SGLT2 Inhibitors: From Molecular Mechanisms to Clinical Outcomes in Cardiology and Diabetology
Abstract
1. Clinical Introduction
2. Materials and Methods
3. The Molecular Structure of Human SGLT2
4. SGLT2 Inhibitor Mechanisms of Action in Diabetic Mellitus
5. Mechanisms of Action of SGLT2 Inhibitors in Heart Failure
5.1. Modulation of Cardiac Energy Metabolism
5.2. Antifibrotic Effects
5.3. Anti-Inflammatory Mechanisms
5.4. Antioxidant Properties
5.5. Hemodynamic and Neurohormonal Modulation
6. Mechanisms of Action of SGLT2 Inhibitors in Acute Coronary Syndrome
7. Comparison of the Mechanisms of Action of SGLT2 Inhibitors in Diabetes, Heart Failure, and Acute Coronary Syndrome
8. Neuroprotective Potential of SGLT2 Inhibitors: Mechanisms and Therapeutic Implications for Central Nervous System Disorders
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Migdalis, I.N. Chronic Complications of Diabetes: Prevalence, Prevention, and Management. J. Clin. Med. 2024, 13, 7001. [Google Scholar] [CrossRef] [PubMed]
- Hinault, C.; Caroli-Bosc, P.; Bost, F.; Chevalier, N. Critical overview on endocrine disruptors in diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 4537. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Ahmed, N.; Tariq, H.; Walgamage, M.; Walgamage, T.; Mohammed, A.; Chou, J.T.-T.; Kałużna-Oleksy, M.; Lesiak, M.; Straburzyńska-Migaj, E. SGLT2 inhibitors in type 2 diabetes mellitus and heart failure—A concise review. J. Clin. Med. 2022, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R. Acute coronary syndromes. Res. J. Pharm. Technol. 2022, 399, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Z.; Mahaffey, K.W.; Matthews, D.R.; Neuen, B.L.; Heerspink, H.J.; Jardine, M.J.; Li, J.; Perkovic, V.; Neal, B.; et al. An exploration of the heterogeneity in effects of SGLT2 inhibition on cardiovascular and all-cause mortality in the EMPA-REG OUTCOME, CANVAS Program, DECLARE-TIMI 58, and CREDENCE trials. Int. J. Cardiol. 2021, 324, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Camilli, M.; Lombardi, M.; Chiabrando, J.G.; Del Buono, M.G.; Montone, R.A.; Biondi-Zoccai, G.; Crea, F.; Minotti, G. Efficacy of sodium-glucose cotransporter-2 inhibitors in heart failure patients treated with dual angiotensin receptor blocker-neprilysin inhibitor: An updated meta-analysis. Eur. Heart J.—Cardiovasc. Pharmacother. 2021, 7, e74–e76. [Google Scholar] [CrossRef] [PubMed]
- Madhok, J.; Vanneman, M. SGLT-2 inhibitors: Proliferating indications and perioperative pitfalls. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Wilczopolski, P.; Buławska, D.; Młynarska, E.; Rysz, J.; Franczyk, B. The importance of SGLT-2 inhibitors as both the prevention and the treatment of diabetic cardiomyopathy. Antioxidants 2022, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Verma, S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Bai, H.; Mather, B.; Hill, M.A.; Jia, G.; Sowers, J.R. Diabetic Vasculopathy: Molecular Mechanisms and Clinical In-sights. Int. J. Mol. Sci. 2024, 25, 804. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Sinha, B.; Mukherjee, R. Heterogeneity in cardiovascular death or hospitalization for heart failure benefits with flozins is linked to weight. ESC Heart Fail. 2023, 10, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.K.; Mistry, N.; Puar, P.; Verma, R.; Anker, S.; Mazer, C.D.; Verma, S. SGLT2 inhibitors and cardiac remodelling: A systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021, 8, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Hoong, C.W.; Chua, M.W. SGLT2 inhibitors as calorie restriction mimetics: Insights on longevity pathways and age-related diseases. Endocrinology 2021, 162, bqab079. [Google Scholar] [CrossRef] [PubMed]
- Pahud de Mortanges, A.; Salvador, D., Jr.; Laimer, M.; Muka, T.; Wilhelm, M.; Bano, A. The role of SGLT2 inhibitors in athero-sclerosis: A narrative mini-review. Front. Pharmacol. 2021, 12, 751214. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Kidney-protective effects of SGLT2 inhibitors. Clin. J. Am. Soc. Nephrol. 2023, 18, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Delgado, A.P.; Herrera, E.A.; Mite, C.T.; Cedeno, P.D.; Van Loon, M.C.; Badimon, J.J. Renal and cardiovascular metabolic impact caused by ketogenesis of the SGLT2 inhibitors. Int. J. Mol. Sci. 2023, 24, 4144. [Google Scholar] [CrossRef] [PubMed]
- Styczkiewicz, K.; Sokołowski, A. The efficacy and safety of sodium-glucose cotransporter inhibitors in cancer patients with heart failure-a single-centre experience. Pol. Heart J. 2024, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Seidu, S.; Kunutsor, S.K.; Topsever, P.; Khunti, K. Benefits and harms of sodium-glucose co-transporter-2 inhibitors (SGLT2-I) and renin–angiotensin–aldosterone system inhibitors (RAAS-I) versus SGLT2-Is alone in patients with type 2 diabetes: A sys-tematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2022, 5, e00303. [Google Scholar] [PubMed]
- Sano, M. Sodium glucose cotransporter (SGLT)-2 inhibitors alleviate the renal stress responsible for sympathetic activation. Ther. Adv. Cardiovasc. Dis. 2020, 14, 1753944720939383. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 Inhibitors beyond Glycaemic Control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Coady, M.J.; El Tarazi, A.; Santer, R.; Bissonnette, P.; Sasseville, L.J.; Calado, J.; Lussier, Y.; Dumayne, C.; Bichet, D.G.; Lapointe, J.-Y. MAP17 Is a Necessary Activator of Renal Na+/Glucose Cotransporter SGLT2. J. Am. Soc. Nephrol. 2017, 28, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Song, D.K.; Kim, M.-S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [PubMed]
- Azizogli, A.R.; Vitti, M.R.; Mishra, R.; Osorno, L.; Heffernan, C.; Kumar, V.A. Comparison of SGLT1, SGLT2, and Dual Inhibitor biological activity in treating Type 2 Diabetes Mellitus. Adv. Ther. 2023, 6, 2300143. [Google Scholar] [CrossRef] [PubMed]
- Lema-Pérez, L. Main organs involved in glucose metabolism. In Sugar Intake—Risks and Benefits and the Global Diabetes Epidemic; IntechOpen: London, UK, 2021; pp. 1–15. [Google Scholar]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Gautier, J.-F.; Chon, S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab. J. 2021, 45, 641. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Testoni, G.; Sullivan, M.A.; López-Soldado, I.; Vilaplana, F.; Gilbert, R.G.; Guinovart, J.J.; Schulz, B.L.; Duran, J. Glycogenin is dispensable for normal liver glycogen metabolism and body glucose homeostasis. Int. J. Biol. Macromol. 2025, 291, 139084. [Google Scholar] [CrossRef]
- Guo, Q.; Hou, X.; Cui, Q.; Li, S.; Shen, G.; Luo, Q.; Wu, H.; Chen, H.; Liu, Y.; Chen, A.; et al. Nutrition, Pectin mediates the mechanism of host blood glucose regulation through intestinal flora. Crit. Rev. Food Sci. Nutr. 2023, 64, 6714–6736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Mahler, G.J. Modelling renal filtration and reabsorption processes in a human glomerulus and proximal tubule microphysiological system. Micromachines 2021, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Beyond SGLT2: Proximal Tubule Transporters as Potential Drug Targets for Chronic Kidney Disease. Nephrol. Dial. Transplant. 2025, 40 (Suppl. S1), i18–i28. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant roles of SGLT2 inhibi-tors in the kidney. Biomolecules 2022, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. SGLT2 inhibitors: Physiology and pharmacology. Kidney360 2021, 2, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Hiraizumi, M.; Akashi, T.; Murasaki, K.; Kishida, H.; Kumanomidou, T.; Torimoto, N.; Nureki, O.; Miyaguchi, I. Transport and inhibition mechanism of the human SGLT2–MAP17 glucose transporter. Nat. Struct. Mol. Biol. 2024, 31, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, R.; Guan, C.; Zhang, Y.; Chen, Z.; Hoerer, S.; Nar, H.; Chen, L. Structural basis of inhibition of the human SGLT2–MAP17 glucose transporter. Nature 2022, 601, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.; Yakovleva, T.; Chu, L.; Tang, W.; Greasley, P.J.; Johansson, S.; Peskov, K.; Helmlinger, G.; Boulton, D.W.; Penland, R.C. Differentiating the sodium-glucose cotransporter 1 inhibition capacity of canagliflozin vs. dapagliflozin and empagliflozin using quantitative systems pharmacology modeling. Pharmacomet. Syst. Pharmacol. 2020, 9, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Cui, W.; Liu, R.; Wang, S.; Ke, H.; Lei, X.; Chen, L. Structural mechanism of SGLT1 inhibitors. Nat. Commun. 2022, 13, 6440. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Niu, Y.; Sun, Z.; Liu, R.; Chen, L. Structures of human SGLT in the occluded state reveal conformational changes during sugar transport. Nat. Commun. 2023, 14, 2920. [Google Scholar] [CrossRef] [PubMed]
- Nelinson, D.S.; Sosa, J.M.; Chilton, R.J. SGLT2 inhibitors: A narrative review of efficacy and safety. J. Am. Osteopath. Assoc. 2021, 121, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vizcaíno, V.; Díez-Fernández, A.; Álvarez-Bueno, C.; Martínez-Alfonso, J.; Cavero-Redondo, I. Safety and efficacy of SGLT2 inhibitors: A multiple-treatment meta-analysis of clinical decision indicators. J. Clin. Med. 2021, 10, 2713. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Hayashi, K.; Ito, S.; Hoshina, Y.; Sakai, M.; Yoshino, K.; Endo, K.; Fujitani, S.; Suzuki, T. Effects of SGLT2 in-hibitors on eGFR in type 2 diabetic patients—The role of antidiabetic and antihypertensive medications. Hypertens. Res. 2021, 44, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, S.; Barillà, F.; Tajmir, R.; Meloni, M.; Della Morte, D.; Bellia, A.; Di Daniele, N.; Lauro, D.; Andreadi, A. The new role of SGLT2 inhibitors in the management of heart failure: Current evidence and future perspective. Pharmaceutics 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rieg, J.A.; Rieg, T. What does sodium-glucose co-transporter 1 inhibition add: Prospects for dual inhibition. Diabetes Obes. Metab. 2019, 21, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.; Sun, Y.; Yu, B.; Tan, X.; Wang, B.; Lu, Y.; Wang, N. SGLT2 inhibition, circulating metabolites, and atrial fibrillation: A Mendelian randomization study. Cardiovasc. Diabetol. 2023, 22, 278. [Google Scholar] [CrossRef] [PubMed]
- Chan-Jiang, E.; Godoy, R.; Mennickent, S.; Vergara, C.; de Diego, M. Determination of the Chemical Stability of Dapagliflozin by LC/DAD and MS/MS Methods. J. Chromatogr. Sci. 2022, 60, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, M.M.; Yao, A.K.; Khanna, A.; Koplowitz, B.; Zhu, M.; Li, W.; Komoroski, B.; Kasichayanula, S.; Discenza, L.; Washburn, W.; et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Am. Soc. Pharmacol. Exp. Ther. 2010, 38, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.D.; Green, P.G.; Lewis, A.J.; Arvidsson, P.; De Maria, G.L.; Arheden, H.; Heiberg, E.; Clarke, W.T.; Rodgers, C.T.; Valkovič, L.; et al. Retained metabolic flexibility of the failing human heart. Circulation 2023, 148, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Capone, F.; Sotomayor-Flores, C.; Bode, D.; Wang, R.; Rodolico, D.; Strocchi, S.; Schiattarella, G.G. Cardiac metabolism in HFpEF: From fuel to signalling. Cardiovasc. Res. 2022, 118, 3556–3575. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free. Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, J.S.; Keung, W.; Wang, W.; Ussher, J.R.; Lopaschuk, G.D. Targeting fatty acid and carbohydrate oxidation—A novel therapeutic intervention in the ischemic and failing heart. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2011, 1813, 1333–1350. [Google Scholar] [CrossRef] [PubMed]
- Wasyluk, W.; Nowicka-Stążka, P.; Zwolak, A. Heart metabolism in sepsis-induced cardiomyopathy—Unusual metabolic dysfunction of the heart. Int. J. Environ. Res. Public Health 2021, 18, 7598. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Actis Dato, V.; Lange, S.; Cho, Y. Metabolic Flexibility of the Heart: The Role of Fatty Acid Metabolism in Health, Heart Fail-ure, and Cardiometabolic Diseases. Int. J. Mol. Sci. 2024, 25, 1211. [Google Scholar] [CrossRef] [PubMed]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Therapy, Inflammatory cytokines and chemokines as therapeutic targets in heart failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and Fibrosis of the Cardiac Muscle in the Course of Obesity—Pathogenesis and Involvement of the Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef] [PubMed]
- Scisciola, L.; Taktaz, F.; Fontanella, R.A.; Pesapane, A.; Surina; Cataldo, V.; Ghosh, P.; Franzese, M.; Puocci, A.; Paolisso, P.; et al. Targeting high glucose-induced epigenetic modifications at cardiac level: The role of SGLT2 and SGLT2 inhibitors. Cardiovas-Cular Diabetol. 2023, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Lee, S.-J.; Lee, J.-J.; Kim, J.-S.; Lee, O.-H.; Kim, C.-K.; Kim, D.; Lee, Y.-H.; Oh, J.; Park, S.; et al. Anti-inflammatory effect for atherosclerosis progression by sodium-glucose cotransporter 2 (SGLT-2) inhibitor in a normoglycemic rabbit model. Korean Circ. J. 2020, 50, 443. [Google Scholar] [CrossRef] [PubMed]
- Nabrdalik-Leśniak, D.; Nabrdalik, K.; Irlik, K.; Janota, O.; Kwiendacz, H.; Szromek-Białek, P.; Maziarz, M.; Stompór, T.; Gumprecht, J.; Lip, G.Y. The influence of SGLT2 inhibitors on oxidative stress in heart failure and chronic kidney disease in patients with type 2 diabetes. Endokrynol. Pol. 2023, 74, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Gager, G.M.; von Lewinski, D.; Sourij, H.; Jilma, B.; Eyileten, C.; Filipiak, K.; Hülsmann, M.; Kubica, J.; Postula, M.; Siller-Matula, J.M. Pharmacotherapy, Effects of SGLT2 inhibitors on ion homeostasis and oxidative stress associated mechanisms in heart failure. Biomed. Pharmacother. 2021, 143, 112169. [Google Scholar] [CrossRef] [PubMed]
- Seidu, S.; Alabraba, V.; Davies, S.; Newland-Jones, P.; Fernando, K.; Bain, S.C.; Diggle, J.; Evans, M.; James, J.; Kanumilli, N.; et al. SGLT2 Inhibitors—The New Standard of Care for Cardiovascular, Re-nal and Metabolic Protection in Type 2 Diabetes: A Narrative Review. Diabetes Ther. 2024, 15, 1099–1124. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Preckel, B.; Hermanides, J.; Hollmann, M.W.; Zuurbier, C.J.; Weber, N.C. Amelioration of endothelial dysfunction by sodium glucose co-transporter 2 inhibitors: Pieces of the puzzle explaining their cardiovascular protection. Br. J. Pharmacol. 2022, 179, 4047–4062. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial nitric oxide synthase (eNOS) and the cardiovascular system: In physiology and in disease states. Am. J. Biomed. Sci. Res. 2022, 15, 153. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon monoxide and nitric oxide as examples of the youngest class of transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef] [PubMed]
- Kinlay, S.; Libby, P.; Ganz, P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001, 12, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Schrottmaier, W.C.; Mussbacher, M.; Salzmann, M.; Assinger, A. Platelet-leukocyte interplay during vascular disease. Ather-Osclerosis 2020, 307, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Grześk, G.; Witczyńska, A.; Węglarz, M.; Wołowiec, Ł.; Nowaczyk, J.; Grześk, E.; Nowaczyk, A. Soluble Guanylyl Cyclase Activators—Promising Therapeutic Option in the Pharmacotherapy of Heart Failure and Pulmonary Hypertension. Molecules 2023, 28, 861. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Park, K.C.; Tokar, S.; Eykyn, T.R.; Fuller, W.; Pavlovic, D.; Swietach, P.; Shattock, M.J. Off-target effects of sodium-glucose co-transporter 2 blockers: Empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+] i in the heart. Cardiovasc. Res. 2021, 117, 2794–2806. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y. SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function. Front. Cardiovasc. Med. 2020, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-J.; Liu, B.-H.; Wan, S.-J.; Cheng, Y.; Zhou, S.-M.; Sun, Y.; Yao, X.-M.; Hua, Q.; Meng, X.-J.; Cheng, J.-H.; et al. A SGLT2 inhibitor dapagliflozin alleviates diabetic cardiomyopathy by suppressing high glucose-induced oxidative stress in vivo and in vitro. Front. Pharmacol. 2021, 12, 708177. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef] [PubMed]
- Alsereidi, F.R.; Khashim, Z.; Marzook, H.; Gupta, A.; Shaaban, A.M.; Ramadan, M.M.; Saleh, M.A. Targeting Inflammato-ry Signaling Pathways with SGLT2 Inhibitors: Insights into Cardiovascular Health and Cardiac Cell Improvement. Curr. Probl. Cardiol. 2024, 49, 102524. [Google Scholar] [CrossRef] [PubMed]
- Hupa-Breier, K.L.; Dywicki, J.; Hartleben, B.; Wellhöner, F.; Heidrich, B.; Taubert, R.; Mederacke, Y.-S.E.; Lieber, M.; Iordanidis, K.; Manns, M.P.; et al. Dulaglutide alone and in combination with empagliflozin attenuate inflammatory pathways and microbiome dysbiosis in a non-diabetic mouse model of NASH. Biomedicines 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Joshi, R.; Borriello, G.; Cacciola, G.; Gonnella, A.; Gigliotti, A.; Nigro, M.; Gigliotti, G. SGLT2 Inhibitors: The First Endothelial-Protector for Diabetic Nephropathy. J. Clin. Med. 2025, 14, 1241. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D. Mechanisms of Diabetic Nephropathy Not Mediated by Hyperglycemia. J. Clin. Med. 2023, 12, 6848. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of sodium-glucose cotransporter 2 inhibitors on water and sodium metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Muto, S.; Fukuda, K.; Watanabe, M.; Ohara, K.; Koepsell, H.; Vallon, V.; Nagata, D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol. Rep. 2020, 8, e14360. [Google Scholar] [CrossRef] [PubMed]
- Shiina, K. Who benefits from the blood pressure-lowering effects of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease?—Obese or non-obese? Hypertens. Res. 2024, 47, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z. SGLT2 inhibitors: The sweet success for kidneys. Annu. Rev. Med. 2023, 74, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: A state-of-the-art review. Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar]
- Fatima, A.; Rasool, S.; Devi, S.; Talha, M.; Waqar, F.; Nasir, M.; Khan, M.R.; Jaffari, S.M.I.A.; Haider, A.; Shah, S.U.; et al. Exploring the Cardiovascular Benefits of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: Expanding Horizons Beyond Diabetes Management. Cureus 2023, 15, e46243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Xu, Y.; Wang, D.; Chen, F.; Tu, Z.; Qian, J.; Xu, S.; Xu, Y.; Hwa, J.; Li, J.; et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell 2022, 13, 336–359. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Diabetes mellitus and heart failure: Epidemiology, pathophysiologic mechanisms, and the role of SGLT2 inhibitors. Life 2023, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, Z.; Li, T.; Chen, K.; Zeng, Z. SGLT2 inhibitor improves the prognosis of patients with coronary heart disease and prevents instent restenosis. Front. Cardiovasc. Med. 2024, 10, 1280547. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Significance of Endothelial Dysfunction Amelioration for Sodium–Glucose Cotransporter 2 Inhibitor-Induced Improvements in Heart Failure and Chronic Kidney Disease in Diabetic Patients. Metabolites 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Brown, K.; Miller, L. Neuroinflammation and Oxidative Stress Modulation by SGLT2 Inhibitors. J. Neurol. Res. 2022, 45, 256–270. [Google Scholar]
- Jones, R.; Patel, M. Cerebral Glucose Metabolism and Cognitive Benefits of SGLT2 Inhibitors. Neurobiol. Insights 2021, 18, 98–112. [Google Scholar]
- Lee, K.; Nguyen, T.; Zhao, R. Restoration of Neurotrophic Levels in Diabetic Models through SGLT2 Inhibition. Brain Res. Ther. 2023, 52, 415–428. [Google Scholar]
- Fauconnier, A.; Melis, M.; Berenbeck, M.; Pio, B.; Croisier, T. Trends in the Drug Target Landscape for Autoimmune Diseases. Nat. Rev. Drug Discov. 2025, 24, 415–416. Available online: https://www.nature.com/articles/d41573-025-00061-7 (accessed on 20 July 2025). [CrossRef] [PubMed]
- Garcia, P.; Wong, H. Acetylcholinesterase Inhibition by SGLT2 Inhibitors in Alzheimer’s Models. Cogn. Neurodegener. Stud. 2020, 11, 33–47. [Google Scholar]
- Heimke, M.; Lenz, F.; Rickert, U.; Lucius, R.; Cossais, F. Anti-Inflammatory Properties of the SGLT2 Inhibitor Empagliflozin in Activated Primary Microglia. Cells 2022, 11, 3107. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Fisher, M. SGLT2 Inhibitors: Mechanisms of Cardiovascular Benefit Beyond Glycaemic Control. Nat. Rev. Cardiol. 2025, 22, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Obata, A.; Kimura, T.; Shimoda, M.; Kinoshita, T.; Matsuoka, T.-A.; Kaku, K. Unexpected pleiotropic effects of SGLT2 inhibitors: Pearls and pitfalls of this novel antidiabetic class. Int. J. Mol. Sci. 2021, 22, 3062. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Kosiborod, M.; Inzucchi, S.E.; Cherney, D.Z. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018, 94, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wijnker, P.J.; Dinani, R.; van der Laan, N.C.; Algül, S.; Knollmann, B.C.; Verkerk, A.O.; Remme, C.A.; Zuurbier, C.J.; Kuster, D.W.; van der Velden, J. Hypertrophic cardiomyopathy dysfunction mimicked in human engineered heart tissue and improved by sodium–glucose cotransporter 2 inhibitors. Cardiovasc. Res. 2024, 120, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Orozco, H.; Voorrips, S.N.; Yurista, S.R.; de Boer, R.A.; Westenbrink, B.D. Atherosclerosis, SGLT2 inhibitors and ketone metabolism in heart failure. J. Lipid Atheroscler. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Zheng, C.-M.; Yen, T.-H.; Lu, K.-C. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int. J. Mol. Sci. 2020, 21, 7833. [Google Scholar] [CrossRef] [PubMed]
- Das, U.S.; Paul, A.; Banerjee, S. SGLT2 inhibitors in heart failure with reduced ejection fraction. Egypt. Heart J. 2021, 73, 93. [Google Scholar] [CrossRef] [PubMed]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Farooq, M.A.; Gaertner, S.; Bruckert, C.; Qureshi, A.W.; Lee, H.-H.; Benrahla, D.; Pollet, B.; Stephan, D.; Ohlmann, P.; et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc. Diabetol. 2020, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef] [PubMed]
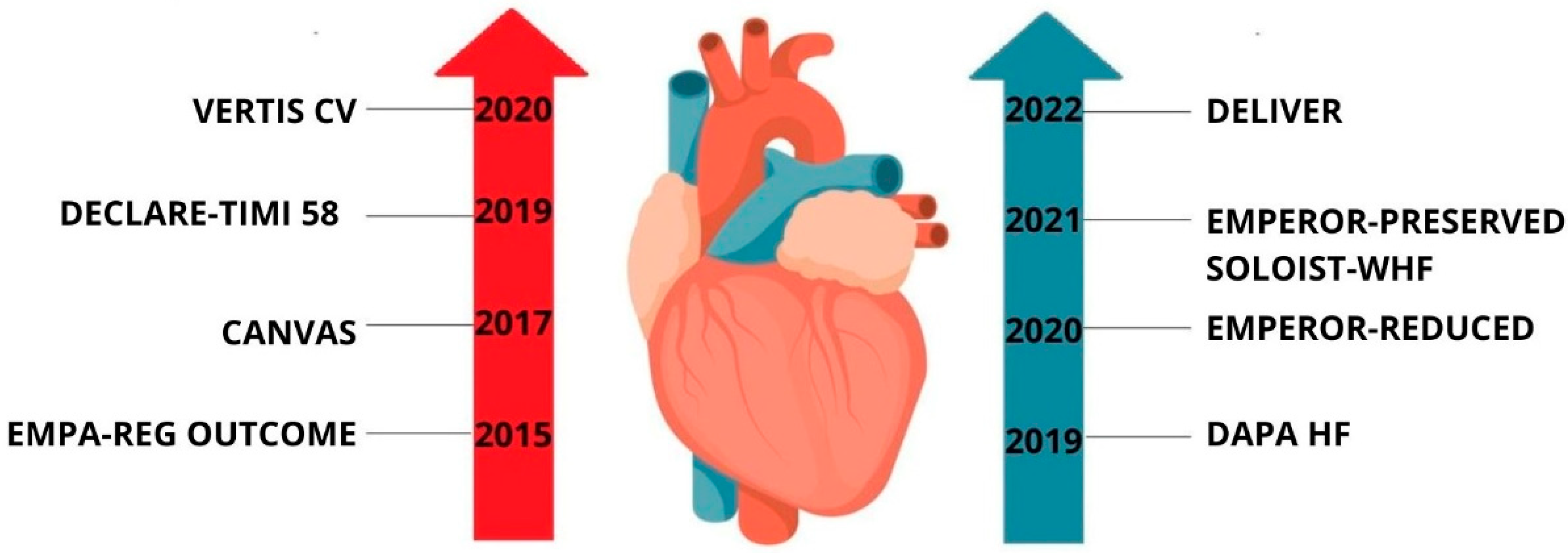

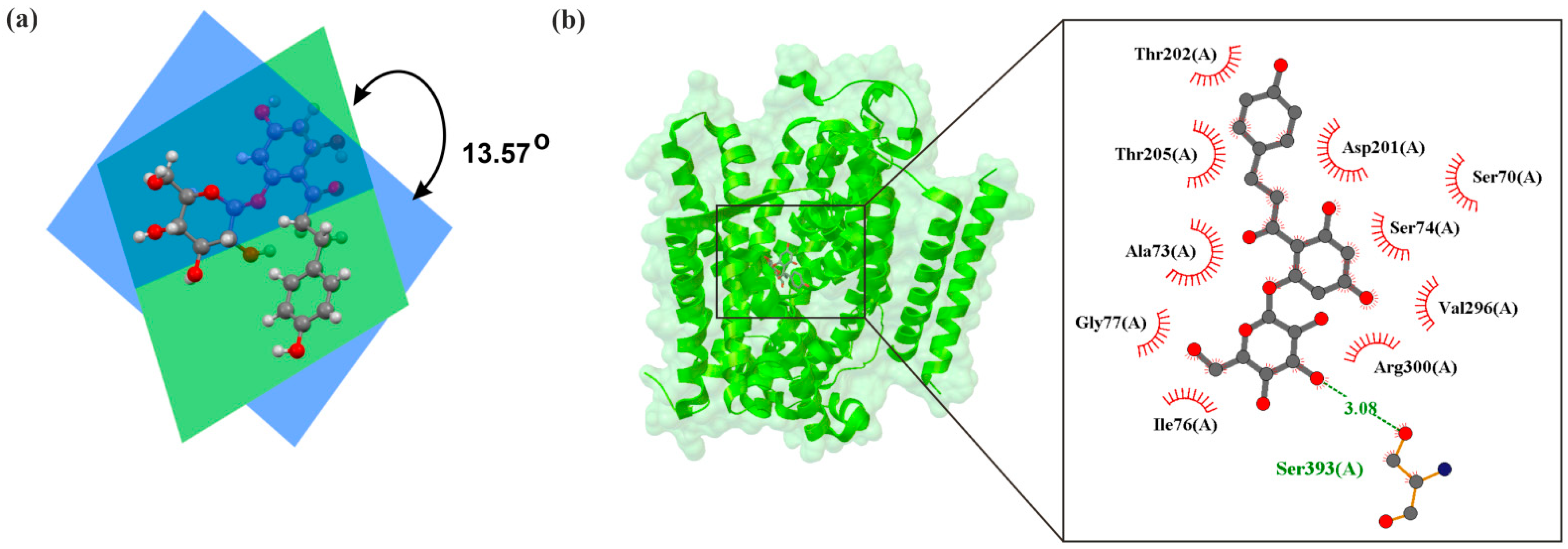
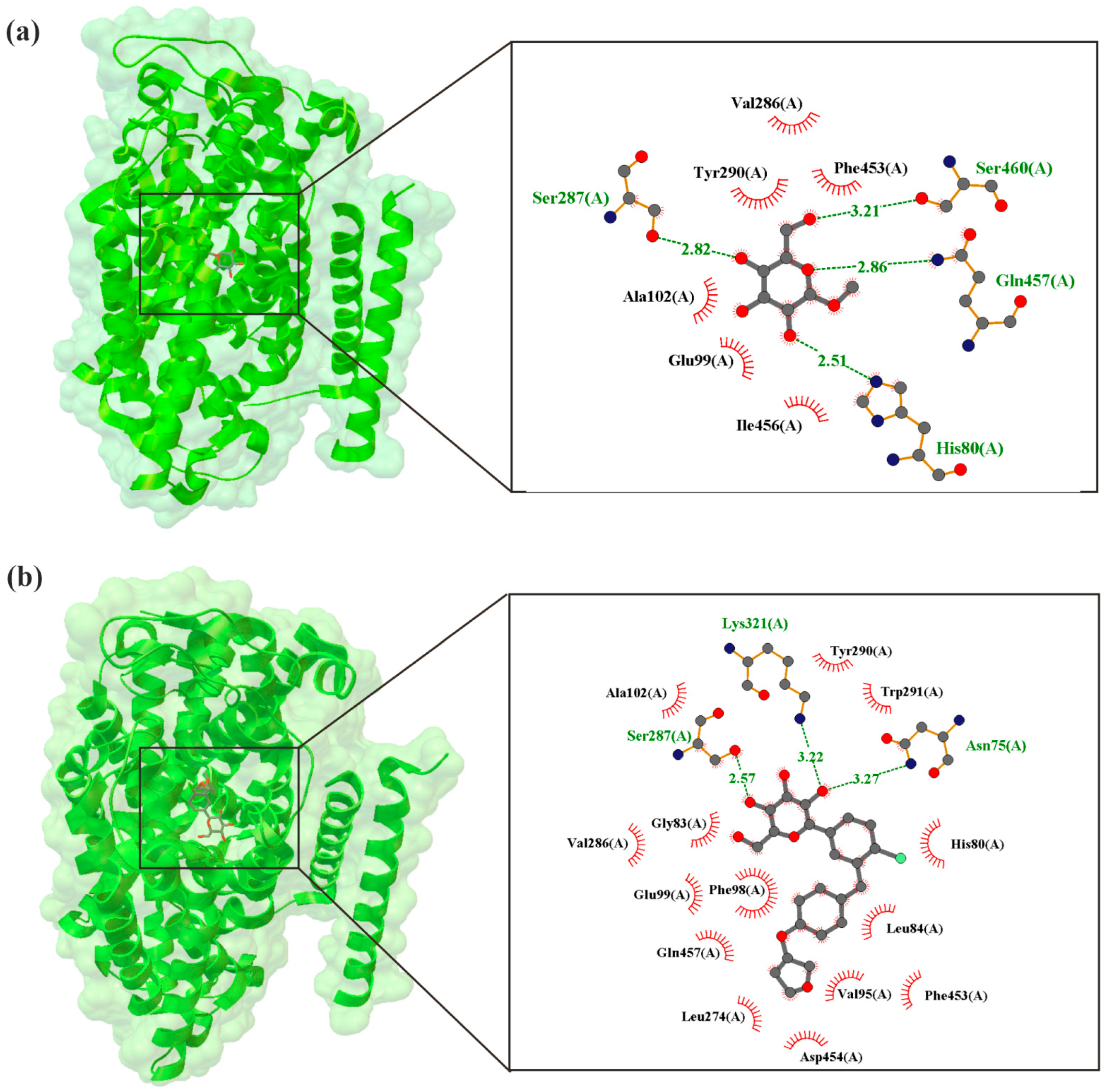
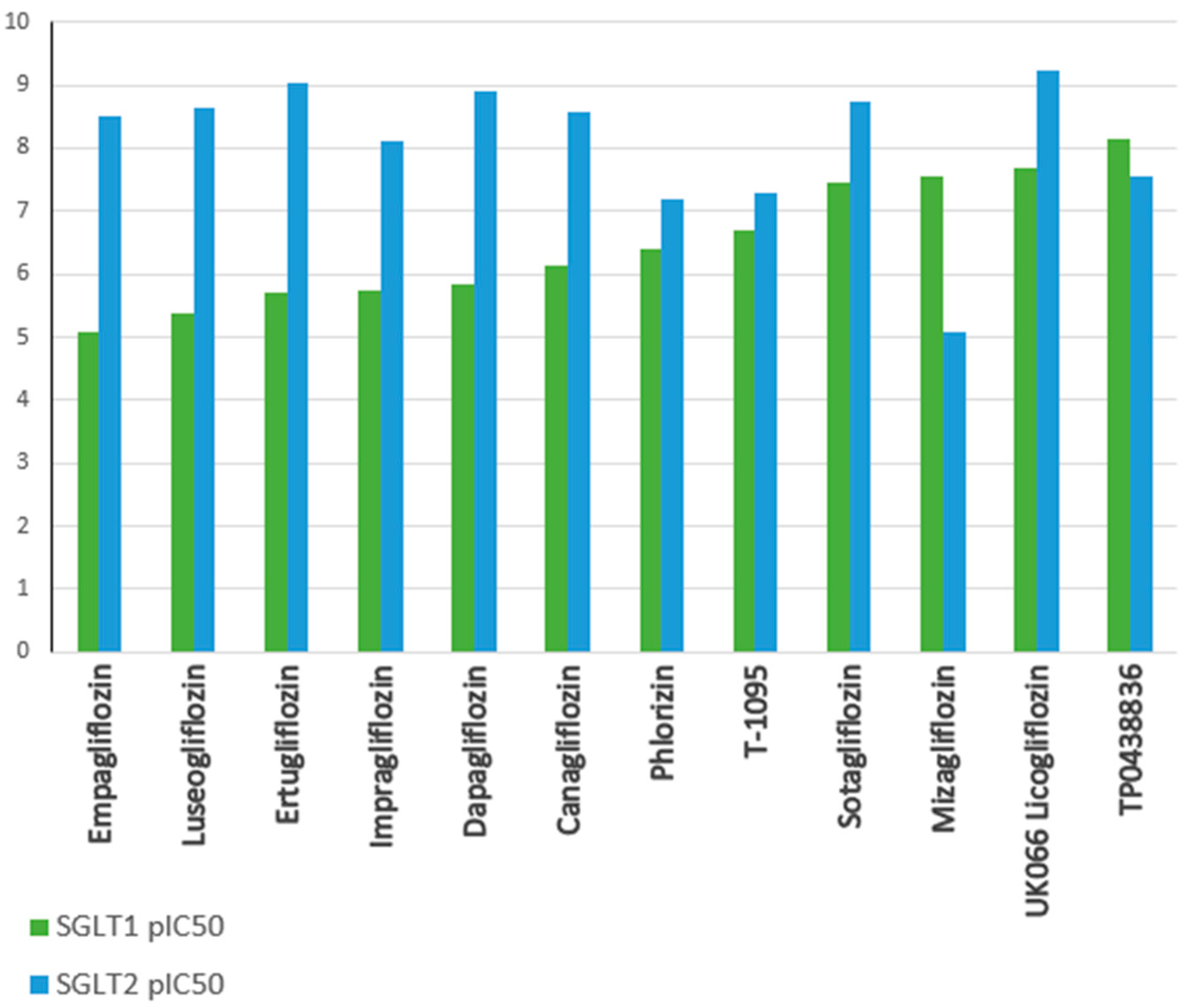

| Aspect | Diabetes | Heart Failure | Acute Coronary Syndrome (ACS) |
|---|---|---|---|
| Basic Mechanism of Action |
|
|
|
| Similarities in Action |
|
|
|
| Differences in Action |
|
|
|
| Therapeutic Synergy |
|
|
|
| Research Perspectives |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stielow, M.; Fijałkowski, Ł.; Alaburda, A.; Grześk, G.; Grześk, E.; Nowaczyk, J.; Nowaczyk, A. SGLT2 Inhibitors: From Molecular Mechanisms to Clinical Outcomes in Cardiology and Diabetology. Molecules 2025, 30, 3112. https://doi.org/10.3390/molecules30153112
Stielow M, Fijałkowski Ł, Alaburda A, Grześk G, Grześk E, Nowaczyk J, Nowaczyk A. SGLT2 Inhibitors: From Molecular Mechanisms to Clinical Outcomes in Cardiology and Diabetology. Molecules. 2025; 30(15):3112. https://doi.org/10.3390/molecules30153112
Chicago/Turabian StyleStielow, Marlena, Łukasz Fijałkowski, Aidas Alaburda, Grzegorz Grześk, Elżbieta Grześk, Jacek Nowaczyk, and Alicja Nowaczyk. 2025. "SGLT2 Inhibitors: From Molecular Mechanisms to Clinical Outcomes in Cardiology and Diabetology" Molecules 30, no. 15: 3112. https://doi.org/10.3390/molecules30153112
APA StyleStielow, M., Fijałkowski, Ł., Alaburda, A., Grześk, G., Grześk, E., Nowaczyk, J., & Nowaczyk, A. (2025). SGLT2 Inhibitors: From Molecular Mechanisms to Clinical Outcomes in Cardiology and Diabetology. Molecules, 30(15), 3112. https://doi.org/10.3390/molecules30153112








