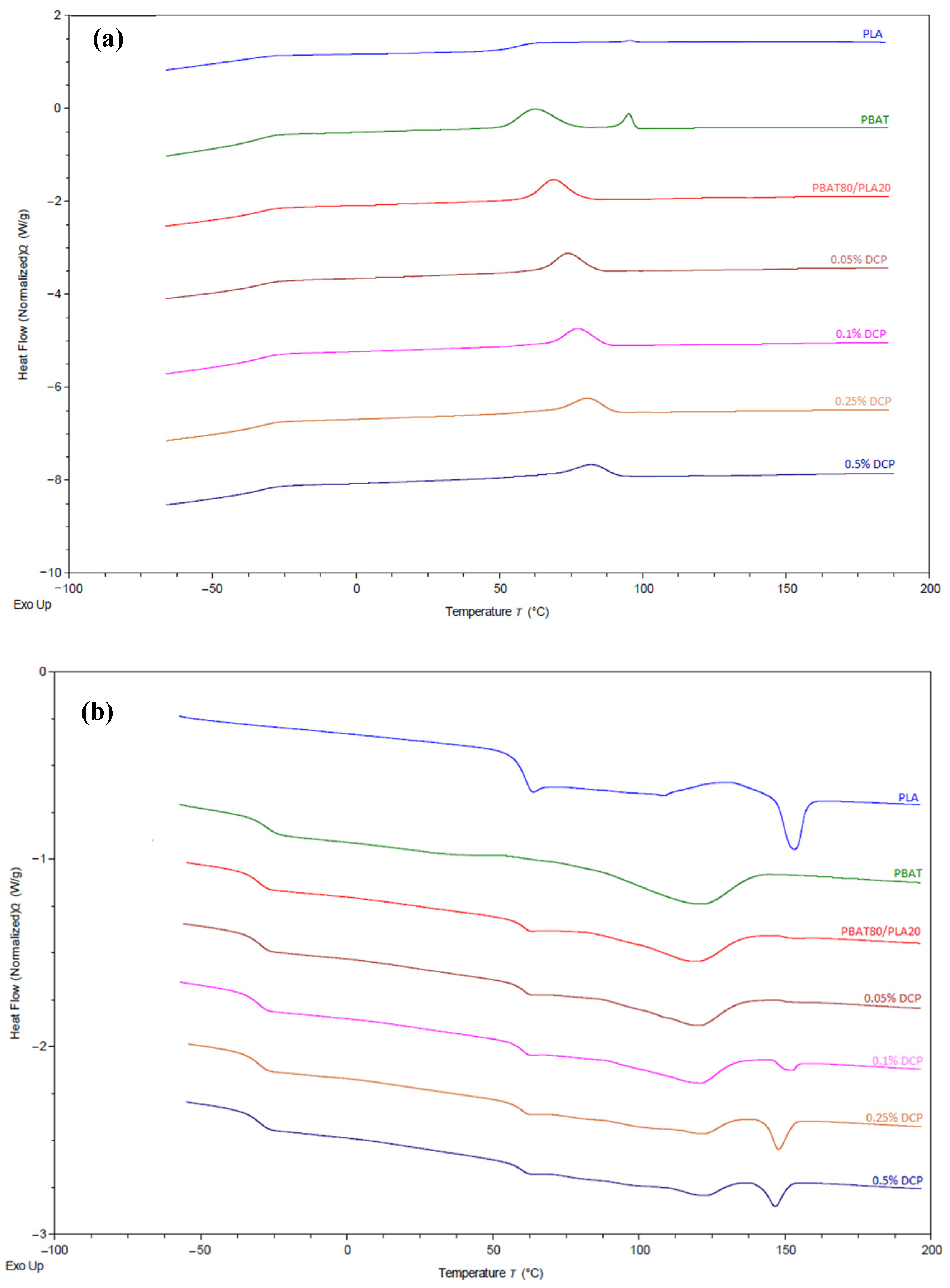
3.1.3. Thermal Analysis
3.1.4. Morphological Study
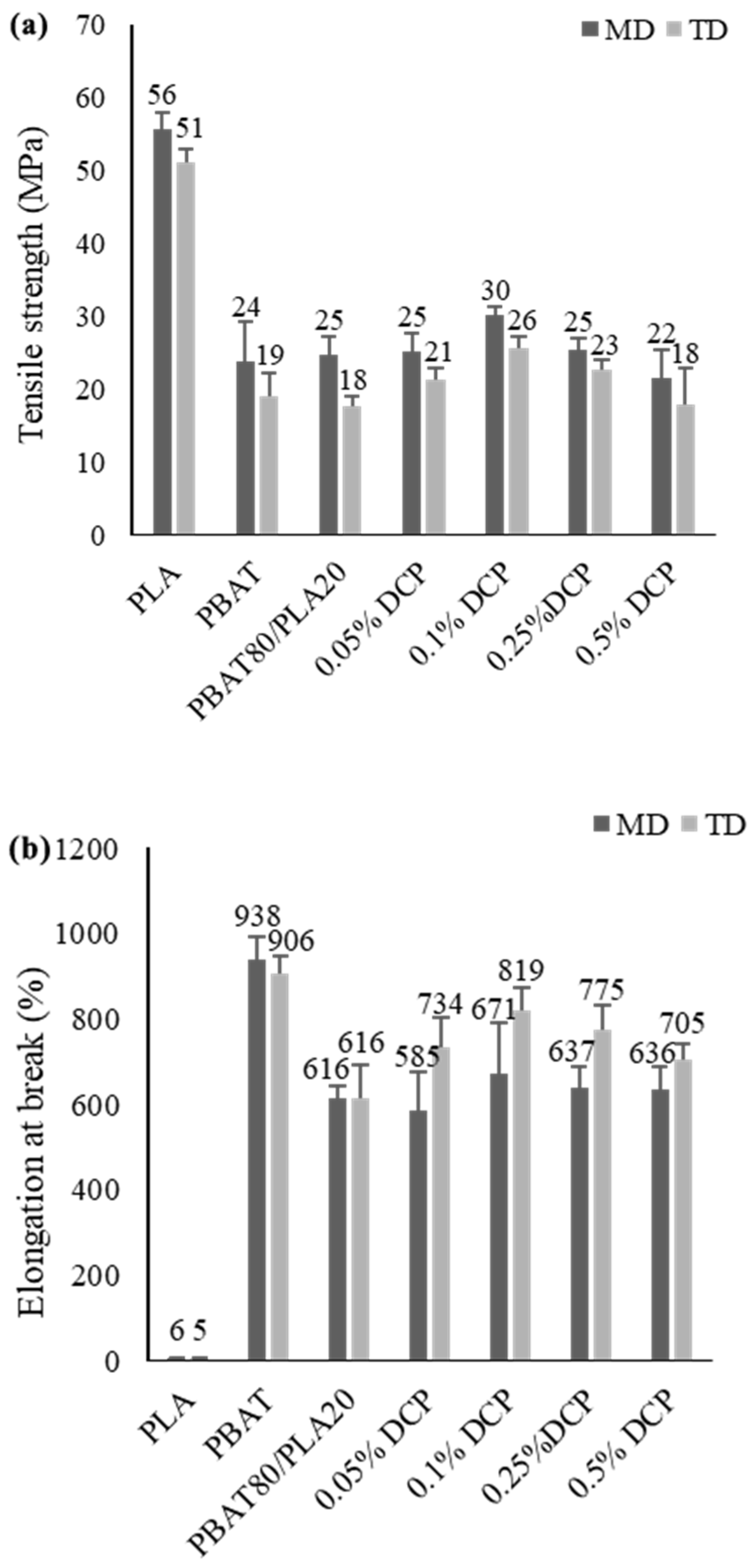
3.1.5. Tensile Properties
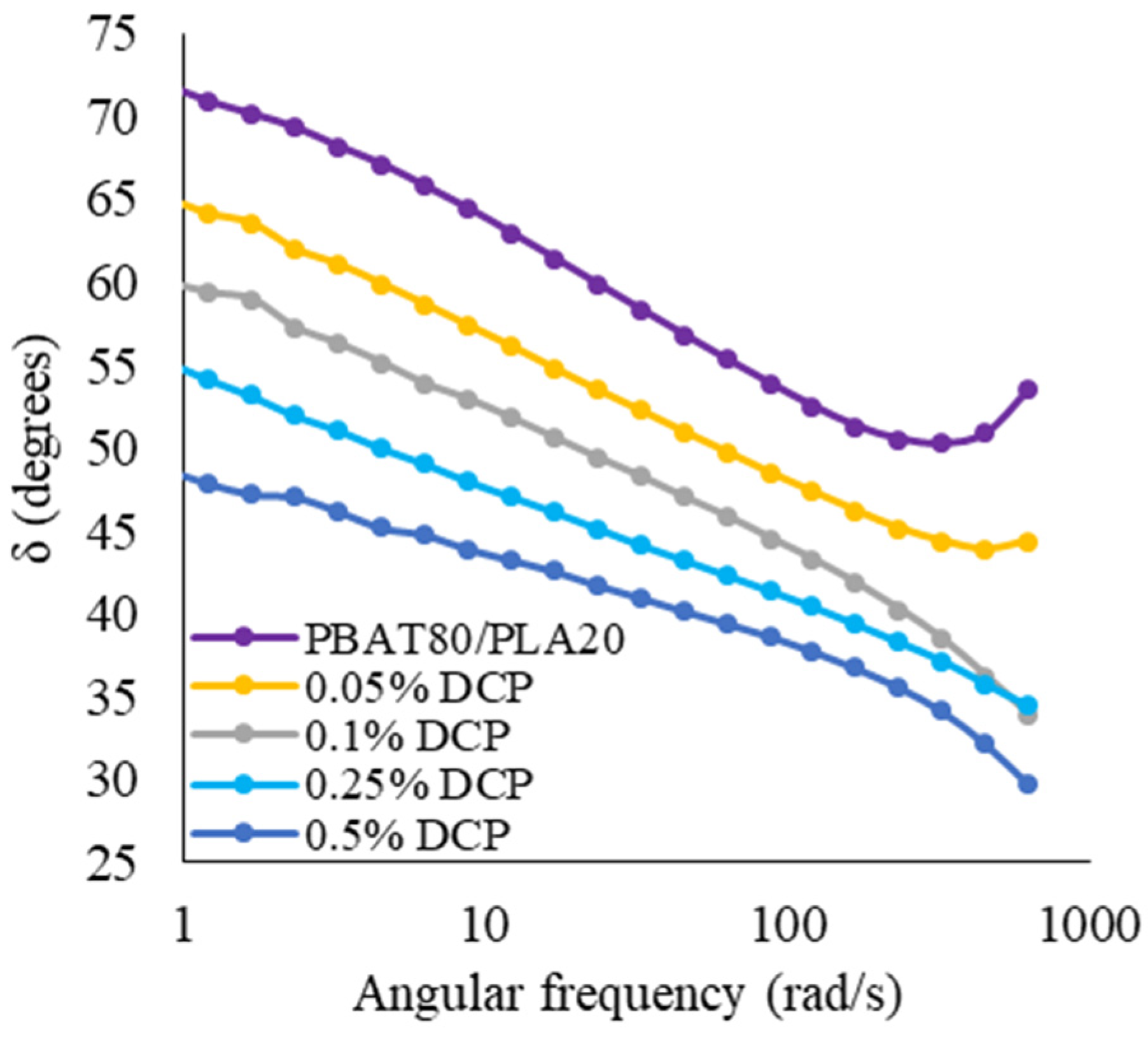
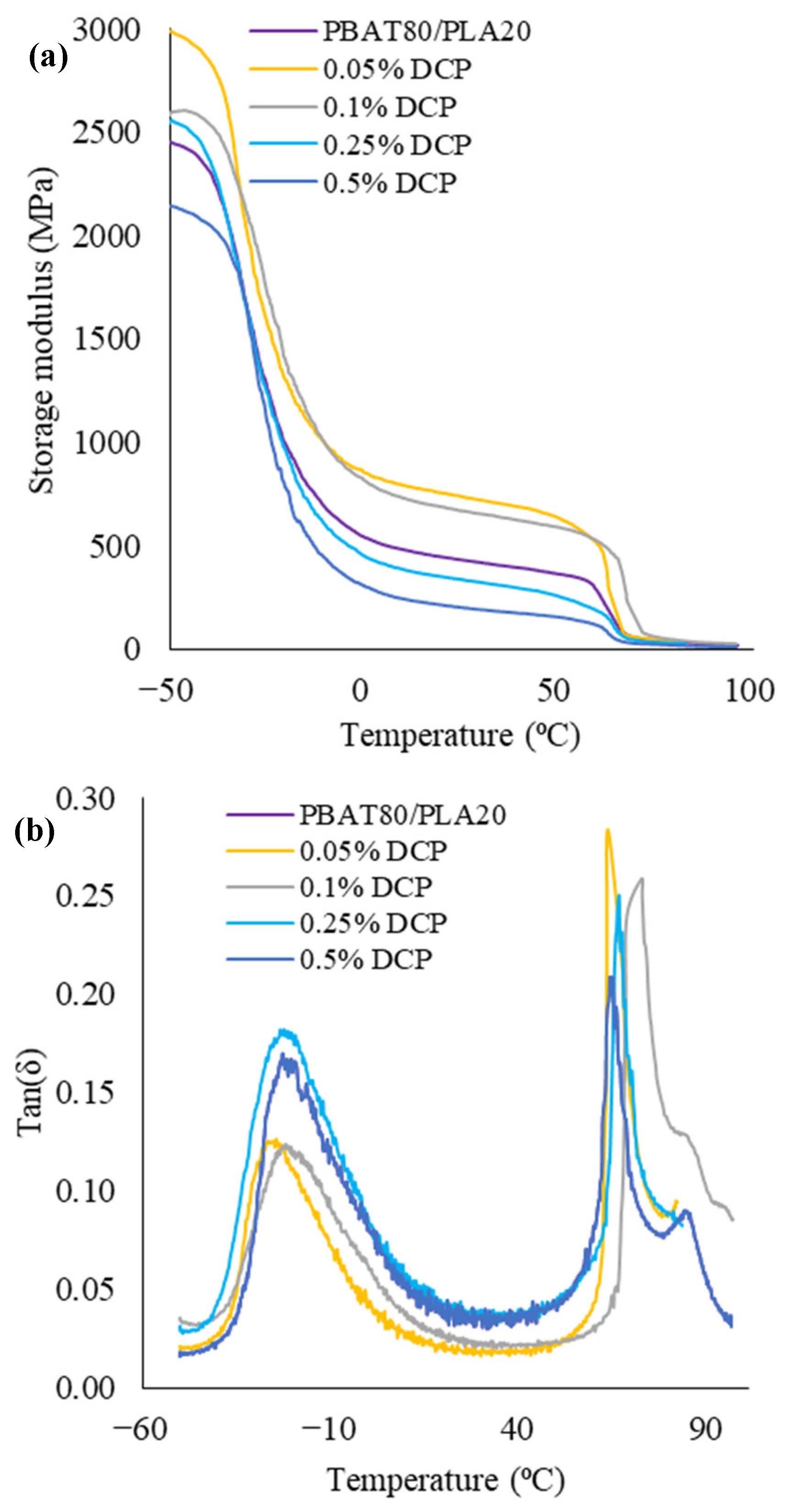
3.1.6. Dynamical-Mechanical Behavior
3.2. Incorporation of Chain Extenders
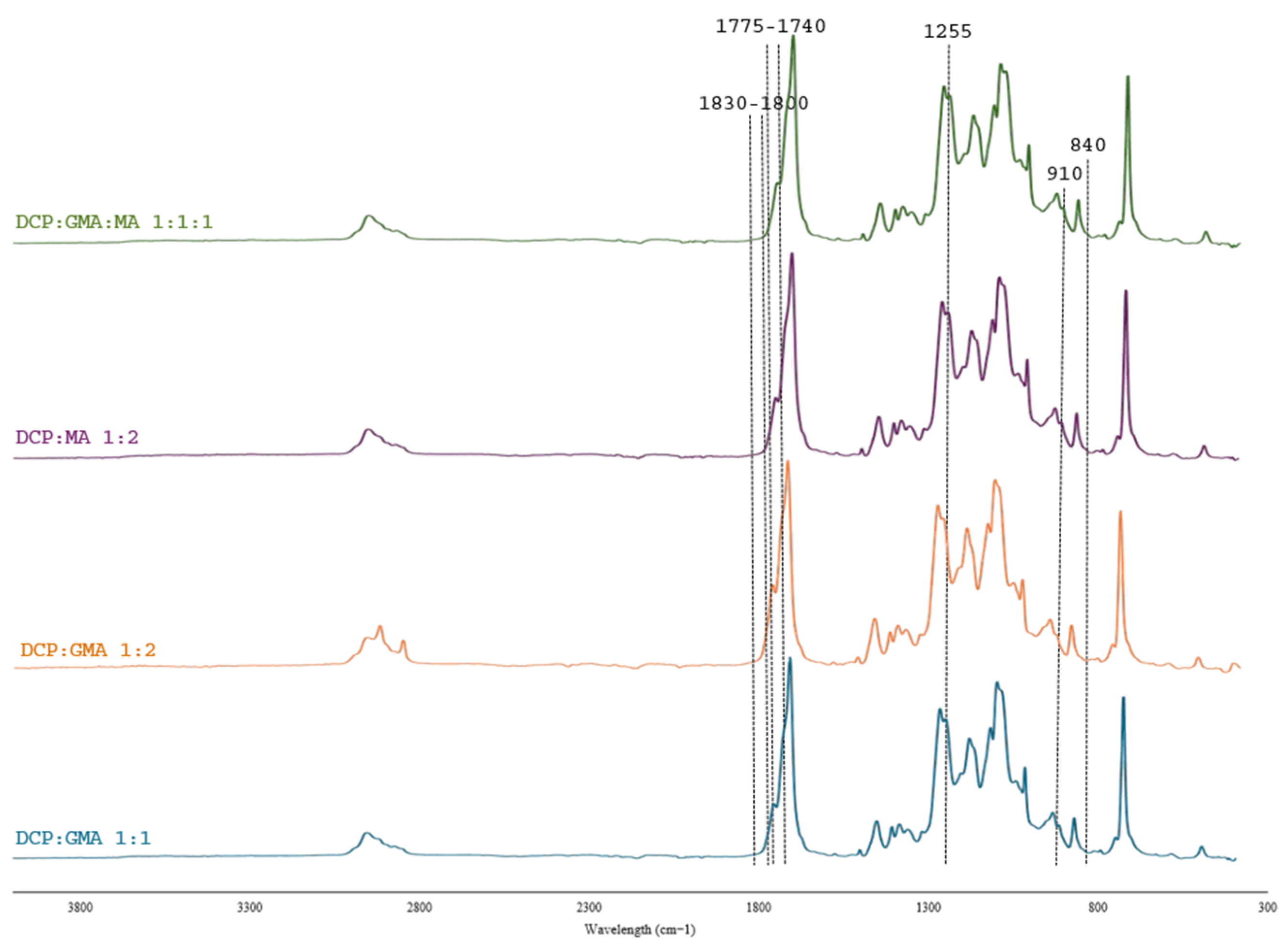
3.2.1. FT-IR Analysis
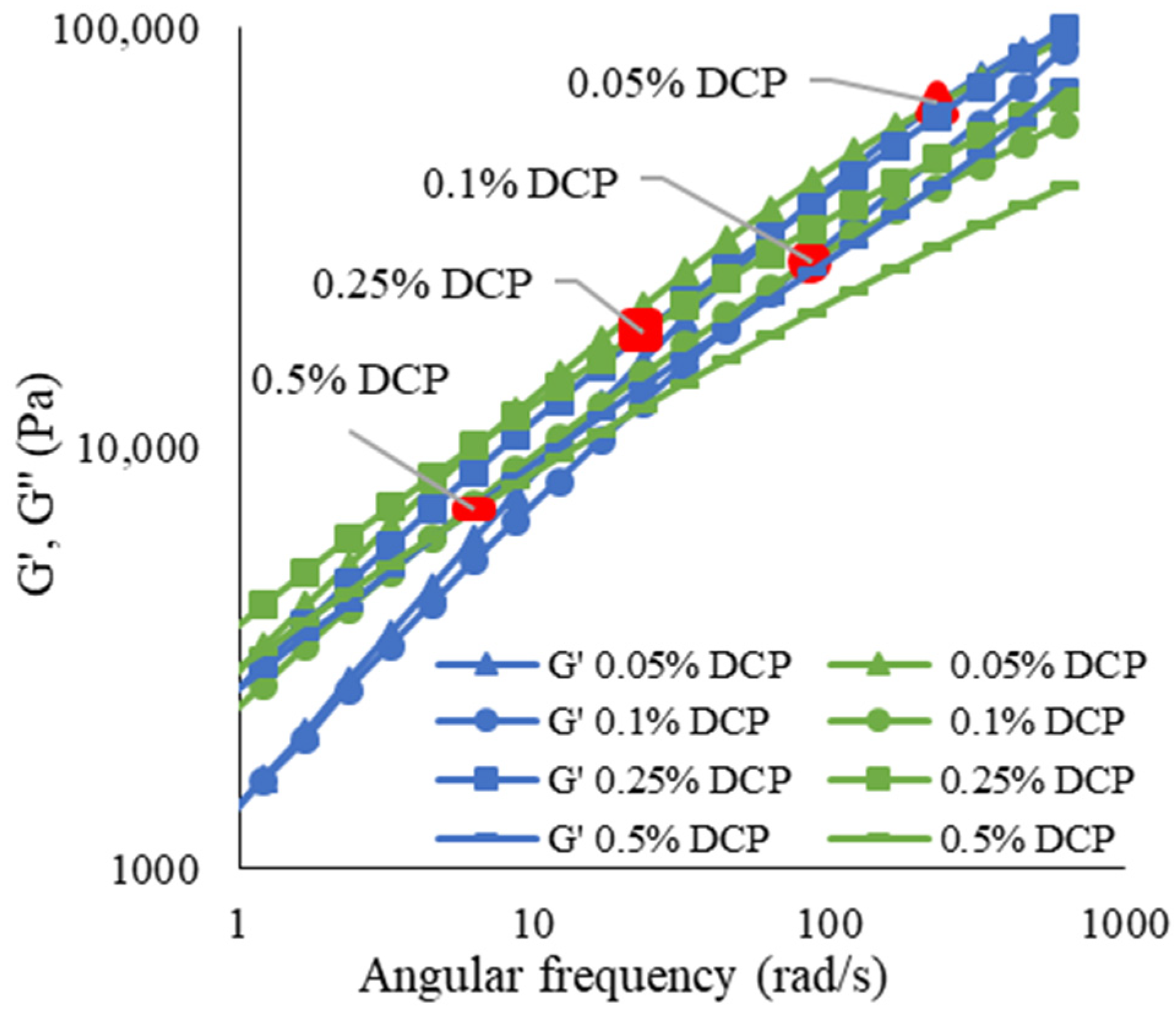
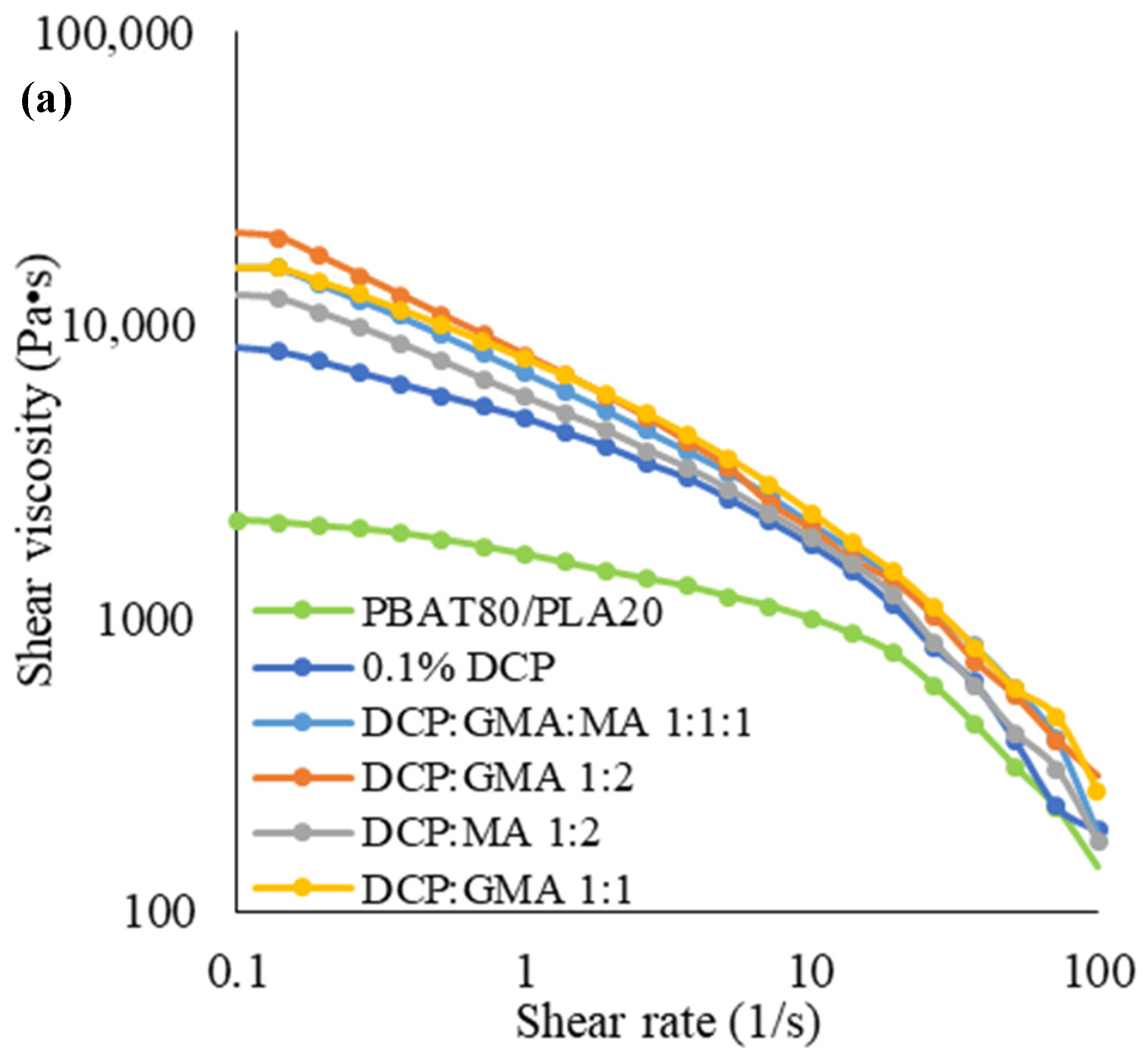
3.2.2. Rheological Properties
3.2.3. Thermal Analysis
3.2.4. Morphological Properties
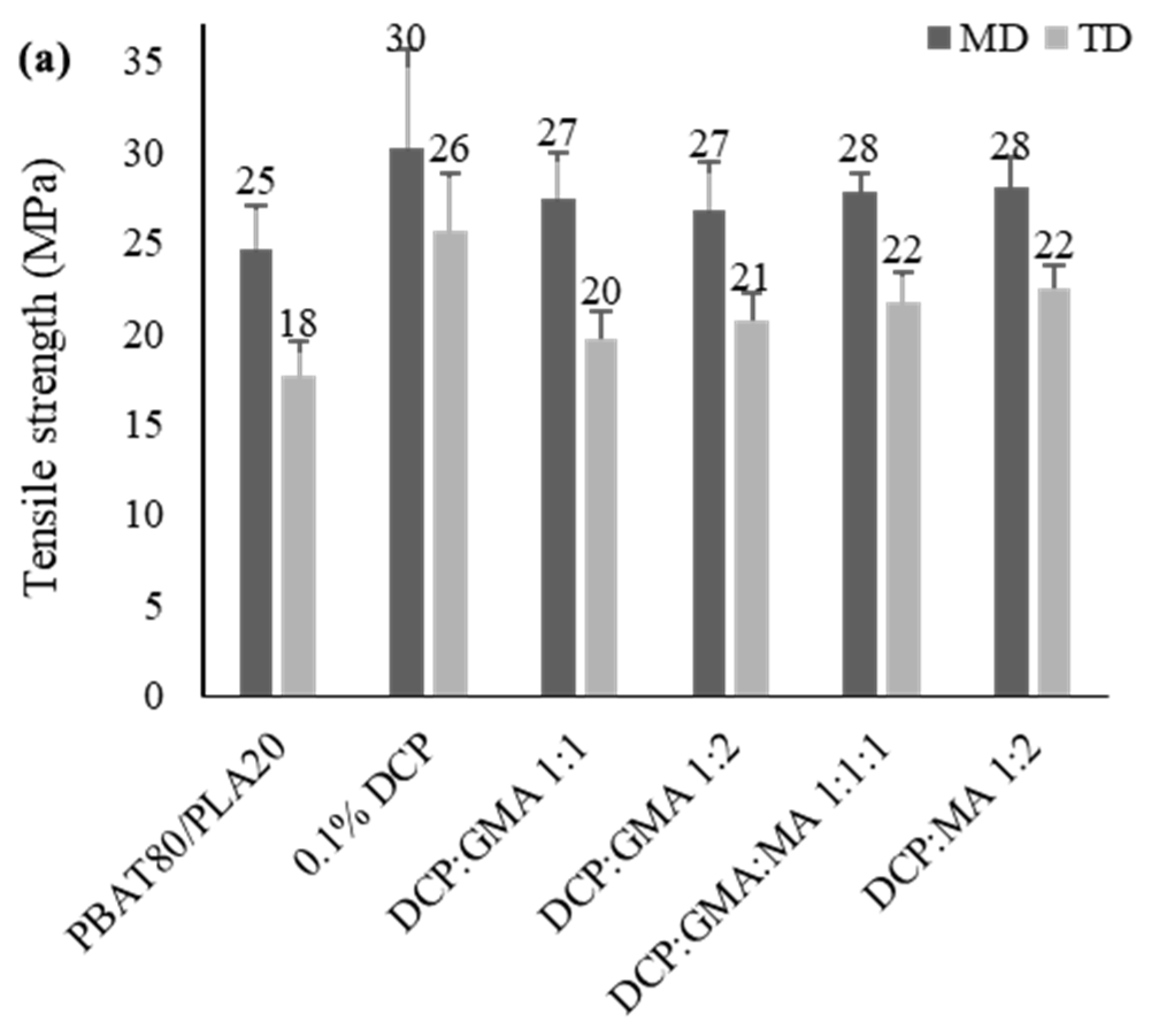
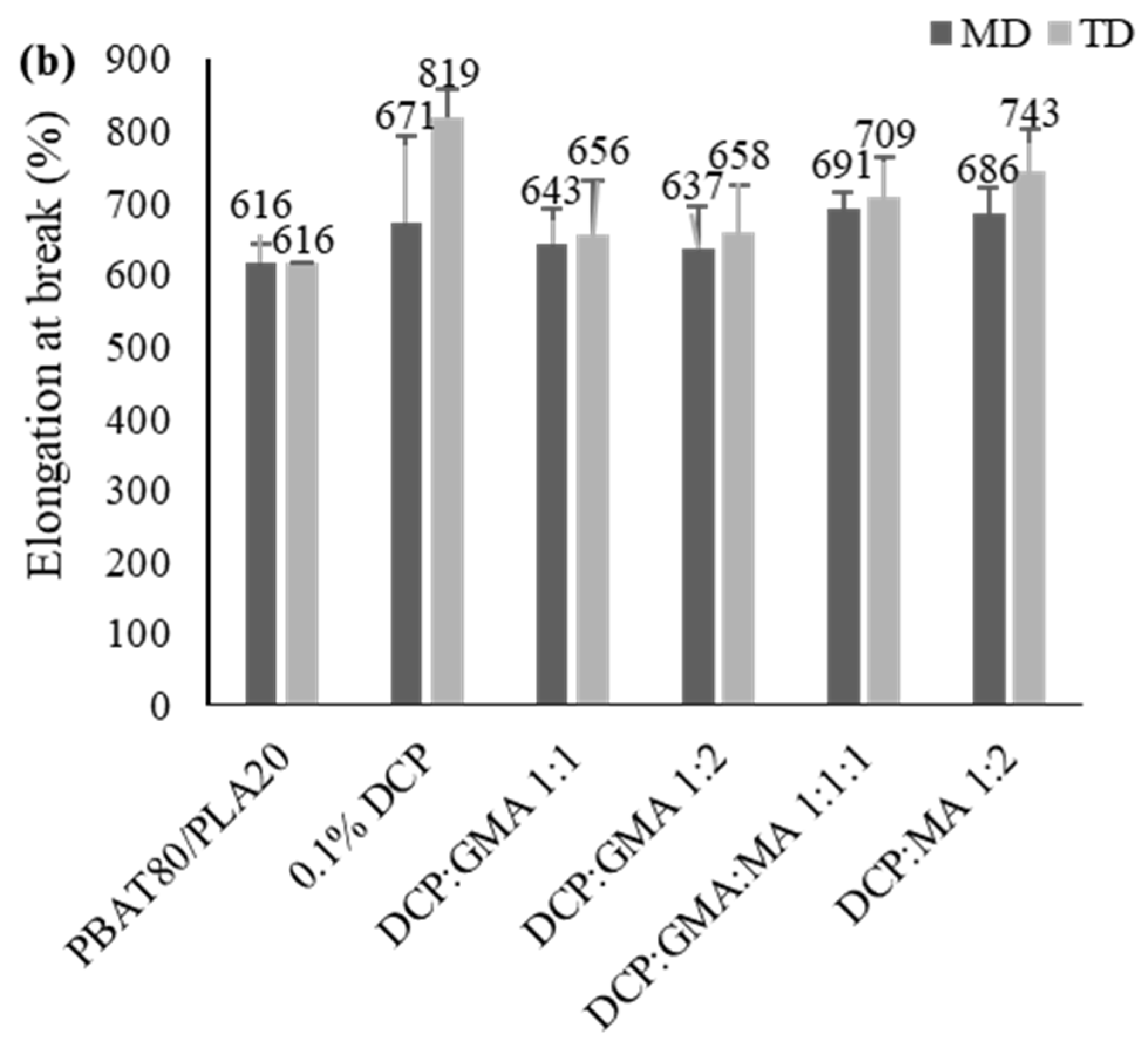
3.2.5. Tensile Properties
3.3. Optimization of Blends with DCP–GMA 1:2
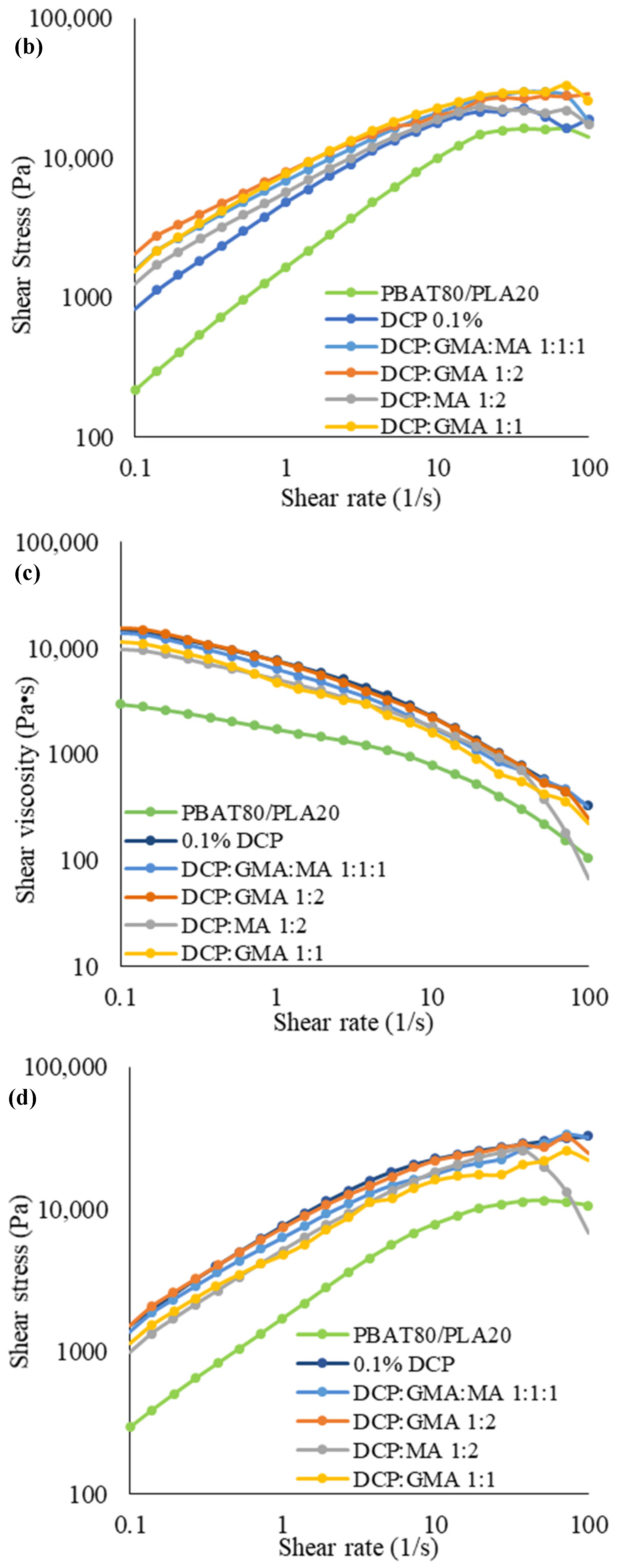
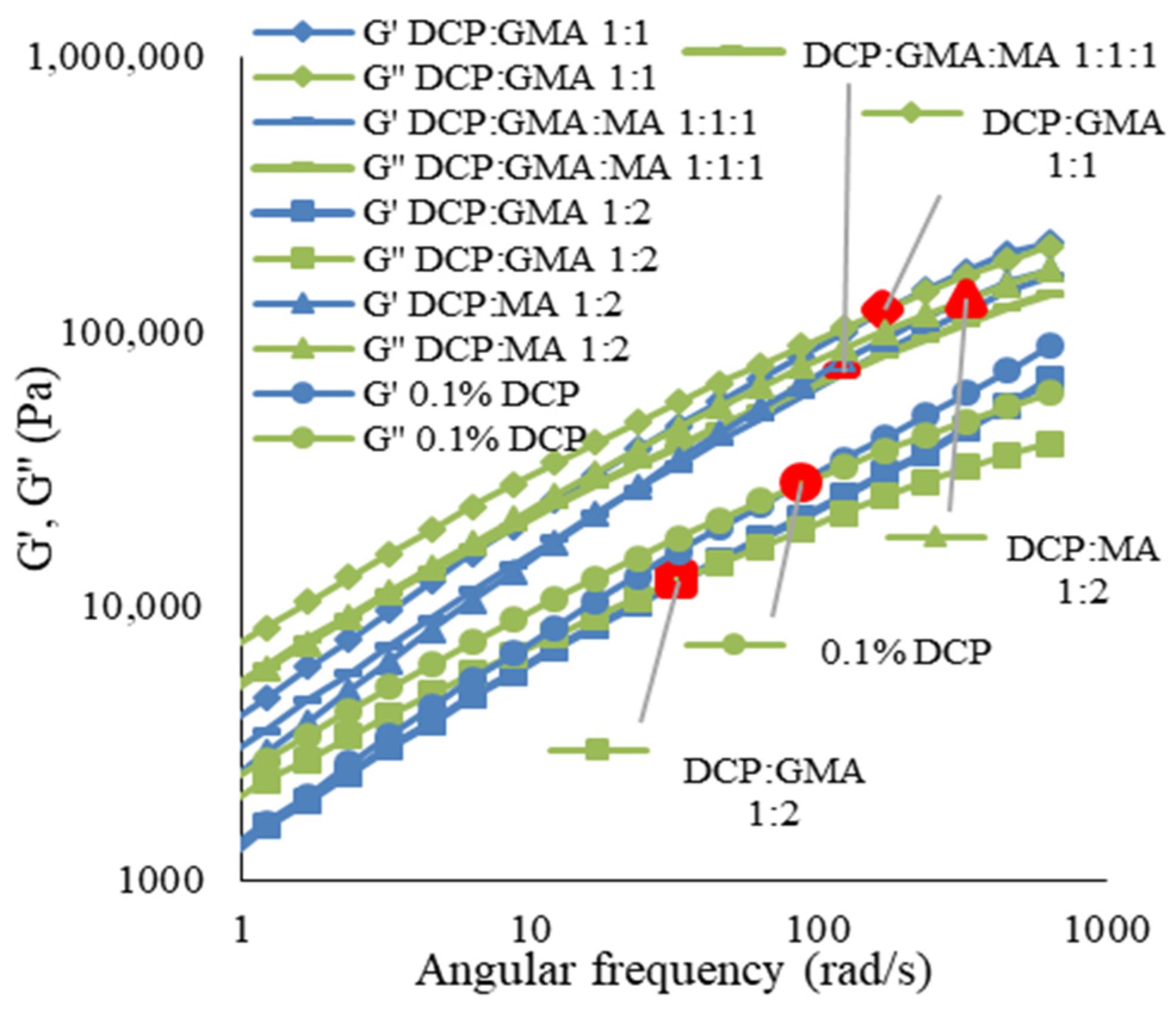
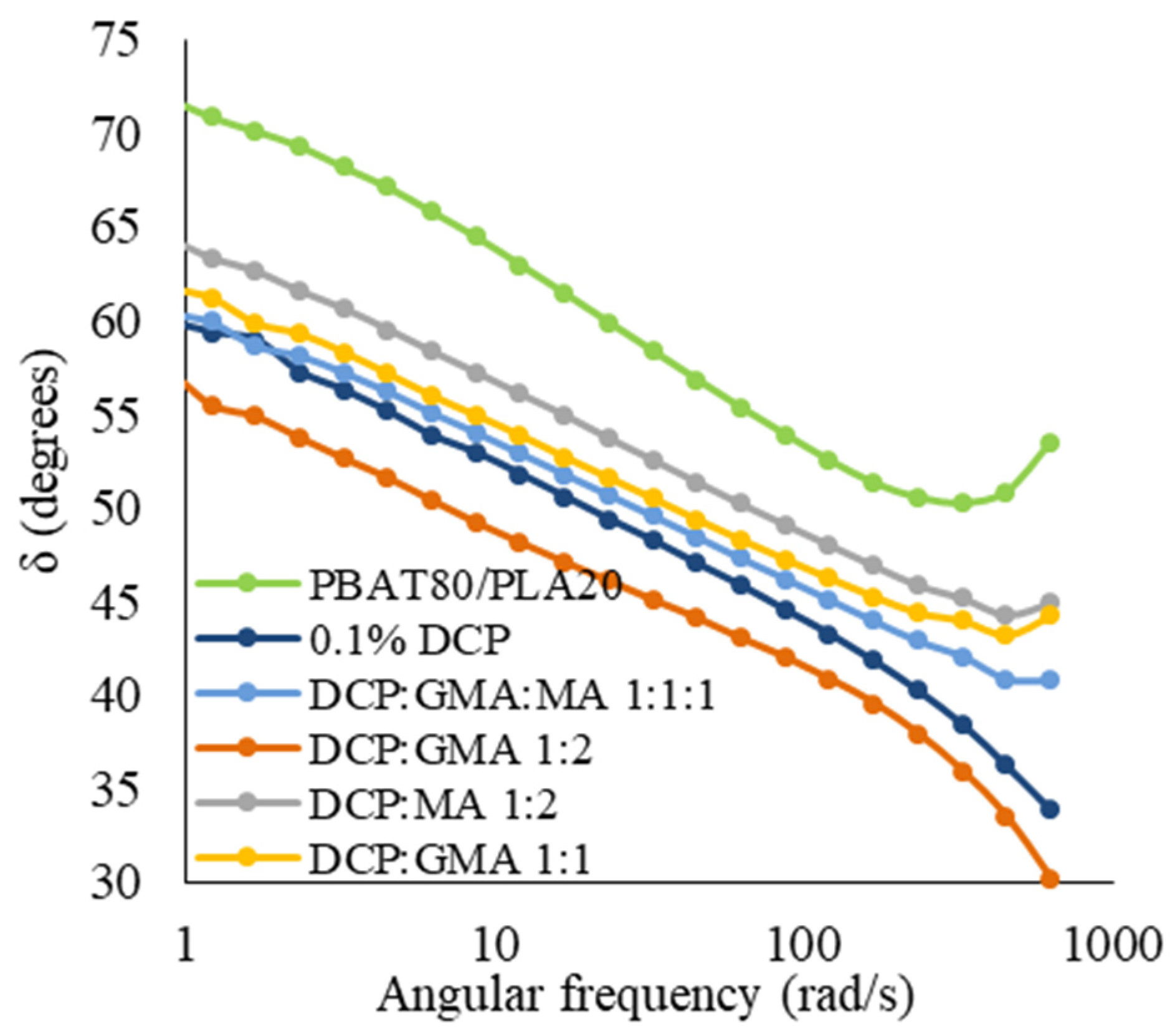
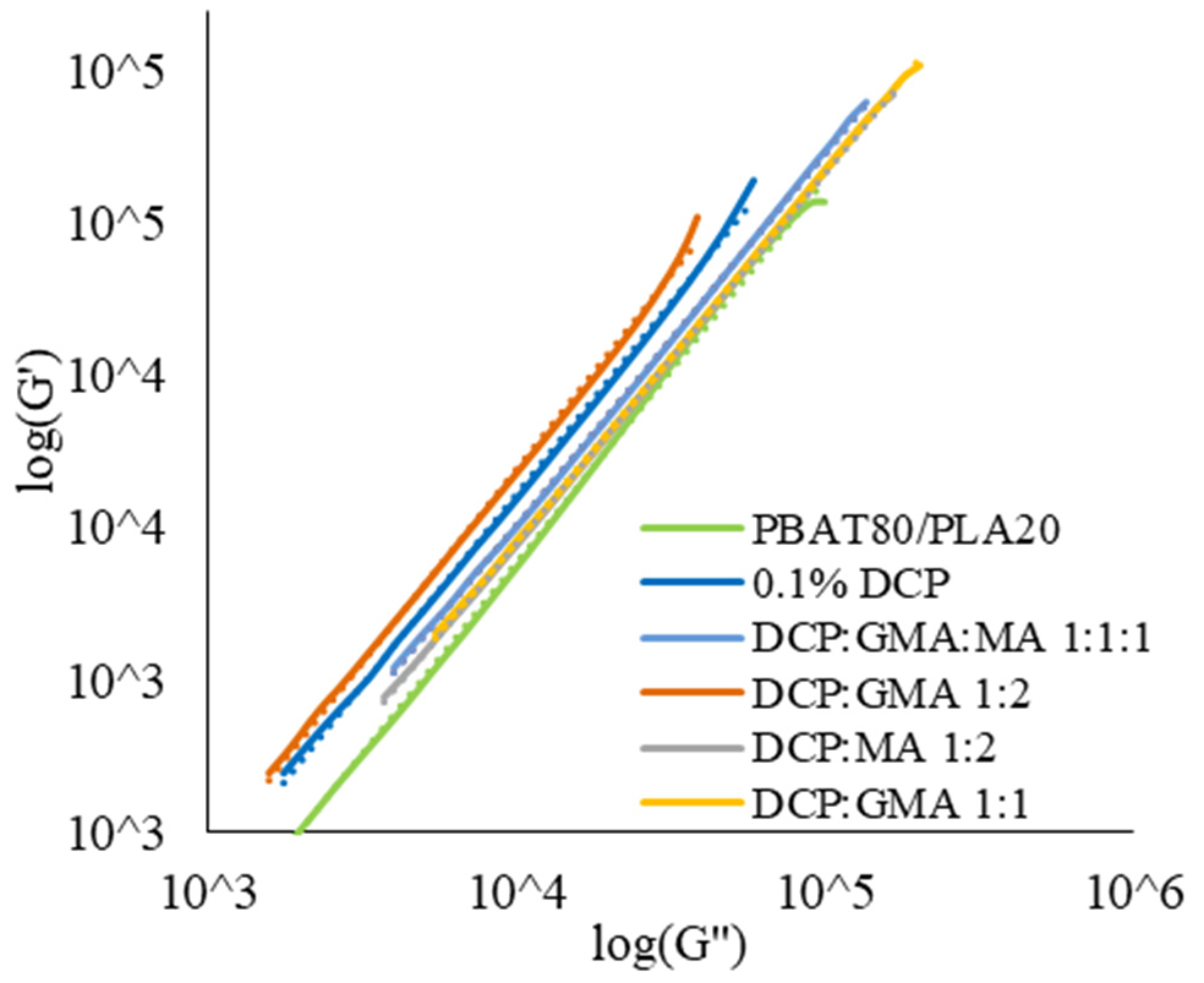
3.3.1. Rheological Properties
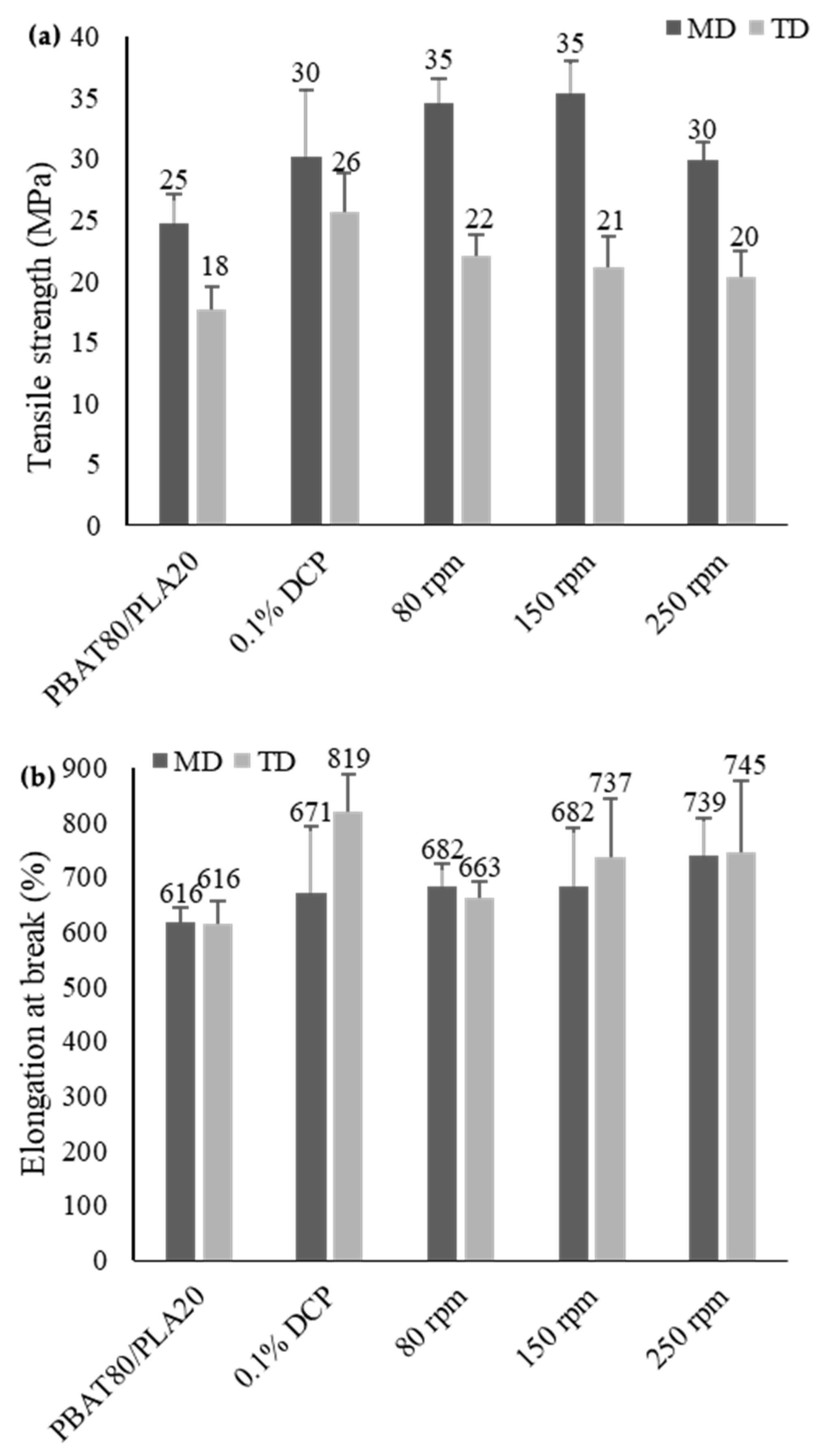
3.3.2. Tensile Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Symbols and Abbreviations
| Symbols and Abbreviations | Definitions |
| LDPE | Low-density Polyethylene |
| PP | Polypropylene |
| PBAT | Poly(butylene adipate-co-terephthalate) |
| PLA | Poly(lactic acid) |
| FIBC | Flexible intermediate bulk container |
| SEM | Scanning Electron Microscopy |
| DMA | Dynamical Mechanical Analysis |
| DSC | Differential Scanning Calorimetry |
| Tg | Glass transition temperature |
| Tc | Melt crystallization temperature |
| Tcc | Cold crystallization temperature |
| Tm | Melting temperature |
| ΔHm | Melt enthalpy |
| ΔHc | Crystallization enthalpy |
| Xc | Degree of crystallinity |
| G′ | Storage modulus |
| G″ | Loss modulus |
| Mw | Molecular weight |
| MWD | Molecular weight Distribution |
| ω | Angular frequency |
| δ | Loss angle |
| wt | Weight |
| η | Shear viscosity |
| L/D | Length/Diameter |
| ASTM | American Society for Testing and Materials |
| g | Grafted |
| STDEV.S | Standard deviation of a sample of the population |
| MD | Machine Direction |
| TD | Transverse Direction |
| S.I. | Supplementary Information |
References
- Houssini, K.; Li, J.; Tan, Q. Complexities of the global plastics supply chain revealed in a trade-linked material flow analysis. Commun. Earth Environ. 2025, 6, 257. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Report of the Intergovernmental Negotiating Committee to Develop an International Legally Binding Instrument on Plastic Pollution, Including in the Marine Environment (UNEP/PP/INC.1/7); UNEP: Montevideo, Uruguay, 2022. [Google Scholar]
- Wakefield, F. Top 25 Recycling Facts and Statistics for 2022; EcoWatch: New York, NY, USA, 2022; Available online: https://www.ecowatch.com/recycling-stats.html (accessed on 22 June 2025).
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising Ductility of Poly(Lactic Acid)/Poly(Butylene Adipate-co-Terephthalate) Blends Through Co-continuous Phase Morphology. J. Polym. Environ. 2018, 26, 3802–3816. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J. Crystallization behavior of fully biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J. Appl. Polym. Sci. 2009, 112, 3754–3763. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(butylene adipate-co-terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Ko, E.; Kim, T.; Ahn, J.; Park, S.; Pak, S.; Kim, M.; Kim, H. Synergic Effect of HNT/oMMT Bi-filler System for the Mechanical Enhancement of PLA/PBAT Film. Fibers Polym. 2021, 22, 2163–2169. [Google Scholar] [CrossRef]
- Santos, T.T.; Almeida, T.G.; Morais, D.D.S.; Magalhães, F.D.; Guedes, R.M.; Canedo, E.L.; Carvalho, L.H. Effect of filler type on properties of PBAT/organoclay nanocomposites. Polym. Bull. 2020, 77, 901–917. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Singh, A.A.; Sharma, S.; Srivastava, M.; Majumdar, A. Modulating the properties of polylactic acid for packaging applications using biobased plasticizers and naturally obtained fillers. Int. J. Biol. Macromol. 2020, 153, 1165–1175. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Mechanical, thermal, and morphology properties of poly(lactic acid) plasticized with poly(ethylene glycol) and epoxidized palm oil hybrid plasticizer. Polym. Eng. Sci. 2016, 56, 1169–1174. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; Zhou, Z.; Li, Q.-F. A green pathway to adjust the mechanical properties and degradation rate of PCL by blending bio-sourced poly(glycerol-succinate) oligoesters. Mater. Chem. Front. 2018, 2, 544–553. [Google Scholar] [CrossRef]
- Jang, J.Y.; Sadeghi, K.; Seo, J. Chain-Extending Modification for Value-Added Recycled PET: A Review. Polym. Rev. 2022, 62, 860–889. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H. Mechanical properties and phase morphology of super-tough PLA/PBAT/EMA-GMA multicomponent blends. Mater. Lett. 2017, 192, 17–20. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical properties, rheological behaviors, and phase morphologies of high-toughness PLA/PBAT blends by in-situ reactive compatibilization. Compos. Part B Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Cheng, J.; Anstey, A.; Mohanty, A.K.; Misra, M. Development of Toughened Blends of Poly(lactic acid) and Poly(butylene adipate-co-terephthalate) for 3D Printing Applications: Compatibilization Methods and Material Performance Evaluation. ACS Sustain. Chem. Eng. 2020, 8, 6576–6589. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Reactive extrusion of biodegradable aliphatic polyesters in the presence of free-radical-initiators: A review. Polym. Degrad. Stab. 2020, 182, 109383. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly(lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Wu, D.; Huang, A.; Fan, J.; Xu, R.; Liu, P.; Li, G.; Yang, S. Effect of blending procedures and reactive compatibilizers on the properties of biodegradable poly(butylene adipate-co-terephthalate)/poly(lactic acid) blends. J. Polym. Eng. 2021, 41, 95–108. [Google Scholar] [CrossRef]
- Monika Pal, A.K.; Bhasney, S.M.; Bhagabati, P.; Katiyar, V. Effect of Dicumyl Peroxide on a Poly(lactic acid) (PLA)/Poly(butylene succinate) (PBS)/Functionalized Chitosan-Based Nanobiocomposite for Packaging: A Reactive Extrusion Study. ACS Omega 2018, 3, 13298–13312. [Google Scholar] [CrossRef]
- Han, Y.; Shi, J.; Mao, L.; Wang, Z.; Zhang, L. Improvement of Compatibility and Mechanical Performances of PLA/PBAT Composites with Epoxidized Soybean Oil as Compatibilizer. Ind. Eng. Chem. Res. 2020, 59, 21779–21790. [Google Scholar] [CrossRef]
- Rigolin, T.R.; Costa, L.C.; Chinelatto, M.A.; Muñoz, P.A.; Bettini, S.H. Chemical modification of poly(lactic acid) and its use as matrix in poly(lactic acid) poly(butylene adipate-co-terephthalate) blends. Polym. Test. 2017, 63, 542–549. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, Y.; Lin, Z.; Zhang, J.; Shi, X. Manipulating phase structure of biodegradable PLA/PBAT system: Effects on dynamic rheological responses and 3D printing. Compos. Sci. Technol. 2020, 200, 108399. [Google Scholar] [CrossRef]
- Galloway, J.A.; Macosko, C.W. Comparison of methods for the detection of cocontinuity in poly(ethylene oxide)/polystyrene blends. Polym. Eng. Sci. 2004, 44, 714–727. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Bronco, S.; Chinea, C. The effect of free radical reactions on structure and properties of poly(lactic acid) (PLA) based blends. Polym. Degrad. Stab. 2010, 95, 332–341. [Google Scholar] [CrossRef]
- Kanzawa, T.; Tokumitsu, K. Mechanical properties and morphological changes of poly(lactic acid)/polycarbonate/poly(butylene adipate-co-terephthalate) blend through reactive processing. J. Appl. Polym. Sci. 2011, 121, 2908–2918. [Google Scholar] [CrossRef]
- Gu, T.; Zhu, D.; Lu, Y.; Lu, S. Effect of PLA-g-GMA on the Thermal, Rheological and Physical Behavior of PLA/PBAT Blends. Polym. Sci. Ser. A 2019, 61, 317–324. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Novel tunable super-tough materials from biodegradable polymer blends: Nano-structuring through reactive extrusion. RSC Adv. 2019, 9, 2836–2847. [Google Scholar] [CrossRef]
- Muller, R.; Bouquey, M.; Mauguière, F.; Schlatter, G.; Serra, C.; Terrisse, J. Rheology of Reactive Polymer Blends: Separation of Mixing and Reatcion Steps. Appl. Rheol. 2001, 11, 141–152. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive extrusion of PLA, PBAT with a multi-functional epoxide: Physico-chemical and rheological properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic properties of polymer solutions. J. Res. Natl. Bur. Stand. 1948, 41, 53–62. [Google Scholar] [CrossRef]
- Khak, M.; Ramazani, A.S.A. Rheological measurement of molecular weight distribution of polymers. E-Polym 2013, 13, 020. [Google Scholar] [CrossRef]
- Chen, C.; Shekh, M.I.; Cui, S.; Stadler, F.J. Rheological Behavior of Blends of Metallocene Catalyzed Long-Chain Branched Polyethylenes. Part I: Shear Rheological and Thermorheological Behavior. Polymers 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, S.M.; McGraw, J.D.; Wood-Adams, P. Short chains enhance slip of highly entangled polystyrenes during thin film dewetting. RSC Adv. 2016, 6, 91163–91170. [Google Scholar] [CrossRef]
- Nadgorny, M.; Gentekos, D.T.; Xiao, Z.; Singleton, S.P.; Fors, B.P.; Connal, L.A. Manipulation of Molecular Weight Distribution Shape as a New Strategy to Control Processing Parameters. Macromol. Rapid Commun. 2017, 38, 1700352. [Google Scholar] [CrossRef] [PubMed]
- Han, C.D.; Kim, J. Rheological technique for determining the order–disorder transition of block copolymers. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1741–1764. [Google Scholar] [CrossRef]
- Fujiyabu, T.; Sakumichi, N.; Katashima, T.; Liu, C.; Mayumi, K.; Chung, U.I.; Sakai, T. Tri-branched gels: Rubbery materials with the lowest branching factor approach the ideal elastic limit. Sci. Adv. 2022, 8, eabk0010. [Google Scholar] [CrossRef]
- Song, J.; Zhou, H.; Wang, X.; Zhang, Y.; Mi, J. Role of chain extension in the rheological properties, crystallization behaviors, and microcellular foaming performances of poly (butylene adipate-co-terephthalate). J. Appl. Polym. Sci. 2019, 136, 47322. [Google Scholar] [CrossRef]
- Su, S.; Duhme, M.; Kopitzky, R. Uncompatibilized PBAT/PLA Blends: Manufacturability, Miscibility and Properties. Materials 2020, 13, 4897. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and properties of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blend with glycidyl methacrylate as reactive processing agent. J. Mater. Sci. 2009, 44, 250–256. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail Parvaiz, M. Effect of glycidyl methacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Nowakowski, M. Development of Toughened Flax Fiber Reinforced Composites. Modification of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends by Reactive Extrusion Process. Materials 2021, 14, 1523. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, M.; Hejna, A.; Haponiuk, J.; Formela, K. Structural and Thermo-Mechanical Properties of Poly(ε-Caprolactone) Modified by Various Peroxide Initiators. Polymers 2019, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Lee, S.B.; Lee, C.K.; Lee, J.Y.; Shim, J.K.; Selke, S.E.; Soto-Valdez, H.; Matuana, L.; Rubino, M.; Auras, R. Grafting of maleic anhydride on poly(L-lactic acid). Effects on physical and mechanical properties. Polym. Test. 2012, 31, 333–344. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Ma, P.; Liu, W.; Zhou, X.; Shi, D.; Ni, Z.; Chen, M. Influence of phthalic anhydride and bioxazoline on the mechanical and morphological properties of biodegradable poly(lactic acid)/poly[(butylene adipate)-co-terephthalate] blends: Influence of PA and BOZ on properties of PLA/PBAT blends. Polym. Int. 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Taylor, S.; Misra, M.; Mohanty, A.K. Thermo-mechanical characterization of bioblends from polylactide and poly(butylene adipate-co-terephthalate) and lignin. Macromol. Mater. Eng. 2015, 300, 299–311. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Zawong, M.; Phusunti, N.; Aht-Ong, D. Toughening and thermal characteristics of plasticized polylactide and poly(butylene adipate-co-terephthalate) blend films: Influence of compatibilization. Int. J. Biol. Macromol. 2021, 183, 346–357. [Google Scholar] [CrossRef]
- Wu, C.-S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar] [CrossRef]
- Barbosa, V.C.S.; Sousa, A.M.F.D.; Silva, A.L.N.D. Influence of processing parameters on mechanical and thermal behavior of PLA/PBAT blend. Polímeros 2022, 32, e2022033. [Google Scholar] [CrossRef]
- Zhu, W.; Jaluria, Y. Residence time and conversion in the extrusion of chemically reactive materials. Polym. Eng. Sci. 2001, 41, 1280–1291. [Google Scholar] [CrossRef]
- Kim, G.-M.; Michler, G.H. Micromechanical deformation processes in toughened and particle filled semicrystalline polymers: Part 2. model representation for micromechanical deformation processes. Polymer 1998, 39, 5699–5703. [Google Scholar] [CrossRef]
- Freitas, A.L.P.D.L.; Tonini Filho, L.R.; Calvão, P.S.; Souza, A.M.C.D. Effect of montmorillonite and chain extender on rheological, morphological and biodegradation behavior of PLA/PBAT blends. Polym. Test. 2017, 62, 189–195. [Google Scholar] [CrossRef]
- Velghe, I.; Buffel, B.; Vandeginste, V.; Thielemans, W.; Desplentere, F. Review on the Degradation of Poly(lactic acid) during Melt Processing. Polymers 2023, 15, 2047. [Google Scholar] [CrossRef]









| GMA | MA |
|---|---|
| 0.25 | 0.25 |
| 0.5 | 0.5 |
| 1 | 1 |
| 2.5 | 2.5 |
| 5 | 5 |
| 0.5 | 0 |
| 1 | 0 |
| 2 | 0 |
| 5 | 0 |
| 10 | 0 |
| 0 | 0.5 |
| 0 | 1 |
| 0 | 2 |
| 0 | 5 |
| 0 | 10 |
| DCP | GMA | MA |
|---|---|---|
| 1 | 1 | 1 |
| 1 | 1 | 0 |
| 1 | 2 | 0 |
| 1 | 0 | 2 |
| Batch-Mixed | Extruded | |||
|---|---|---|---|---|
| Sample | Angular Frequency (rad/s) | Modulus (kPa) | Angular Frequency (rad/s) | Modulus (kPa) |
| PBAT80/PLA20 | 325.4 | 91.8 | - | - |
| 0.05% DCP | 168.6 | 120.9 | 234.2 | 66.0 |
| 0.1% DCP | 121.3 | 63.7 | 87.3 | 27.7 |
| 0.25% DCP | 23.4 | 64.1 | 23.4 | 18.8 |
| 0.5% DCP | 4.5 | 21.5 | 6.3 | 7.1 |
| Sample | Slope | Intercept |
|---|---|---|
| PBAT80/PLA20 | 1.2558 | −1.3386 |
| 0.05% DCP | 1.2366 | −1.1534 |
| 0.1% DCP | 1.2165 | −0.8206 |
| 0.25% DCP | 1.2361 | −1.0038 |
| 0.5% DCP | 1.2636 | −1.1472 |
| Tg2 PBAT (°C) | Tg2 PLA (°C) | Tm2 PBAT (°C) | ΔHm2 PBAT (J/g) | Tm2 PLA (°C) | ΔHm2 PLA (J/g) | ΔHcc1 (J/g) | Xc2 of PLA (%) | Xc2 of PBAT (%) | Tc1 (°C) | ΔHc1 (J/g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | - | 57.8 | - | - | 153.2 | 5.1 | 4.9 | 0.2 | - | 95.4 | 0.4 |
| PBAT | −27.5 | - | 121.9 | 13.6 | - | - | - | - | 11.9 | 30.3 | 20.1 |
| PBAT80/PLA20 | −30.6 | 59.7 | 121.3 | 8.4 | - | - | - | - | 9.2 | 66.3 | 14.6 |
| 0.05% DCP | −30.2 | 59.2 | 120.4 | 11.1 | - | - | - | - | 12.2 | 73.8 | 16.8 |
| 0.1% DCP | −31.3 | 58.8 | 120.9 | 9.4 | 151.1 | 0.8 | - | - | 10.3 | 77.0 | 16.0 |
| 0.25% DCP | −31.2 | 57.6 | 122.4 | 5.5 | 148.0 | 2.7 | - | - | 6.0 | 80.4 | 15.6 |
| 0.5% DCP | −30.8 | 59.1 | 123.0 | 3.3 | 146.6 | 2.3 | - | - | 3.6 | 81.8 | 14.8 |
| Sample | Mean Pore Size (nm) | STDEV.S (nm) |
|---|---|---|
| PBAT80/PLA20 | 611.74 | 286.54 |
| 0.05% DCP | 512.04 | 131.74 |
| 0.1% DCP | 420.1 | 194.32 |
| 0.25% DCP | 732.33 | 374.38 |
| 0.5% DCP | 275.7 | 63.16 |
| Sample | Slope | Intercept |
|---|---|---|
| PBAT80/PLA20 | 1.2558 | −1.3386 |
| 0.1% DCP | 1.2636 | −1.1472 |
| DCP–GMA–MA 1:1:1 | 1.2238 | −1.0884 |
| DCP–GMA 1:2 | 1.2747 | 1.1059− |
| DCP–MA 1:2 | 1.2171 | 1.1213− |
| DCP–GMA 1:1 | 1.2114 | 1.0867− |
| Tg2 PBAT (°C) | Tg2 PLA (°C) | Tm2 PBAT (°C) | ΔHm2 PBAT (J/g) | Tm2 PLA (°C) | ΔHm2 PLA (J/g) | Xc2 of PLA (%) | Xc2 of PBAT (%) | Tc1 (°C) | ΔHc1 (J/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| DCP–MA 1:2 | −30.0 | 59.3 | 121.8 | 6.7 | - | - | - | 9.2 | 74.1 | 13.9 |
| DCP–GMA 1:2 | −30.2 | 58.2 | 121.9 | 6.9 | - | - | - | 9.4 | 78.2 | 12.1 |
| DCP–GMA 1:1 | −31.9 | 58.9 | 122.9 | 6.8 | - | - | - | 9.3 | 76.7 | 13.1 |
| DCP–GMA–MA 1:1:1 | −29.3 | 61.1 | 123.8 | 5.9 | - | - | - | 8.0 | 74.6 | 13.7 |
| Sample | Mean Pore Size (nm) | STDEV.S (nm) |
|---|---|---|
| DCP–GMA 1:1 | 1383.32 | 749.13 |
| DCP–GMA–MA 1:1:1 | 1931.48 | 1160.79 |
| DCP–GMA 1:2 | 1302.35 | 492.30 |
| DCP–MA 1:2 | 780.98 | 390.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laevsky, K.D.; Zilberfarb, A.; Ophir, A.; Dotan, A.L. Stretchability and Melt Strength Enhancement of Biodegradable Polymer Blends for Packaging Solutions. Molecules 2025, 30, 3211. https://doi.org/10.3390/molecules30153211
Laevsky KD, Zilberfarb A, Ophir A, Dotan AL. Stretchability and Melt Strength Enhancement of Biodegradable Polymer Blends for Packaging Solutions. Molecules. 2025; 30(15):3211. https://doi.org/10.3390/molecules30153211
Chicago/Turabian StyleLaevsky, Katy D., Achiad Zilberfarb, Amos Ophir, and Ana L. Dotan. 2025. "Stretchability and Melt Strength Enhancement of Biodegradable Polymer Blends for Packaging Solutions" Molecules 30, no. 15: 3211. https://doi.org/10.3390/molecules30153211
APA StyleLaevsky, K. D., Zilberfarb, A., Ophir, A., & Dotan, A. L. (2025). Stretchability and Melt Strength Enhancement of Biodegradable Polymer Blends for Packaging Solutions. Molecules, 30(15), 3211. https://doi.org/10.3390/molecules30153211







