Natural Neuroinflammatory Modulators: Therapeutic Potential of Fungi-Derived Compounds in Selected Neurodegenerative Diseases
Abstract
1. Introduction
- Antibiotics: penicillins, cephalosporins, and fusidic acid;
- Antifungal drugs: griseofulvin, strobilurins, and echinocandins;
- Statins (cholesterol-lowering drugs): mevinoline, lowastatin, and simvastatin;
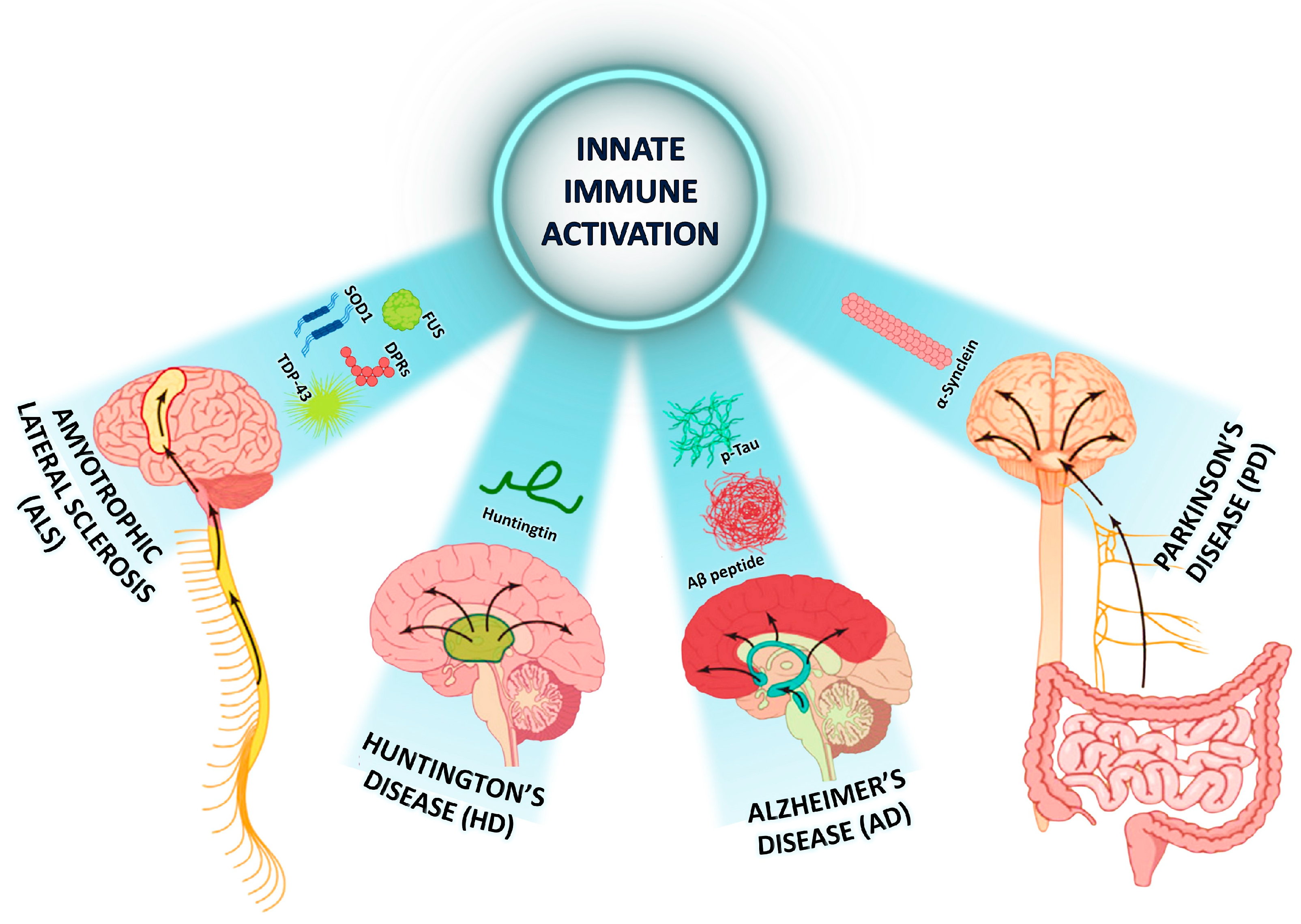
2. Pathogenesis of Neurodegenerative Diseases
2.1. General Definition of the Inflammatory Response in the Central Nervous System
2.2. Role of Blood–Brain Barrier
2.3. Microglia and Astrocytes—Main Cells Involved in the Inflammatory Response in the Central Nervous System
2.4. Role of Inflammatory Mediators
2.5. Specificity of the Inflammatory Response in Selected Neurodegenerative Diseases (NDDs)
3. Bioactive Compounds in Mushrooms with Health-Promoting Properties and Their Mechanisms of Action on the Inflammatory Response
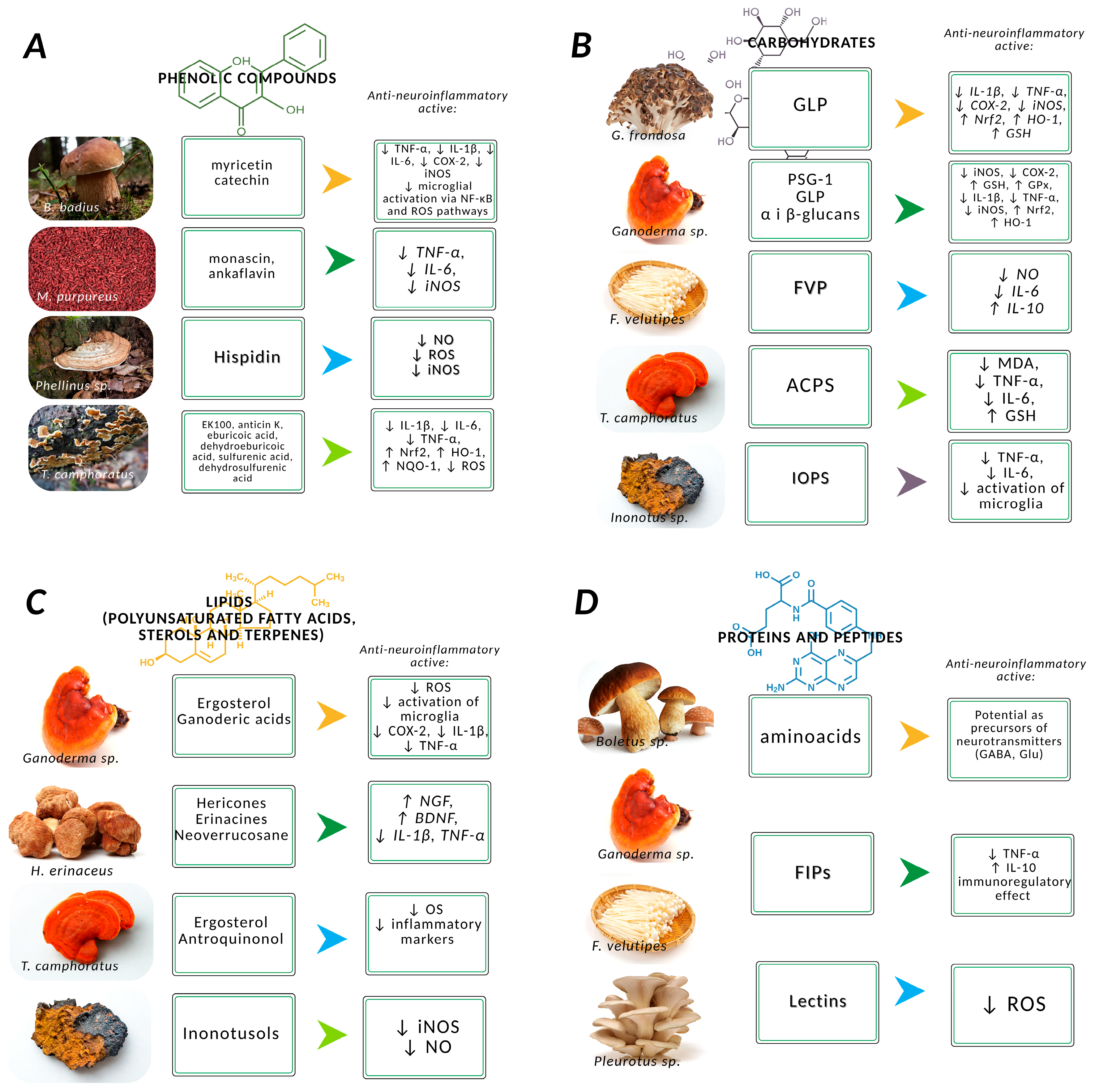
3.1. Phenolic Compounds
3.2. Carbohydrates
- Antioxidant activity;
- Anti-amyloidogenic activity;
- Anti-neuroinflammatory effects;
- Anticholinesterase activity;
- Anti-apoptotic effects;
- Anti-neurotoxic effects;
- Anti-ferroptotic effects.
3.3. Proteins and Peptides
3.4. Lipids
4. Future Prospects
4.1. Formulation, Administration and Safety of Bioactive Mushroom Products
4.2. Synergistic Actions and Integration with Conventional Therapies
5. Conclusions
- Bioactive compounds derived from mushrooms—particularly polysaccharides, phenols, and terpenoids—exert significant anti-inflammatory and neuroprotective effects, giving them therapeutic potential in the treatment of neurodegenerative and other diseases (e.g., cardiovascular disease, cancer).
- Certain fungal components modulate key molecular targets and signaling pathways (e.g., NGF/TrkA, BDNF/TrkB, iNOS, Nrf2/HO-1), enabling precise regulation of cellular processes.
- Unique functional groups in fungal compounds may underlie their specific mechanisms of action.
- Some fungal metabolites are active at very low concentrations and demonstrate lower toxicity compared to synthetic neuroprotective agents.
- Continued research is essential to identify new bioactive compounds, elucidate their mechanisms of action, and explore their potential for therapeutic application in neurodegenerative disease treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| ACPS | Polysaccharides from Amanita caesarea |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| APC | Polysaccharides from Tajwanofungus camphoratus (Amanita camphorata) |
| Aβ | Amyloid-beta |
| BBB | Blood–brain barrier |
| CAT | Catalase |
| CCL2 | C-C motif chemokine Ligand 2 |
| CD | Cluster of differentation |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| FIPs | Fungal immunomodulatory proteins |
| FUS | Fused in sarcoma gene |
| FVP | Polysaccharides from Flammulina velutipes |
| GFP | Polisaccharides from Grifola frondosa |
| GLP | Polisaccharides from Ganoderma lucidum |
| HD | Huntington’s disease |
| HTT | Huntingtin gene |
| HO-1 | Heme oxygenase-1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IOPS | Inonotus obliquus polysaccharide |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| NDDs | Neurodegenerative diseases |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve growth factory |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NLRP3 | Nucleotide-binding oligomerization domain—leucine-rich repeats—and pyrin domain-containing protein 3 |
| NO | Nitric oxide |
| NQO-1 | NAD(P)H:quinone oxidoreductase 1 |
| NVU | Neurovascular unit |
| OS | Oxidative stress |
| PD | Parkinson’s disease |
| PSG-1 | Polysaccharide from Ganoderma atrum |
| ROS | Reactive oxygen species |
| SOD1 | Superoxide dismutase 1 |
| TLH-3 | Polysaccharide from Tricholoma lobayense |
References
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- McGirr, S.; Venegas, C.; Swaminathan, A. Alzheimer’s disease: A Brief Review. J. Exp. Neurol. 2020, 1, 89–98. [Google Scholar] [CrossRef]
- Su, M.; Wang, T.; Zou, C.; Cao, K.; Liu, F. Global, regional, and national burdens of Alzheimer’s disease and other forms of dementia in the elderly population from 1999 to 2019: A trend analysis based on the Global Burden of Disease Study 2019. Ibrain 2024, 10, 488–499. [Google Scholar] [CrossRef]
- Ilic, I.; Jakovljevic, V.; Zivanovic Macuzic, I.; Ravic-Nikolic, A.; Ilic, M.; Sorak, M.; Milicic, V. Trends in global burden of Alzheimer’s disease and other dementias attributable to high fasting plasma glucose, 1990–2021. Medicina 2024, 60, 1783. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, L.; Li, M.; Wen, X.; Zhang, W.; Li, X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. Front. Aging Neurosci. 2025, 16, 1498756. [Google Scholar] [CrossRef]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Ajitkumar, A.; Lui, F.; De Jesus, O. Huntington Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ruffo, P.; Traynor, B.J.; Conforti, F.L. Advancements in genetic research and RNA therapy strategies for amyotrophic lateral sclerosis (ALS): Current progress and future prospects. J. Neurol. 2025, 272, 233. [Google Scholar] [CrossRef]
- Xu, L.; Liu, T.; Liu, L.; Yao, X.; Chen, L.; Fan, D.; Zhan, S.; Wang, S. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 2020, 267, 944–953. [Google Scholar] [CrossRef]
- Nogueira-Machado, J.A.; das Chagas Lima e Silva, F.; Rocha-Silva, F.; Gomes, N. Amyotrophic Lateral Sclerosis (ALS): An overview of genetic and metabolic signaling mechanisms. CNS Neurol. Disord. Drug Targets 2025, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gomez, S.; Heneka, M.T. Innate immune activation in neurodegenerative diseases. Immunity 2024, 57, 790–814. [Google Scholar] [CrossRef]
- Shusharina, N.; Yukhnenko, D.; Botman, S.; Sapunov, V.; Savinov, V.; Kamyshov, G.; Sayapin, D.; Voznyuk, I. Modern methods of diagnostics and treatment of neurodegenerative diseases and depression. Diagnostics 2023, 13, 573. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.; Aisyah Alias, S.; Hyde, K.D. Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fungal Divers. 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Jiamworanunkul, S.; Chomcheon, P. Screening of antimicrobial and antioxidant properties of ethyl acetate extracts from wild edible mushrooms. Thai J. Pharm. Sci. 2019, 43, 7. [Google Scholar] [CrossRef]
- Guo, D.; Liu, C.; Zhu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Jiang, J.; Qian, H. Advanced insights into mushroom polysaccharides: Extraction methods, structure-activity, prebiotic properties, and health-promoting effects. Int. J. Biol. Macromol. 2025, 308, 142319. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant compounds from edible mushrooms as potential candidates for treating age-related neurodegenerative diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Sevindik, M.; Akgul, H.; Bal, C.; Selamoglu, Z. Phenolic contents, oxidant/antioxidant potential and heavy metal levels in Cyclocybe cylindracea. IJPER 2018, 52, 437–441. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Well. 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Frljak, J.; Mulabećirović, A.H.; Isaković, S.; Karahmet, E.; Toroman, A. Biological active components of selected medical fungi. Open J. Prev. Med. 2021, 11, 9–22. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A mechanistic review on medicinal mushrooms-derived bioactive compounds: Potential mycotherapy candidates for alleviating neurological disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Gorji, A. Neuroinflammation: The pathogenic mechanism of neurological disorders. Int. J. Mol. Sci. 2022, 23, 5744. [Google Scholar] [CrossRef]
- Konsman, J.P. Cytokines in the brain and neuroinflammation: We didn’t starve the fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Ahmad, A.; Patel, V.; Xiao, J.; Khan, M.M. The role of neurovascular system in neurodegenerative diseases. Mol. Neurobiol. 2020, 57, 4373–4393. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Neurodegenerative Diseases. Available online: https://taylorandfrancis.com/knowledge/medicine-and-healthcare/neurodegenerative-diseases/ (accessed on 12 May 2025).
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood–brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood–brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.-Y.; Huang, T.-T.; Zhou, Y.-X.; Wang, X.; Yang, L.-Q.; Chen, Z.-A.; Yu, W.-F.; Li, P.-Y. The peripheral immune response after stroke—A double edge sword for blood-brain barrier integrity. CNS Neurosci. Ther. 2018, 24, 1115–1128. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Geng, P.; Du, W.; Guo, C.; Wang, Q.; Zheng, G.; Jin, X. Role of crosstalk between glial cells and immune cells in blood-brain barrier damage and protection after acute ischemic stroke. Aging Dis. 2023, 15, 2507–2525. [Google Scholar] [CrossRef]
- Fakhoury, M. Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener. Dis. 2015, 15, 63–69. [Google Scholar] [CrossRef]
- Bendig, J.; Frank, A.; Reichmann, H. Aging and Parkinson’s disease: A complex interplay of vulnerable neurons, the immune system and the blood-brain barrier. Ageing Neur. Dis. 2024, 4, 5. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 2022, 19, 132. [Google Scholar] [CrossRef]
- Xu, L.; He, D.; Bai, Y. Microglia-mediated inflammation and neurodegenerative disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Liu, B.; Hong, J.-S. Role of microglia in inflammation-mediated neurodegenerative diseases: EcMhanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Turniak-Kusy, M.; Pacan, I.; Glabinski, A. Human primary astrocytes differently respond to pro- and anti-inflammatory stimuli. Biomedicines 2022, 10, 1769. [Google Scholar] [CrossRef] [PubMed]
- Edison, P. Astroglial activation: Current concepts and future directions. Alzheimers Dement. 2024, 20, 3034–3053. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The role of astrocytes in CNS inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-astrocyte crosstalk: An intimate molecular Conversation. Neuroscientist. 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk between astrocytes and microglia: An overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- González, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274, 1–13. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro- and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010, 20, 357–367. [Google Scholar] [CrossRef]
- Sheremeta, C.-L.; Yarlagadda, S.; Smythe, M.L.; Noakes, P.G. Prostaglandins in the inflamed central nervous system: Potential therapeutic targets. Curr. Drug Targets 2024, 25, 885–908. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J. Microglia-mediated neuroinflammation is an amplifier of virus-induced neuropathology. J. Neurovirol. 2014, 20, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 6, 575–590. [Google Scholar] [CrossRef]
- Guan, P.-P.; Cao, L.-L.; Wang, P. Elevating the levels of calcium ions exacerbate Alzheimer’s Disease via inducing the production and aggregation of β-amyloid protein and phosphorylated Tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef]
- Twarowski, B.; Herbet, M. Inflammatory processes in Alzheimer’s Disease—Pathomechanism, diagnosis and treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S. Inflammation and Parkinson’s disease pathogenesis. Mov. Disord. 2010, 25, 55–57. [Google Scholar] [CrossRef]
- Goleij, P.; Amini, A.; Tabari, M.A.K.; Hadipour, M.; Sanaye, P.M.; Alsharif, K.F.; Daglia, M.; Larsen, D.S.; Khan, H. The role of interleukin (IL)-2 cytokine family in Parkinson’s disease. Cytokine 2025, 191, 156954. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Miliukhina, I.V.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef]
- Khaboushan, A.S.; Moeinafshar, A.; Ersi, M.H.; Teixeira, A.L.; Zolbin, M.M.; Kajbafzadeh, A.-M. Circulating levels of inflammatory biomarkers in Huntington’s disease: A systematic review and meta-analysis. J. Neuroimmunol. 2023, 385, 578243. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Charron, O.; Latham, L.B.; Colpo, G.D.; Zanotti-Fregonara, P.; Yu, M.; Freeman, L.; Furr Stimming, E.; Teixeira, A.L. Microglia activation in basal ganglia is a late event in huntington disease pathophysiology. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e984. [Google Scholar] [CrossRef]
- Moghaddam, M.H.; Bayat, A.-H.; Eskandari, N.; Abdollahifar, M.; Fotouhi, F.; Forouzannia, A.; Rafiei, R.; Hatari, S.; Seraj, A.; Shahidi, A.M.E.J.; et al. Elderberry diet ameliorates motor function and prevents oxidative stress-induced cell death in rat models of Huntington disease. Brain Res. 2021, 1762, 147444. [Google Scholar] [CrossRef]
- Staats, K.A.; Borchelt, D.R.; Tansey, M.G.; Wymer, J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol. Neurodegener. 2022, 17, 11. [Google Scholar] [CrossRef]
- Valadão, P.A.C.; Santos, K.B.S.; Ferreira E Vieira, T.H.; Macedo Cordeiro, T.; Teixeira, A.L.; Guatimosim, C.; de Miranda, A.S. Inflammation in Huntington’s disease: A few new twists on an old tale. J. Neuroimmunol. 2020, 348, 577380. [Google Scholar] [CrossRef] [PubMed]
- Femiano, C.; Bruno, A.; Gilio, L.; Buttari, F.; Dolcetti, E.; Galifi, G.; Azzolini, F.; Borrelli, A.; Furlan, R.; Finardi, A.; et al. Inflammatory signature in amyotrophic lateral sclerosis predicting disease progression. Sci. Rep. 2024, 14, 19796. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xu, Y.; Liu, M.; Cui, L. The Inflammatory Puzzle: Piecing together the Links between Neuroinflammation and Amyotrophic Lateral Sclerosis. Aging Dis. 2024, 15, 96. [Google Scholar] [CrossRef]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef]
- Abitbol, A.; Mallard, B.; Tiralongo, E.; Tiralongo, J. Mushroom natural products in neurodegenerative disease drug discovery. Cells 2022, 11, 3938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, S.; Feng, X.; Li, L.; Hao, J.; Wang, D.; Wang, Q. Mushroom polysaccharides as potential candidates for alleviating neurodegenerative diseases. Nutrients 2022, 14, 4833. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Tong, Z.; Chu, G.; Wan, C.; Wang, Q.; Yang, J.; Meng, Z.; Du, L.; Yang, J.; Ma, H. Multiple metabolites derived from mushrooms and their beneficial effect on Alzheimer’s Diseases. Nutrients 2023, 15, 2758. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, Y.; Guo, W.-H.; Wang, D.-C.; Zhang, Q.; Li, D.; Rong, J.; Gao, J.-M. Molecular diversity and potential anti-neuroinflammatory activities of cyathane diterpenoids from the basidiomycete Cyathus africanus. Sci. Rep. 2017, 7, 8883. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Chen, D.-Q.; Jin, M.-H.; Jin, Y.-H.; Li, J.; Shen, G.-N.; Li, W.-L.; Gong, Y.-X.; Mao, Y.-Y.; Xie, D.-P.; et al. Anti-inflammatory effect of hispidin on LPS induced macrophage inflammation through MAPK and JAK1/STAT3 signaling pathways. Appl. Biol. Chem. 2020, 63, 21. [Google Scholar] [CrossRef]
- Jin, M.-H.; Chen, D.-Q.; Jin, Y.-H.; Han, Y.-H.; Sun, H.-N.; Kwon, T. Hispidin inhibits LPS-induced nitric oxide production in BV-2 microglial cells via ROS-dependent MAPK signaling. Exp. Ther. Med. 2021, 22, 970. [Google Scholar] [CrossRef]
- Lai, M.-C.; Liu, W.-Y.; Liou, S.-S.; Liu, I.-M. Hispidin in the medicinal fungus protects dopaminergic neurons from JNK activation-regulated mitochondrial-dependent apoptosis in an MPP+-Induced in vitro model of Parkinson’s Disease. Nutrients 2023, 15, 549. [Google Scholar] [CrossRef]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Palkina, K.A.; Ipatova, D.A.; Shakhova, E.S.; Balakireva, A.V.; Markina, N.M. Therapeutic potential of hispidin—Fungal and plant polyketide. J. Fungi 2021, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.-W.; David, P.; Naidu, M.; Wong, K.-H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wan, P.; Qiao, J.; Liu, Y.; Peng, Q.; Zhang, Z.; Shu, X.; Xia, Y.; Sun, B. Current and further outlook on the protective potential of Antrodia camphorata against neurological disorders. Front. Pharmacol. 2024, 15, 1372110. [Google Scholar] [CrossRef]
- Sun, P.; Li, W.; Guo, J.; Peng, Q.; Ye, X.; Hu, S.; Liu, Y.; Liu, W.; Chen, H.; Qiao, J.; et al. Ergosterol isolated from Antrodia camphorata suppresses LPS-Induced neuroinflammatory responses in microglia cells and ICR mice. Molecules 2023, 28, 2406. [Google Scholar] [CrossRef]
- Tsay, H.-J.; Liu, H.-K.; Kuo, Y.-H.; Chiu, C.-S.; Liang, C.-C.; Chung, C.-W.; Chen, C.-C.; Chen, Y.-P.; Shiao, Y.-J. EK100 and antrodin c improve brain amyloid pathology in APP/PS1 transgenic mice by promoting microglial and perivascular clearance pathways. Int. J. Mol. Sci. 2021, 22, 10413. [Google Scholar] [CrossRef]
- Bonetto, V.; Ferraresi, A.; Sampò, S.; Isidoro, C. Fungal bioactive compounds as emerging therapeutic options for age-related neurodegenerative disorders. Int. J. Mol. Sci. 2025, 26, 4800. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Chen, T.-H.; Lee, B.-H.; Hsu, Y.-W.; Pan, T.-M. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014, 64, 94–103. [Google Scholar] [CrossRef]
- Shi, Y.-X.; Chen, W.-S. Monascin ameliorate inflammation in the lipopolysaccharide-induced BV-2 microglial cells via suppressing the NF-κB/p65 pathway. Iran. J. Basic Med. Sci. 2020, 23, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, D.; Zhang, W.; Lu, Y.; Zhang, M.; Wang, L.; Li, X.; Zhou, L.; Wu, Q.; Pan, W.; et al. Antioxidant and anti-aging activities of the polysaccharide TLH-3 from Tricholoma lobayense. Int. J. Biol. Macromol. 2016, 85, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, X.; Wang, Y.; Wang, D.; Li, W.; Chen, L.; Pan, W.; Mehmood, S.; Chen, Y. Immunomodulating activity of the polysaccharide TLH-3 from Tricholoma lobayense in RAW264.7 macrophages. Int. J. Biol. Macromol. 2018, 107, 2679–2685. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Zhang, Y.; Liu, X.; Wang, C.; Teng, L.; Wang, D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int. J. Biol. Macromol. 2019, 121, 29–37. [Google Scholar] [CrossRef]
- Han, Y.; Nan, S.; Fan, J.; Chen, Q.; Zhang, Y. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Biol. Macromol. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, L.; Chen, Y.; Chen, X.; Dong, Y.; Zheng, S.; Zhang, L.; Li, W.; Du, J.; Li, H. A Maitake (Grifola frondosa) polysaccharide ameliorates Alzheimer’s disease-like pathology and cognitive impairments by enhancing microglial amyloid-β clearance. RSC Adv. 2019, 9, 37127–37135. [Google Scholar] [CrossRef]
- Behrad, S.; Pourranjbar, S.; Pourranjbar, M.; Abbasi-Maleki, S.; Mehr, S.R.; Salmani, R.H.G.; Moradikor, N. Grifola frondosa polysaccharides alleviate Alzheimer’s disease in rats. Asian Pac. J. Trop. Biomed. 2024, 14, 500–506. [Google Scholar] [CrossRef]
- Li, N.; Li, H.; Liu, Z.; Feng, G.; Shi, C.; Wu, Y. Unveiling the therapeutic potentials of mushroom bioactive compounds in Alzheimer’s Disease. Foods 2023, 12, 2972. [Google Scholar] [CrossRef]
- Seliman, T.M.H.; Ülbegi, G.A.; Özsoy, N.; Kavlo, H.A.; Sağirli, P.A. Anti-inflammatory activity of a novel lectin isolated from Pleurotus eryngii var. Ferulae mushroom. İstanbul J. Pharm. 2024, 54, 195–204. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chang, C.-Y.; Li, J.-R.; Wang, J.-D.; Wu, C.-C.; Kuan, Y.-H.; Liao, S.-L.; Wang, W.-Y.; Chen, C.-J. Anti-inflammatory and neuroprotective effects of fungal immunomodulatory protein involving microglial inhibition. Int. J. Mol. Sci. 2018, 19, 3678. [Google Scholar] [CrossRef]
- Ejike, U.C.; Chan, C.J.; Okechukwu, P.N.; Lim, R.L.H. New advances and potentials of fungal immunomodulatory proteins for therapeutic purposes. Crit. Rev. Biotechnol. 2020, 40, 1172–1190. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Paloi, B.P.; Srinuanpan, S.; Hoijang, S.; Karunarathna, S.C.; Acharya, K.; Suwannarach, N.; Lumyong, S. Termite mushrooms (Termitomyces), a potential source of nutrients and bioactive compounds exhibiting human health benefits: A review. J. Fungi 2023, 9, 112. [Google Scholar] [CrossRef]
- Qi, J.; Ojika, M.; Sakagami, Y. Termitomycesphins A–D, novel neuritogenic cerebrosides from the edible chinese mushroom Termitomyces albuminosus. Tetrahedron 2000, 56, 5835–5841. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, K.; Gao, L.; Sakagami, Y.; Kawagishi, H.; Ojika, M.; Qi, J. Termitomycesphins G and H, Additional cerebrosides from the edible chinese mushroom Termitomyces albuminosus. Biosci. Biotechnol. Biochem. 2012, 76, 791–793. [Google Scholar] [CrossRef]
- Tee, P.Y.E.; Krishnan, T.; Cheong, X.T.; Maniam, S.A.P.; Looi, C.Y.; Ooi, Y.Y.; Chua, C.L.L.; Fung, S.-Y.; Chia, A.Y.Y. A review on the cultivation, bioactive compounds, health-promoting factors and clinical trials of medicinal mushrooms Taiwanofungus camphoratus, Inonotus obliquus and Tropicoporus linteus. Fungal Biol. Biotechnol. 2024, 11, 7. [Google Scholar] [CrossRef]
- Xu, C.; Xie, Q.; Kuo, C.-L.; Yang, X.; Huang, D. Evidence-Based Nutraceuticals Derived from Antrodia cinnamomea. Foods 2025, 14, 1212. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and neuroprotective effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- Bui, V.T.; Wu, K.; Chen, C.; Nguyen, A.T.; Huang, W.; Lee, L.; Chen, W.; Huang, C.; Shiao, Y. Exploring the synergistic effects of erinacines on microglial regulation and Alzheimer’s Pathology under metabolic stress. CNS Neurosci. Ther. 2024, 30, e70137. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Feng, X.; Zhang, Y.; Hu, X.; Hui, H.; Xue, X.; Qi, J. Unprecedented neoverrucosane and cyathane diterpenoids with anti-neuroinflammatory activity from cultures of the culinary-medicinal mushroom Hericium erinaceus. Molecules 2023, 28, 6380. [Google Scholar] [CrossRef]
- Yin, X.; Wei, J.; Wang, W.-W.; Gao, Y.-Q.; Stadler, M.; Kou, R.-W.; Gao, J.-M. New cyathane diterpenoids with neurotrophic and anti-neuroinflammatory activity from the bird’s nest fungus Cyathus africanus. Fitoterapia 2019, 134, 201–209. [Google Scholar] [CrossRef]
- Skubel, T.; Budzyńska, J.; Czarnota, J.; Dobrzyński, M.; Rybak, N.; Dudek, I. Therapeutic potential of Lion’s Mane (Hericium erinaceus) in neurological and cognitive disorders—A review of the literature. J. Edu. Health Sport 2022, 12, 498–504. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process Preserv. 2017, 42, e13386. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Moreno-Pérez, M.A. Identification of phenolic compounds by liquid chromatography-mass spectrometry in seventeen species of wild mushrooms in Central Mexico and determination of their antioxidant activity and bioactive compounds. Food Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef]
- Shao, H.J.; Jeong, J.B.; Kim, K.-J.; Lee, S.-H. Anti-inflammatory activity of mushroom-derived hispidin through blocking of NF-κB activation. J. Sci. Food Agric. 2015, 95, 2482–2486. [Google Scholar] [CrossRef]
- Chao, T.-Y.; Hsieh, C.-C.; Hsu, S.-M.; Wan, C.-H.; Lian, G.-T.; Tseng, Y.-H.; Kuo, Y.-H.; Hsieh, S.-C. Ergostatrien-3β-ol (EK100) from Antrodia camphorata attenuates oxidative stress, inflammation, and liver injury in vitro and in vivo. Prev. Nutr. Food Sci. 2021, 26, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-H.; Lai, K.-S.; Huang, Y.-Y.; Chen, H.-Y.; Xiong, L.-Q.; Guo, H.-K.; Yang, Q.-Q.; Zhang, B.-B. Efficient production of Antrodin C by microparticle-enhanced cultivation of medicinal mushroom Antrodia cinnamomea. J. Biosci. Bioeng. 2023, 135, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Botić, T.; Gregori, A.; Knez, Z.; Gregori, A.; Pohleven, F. The effects of different solvents on bioactivemetabolites and “in vitro” antioxidant and anti-acetylcholinesterase activity of Ganoderma lucidum fruiting body and primordia extracts. Maced. J. Chem. Chem. Eng. 2017, 36, 129–141. [Google Scholar] [CrossRef]
- Villares, A.; Mateo-Vivaracho, L.; Guillamón, E. Structural features and healthy properties of polysaccharides occurring in mushrooms. Agriculture 2012, 2, 452–471. [Google Scholar] [CrossRef]
- Badalyan, S.M. Potential of mushroom bioactive molecules to develophealthcare biotech products. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; pp. 373–378. [Google Scholar]
- Lin, C.-H.; Sheu, G.-T.; Lin, Y.-W.; Yeh, C.-S.; Huang, Y.-H.; Lai, Y.-C.; Chang, J.-G.; Ko, J.-L. A new immunomodulatory proteinfrom Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Ko, J.-L.; Hsu, C.-I.; Lin, R.-H.; Kao, C.-L.; Lin, J.-Y. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. FEBS J. 1995, 228, 244–249. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.; Oliveira, M.B.P.; Ferreira, I.C. Development of a novel methodology for the analysis of ergosterol in mushrooms. Food Anal. Method 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.G.; Rinker, D.L.; Tsao, R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D´Arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Öztürk, M.; Tel-Ҫayan, G.; Öztürk, F.A.; Duru, M.E. The cooking effect on two edible mushrooms in Anatolia: Fatty acid composition, total bioactive compounds, antioxidant and anticholinesterase activities. Rec. Nat. Prod. 2014, 8, 189–194. [Google Scholar]
- Tel-Ҫayan, G.; Öztürk, M.; Duru, M.E.; Turkoglu, A. Antioxidant and anticholinesterase activities of five wild mushroom species with total bioactive contents. Pharm. Biol. 2015, 53, 824–830. [Google Scholar] [CrossRef]
- Duru, M.E.; Tel-Ҫayan, G. Biologically active terpenoids from mushroom origin: A review. Rec. Nat. Prod. 2015, 9, 456–483. [Google Scholar]
- Dündar, A.; Okumuş, V.; Özdemir, S.; Ҫelik, K.S.; Boga, M.; Ozcagli, E.; Ozhan, G.; Yildiz, A. Antioxidant, antimicrobial, cytotoxic and anticholinesterase activities of seven mushroom species with their phenolic acid composition. J. Hortic. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Wang, S.; Bao, L.; Zhao, F.; Wang, Q.; Li, S.; Ren, J.; Li, L.; Wen, H.; Guo, L.; Liu, H. Isolation, Identification, and bioactivity of monoterpenoids and sesquiterpenoids from the mycelia of edible mushroom Pleurotus cornucopiae. J. Agric. Food Chem. 2013, 61, 5122–5129. [Google Scholar] [CrossRef]
- Sabaratnam, V.; Kah-Hui, W.; Naidu, M.; David, P.R. Neuronal health–Can culinary and medicinal mushrooms help? J. Trad. Compl. Med. 2013, 3, 62–68. [Google Scholar] [CrossRef]
- Jiang, X.; Song, Y.; Lv, C.; Li, Y.; Feng, X.; Zhang, H.; Chen, Y.; Wang, Q. Mushroom-derived bioactive components with definite structures in alleviating the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2024, 15, 1373660. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Xu, Y.Z.; Wang, W.W.; Yang, Z.; Liu, B.; Stadler, M.; Liu, L.L.; Gao, J.M. Cyathane diterpenes from cultures of the Bird’s Nest Fungus Cyathus hookeri and their neurotrophic and anti-neuroinflammatory activities. J. Nat. Prod. 2019, 82, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Seo, J.; Kim, J.; Kim, H.; Youn, U.; Lee, J.; Jung, H.; Na, M.; Hattori, M.; Min, B.; et al. Lanostane triterpenes from the fruiting bodies of Ganoderma lucidum and their inhibitory effects on adipocyte differentiation in 3T3-L1 cells. J. Nat. Prod. 2010, 73, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.-W.; Han, R.; Gao, Y.-Q.; Li, D.; Yin, X.; Gao, J.-M. Anti-neuroinflammatory polyoxygenated lanostanoids from Chaga mushroom Inonotus obliquus. Phytochemistry 2021, 184, 112647. [Google Scholar] [CrossRef] [PubMed]

| Category | Examples | Key Bioactive Compounds | Main Biological Activities | Applications/Remarks |
|---|---|---|---|---|
| Edible mushrooms | Pleurotus ostreatus, Lentinula edodes, Agaricus bisporus, Cantharellus cibarius | Polysaccharides (β-glucans), proteins, ergosterol, phenolic compounds | Antioxidant, mild immunomodulatory, cholesterol-lowering, prebiotic | Human nutrition, functional foods; safe for regular consumption |
| Medicinal mushrooms | Ganoderma lucidum, Hericium erinaceus, Cordyceps militaris, Inonotus obliquus | Triterpenoids, polysaccharides, peptides (FIPs), nucleosides (cordycepin) | Strong anti-inflammatory, immunomodulatory, neuroprotective, anticancer, hypoglycemic | Nutraceuticals, dietary supplements, drug development; often bitter or inedible as food |
| Toxic mushrooms | Amanita phalloides, Cortinarius orellanus, Gyromitra esculenta | Amatoxins, orellanine, gyromitrin | Cytotoxic, hepatotoxic, nephrotoxic; some act as neurotoxins | Dangerous to health; no therapeutic application; require strict toxicological monitoring |
| Industrial/fermentation-related mushrooms | Monascus purpureus | Monascin, ankaflavin | Anti-inflammatory, antioxidant, cholesterol-lowering | Used in traditional fermentation (e.g., red yeast rice) subject to quality and safety limits |
| Neurodegenerative Disease | Main Neuroinflammatory Abnormalities | Major Genetic Causes |
|---|---|---|
| Alzheimer’s disease (AD) | ||
| Parkinson’s disease (PD) |
| |
| Huntington’s disease (HD) |
|
|
| Amyotrophic lateral sclerosis (ALS) |
|
| Group of Compounds | Bioactive Substance | Mushroom Species | Experimental Model | Clinical Potencial | Reference |
|---|---|---|---|---|---|
| Phenolic compounds | myricetin | Boletus badius Cantharellus cibarius Pleurotus ostreatus | n.d. | n.d. | [71,73] |
| Catechin | Boletus badius Cantharellus cibarius Pleurotus ostreatus | n.d. | n.d. | [71,73] | |
| Hispidin | Inonotus sp. Phellinus sp. Gymnopilus spectabilis | in vitro (BV2, PC12), in vivo (MPTP mice) | AD, PD | [20,72,75,76,77,78,79,80] | |
| EK100 (Ergostatrien-3β-ol) | Taiwanofungus camphoratus (formerly known as Antrodia cinnamomea) | in vitro (BV2), in vivo (APP/PS1 mice), | AD | [81,82,83] | |
| antrodin C | Taiwanofungus camphoratus | in vivo (APP/PS1 mice) | AD | [81,82,83] | |
| monascin | Monascus purpureus | in vitro, in vivo (LPS-induced) | Experimental model of inflammation and neurotoxicity | [84,85,86] | |
| Ankaflavin | Monascus purpureus | in vitro, in vivo (LPS-induced) | Experimental model of inflammation and neurotoxicity | [84,85] | |
| Carbohydrates | α i β-glucans | Ganoderma lucidum Pleurotus sp. Lentinula edodes | n.d. | n.d. | [16,72] |
| Cordycepin | Cordyceps militaris | n.d. | n.d. | [72,87] | |
| FVP | Flammulina velutipes | in vitro (BV2) | neuroinflamation model | [17,68,72] | |
| TLH-3 | Tricholoma labayense | in vivo (MPTP mice) | PD | [69,88,89] | |
| PSG-1 | Ganoderma atrum | in vivo (MPTP mice) | PD | [69,72] | |
| ACPS | Amanita caesarea | in vivo (AlCl3-induced) | AD | [69,72,90] | |
| IOPS | Inonotus obliquus | in vitro (BV2), in vivo (Aβ-induced) | AD | [72,91] | |
| GLP | Ganoderma lucidum | in vitro (BV2), in vivo (Aβ, MPTP) | AD, PD | [69,72,87,92] | |
| GFP | Grifola frondosa | in vitro (PC12) | AD | [68,72,93,94] | |
| ACP | Taiwanofungus camphoratus (formerly known as Antrodia camphorata) | n.d. | n.d. | [69,81] | |
| Proteins, Peptides | aminoacids (e.g., threonine, serine, glicine, leucine) | Trametes versicolor Pleorotus geesteranus | n.d. | n.d. | [23,95] |
| Lectins | Boletus edulis Pleurotus eryngii | n.d. | n.d. | [71,96] | |
| FIPs | Ganoderma microsporum Ganoderma tsugae Ganoderma lucidum Flammulina velutipes | in vitro immunological model | AD, PD | [20,97,98] | |
| Lipids | Cerebrosides | Termitomyces sp. | n.d. | n.d. | [95,99,100,101] |
| Ergosterol | Taiwanofungus camphoratus Ganoderma lucidum Auricularia polytricha, Cordyceps militaris | in vitro (BV2), in vivo | AD, PD | [20,81,82,83,87,102] | |
| Antroquinonol | Taiwanofungus camphoratus | in vivo (APP/PS1), | AD | [81,84,87,103] | |
| Hericones | Hericium erinaceus | in vitro (PC12), in vivo (Aβ, MPTP) | AD, PD | [20,72,104] | |
| Erinacines | Hericium erinaceus | in vitro (PC12), in vivo (Aβ, MPTP) | AD. PD | [20,72,87,104,105,106] | |
| neoverrucosane | Hericium erinaceus | n.d. | n.d. | [106] | |
| Scarboines | Sarcodon cyrneus | n.d. | n.d. | [74] | |
| Sarcodonins | Sarcodon cyrneus | n.d. | n.d. | [74] | |
| Ribisins | Phellinus ribis | n.d. | n.d. | [14] | |
| Cyafricanins (neocyathins, cyathens, cyathans) | Cyathus africanus | n.d. | n.d. | [68,74,107] | |
| cyahookerrin B | Cyathus hookeri | n.d. | n.d. | [108] | |
| ganoderic acids | Ganoderma sp. | n.d. | n.d. | [20,68,72,80] | |
| inonotusols | Inonotus obliquus | in vitro (BV2) | AD, PD | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godela, A.; Rogacz, D.; Pawłowska, B.; Biczak, R. Natural Neuroinflammatory Modulators: Therapeutic Potential of Fungi-Derived Compounds in Selected Neurodegenerative Diseases. Molecules 2025, 30, 3158. https://doi.org/10.3390/molecules30153158
Godela A, Rogacz D, Pawłowska B, Biczak R. Natural Neuroinflammatory Modulators: Therapeutic Potential of Fungi-Derived Compounds in Selected Neurodegenerative Diseases. Molecules. 2025; 30(15):3158. https://doi.org/10.3390/molecules30153158
Chicago/Turabian StyleGodela, Agnieszka, Diana Rogacz, Barbara Pawłowska, and Robert Biczak. 2025. "Natural Neuroinflammatory Modulators: Therapeutic Potential of Fungi-Derived Compounds in Selected Neurodegenerative Diseases" Molecules 30, no. 15: 3158. https://doi.org/10.3390/molecules30153158
APA StyleGodela, A., Rogacz, D., Pawłowska, B., & Biczak, R. (2025). Natural Neuroinflammatory Modulators: Therapeutic Potential of Fungi-Derived Compounds in Selected Neurodegenerative Diseases. Molecules, 30(15), 3158. https://doi.org/10.3390/molecules30153158








