Lipase-Catalyzed Cyclization of β-Ketothioamides with β-Nitrostyrene for the Synthesis of Tetrasubstituted Dihydrothiophenes
Abstract
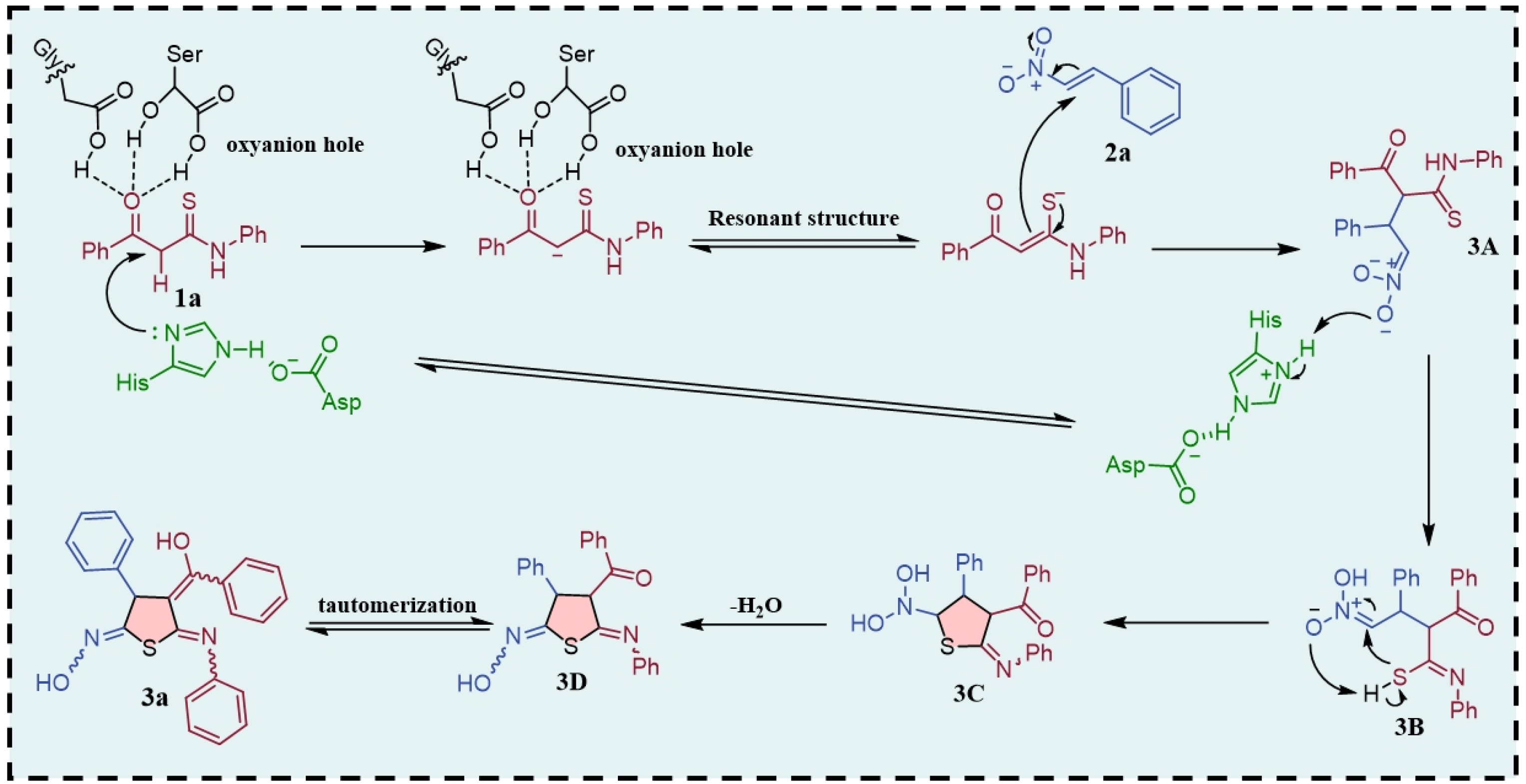
1. Introduction
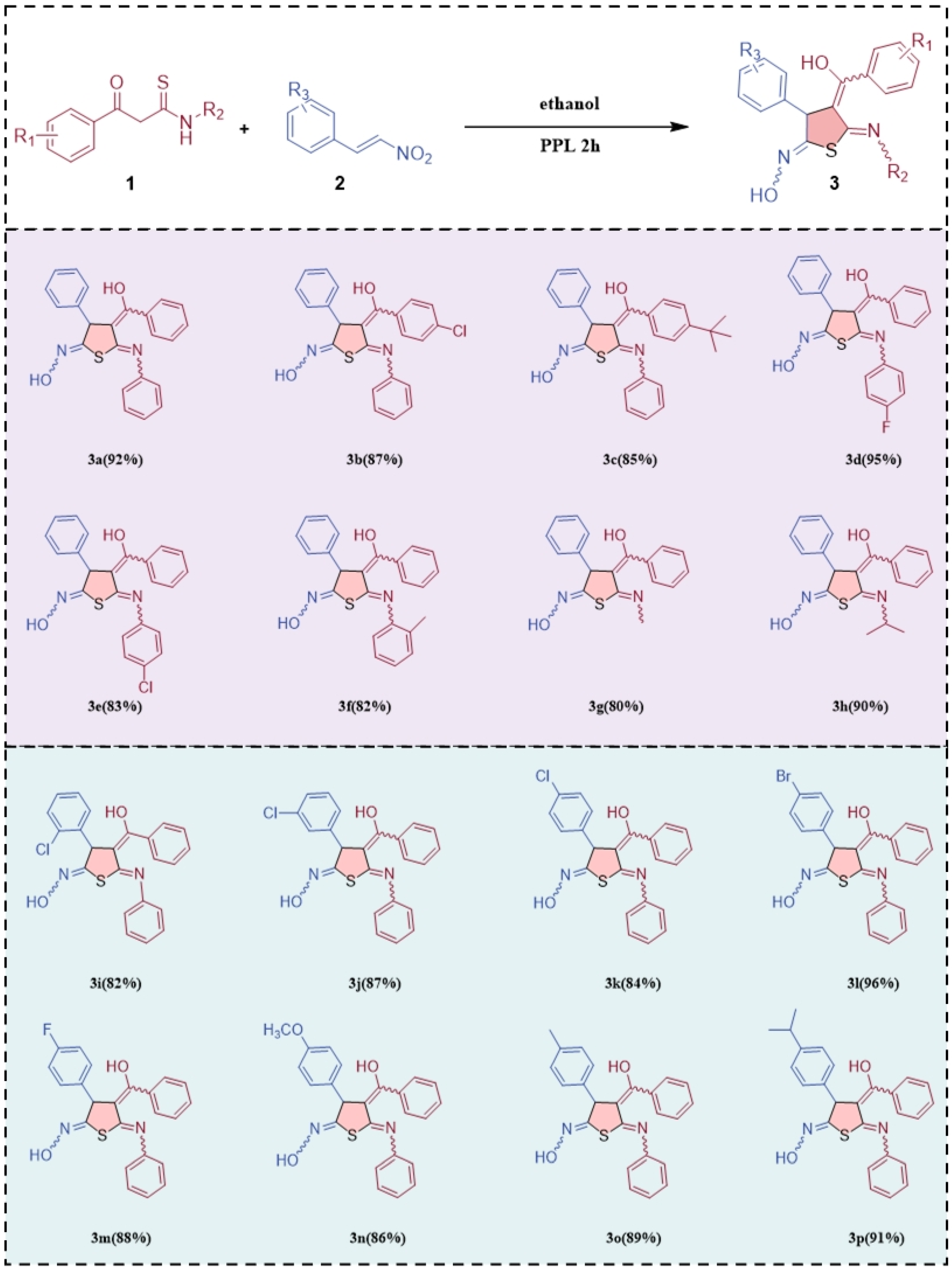
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for Synthesis of β-Ketothioamide (1)
3.3. General Procedure for Lipase-Catalyzed Synthesis of Tetrasubstituted Dihydrothiophenes (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molvi, K.I.; Mansuri, M.; Sudarsanam, V.; Patel, M.M.; Andrabi, S.M.; Haque, N. Synthesis, anti-inflammatory, analgesic and antioxidant activities of some tetrasubstituted thiophenes. J. Enzyme Inhib. Med. Chem. 2008, 23, 829–838. [Google Scholar] [CrossRef]
- Yao, C.H.; Shen, Z.Q.; Rajan, Y.C.; Huang, Y.W.; Lin, C.Y.; Song, J.S.; Shiao, H.Y.; Ke, Y.Y.; Fan, Y.S.; Tsai, C.H.; et al. Discovery of tetrasubstituted thiophenes as Cisd2 activators: A potential novel therapeutic option in nonalcoholic fatty liver disease. Eur. J. Med. Chem. 2023, 258, 115583. [Google Scholar] [CrossRef]
- Li, L.-X.; Xie, Z.-H.; Fernandez, C.; Wu, L.; Cheng, D.; Jiang, X.-H.; Zhong, C.-J. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; Garcia-Benito, I.; Zimmermann, I.; Arago, J.; Molina-Ontoria, A.; Orti, E.; Martin, N.; Nazeeruddin, M.K. Tetrasubstituted Thieno[3,2-b]thiophenes as Hole-Transporting Materials for Perovskite Solar Cells. J. Org. Chem. 2020, 85, 224–233. [Google Scholar] [CrossRef]
- Molvi, K.I.; Vasu, K.K.; Yerande, S.G.; Sudarsanam, V.; Haque, N. Syntheses of new tetrasubstituted thiophenes as novel anti-inflammatory agents. Eur. J. Med. Chem. 2007, 42, 1049–1058. [Google Scholar] [CrossRef]
- Molvi, K.I.; Sudarsanam, V.; Patel, M.M.; Haque, N. Design, synthesis and pharmacological evaluation of novel tetrasubstituted thiophene analogues as anti-inflammatory agents. J. Enzyme Inhib. Med. Chem. 2008, 23, 819–828. [Google Scholar] [CrossRef]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.S.; Wen, L.R.; Li, M. beta-ketothioamides: Efficient reagents in the synthesis of heterocycles. Org. Biomol. Chem. 2015, 13, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Yadav, D.; Singh, M.S. Rhodium(II)-Catalyzed Annulative Coupling of beta-Ketothioamides with alpha-Diazo Compounds: Access to Highly Functionalized Thiazolidin-4-ones and Thiazolines. J. Org. Chem. 2020, 85, 8320–8329. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Wang, N.N.; Du, W.B.; Zhu, M.Z.; Pan, C.; Zhang, L.B.; Li, M. Electrochemical Selective Oxidative Synthesis of Diversified Sulfur Heterocycles from β-Ketothioamides. Chin. J. Chem. 2021, 39, 1831–1837. [Google Scholar] [CrossRef]
- Bogdanowicz-Szwed, K.; Palasz, A.; Rys, B.; Soja, D.; Grochonski, J.; Serda, P. The Conjugate Addition of Benzoyl(thioacetanilides) to Nitroalkenes Synthesis of Functionalized Thiophenes and Pyrroles. Liebigs Ann. 2006, 1996, 1457–1462. [Google Scholar] [CrossRef]
- Bogdanowicz-Szwed, K.; Grochowski, J.; Obara, A.; Rys, B.; Serda, P. Stereoselective Synthesis of Bridged, Azepine Derivatives via Polyfunctionalized, Spiroannulated Thiophene. Novel Rearrangement of Oxime Esters. J. Org. Chem. 2001, 66, 7205–7208. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz-Szwed, K.; Gil, R.; Serda, P. The Conjugate Addition-Cyclization of 3-Oxoacid Thioanilides to β-Nitrostyrenes. An Efficient Synthesis of Functionalized Thiophenes and their Transformation to Pyrroles. Monatsh. Chem. 2006, 137, 219–229. [Google Scholar] [CrossRef]
- Bogdanowicz-Szwed, K.; Gil, R. Synthesis of Functionalized Spiro[cycloalkanono-2,3-thiophenes] via Tandem Conjugate Addition-Cyclization of 3-Oxoacid Thioanilides to Nitroalkenes. Monatsh. Chem. 2004, 135, 1415–1425. [Google Scholar] [CrossRef]
- Bogdanowicz-Szwed, K.; Czarny, A. Synthesis of functionalised polythiophenes and(2-thienylcarbonyl)pyrroles viaconjugate addition of(2-thienylcarbonyl)thioacetanilides to nitroakenes. J. Chem. Res. 2003, 2003, 51–53. [Google Scholar] [CrossRef]
- Wen, L.R.; He, T.; Lan, M.C.; Li, M. Three-component cascade annulation of beta-ketothioamides promoted by CF3CH2OH: A regioselective synthesis of tetrasubstituted thiophenes. J. Org. Chem. 2013, 78, 10617–10628. [Google Scholar] [CrossRef]
- Zeng, X.M.; Meng, C.Y.; Bao, J.X.; Xu, D.C.; Xie, J.W.; Zhu, W.D. Enantioselective Construction of Polyfunctionalized Spiroannulated Dihydrothiophenes via a Formal Thio [3+2] Cyclization. J. Org. Chem. 2015, 80, 11521–11528. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, X.; Cheng, B.; Li, H.; Li, Y.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Synthesis of tetrasubstituted thiophenes via a [3+2] cascade cyclization reaction of pyridinium 1,4-zwitterionic thiolates and activated allenes. Chem. Commun. 2020, 56, 3085–3088. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.; Tao, Q.; Xu, W.; Sun, H.; Wu, P.; Cheng, B.; Zhai, H. Synthesis of tetrasubstituted thiophenes from pyridinium 1,4-zwitterionic thiolates and modified activated alkynes. Chin. Chem. Lett. 2021, 32, 3972–3975. [Google Scholar] [CrossRef]
- He, Y.; Lou, J.; Wu, P.; Zhou, Y.G.; Yu, Z. Copper-Catalyzed Annulative Coupling of S,S-Disubstituted Enones with Diazo Compounds to Access Highly Functionalized Thiophene Derivatives. J. Org. Chem. 2020, 85, 1044–1053. [Google Scholar] [CrossRef]
- Alizadeh, A.; Vahabi, A.H.; Bazgir, A.; Khavasi, H.R.; Zhu, Z.; Ng, S.W. Highly mild approach towards synthesis of tetrasubstituted thiophenes by an organic salt afforded by cyclic thioureas and ketene dithioacetals. RSC Adv. 2015, 5, 85028–85034. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Cao, F.; Zhang, P.; Ou, J.; Tang, K. Immobilization of lipase onto metal–organic frameworks for enantioselective hydrolysis and transesterification. AIChE J. 2020, 66, 16292–16294. [Google Scholar] [CrossRef]
- Ou, J.; Yuan, X.; Liu, Y.; Zhang, P.; Xu, W.; Tang, K. Lipase from pseudomonas cepacia immobilized into ZIF-8 as bio-catalyst for enantioselective hydrolysis and transesterification. Process Biochem. 2021, 102, 132–140. [Google Scholar] [CrossRef]
- Mateos, P.S.; Navas, M.B.; Morcelle, S.R.; Ruscitti, C.; Matkovic, S.R.; Briand, L.E. Insights in the biocatalyzed hydrolysis, esterification and transesterification of waste cooking oil with a vegetable lipase. Catal. Today 2021, 372, 211–219. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, C.; Hao, M.; Wang, H.; Wang, Z.; Shen, C.; Cheong, L.Z. Enhanced catalytic stability of lipase immobilized on oxidized and disulfide-rich eggshell membrane for esters hydrolysis and transesterification. Int. J. Biol. Macromol. 2017, 105 Pt 1, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.D.; Pereira, M.G.; Vici, A.C.; Filice, M.; Pessela, B.C.; Guisan, J.M.; Fernandez-Lorente, G.; Polizeli, M.D. Immobilization Effects on the Catalytic Properties of Two Fusarium Verticillioides Lipases: Stability, Hydrolysis, Transesterification and Enantioselectivity Improvement. Catalysts 2018, 8, 84. [Google Scholar] [CrossRef]
- Siar, E.-H.; Arana-Peña, S.; Barbosa, O.; Zidoune, M.; Fernandez-Lafuente, R. Immobilization/Stabilization of Ficin Extract on Glutaraldehyde-Activated Agarose Beads. Variables That Control the Final Stability and Activity in Protein Hydrolyses. Catalysts 2018, 8, 149. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, D.; Wang, X.; Yang, L. Ionic Liquids: Efficient Media for the Lipase-Catalyzed Michael Addition. Molecules 2018, 23, 2154. [Google Scholar] [CrossRef]
- Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.; Scott, E.L.; Franssen, M.C. Lipase-catalyzed aza-Michael reaction on acrylate derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef]
- Samsonowicz-Gorski, J.; Koszelewski, D.; Kowalczyk, P.; Smigielski, P.; Hrunyk, A.; Kramkowski, K.; Wypych, A.; Szymczak, M.; Lizut, R.; Ostaszewski, R. Promiscuous Lipase-Catalyzed Knoevenagel-Phospha-Michael Reaction for the Synthesis of Antimicrobial beta-Phosphono Malonates. Int. J. Mol. Sci. 2022, 23, 8819. [Google Scholar] [CrossRef]
- González-Martínez, D.; Gotor, V.; Gotor-Fernández, V. Application of Deep Eutectic Solvents in Promiscuous Lipase-Catalysed Aldol Reactions. Eur. J. Org. Chem. 2016, 2016, 1513–1519. [Google Scholar] [CrossRef]
- Du, L.-H.; Long, R.-J.; Xue, M.; Chen, P.-F.; Yang, M.-J.; Luo, X.-P. Continuous-Flow Synthesis of β-Amino Acid Esters by Lipase-Catalyzed Michael Addition of Aromatic Amines. Catalysts 2020, 10, 432. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme production ofd-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Long, C.-J.; Pu, H.-P.; He, Y.-H.; Guan, Z. Direct enantioselective α-alkylation of secondary acyclic amines with ketones by combining photocatalysis and lipase catalytic promiscuity. Org. Chem. Front. 2023, 10, 5108–5116. [Google Scholar] [CrossRef]
- Dutt, S.; Mohapatra, A.; Pandey, S.; Tyagi, V. A decade update on the promiscuity of α-amylase in organic synthesis. Tetrahedron 2024, 155, 133905. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Fernandez-Lafuente, R.; Tardioli, P.W. Ethanolysis of soybean oil catalyzed by magnetic CLEA of porcine pancreas lipase to produce ecodiesel. Efficient separation of ethyl esters and monoglycerides. Renew. Energy 2022, 198, 455–462. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T.; Guo, Z.; Zhou, L.; Liu, G.; He, Y.; Ma, L.; Gao, J.; Bai, J.; Hollmann, F.; et al. Asymmetric alpha-benzylation of cyclic ketones enabled by concurrent chemical aldol condensation and biocatalytic reduction. Nat. Commun. 2024, 15, 71. [Google Scholar] [CrossRef]
- Long, C.J.; Cao, H.; Zhao, B.K.; Tan, Y.F.; He, Y.H.; Huang, C.S.; Guan, Z. Merging the Non-Natural Catalytic Activity of Lipase and Electrosynthesis: Asymmetric Oxidative Cross-Coupling of Secondary Amines with Ketones. Angew. Chem. 2022, 61, 3666. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, K.; Xu, Y.; Ma, J.; Xie, H.; Zhang, H.; Jiang, Y.; Zhao, R.; Wang, L. Lipase-catalyzed one-pot four-component reaction in water: Green construction of substituted 2,3-dihydrothiophenes. New J. Chem. 2023, 47, 20316–20321. [Google Scholar] [CrossRef]
- Xie, H.; Li, F.; Xu, Y.; Wang, C.; Xu, Y.; Wu, J.; Li, Z.; Wang, Z.; Wang, L. Vitreoscillahemoglobin: A natural carbene transfer catalyst for diastereo- and enantioselective synthesis of nitrile-substituted cyclopropanes. Green. Chem. 2023, 25, 6853–6858. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Wang, C.; Wang, C.; Xie, H.; Xu, Y.; Chen, P.; Wang, L. Efficient synthesis of substituted pyrazoles Via [3+2] cycloaddition catalyzed by lipase in ionic liquid. Process Biochem. 2023, 124, 253–258. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Xu, Y.; Wang, C.; Du, C.; Wang, Z.; Wang, L. A Practical Synthesis of 2-Arylimino-2H-Chromenes via Biocatalytic System in Water. ChemistrySelect 2023, 8, 851. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, C.; Xie, H.; Xu, Y.; Wang, C.; Du, C.; Wang, Z.; Wang, L. Green Synthesis of Spirooxindoles via Lipase-Catalyzed One-Pot Tandem Reaction in Aqueous Media. Catalysts 2023, 13, 143. [Google Scholar] [CrossRef]
- Mendes, A.A.; Oliveira, P.C.; de Castro, H.F. Properties and biotechnological applications of porcine pancreatic lipase. J. Mol. Catal. B Enzym. 2012, 78, 119–134. [Google Scholar] [CrossRef]
- Feng, X.; Wang, J.-J.; Xun, Z.; Huang, Z.-B.; Shi, D.-Q. Multicomponent Strategy to Indeno[2,1-c]pyridine and Hydroisoquinoline Derivatives through Cleavage of Carbon–Carbon Bond. J. Org. Chem. 2015, 80, 1025–1033. [Google Scholar] [CrossRef]

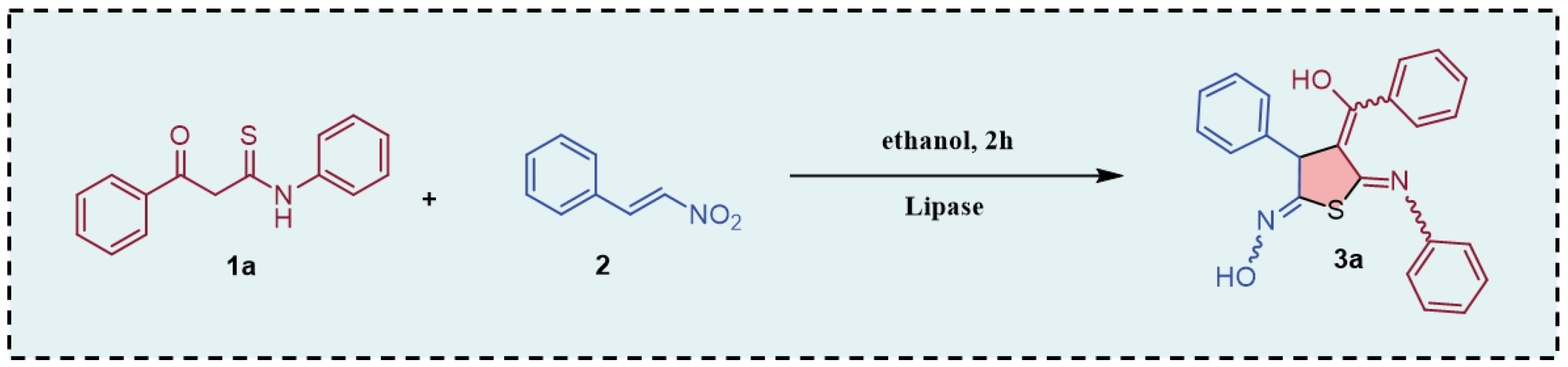
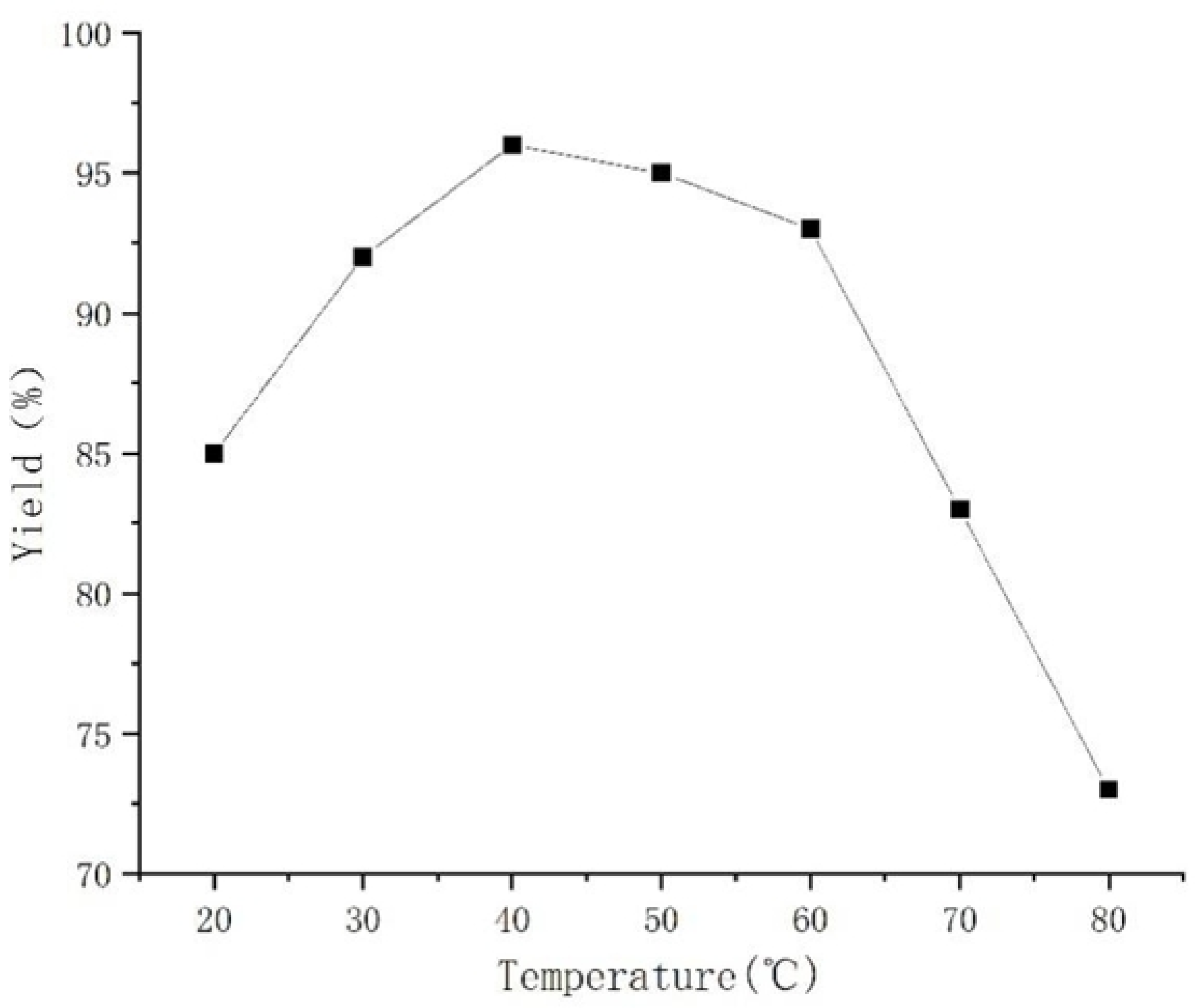
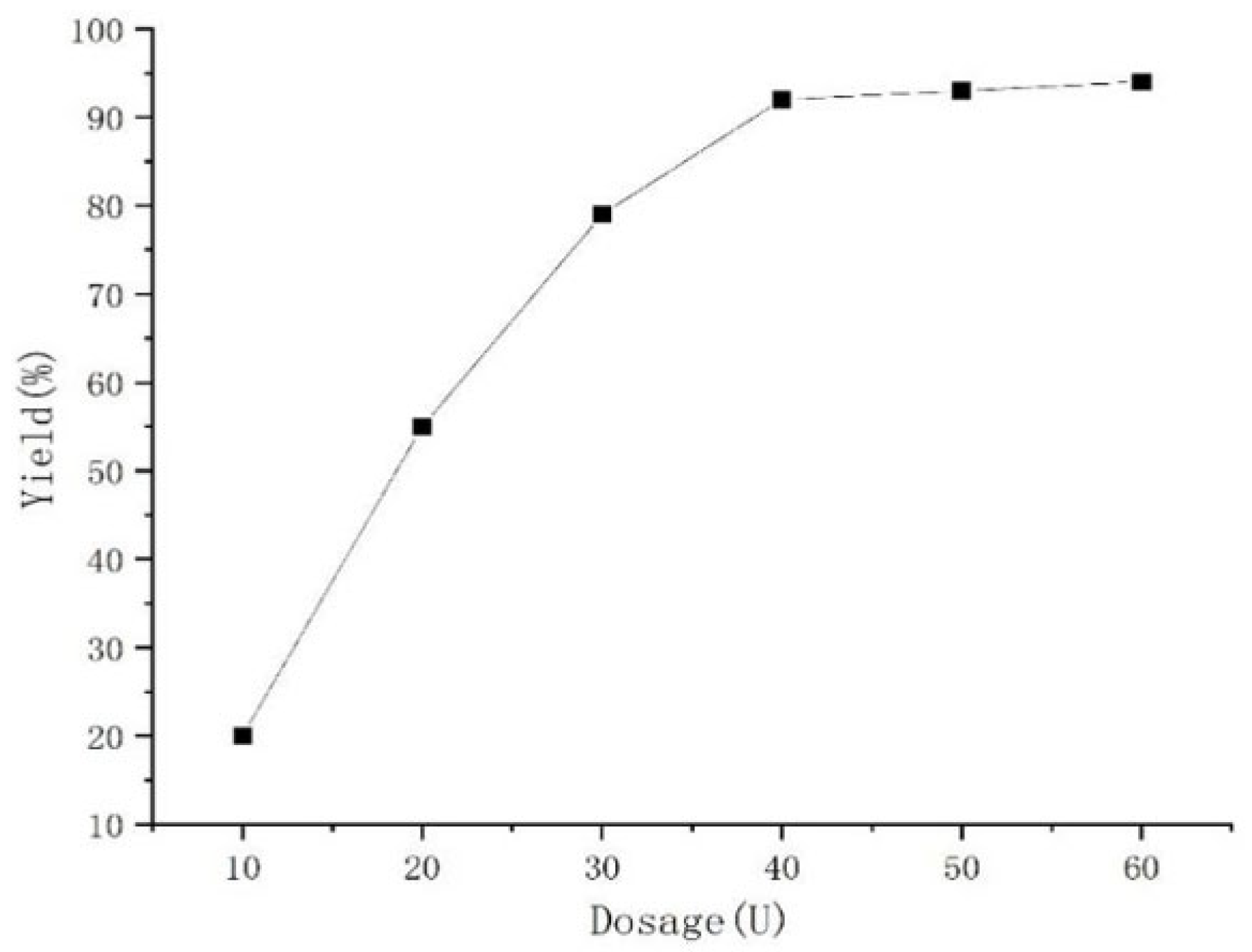
| Entry | Catalyst | Yield (%) |
|---|---|---|
| 1 | PPL 1 | 92 |
| 2 | CSL | 46 |
| 3 | CRL | 55 |
| 4 | MML | 52 |
| 5 | Cal-B | 68 |
| 6 | Movozym-435 | 76 |
| 7 | BSA | N.D. |
| 8 | PPL 2 | N.D. |
| 9 | PPL 3 | N.D. |
| 10 | None | N.D. |
| 11 | Triethylamine 4 | 53 |
| Entry | Solvent | Yield (%) |
|---|---|---|
| 1 | Water | 80 |
| 2 | Ethanol | 92 |
| 3 | Acetonitrile | 94 |
| 4 | Dimethyl sulfoxide | 67 |
| 5 | N, N-dimethylformamide | 76 |
| 6 | Ethyl acetate | 84 |
| 7 | Dichloromethane | 50 |
| 8 | n-Hexane | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Piao, Y.; Kan, W.; Wang, L.; Li, Y. Lipase-Catalyzed Cyclization of β-Ketothioamides with β-Nitrostyrene for the Synthesis of Tetrasubstituted Dihydrothiophenes. Molecules 2025, 30, 3202. https://doi.org/10.3390/molecules30153202
Dai Y, Piao Y, Kan W, Wang L, Li Y. Lipase-Catalyzed Cyclization of β-Ketothioamides with β-Nitrostyrene for the Synthesis of Tetrasubstituted Dihydrothiophenes. Molecules. 2025; 30(15):3202. https://doi.org/10.3390/molecules30153202
Chicago/Turabian StyleDai, Yihang, Yuming Piao, Wenbo Kan, Lei Wang, and Yazhuo Li. 2025. "Lipase-Catalyzed Cyclization of β-Ketothioamides with β-Nitrostyrene for the Synthesis of Tetrasubstituted Dihydrothiophenes" Molecules 30, no. 15: 3202. https://doi.org/10.3390/molecules30153202
APA StyleDai, Y., Piao, Y., Kan, W., Wang, L., & Li, Y. (2025). Lipase-Catalyzed Cyclization of β-Ketothioamides with β-Nitrostyrene for the Synthesis of Tetrasubstituted Dihydrothiophenes. Molecules, 30(15), 3202. https://doi.org/10.3390/molecules30153202






