Research Progress and Prospects of Flavonoids in the Treatment of Hyperlipidemia: A Narrative Review
Abstract
1. Introduction
2. Flavonoids
2.1. Flavones
2.2. Flavonols
2.3. Dihydroflavones
2.4. Isoflavones
2.5. Flavanols
2.6. Chalcones
2.7. Anthocyanidins
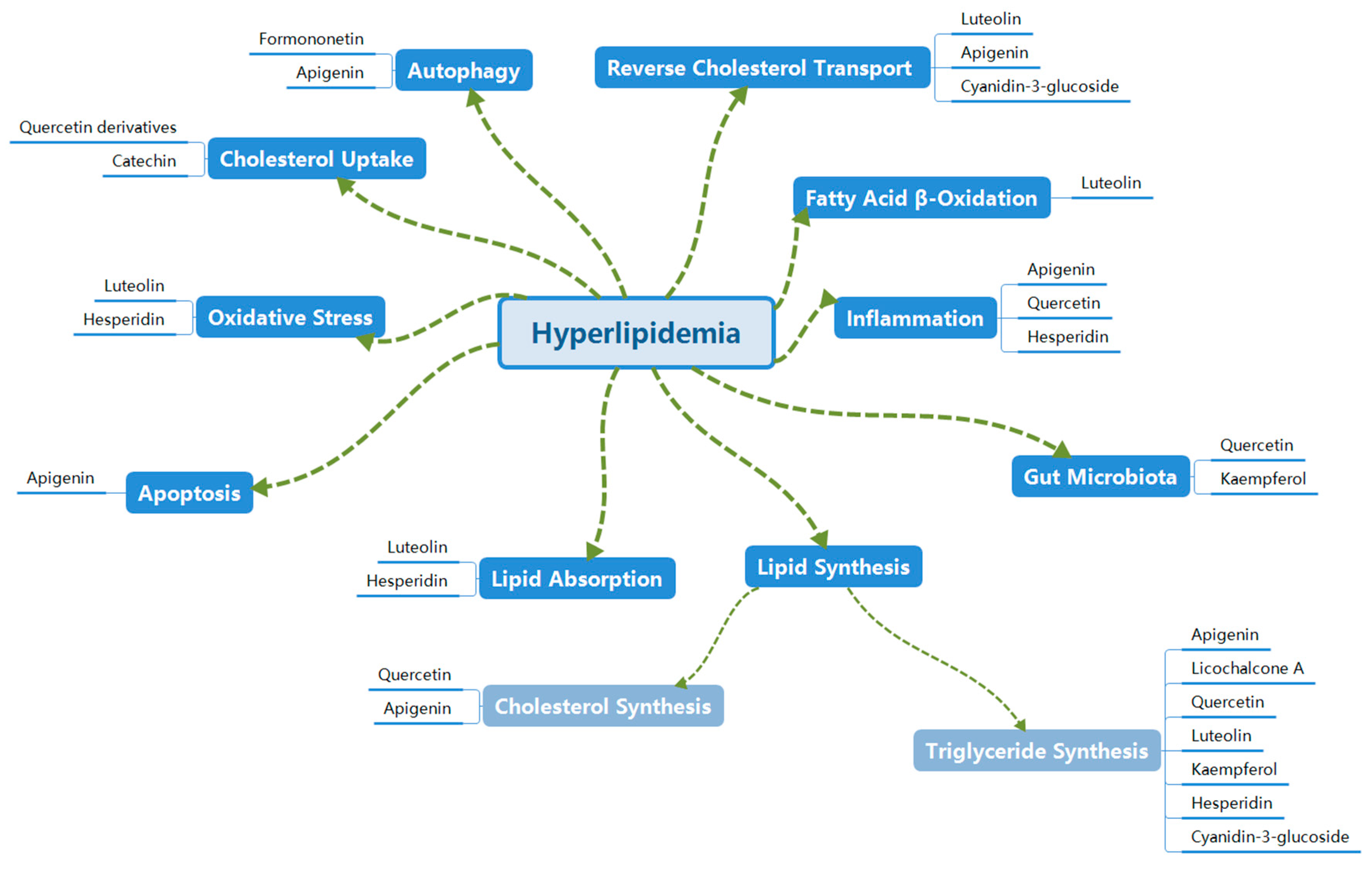
3. Mechanisms of Flavonoids in Treating Hyperlipidemia
3.1. Inhibition of Lipid Synthesis
3.1.1. Inhibition of Triglyceride Synthesis
3.1.2. Inhibition of Cholesterol Synthesis
3.2. Inhibition of Lipid Absorption
3.3. Promotion of Cholesterol Uptake
3.4. Promotion of Reverse Cholesterol Transport (RCT)
3.5. Inhibition of Oxidative Stress
3.6. Promotion of Fatty Acid β-Oxidation
3.7. Regulation of Autophagy
3.8. Inhibition of Apoptosis
3.9. Inhibition of Inflammation
3.10. Regulation of Gut Microbiota
4. Bioavailability of Flavonoids
5. Safety of Flavonoids
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.X.; Xu, W.J.; Zhang, F.L.; Ding, M.Z.; Tan, X.M.; Xia, T. Investigation of Simiao Pill in Improving Lipid Deposition in Free Fatty Acid-Induced Hyperlipidemic HepG2 Cell Model Based on PIK3CA/AKT1/PPARG/CYP7A1 Signaling Pathway. Drug Eval. Res. 2025, 48, 1425–1437. [Google Scholar]
- Tian, Q.S.; Li, Y.L.; Pei, H.Y.; Tian, Y.Y.; Zuo, Z.P.; Zhao, X.Y.; Liu, C.; Wang, Z.B. Pathogenesis of Hyperlipidemia and Drug Therapy. Chem. Life 2022, 42, 2237–2247. [Google Scholar] [CrossRef]
- Zhou, Z.; Curtis, A.J.; Breslin, M.; Nelson, M. Regarding Article, Statin Toxicity: Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, e120. [Google Scholar] [CrossRef] [PubMed]
- Shuai, P.; Cao, N.N.; Li, X.; Zang, W.J.; Yang, W.J.; Xiao, X.F. Research Progress on Signal Pathways Involved in Hyperlipidemia Intervened by Traditional Chinese Medicine. Drug Eval. Res. 2022, 45, 177–185. [Google Scholar]
- Nei, Y.; Zhang, M.; Huang, Y.W. Research Progress on Natural Compounds Inhibiting HMG-CoA Reductase for Improving Cardiovascular and Cerebrovascular Diseases. J. Food Saf. Qual. 2021, 12, 7333–7341. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V. Special Issue “Flavonoids and Their Disease Prevention and Treatment Potential”: Recent Advances and Future Perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 29, e47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laveriano-Santos, E.P.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Castro-Barquero, S.; Ruiz-León, A.M.; Estruch, R.; Casas, R.; Bodega, P.; de Miguel, M.; de Cos-Gandoy, A.; et al. Flavonoid Intake From Cocoa-Based Products and Adiposity Parameters in Adolescents in Spain. Front. Nutr. 2022, 6, 931171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 2012, 223, 1635–1701. [Google Scholar]
- Chen, Z.; Zhang, M.; He, R.R.; Xu, J.; Nie, H. Research progress on the prevention and treatment of hyperlipidemia by plant flavones of medicinal and food homology. J. Liaoning Univ. Tradit. Chin. Med. 2024, 26, 215–220. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mo, X.X.; Chang, G.X.; Yuan, Y.; Yu, J.J.; Zheng, Y.G.; Wang, Y. The Research Progress on Flavonoid Chemical Constituents, Content Determination and Pharmacological Effects of Plants in Leonurus Genus. J. Yunnan Natl. Univ. Nat. Sci. Ed. 2025, 1–18. Available online: http://kns.cnki.net/kcms/detail/53.1192.n.20250319.1524.002.html (accessed on 19 March 2025).
- Wu, M.J.; Ge, J.X.; Yang, Y.F.; Wu, H.Z. The Research Progress on Chemical Constituents and Pharmacological Effects of Olea europaea Leaves and Predictive Analysis of Its Quality Markers. Cent. South Pharm. 2025, 23, 1726–1734. Available online: http://kns.cnki.net/kcms/detail/43.1408.R.20250427.1642.007.html (accessed on 19 March 2025).
- Li, J.J.; Chen, S.Y.; Lan, W. The Research Progress on Chemical Constituents and Pharmacological Effects of Chamomile and Prediction Analysis of Its Quality Markers. Chin. Pharm. J. 2024, 59, 664–675. [Google Scholar]
- Majma Sanaye, P.; Mojaveri, M.R.; Ahmadian, R.; Sabet Jahromi, M.; Bahramsoltani, R. Apigenin and its dermatological applications: A comprehensive review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Ahmed, M.M.; Yasir, M.; Warsi, M.H.; Alquraini, A.; Ghoneim, M.M.; Alshehri, S. Development and Optimization of Hybrid Polymeric Nanoparticles of Apigenin: Physicochemical Characterization, Antioxidant Activity and Cytotoxicity Evaluation. Sensors 2022, 22, 1364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Tang, R.Y.; Zhang, J.N.; Chen, W.T.; Jia, D.Z.; Xiao, G.L. The Exploration of Apigenin on Hepatic Protection and Mechanisms in Hyperlipidemic Mice Based on Metabolomics Approach. Chin. J. New Drugs Clin. Pharmacol. 2024, 35, 1344–1351. [Google Scholar] [CrossRef]
- Ren, Y.R. The Study on Chemical Characterization of Taraxacum mongolicum in Vitro and in Vivo and Its Potential Mechanisms for Improving Hyperlipidemia. Beijing Univ. Chin. Med. 2023. [Google Scholar] [CrossRef]
- Yang, S.B.; Lu, J.Q.; Dong, L.; Xiao, X.; Mao, M.L.; Pang, Y.; Chen, J.Y.; Zhang, Y.L.; Chen, W.; Chen, L.D.; et al. The Research Progress on Chemical Components of Chrysanthemum and Its Prevention and Treatment of Cardiovascular Diseases. J. Liaoning Univ. Tradit. Chin. Med. 2025, 27, 95–99. [Google Scholar]
- Zhou, C.S.; Rao, G.X. The Study on Chemical Constituents of Nelumbo nucifera Stem. Chin. J. Ethnomed. Ethnopharm. 2024, 33, 28–31. [Google Scholar]
- Feng, W.S.; Chen, X.; Zheng, X.K.; Zhang, C.L.; Li, D.D. Study on Chemical Constituents of Honeysuckle. J. Chin. Pharm. Sci. 2011, 46, 338–340. [Google Scholar]
- Shuang, F.; Yaling, J.; Jinhai, L.; Cui, G.Y. Research progress on luteolin derivatives. Chem. Bull. 2024, 87, 300–309. [Google Scholar] [CrossRef]
- Xin, Z.; Fan, X.; Jun, Z.; He, Y.F.; Pei, X.J. Research progress on physiological functions and preparations of luteolin. China Surfactant Deterg. Cosmet. 2023, 53, 437–444. [Google Scholar]
- Rath, P.; Chauhan, A.; Ranjan, A.; Aggarwal, D.; Rani, I.; Choudhary, R.; Shahwan, M.; Ramniwas, S.; Joshi, H.; Haque, S.; et al. Luteolin: A promising modulator of apoptosis and survival signaling in liver cancer. Pathol. Res. Pract. 2024, 260, 155430. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kim, M.Y.; Cho, J.Y. Immunopharmacological Activities of Luteolin in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 2136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.L. Screening and Mechanistic Study of Active Components from Astragalus membranaceus Against Gastric Cancer. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2024. [Google Scholar] [CrossRef]
- Li, X.; Cheng, B.B.; Tan, J.L.; Wan, J.Q.; Wang, H.Y.; Dai, A.G. Study on Quercetin, the Main Component of Astragalus membranaceus, in Regulating Ferroptosis of PASMCs via MAPK Pathway for Anti-Hypoxic Pulmonary Hypertension. J. Chin. Pharm. Sci. 2024, 33, 714–729. [Google Scholar]
- Sun, Y.N.; Yang, K.; Zhang, Z.Q. Study on Content Changes of Five Chemical Constituents in Trichosanthes kirilowii Before and After Compatibility with Aconitum kusnezoffii. Chin. Arch. Tradit. Chin. Med. 2022, 40, 240–246. [Google Scholar] [CrossRef]
- Li, R.; Tian, J.F.; Luo, X.F.; Li, M.R. Research Progress on Chemical Constituents and Pharmacological Effects of Albizia julibrissin Flowers. Tianjin Pharm. 2022, 34, 66–71. [Google Scholar]
- Lin, Q.F.; Zhang, N.; Chen, Z.W.; Zhang, L. Study on Chemical Constituents of Red Mulberry Leaves. J. Chin. Med. Mater. 2020, 43, 1364–1367. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Changjiang, G.; Jing, X.; Jingyu, W.; Yang, J.J.; Wu, J.Q.; Gao, W.N. Contents of flavonoids in common vegetables in China. Acta Nutr. Sinica 2009, 31, 185–190. [Google Scholar]
- Junjie, Z. Identification and Metabolic Diversity of Flavonoids in Citrus Fruits. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Jingfang, W.; Ke, L.; Jinhai, L.; Cui, G.Y. Natural antioxidant-quercetin. Chem. Adhes. 2024, 46, 611–615+630. [Google Scholar]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 2023, 938, 175407. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Liu, Y.; Zhang, X.; Sun, X.; Wang, N.; Zhang, C.; Deng, H.; Yao, X.; Wang, S.; Yang, G. Unraveling Quercetin’s Potential: A Comprehensive Review of Its Properties and Mechanisms of Action, in Diabetes and Obesity Complications. Phytother. Res. 2024, 38, 5641–5656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Zhou, J.; Cai, Y.; Li, Z.; Sun, J.; Xie, Z.; Hao, G. Metabolic effects of quercetin on inflammatory and autoimmune responses in rheumatoid arthritis are mediated through the inhibition of JAK1/STAT3/HIF-1α signaling. Mol. Med. 2024, 30, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alía, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Liang, Y.; Hu, G.R.; Tian, Y. Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J. Pharmacol. 2016, 48, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, H.L. Study on Flavonoid Glycosides in the Bulk Drug of Ginkgo Leaf Injection. Master’s Thesis, Ningxia Medical University, Yinchuan, China, 2020. [Google Scholar] [CrossRef]
- Tursun, E.; Zhou, Z.B.; Wan, C.X. Isolation and Identification of Chemical Constituents from Xinjiang Safflower and Its Seed Oil. J. Tarim. Univ. 2017, 29, 1–6. [Google Scholar]
- Zheng, D.D. Study on Chemical Constituents of Cnidium monnieri. Master’s Thesis, Tianjin University of Traditional Chinese Medicine, Tianjin, China, 2020. [Google Scholar] [CrossRef]
- Jiao, H.Y.; Liu, H.; Xue, X.; Chen, X.F. Research Progress on Chemical Constituents and Biological Activities of Polyphenols from Blueberry Leaves. Guangzhou Chem. Ind. 2022, 50, 10–12+20. [Google Scholar]
- Dong, L.M.; Kong, L.X.; Chen, D.X.; Lan, L.P.; Wu, X.Y.; Zhao, T. Study on Flavonoid Chemical Constituents and Antioxidant Activity of Ficus carica. China Food Saf. Mag. 2023, 34, 93–95+99. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini. Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Front. Endocrinol. 2022, 13, 990299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nejabati, H.R.; Roshangar, L. Kaempferol: A potential agent in the prevention of colorectal cancer. Physiol. Rep. 2022, 10, e15488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, M.S.; Altamimi, A.S.A.; Afzal, M.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Gupta, G.; Shahwan, M.; Kukreti, N.; Wong, L.S.; et al. Kaempferol: Paving the path for advanced treatments in aging-related diseases. Exp. Gerontol. 2024, 188, 112389. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Cho, E.; Sampson, L.; Katan, M.B.; Spiegelman, D.; Willett, W.C.; Holmes, M.D. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int. J. Cancer 2005, 114, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhou, S.; Nao, J. Kaempferol as a therapeutic agent in Alzheimer’s disease: Evidence from preclinical studies. Ageing Res. Rev. 2023, 87, 101910. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Shu, J.B.; Chen, J.P.; Su, W.Y.; Wu, Q.; Zhu, W.D. Quality Evaluation of Tangerine Peel from Different Botanical Origins. Chin. Arch. Tradit. Chin. Med. 2025, 1–21. Available online: http://kns.cnki.net/kcms/detail/21.1546.R.20250609.1519.013.html (accessed on 10 June 2025).
- Wang, Q.R.; Liu, R.X.; He, X.; Zhang, T.; Zhao, X.H. Research Progress on Pharmacological Effects and Solubility-Enhancing Technologies of Hesperidin. Prog. Vet. Med. 2025, 46, 91–96. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buzdağlı, Y.; Eyipınar, C.D.; Kacı, F.N.; Tekin, A. Effects of hesperidin on anti-inflammatory and antioxidant response in healthy people: A meta-analysis and meta-regression. Int. J. Environ. Health Res. 2023, 33, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, W.; Tan, R.; Xu, C.; Chen, X.; Li, S.; Liu, Y.; Qiu, H.; Cao, H.; Cheng, Q. The benefits of hesperidin in central nervous system disorders, based on the neuroprotective effect. Biomed. Pharmacother. 2023, 159, 114222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, S.J.; Zhang, Y.G.; Cui, X.H.; Yu, W.B. Study on Extraction, Isolation and Action Mechanism of Anti-Inflammatory Active Components from Pueraria lobata. J. Tonghua Norm. Univ. 2025, 46, 68–80. [Google Scholar] [CrossRef]
- Lu, H.Y. Study on Formononetin from Astragalus membranaceus Combined with Intermittent Exercise in Alleviating Myocardial Injury in Myocardial Infarction Rats. Mol. Plant Breed. 2024, 22, 7556–7563. [Google Scholar] [CrossRef]
- Tian, J.M.; Liu, H.; Bai, X.M.; Jia, T.J. Determination of Formononetin Content in Flavonoids of Glycyrrhiza uralensis by High Performance Liquid Chromatography. Chin. Tradit. Pat. Med. 2007, 8, 1232–1233. [Google Scholar]
- Aliya, S.; Alhammadi, M.; Park, U.; Tiwari, J.N.; Lee, J.H.; Han, Y.K.; Huh, Y.S. The potential role of formononetin in cancer treatment: An updated review. Biomed. Pharmacother. 2023, 168, 115811. [Google Scholar] [CrossRef] [PubMed]
- Machado Dutra, J.; Espitia, P.J.P.; Andrade Batista, R. Formononetin: Biological effects and uses—A review. Food Chem. 2021, 359, 129975. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Hu, F.; Lu, D.; Jin, H.; Lu, H.; Xue, E.; Wu, D. Formononetin inhibits IL-1β-induced inflammation in human chondrocytes and slows the progression of osteoarthritis in rat model via the regulation of PTEN/AKT/NF-κB pathway. Int. Immunopharmacol. 2022, 113 Pt A, 109309. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Niu, Q.Y.; Zhang, X.; Wen, X.L.; Huang, S.H.; Chen, L.P.; Huang, L.P.; Liu, N.H. Research Progress on Chemical Constituents, Pharmacological Effects, Medicinal Property and Prediction of Quality Markers (Q-Markers) of Ampelopsis japonica. Pharmacol. Clin. Chin. Mater. Medica 2025, 1–16. [Google Scholar] [CrossRef]
- Mi, J.; Wei, J.Y.; Lu, L.; Zhang, L.T.; Jin, B.; Li, X.Y.; Yan, Y.M. Comparison of Total Flavonoid Content and Antioxidant Activity in Lycium barbarum Determined by Different Colorimetric Methods. Food Res. Dev. 2025, 46, 155–162. [Google Scholar]
- Kang, Y.S.; Ryu, C.; Suguri, M.; Park, S.B.; Kishino, S.; Onoyama, H. Estimating the catechin concentrations of new shoots in green tea fields using ground-based hyperspectral imagery. Food Chem. 2022, 370, 130987. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Long, Y.; Fang, J.; Liu, G. Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate. Nutrients 2024, 16, 2074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Yang, J.Y.; Wang, X.X.; Wang, Y.; Liu, R.X.; Chen, W.; Li, H. Determination of Total Flavonoids and Liquiritigenin A in Licorice Residue and Optimization of Extraction-Purification Process. Chem. Bio-Eng. 2024, 41, 55–61. [Google Scholar]
- Li, M.T.; Xie, L.; Jiang, H.M.; Huang, Q.; Tong, R.S.; Li, X.; Xie, X.; Liu, H.M. Role of Licochalcone A in Potential Pharmacological Therapy: A Review. Front. Pharmacol. 2022, 13, 878776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shu, J.; Cui, X.; Liu, X.; Yu, W.; Zhang, W.; Huo, X.; Lu, C. Licochalcone A inhibits IgE-mediated allergic reaction through PLC/ERK/STAT3 pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221135462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.J.; Du, H.Z.; Miao, Y.H.; Fang, Y.; Zhao, T.T.; Liu, D.H. Predictive Analysis of Quality Markers and Resource Evaluation of Artemisia argyi Based on Chemical Components and Network Pharmacology. China J. Chin. Mater. Medica 2023, 48, 5474–5486. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, L.L.; Lan, Y.Y. Research Progress on Chemical Constituents, Pharmacological Effects and Quality Markers of Fructus Mori. Chongqing Med. 2021, 50, 1063–1067. [Google Scholar]
- Gan, X.N.; Wang, H.J.; Li, Y.Z.; Li, B. UPLC-Triple TOF/MS Analysis of Chemical Components and Determination of Total Anthocyanins in Lycium ruthenicum. Food Sci. 2021, 42, 185–190. [Google Scholar]
- Safdar, M.A.; Aslam, R.M.N.; Shakeel, A.; Shiza, W.M.; Jmail, A.; Mehmood, M.H.; Gul, H. Cyanidin as potential anticancer agent targeting various proliferative pathways. Chem. Biol. Drug. Des. 2023, 101, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, Z.; Zhu, Y.; Shen, T.; Wang, H.; Shui, G.; Loor, J.J.; Fang, Z.; Chen, M.; Wang, X.; et al. Cyanidin-3-O-glucoside improves non-alcoholic fatty liver disease by promoting PINK1-mediated mitophagy in mice. Br. J. Pharmacol. 2020, 177, 3591–3607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X.; et al. Cyanidin-3-O-glucoside and its phenolic metabolites ameliorate intestinal diseases via modulating intestinal mucosal immune system: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, T.; Su, J.; Zhao, Y.; Wang, C.C.; Li, X. Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 2017, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin Ameliorates Insulin Resistance and Lipid Accumulation by Endoplasmic Reticulum Stress and SREBP-1c/SREBP-2 Pathway in Palmitate-Induced HepG2 Cells and High-Fat Diet-Fed Mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Ma, Y.; Wang, Y.; Du, Z.Y.; Shen, J.K.; Peng, H.L. Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother. Res. 2011, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.W.; Chen, P.N.; Wu, H.C.; Wu, S.W.; Tsai, P.Y.; Hsieh, Y.S.; Chang, H.R. Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Wang, X.; Ying, T.; Li, X.; Tang, Y.; Wang, Y.; Yu, T.; Sun, M.; Zhao, J.; Du, Y.; et al. Kaempferol alleviates the inflammatory response and stabilizes the pulmonary vascular endothelial barrier in LPS-induced sepsis through regulating the SphK1/S1P signaling pathway. Chem. Biol. Interact. 2022, 368, 110221. [Google Scholar] [CrossRef] [PubMed]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural. Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Nie, T.; Zhang, P.; Ma, J.; Shan, A. Hesperidin attenuates hepatic lipid accumulation in mice fed high-fat diet and oleic acid induced HepG2 via AMPK activation. Life Sci. 2022, 296, 120428. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.; Gadalla, H.; Filimban, W.A.; Albukhari, T.A.; Sembawa, H.; Bagadood, R.M.; Sindi, G.; Abdelhamid, F.M.; El-Boshy, M.E.; Risha, E.F. Hesperidin ameliorates thioacetamide-induced liver fibrosis via antioxidative and anti-inflammatory mechanisms targeting TGF-β/α-SMA pathways in rats. Int. J. Immunopathol. Pharmacol. 2024, 38, 3946320241309004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yurtal, Z.; Altug, M.E.; Unsaldi, E.; Secinti, I.E.; Kucukgul, A. Investigation of Neuroprotective and Therapeutic Effects of Hesperidin in Experimental Spinal Cord Injury. Turk. Neurosurg. 2020, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xia, R.; Yang, S.; Liu, L.; Zhang, J.; Feng, K.; Shang, Y.; Qu, J.; Li, L.; Chen, N.; et al. Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE mice. Theranostics 2020, 10, 1090–1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, T.K.; Chu, K.O.; Wang, C.C.; Pang, C.P. Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment. Antioxidants 2023, 12, 1320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Z.; Guo, Z.; Xiao, T.; Liu, H.; Su, G.; Zhao, Y. Enrichment of total flavones and licochalcone A from licorice residues and its hypoglycemic activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1114–1115, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Kim, S.J.; Kim, D.Y.; Jo, H.K.; Kim, G.W.; Chung, S.H. Licochalcone A regulates hepatic lipid metabolism through activation of AMP-activated protein kinase. Fitoterapia 2013, 86, 208. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Wu, C.Y.; Lin, C.F.; Liu, Y.W.; Lin, T.C.; Liao, H.J.; Chang, G.R. The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. Pharmacother. 2022, 151, 113128. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, G.; Lv, S.; Xiao, Y.; Fan, C.; Zou, M.; Wang, Y.; Guo, Q.; Kabir, M.A.; Peng, X. Avian safety guardian: Luteolin restores Mycoplasma gallisepticum-induced immunocompromise to improve production performance via inhibiting the IL-17/NF-kB pathway. Int. Immunopharmacol. 2023, 124 Pt B, 110946. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhang, S.J.; Ma, P.; Dan, J.H. Research Progress on the Regulation of SREBP2 Expression and Activity. Chin. J. Cell. Biol. 2021, 43, 2441–2448. [Google Scholar]
- Zhang, S.J.; Wang, S.; Zhang, T.T.; Jia, S.T.; Dan, J.H. Research Progress on the Regulation of HMGCR Expression and Activity. Chin. J. Cell. Biol. 2023, 45, 990–996. [Google Scholar]
- Wang, L.C. Mechanistic Study on Apigenin Inhibiting Non-Alcoholic Fatty Liver Disease. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2023. [Google Scholar] [CrossRef]
- Oh, G.S.; Lee, G.G.; Yoon, J.; Oh, W.K.; Kim, S.W. Selective inhibition of liver X receptor α-mediated lipogenesis in primary hepatocytes by licochalcone A. Chin. Med. 2015, 10, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; He, B.; Chen, E.; Lu, J.; Wang, J.; Cao, H.; Li, L. The aryl hydrocarbon receptor ligand ITE inhibits cell proliferation and migration and enhances sensitivity to drug-resistance in hepatocellular carcinoma. J. Cell. Physiol. 2021, 236, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Park, H.J.; Woo, J.H.; Kim, M.K.; Koh, P.O.; Min, W.; Ko, Y.G.; Kim, C.H.; Won, C.K.; Cho, J.H. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement Altern. Med. 2012, 12, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell. Metab. 2006, 4, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020, 131, 110769. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sharen, G.; Yuan, F.; Peng, Y.; Chen, R.; Zhou, X.; Wei, H.; Li, B.; Jing, W.; Zhao, J. TIP30 regulates lipid metabolism in hepatocellular carcinoma by regulating SREBP1 through the Akt/mTOR signaling pathway. Oncogenesis 2017, 6, e347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.; Wang, T.; Shi, Y.; Deng, J.; Yan, X.; Zhang, C.; Yin, X.; Liu, W. Integrated network pharmacology, metabolomics and molecular docking analysis to reveal the mechanisms of quercetin in the treatment of hyperlipidemia. J. Pharm. Biomed. Anal. 2025, 252, 116507. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T.; Kadowaki, T. Adiponectin/AdipoR Research and Its Implications for Lifestyle-Related Diseases. Front. Cardiovasc. Med. 2019, 6, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoang, M.H.; Jia, Y.; Lee, J.H.; Kim, Y.; Lee, S.J. Kaempferol reduces hepatic triglyceride accumulation by inhibiting Akt. J. Food Biochem. 2019, 43, e13034. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nonaka, M.; Mochizuki, H.; Handa, K.; Hanada, H.; Hirota, K. Naringenin and hesperetin induce growth arrest, apoptosis, and cytoplasmic fat deposit in human preadipocytes. J. Agric. Food Chem. 2008, 56, 11030. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.Y.; Baek, N.I.; Chung, S.H. Licochalcone A prevents adipocyte differentiation and lipogenesis via suppression of peroxisome proliferator-activated receptor γ and sterol regulatory element-binding protein pathways. J. Agric. Food Chem. 2012, 60, 5112. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040. [Google Scholar] [CrossRef] [PubMed]
- Sannappa Gowda, N.G.; Shiragannavar, V.D.; Karunakara, S.H.; Veeranna, R.P.; Suvarna, D.; Kumar, D.P.; Santhekadur, P.K. Novel role of Quercetin in ameliorating metabolic syndrome via VDR mediated activation of adiponectin/AdipoR2 signaling. Biochem. Biophys. Rep. 2024, 39, 101754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, S.; Shu, L.; Cao, Q.Y.; Hu, H.M.; Zhu, Y.C. Hawthorn leaf flavonoids alleviate the deterioration of atherosclerosis by inhibiting SCAP-SREBP2-LDLR pathway through sPLA2-ⅡA signaling in macrophages in mice. J. Ethnopharmacol. 2024, 327, 118006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Yang, J.B.; Zhou, Y.Y.; Yang, S.H.; Yang, R.H.; Fang, S.J.; Ma, Y.X.; Niu, W.Y. Research Progress on the Regulatory Mechanisms of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. Chin. J. Pathophysiol. 2025, 41, 791–797. [Google Scholar]
- Clarke, P.R.; Hardie, D.G. Regulation of HMG-CoA reductase: Identification of the site phosphorylated by the AMP—Activated protein kinase in vitro and in intact rat liver. EMBO J. 1990, 9, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Puthanveetil, P.; Kong, X.; Bräse, S.; Voros, G.; Peer, W.A. Transcriptome analysis of two structurally related flavonoids; Apigenin and Chrysin revealed hypocholesterolemic and ketogenic effects in mouse embryonic fibroblasts. Eur. J. Pharmacol. 2021, 893, 173804. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxid. Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, S.; Ye, J. 7-Dehydrocholesterol: A sterol shield against an iron sword. Mol. Cell 2024, 84, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Cochran, C.; Martin, K.; Rafferty, D.; Choi, J.; Leontyev, A.; Shetty, A.; Kurup, S.; Puthanveetil, P. Untargeted Metabolomics Based Prediction of Therapeutic Potential for Apigenin and Chrysin. Int. J. Mol. Sci. 2023, 24, 4066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, R.; Liu, W.; Zeng, J.; Meng, J.; Shi, L.; Yang, S.; Chang, J.; Wang, C.; Xing, K.; Wen, J.; et al. Recent advances in the screening methods of NPC1L1 inhibitors. Biomed. Pharmacother. 2022, 155, 113732. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Dong, L.W.; Liu, S.; Meng, F.H.; Xie, C.; Lu, X.Y.; Zhang, W.J.; Luo, J.; Song, B.L. Bile acids-mediated intracellular cholesterol transport promotes intestinal cholesterol absorption and NPC1L1 recycling. Nat. Commun. 2023, 14, 6469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter niemann-pick c1-like 1 in caco-2 cells and rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudhia, Z.; Louw, J.; Muller, C.; Joubert, E.; de Beer, D.; Kinnear, C.; Pheiffer, C. Cyclopia maculata and Cyclopia subternata (honeybush tea) inhibits adipogenesis in 3T3-L1 pre-adipocytes. Phytomedicine 2013, 20, 401. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wu, L.; Yin, Q.; Li, L.; Zheng, X.; Du, S.; Huang, X.; Bai, L.; Wang, Y.; Bian, Y. A promising therapy for fatty liver disease: PCSK9 inhibitors. Phytomedicine 2024, 128, 155505. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Fan, D.; Wang, L.; Guo, L.; Jiang, H.; Wang, L. Mechanisms of action and therapeutic potential of PCSK9-regulating drugs. Pharm. Biol. 2025, 63, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Mayne, J.; Sirois, F.; Fedoryak, O.; Raymond, A.; Noad, J.; Chrétien, M. Mice Fed a High-Cholesterol Diet Supplemented with Quercetin-3-Glucoside Show Attenuated Hyperlipidemia and Hyperinsulinemia Associated with Differential Regulation of PCSK9 and LDLR in their Liver and Pancreas. Mol. Nutr. Food Res. 2018, 62, e1700729. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.K.; Majid, N.I.; Yusof, H.M.; Zainol, K.M.; Mohamad, H.; Mohd Zin, Z. Catechin profile and hypolipidemic activity of Morinda citrifolia leaf water extract. Heliyon 2020, 6, e04337. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Maderna, P.; Sirtori, C.R. Reverse cholesterol transport: Physiology and pharmacology. Atherosclerosis 1991, 88, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Jiang, T.; Zhou, H.F.; Liang, Y.; Zhao, G.J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell Physiol. Biochem. 2018, 47, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Dergunov, A.D.; Savushkin, E.V.; Dergunova, L.V.; Litvinov, D.Y. Significance of Cholesterol-Binding Motifs in ABCA1, ABCG1, and SR-B1 Structure. J. Membr. Biol. 2019, 252, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, K.; Kim, S.H.; Hong, M.J.; Jeong, N.J.; Kim, M.S. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J. Food Biochem. 2020, 44, e13358. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sheng, F.; Zou, L.; Xiao, J.; Li, P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 2021, 34, 109–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, M.Y.C.; Kiat Ho, H. Pharmacological modulation of cholesterol 7α-hydroxylase (CYP7A1) as a therapeutic strategy for hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, M.; Gao, S.; Li, D.; Zhang, Y.; Jin, T.; Ling, W. Cyanidin-3-O-β-glucoside upregulates hepatic cholesterol 7α-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012, 56, 610. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.P. Effects of Acupuncture Therapy on Hepatic Oxidative Stress and Nrf2/Keap1/HO-1 Signaling Pathway in Hyperlipidemic Rats. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2024. [Google Scholar] [CrossRef]
- Yang, J.B.; Chen, X.T.; Zhou, Y.Y.; Yang, S.H.; Yang, R.H.; Wang, Q.; Xue, S.; Niu, W.Y. Research Progress on the Preventive and Therapeutic Effects of Traditional Chinese Medicine on Metabolic-Associated Fatty Liver Disease Based on Nrf2 Signaling Pathway. Drug Eval. Res. 2024, 47, 2681–2687. [Google Scholar]
- Yang, J.T.; Wang, J.; Zhou, X.R.; Xiao, C.; Lou, Y.Y.; Tang, L.H.; Zhang, F.J.; Qian, L.B. Luteolin alleviates cardiac ischemia/reperfusion injury in the hypercholesterolemic rat via activating Akt/Nrf2 signaling. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Akhtar, F.; Rizvi, S.I. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin. Exp. Pharmacol. Physiol. 2020, 47, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khan, M.I.; Ashfaq, F.; Alsayegh, A.A.; Khatoon, F.; Altamimi, T.N.; Rizvi, S.I. Hesperidin Supplementation Improves Altered PON -1, LDL Oxidation, Inflammatory Response and Hepatic Function in an Experimental Rat Model of Hyperlipidemia. Indian J. Clin. Biochem. 2024, 39, 257–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cherkaoui-Malki, M.; Surapureddi, S.; El-Hajj, H.I.; Vamecq, J.; Andreoletti, P. Hepatic steatosis and peroxisomal fatty acid beta-oxidation. Curr. Drug. Metab. 2012, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.B.; Fu, S.X.; Wu, C.C.; Wang, X.X.; Yu, G.J.; Long, M.; Wang, Z.; Liu, G.W. Alterations of fatty acid β-oxidation capability in the liver of ketotic cows. J. Dairy Sci. 2012, 95, 1759. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic Effects and Preliminary Mechanism of Chrysanthemum Flavonoids, Its Main Components Luteolin and Luteoloside in Hyperlipidemia Rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sathyanarayan, A.; Mashek, M.T.; Mashek, D.G. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pastore, N.; Vainshtein, A.; Klisch, T.J.; Armani, A.; Huynh, T.; Herz, N.J.; Polishchuk, E.V.; Sandri, M.; Ballabio, A. TFE3 regulates whole-body energy metabolism in cooperation with TFEB. EMBO Mol. Med. 2017, 9, 605–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Zhao, Y.; Wang, G.; Feng, S.; Ge, X.; Ye, W.; Wang, Z.; Zhu, Y.; Cai, W.; Bai, J.; et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox. Biol. 2022, 53, 102344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Zhao, H.; Li, X.; Wang, Q.; Yan, M.; Zhang, H.; Zhao, T.; Zhang, N.; Zhang, P.; Peng, L.; et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J. Nutr. Biochem. 2019, 73, 108214. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, Y.; Yao, N.; Gan, H.; Zeng, Q.; Huang, X.; Huang, D.; Cai, D.; Chen, Y. Apigenin attenuates the atherosclerotic lesions through enhancing selective autophagy/lipophagy and promoting RCT process. Pharm. Biol. 2025, 63, 387–401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, M.; Mehta, J.L.; Hu, C. LOX-1 and obesity. Cardiovasc. Drugs Ther. 2011, 25, 469. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Chen, W.B.; Shi, L.; Jiang, X.; Li, K.; Wang, Y.X.; Liu, Y.Q. The Underlying Mechanisms of Curcumin Inhibition of Hyperglycemia and Hyperlipidemia in Rats Fed a High-Fat Diet Combined With STZ Treatment. Molecules 2020, 25, 271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Q.; Li, Y.C.; Du, C.; Wang, L.N.; Xiao, Y.H. Effects of Apigenin on the Expression of LOX-1, Bcl-2, and Bax in Hyperlipidemia Rats. Chem. Biodivers. 2021, 18, e2100049. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Song, Y.; Yang, Y.; Zeng, C. NLRP3 inflammasome and pyroptosis in cardiovascular diseases and exercise intervention. Front. Pharmacol. 2024, 15, 1368835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramachandran, R.; Manan, A.; Kim, J.; Choi, S. NLRP3 inflammasome: A key player in the pathogenesis of life-style disorders. Exp. Mol. Med. 2024, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Z.; Liu, L.; Zhao, S.; Zhao, J.; Li, S.; Li, M. Apigenin attenuates atherosclerosis and non-alcoholic fatty liver disease through inhibition of NLRP3 inflammasome in mice. Sci. Rep. 2023, 13, 7996. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, L.; Chi, X.; Wang, H.; Dai, A.; Dong, J.; Liu, S.; Zhang, D. Mechanism of action of buckwheat quercetin in regulating lipid metabolism and intestinal flora via Toll-like receptor 4 or nuclear factor κB pathway in rats on a high-fat diet. Nutrition 2023, 115, 112148. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent For Obesity. Drug Des. Devel. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.X.; Ma, J.J.; Li, Y.J.; Dong, S.Q.; Yang, B.; Fan, H.R. Research Progress on the Mechanisms of Traditional Chinese Medicine in Treating Hyperlipidemia Based on Intestinal Microbiota. Chin. Herb. Med. 2025, 56, 2171–2183. [Google Scholar]
- Liu, Z.X.; Kui, L.L. Exploring the Prevention and Treatment of Hyperlipidemia by Integrative Chinese and Western Medicine Based on Intestinal Microbiota. Asia-Pac. Tradit. Med. 2024, 20, 191–197. [Google Scholar]
- Wang, T.; Liu, L.; Deng, J.; Jiang, Y.; Yan, X.; Liu, W. Analysis of the mechanism of action of quercetin in the treatment of hyperlipidemia based on metabolomics and intestinal flora. Food Funct. 2023, 14, 2112–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. Biomed. Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.J.; Liu, D.P.; Huang, Y.T.; Yuan, G.; Shuai, Q. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Robinson, D.H.; Birt, D.F. Evaluation of properties of apigenin and [G-3H]apigenin and analytic method development. J. Pharm. Sci. 1997, 86, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, M. Absorption and Metabolism of Flavonoids in the Caco-2 Cell Culture Model and a Perused Rat Intestinal Model, Drug Metabolism and Disposition. Drug. Metab. Dispos. 2002, 30, 370. [Google Scholar] [CrossRef] [PubMed]
- Gradolatto, A.; Basly, J.P.; Berges, R.; Teyssier, C.; Chagnon, M.C.; Siess, M.H.; Canivenc-Lavier, M.C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, X.; Lin, Z.; Zhu, Y.; Jiang, X.; Zhao, L.; Zeng, F.; Chen, L.; Xu, W.; Liu, T.; et al. 6,8-(1,3-Diaminoguanidine) luteolin and its Cr complex show hypoglycemic activities and alter intestinal microbiota composition in type 2 diabetes mice. Food Funct. 2022, 13, 3572–3589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Pan, H.; Lu, Y.; Ding, L. An HPLC-MS/MS method for the simultaneous determination of luteolin and its major metabolites in rat plasma and its application to a pharmacokinetic study. J. Sep. Sci. 2018, 41, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Pai, Y.F.; Tsai, T.H. Isolation of Luteolin and Luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and Their Pharmacokinetics in Rats. J. Agric. Food Chem. 2015, 63, 7700. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, A.; Ito, J.; Parida, I.S.; Syoji, N.; Fujii, T.; Takahashi, H.; Nakagawa, K. Improving water dispersibility and bioavailability of luteolin using microemulsion system. Sci. Rep. 2022, 12, 11949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaci, H.; Bodnárová, S.; Fliszár-Nyúl, E.; Lemli, B.; Pelantová, H.; Valentová, K.; Bakos, É.; Özvegy-Laczka, C.; Poór, M. Interaction of luteolin, naringenin, and their sulfate and glucuronide conjugates with human serum albumin, cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) enzymes and organic anion transporting polypeptide (OATP1B1 and OATP2B1) transporters. Biomed. Pharmacother. 2023, 157, 114078. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Sun, Z.; Liu, Y.; Xu, J.; Liu, S.; Huang, B.; Ma, L.; Yu, Z.; Bi, K. Pharmacokinetics of luteolin and tetra-acetyl-luteolin assayed by HPLC in rats after oral administration. Biomed. Chromatogr. 2010, 24, 826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, L.P.; Luo, S.Q.; Jiang, H.D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008, 56, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Dai, S.; Wang, C.; Fu, K.; Wu, R.; Zhao, X.; Yao, Y.; Li, Y. Luteolin as a potential hepatoprotective drug: Molecular mechanisms and treatment strategies. Biomed. Pharmacother. 2023, 167, 115464. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Barber, E.; Kellow, N.J.; Williamson, G. Improving quercetin bioavailability: A systematic review and meta-analysis of human intervention studies. Food Chem. 2025, 477, 143630. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.H. Preparation and in Vitro and in Vivo Evaluation of Quercetin Nanoemulsion. Master’s Thesis, Anhui Medical University, Hefei, China, 2022. [Google Scholar] [CrossRef]
- Arts, I.C.; Sesink, A.L.; Faassen-Peters, M.; Hollman, P.C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omiecinski, C.J.; Vanden Heuvel, J.P.; Perdew, G.H.; Peters, J.M. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011, 120 (Suppl. S1), S49–S75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Boer, V.C.; Dihal, A.A.; van der Woude, H.; Arts, I.C.; Wolffram, S.; Alink, G.M.; Rietjens, I.M.; Keijer, J.; Hollman, P.C. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005, 135, 1718. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Rouanet, J.M.; Auger, C.; Teissèdre, P.L.; Caldwell, S.T.; Hartley, R.C.; Lean, M.E.; Edwards, C.A.; Crozier, A. Bioavailability of [2-(14)C]quercetin-4’-glucoside in rats. J. Agric. Food Chem. 2008, 56, 12127. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Safety 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1387665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin-Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)-Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.; Mendes, T.; Bronze, M.R.; Simplício, A.L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg. Med. Chem. 2008, 16, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, S230–S242. [Google Scholar] [CrossRef] [PubMed]
- Makarova, N.M. Bioavailability and metabolism of flavonoids. Eksp. Klin. Farmakol. 2011, 74, 33–40. [Google Scholar] [PubMed]
- Sharma, N.; Kabra, A. Formononetin: Pharmacological properties and therapeutic potential. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; Fan, M.X.; Zhao, H.Y.; Li, M.X.; Wu, X.; Gao, W.Y. Pharmacokinetics and Bioavailability of the Isoflavones Formononetin and Ononin and Their in vitro Absorption in Using Chamber and Caco-2 Cell Models. J. Agric. Food Chem. 2018, 66, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Wahajuddin Tewari, D.; Pradhan, T.; Jain, G.K. PAMPA permeability, plasma protein binding, blood partition, pharmacokinetics and metabolism of formononetin, a methoxylated isoflavone. Food Chem. Toxicol. 2011, 49, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Formononetin, a Type of Methoxylated Isoflavone, and Its Role in Cancer Therapy through the Modulation of Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 9719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, Z.; Wang, Y.; Guo, J.; Tian, C.; Pan, W.; Wang, H.; Yan, J. Prostate Cancer Therapy Using Docetaxel and Formononetin Combination: Hyaluronic Acid and Epidermal Growth Factor Receptor Targeted Peptide Dual Ligands Modified Binary Nanoparticles to Facilitate the in vivo Anti-Tumor Activity. Drug. Des. Dev. Ther. 2022, 16, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Lan, Q.; Liang, X.; Zhao, J.; Huang, H.; Zhan, Y.; Qin, Z.; Jiang, X.; Zheng, L. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J. Nanobiotechnol. 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manach, C.; Texier, O.; Morand, C.; Crespy, V.; Régérat, F.; Demigné, C.; Rémésy, C. Comparison of the bioavailability of quercetin and catechin in rats. Free Radic. Biol. Med. 1999, 27, 1259. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, J.S.; Choi, D.H. Effects of licochalcon A on the pharmacokinetics of losartan and its active metabolite, EXP-3174, in rats. Pharmazie 2013, 68, 882. [Google Scholar] [PubMed]
- Li, M.; Yang, S.; Yi, H.; Wang, Z.; Xu, B.; Li, G.; Ma, C.; Yuan, C.; Wang, Z. Preparation and evaluation of licochalcone A-integrated casein-pectin nanodelivery system: Insights into its gastrointestinal digestibility and bioavailability. Food Chem. 2025, 486, 144633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, J.; Yang, Y.; Adu-Frimpong, M.; Ji, H.; Toreniyazov, E.; Wang, Q.; Yu, J.; Xu, X. SMEDDS for improved oral bioavailability and anti-hyperuricemic activity of licochalcone A. J. Microencapsul. 2021, 38, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Thomasset, S.C.; P-Berry, D.; Garcea, G.; Brown, K.; Steward, W.P.; Gescher, A.J. Determination of anthocyanins in the urine of patients with colorectal liver metastases after administration of bilberry extract. Biomed. Chromatogr. 2011, 25, 660. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Abdullah, A.N.; Hussain, M.; Lu, X.; Xu, J.; Zhong, H.; Guan, R. A review of recent advances on cyanidin-3-glucoside: The biotransformation, absorption, bioactivity and applications of nano-encapsulation. Food Funct. 2023, 14, 6320–6345. [Google Scholar] [CrossRef] [PubMed]
- Shoubaky, G.A.E.; Abdel-Daim, M.M.; Mansour, M.H.; Salem, E.A. Isolation and identification of a flavone apigenin from marine red alga Acanthophora spicifera with antinociceptive and anti-Inflammatory activities. J. Exp. Neurosci. 2016, 10, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroudi, A.; Alharbi, H.O.A.; AlSuhaymi, N.; Alsugoor, M.H.; Aldakheel, F.M.; Khan, A.A.; Rahmani, A.H. Apigenin: A Bioflavonoid with a Promising Role in Disease Prevention and Treatment. Biomedicines 2024, 12, 1353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Li, Q.; Xiao, B.; Fang, H.; Huang, B.; Huang, F.; Wang, Y. Luteolin enhances the antitumor efficacy of oncolytic vaccinia virus that harbors IL-24 gene in liver cancer cells. J. Clin. Lab. Anal. 2021, 35, e23677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Im, E.; Yeo, C.; Lee, E.O. Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018, 209, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Hu, A.; Hu, Y.; Ma, J.; Weng, P.; Dai, J. Anti-hepatoma cells function of luteolin through inducing apoptosis and cell cycle arrest. Tumour. Biol. 2014, 35, 3053. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhao, P.; Li, X.; Pan, H.; Ma, S.; Ding, L. Cytotoxicity of luteolin in primary rat hepatocytes: The role of CYP3A-mediated ortho-benzoquinone metabolite formation and glutathione depletion. J. Appl. Toxicol. 2015, 35, 1372. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2016, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Ruscinc, N.; Massarico Serafim, R.A.; Almeida, C.; Rosado, C.; Baby, A.R. Challenging the safety and efficacy of topically applied chlorogenic acid, apigenin, kaempferol, and naringenin by HET-CAM, HPLC-TBARS-EVSC, and laser Doppler flowmetry. Front. Chem. 2024, 12, 1400881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phyther. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Pingale, T.D.; Gupta, G.L. Acute and sub-acute toxicity study reveals no dentrimental effect of formononetin in mice upon repeated i.p. dosing. Toxicol. Mech. Methods 2023, 33, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity 2008, 16, 1338. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, K.S.; Squarisi, I.S.; Acésio, N.O.; Nicolella, H.D.; Ozelin, S.D.; Reis Santos de Melo, M.; Guissone, A.P.P.; Fernandes, G.; Silva, L.M.; da Silva Filho, A.A.; et al. Licochalcone A a licorice flavonoid: Antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J. Toxicol. Environ. Health A 2020, 83, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Han, S.; Lee, J.; Hong, T.; Yim, D.S. The safety and pharmacokinetics of cyanidin-3-glucoside after 2-week administration of black bean seed coat extract in healthy subjects. Korean J. Physiol. Pharmacol. 2012, 16, 249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.S.; Li, X.C.; Wang, X.M.; Guo, J.F.; Wu, L.L.; Qin, L.L.; Liu, T.H. Research Progress on Traditional Chinese Medicine Intervening in PPARγ Signaling Pathway for Improving Hyperlipidemia. China J. Tradit. Chin. Med. Pharm. 2025, 40, 1851–1855. [Google Scholar]
| Category | HCAs | Structure | Source | Biological Activity/Application | Experimental Model | Dose Ranges | Ref. |
|---|---|---|---|---|---|---|---|
| Flavones | Apigenin (C15H10O6) | 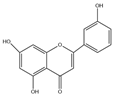 | Leonurus japonicus, olive leaves, chamomile, parsley, celery, Basella alba L., artichoke, etc. | anti-inflammatory | in vitro | 10, 20, 30 μM | [17] |
| antioxidation | in vivo | 20 mg/kg | [90] | ||||
| anticancer | in vivo | 20–200 mg/kg | [19] | ||||
| anti-apoptosis | in vivo | 10 mg/kg | [91] | ||||
| improve lipid metabolism | in vitro | 40 μmol/L | [21] | ||||
| insulin resistance | in vivo | 10 mg/kg | [92] | ||||
| Luteolin (C15H10O7) | 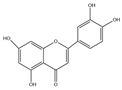 | Taraxacum mongolicum, Chrysanthemum morifolium, lotus stems, Lonicera japonica, cabbage, celery, apples, oranges, pomegranates, lemons, etc. | including anticancer | in vivo | 30 mg/kg | [93] | |
| lipid-lowering | in vitro | 10–20 mm | [94] | ||||
| anti-inflammatory | in vitro | 10 μm, 100 μm | [29] | ||||
| modulation of intestinal microbiota | in vivo | 17.3 mg/mL | [30] | ||||
| immunoregulatory functions | in vivo | 100 mg/kg | [95] | ||||
| Flavonols | Quercetin (C15H10O7) |  | Astragalus membranaceus, Trichosanthes kirilowii, Albizia julibrissin flowers, and Morus alba leaves, as well as in fruits and vegetables including grapes, onions, carrots, and potatoes | ameliorating metabolic syndrome | in vivo | 5, 10 mg/kg | [41] |
| antibacterial effects | in vitro | 1–8 mg/mL | [42] | ||||
| neuroprotection | in vivo | 50, 100 mg/kg | [43] | ||||
| anti-ferroptotic activity | in vivo | 25 mg/kg | [44] | ||||
| hypoglycemic | clinical | 400 mg/kg | [41] | ||||
| anti-obesity properties | in vivo | 240 mg/kg | [41] | ||||
| antirheumatic effects | in vitro | 20, 40, 80 μmol/L | [46] | ||||
| Kaempferol (C15H10O6) | 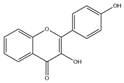 | Ginkgo biloba leaves, Carthamus tinctorius, Cnidium monnieri, blueberry leaves, figs, legumes, cauliflower, cabbage, strawberries, tea, and tomatoes | including anticancer | in vitro | 0, 25, 50, 75, 100 μM | [96] | |
| anti-inflammatory | in vivo | 3, 5, 9 mg/kg | [97] | ||||
| antioxidant | in vivo | 10 mg/kg | [98] | ||||
| Alzheimer’s disease | in vivo | 10 mg/kg | [60] | ||||
| Dihydroflavones | Hesperidin (C28H34O15) | 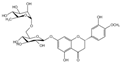 | citrus fruits such as Citrus sinensis and Citrus reticulata | lipid-lowering | in vivo | 150, 300 mg/kg | [99] |
| antioxidant | in vivo | 50 mg/kg | [64] | ||||
| anti-inflammatory | in vivo | 200 mg/kg | [100] | ||||
| neuroprotective effects | in vivo | 200 mg/kg | [101] | ||||
| Isoflavones | Formononetin (C16H12O4) | 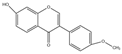 | leguminous plants (such as Astragalus membranaceus, Pueraria lobata, Glycyrrhiza uralensis, etc.), coffee beans, strawberries, and grapes | including anticancer | in vivo | 10 mg | [71] |
| anti-obesity | clinical | 30 | [71] | ||||
| lipid-lowering | in vivo | 10 mg/kg | [102] | ||||
| anti-osteoarthritis | in vitro | 200 μM | [72] | ||||
| neuroprotective effects | in vitro | 10 µM | [71] | ||||
| Flavanols | Catechin (C15H14O6) | 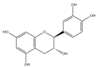 | Ampelopsis japonica, Lycium barbarum, green tea, kiwifruit, etc. | antioxidation | in vitro | 50 μM | [103] |
| including anticancer | in vitro | 25 μMol/L | [79] | ||||
| lipid-lowering | clinical | 400 mg/d | [80] | ||||
| Chalcones | Licochalcone A(C21H22O4) | 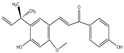 | Glycyrrhiza uralensis | lower blood sugar | in vivo | 100, 200, 300 mg/kg | [104] |
| lipid-lowering | in vivo | 5, 10 mg/kg | [105] | ||||
| anti-allergic properties | in vivo | 20, 40, 80 mg/kg | [83] | ||||
| Anthocyanidins | Cyanidin-3-glucoside (C21H21ClO11) | 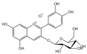 | Artemisia argyi, mulberries, black wolfberries, purple cabbage, purple sweet potatoes, grapes, etc. | including anticancer | in vivo | 5 mg/kg | [106] |
| lipid-lowering | in vitro | 100 μM | [88] | ||||
| modulation of intestinal microbiota | in vivo | 250 mg/kg | [89] |
| Category | HCAs | Mechanisms of Action | Classification of Mechanisms | Experimental Model | Dose Ranges | Ref. |
|---|---|---|---|---|---|---|
| Flavones | Apigenin (C15H10O6) | miR-363-3p↓, INSIG1↑, SREBP1, ACC1, FASN, SCD↓ | Inhibition of Triglyceride Synthesis | HepG2 cells C57BL/6J mice | 20, 40 μmol/L 150, 250 mg/kg | [109] |
| PI3K, p-Akt, PPARγ↓ | 3T3-L1 preadipocytes | 20, 40 μM | [114] | |||
| SREBP2, HMGCR↓ | Inhibition of Cholesterol Synthesis | mouse embryonic fibroblasts | 50 μM | [127] | ||
| 7-Dehydrocholesterol, Xanthine↓ | mouse embryonic fibroblasts | 25 μM | [130] | |||
| miR-33↓, ABCA1↑ | Promotion of Cholesterol Uptake | RAW264.7 cells ApoE–/–mice | 10, 20, 40 μM 10 mg/kg | [141] | ||
| ULK1, UVRAG, beclin-1↑ | Promote Autophagy | C57BL/6J mice | 6.25, 12.5, 25 mg/kg | [160] | ||
| LOX-1↑, Bcl-2, Bax↓ | Inhibition of Apoptosis | Sprague Dawley rats | 20, 40, 80 mg/kg | [163] | ||
| NLRP3, NF-κB↓ | Inhibition of Inflammation | HepG2 cells Ldlr−/−mice | 25, 50 μM 5 mg/kg | [166] | ||
| Luteolin (C15H10O7) | PI3K, p-Akt, PPARγ↓ | Inhibition of Triglyceride Synthesis | 3T3-L1 preadipocytes | 10 μM | [114] | |
| NPC1L1↓ | Inhibition of Lipid Absorption | Caco-2 cells | 25–100 μM | [133,134] | ||
| LXRα, ABCG1, SRB1↑ | Promotion of Cholesterol Uptake | HepG2 cells C57BL/6J mice | 10–50 μM | [144] | ||
| Akt, Nrf2, NQO1, HO-1↑, mPTP↓ | Inhibition of Oxidative Stress | Sprague Dawley rats | 100 mg/kg | [150] | ||
| FAβO↑, FAS↓ | Promotion of Fatty Acid β-Oxidation | Sprague Dawley rats | 50 mg/kg | [155] | ||
| Flavonols | Quercetin (C15H10O7) | PI3K, p-Akt, PPARγ↓ | Inhibition of Triglyceride Synthesis | 3T3-L1 preadipocytes | 10 μM | [114] |
| AKT, mTOR, SREBP1↓ | Sprague Dawley rats | 150 mg/kg | [116] | |||
| Adipo, AdipoR2↑ | 3T3-L1 preadipocytes Swiss albino mice | 5, 10, 20 μM 100 mg/kg | [122] | |||
| HMGCR↓ | Inhibition of Cholesterol Synthesis | C57BL/6J mice | 5 mg/kg | [128] | ||
| TLR4, NF-κB↓ | Inhibition of Inflammation | Sprague Dawley rats | 200 mg/kg | [138] | ||
| beneficial bacteria↑, pathogenic bacteria↓, Firmicutes/Bacteroidetes↓ | Regulation of Gut Microbiota | Sprague Dawley rats | 10, 100, 200 mg/kg | [171] | ||
| Kaempferol (C15H10O6) | AKT, SREBP1↓ | Inhibition of Triglyceride Synthesis | HepG2, THP-1, cco2 cells | 10, 20 μM | [118] | |
| relative abundance of Firmicutes↓, relative abundance of Bacteroidetes↑ | Regulation of Gut Microbiota | C57BL/6J mice | 200 mg/kg | [172] | ||
| Dihydroflavones | Hesperidin (C28H34O15) | p-Akt, NF-κB, Bcl-2↓ | Inhibition of Triglyceride Synthesis | AML-1 human preadipocyte cell line | 100, 500 µM | [119] |
| STC-1, CCK↑ | Inhibition of Lipid Absorption | 3T3-L1 preadipocytes | 0–1600 μg/mL | [135] | ||
| LDL oxidation, PON-1↓ | Inhibition of Oxidative Stress | Wistar rat | 100 mg/kg | [151] | ||
| IL-6↓; TNF-α↓ | Inhibition of Inflammation | Sprague Dawley rats | 50 mg/kg | [168] | ||
| Isoflavones | Formononetin (C16H12O4) | AMPK↑, TFEB↓ | Promote Autophagy | HepG2 cells C57BL/6J mice | 40 μM 100 mg/kg | [159] |
| Flavanols | Catechin (C15H14O6) | LDL-c uptake↑ | Promotion of Cholesterol Uptake | HepG2 cells | 4 μg/mL | [139] |
| Chalcones | Licochalcone A(C21H22O4) | LXRα, SREBP1↓ | Inhibition of Triglyceride Synthesis | HepG2 cells | 10 μg/mL | [110] |
| PPARγ, SREBP1↓ | 3T3-L1 preadipocytes ICR mouse | 5, 10 μM 5, 10 mg/kg | [120] | |||
| Anthocyanidins | Cyanidin-3-glucoside (C21H21ClO11) | O-glycosylation of FoxO1↑, FFAs, glycerol, ATGL↓ | Inhibition of Triglyceride Synthesis | 3T3-L1 preadipocytes | 50 μM | [121] |
| pAMPK↑, LPL↓ | skeletal muscle cell, adipocyte KK-Ay mouse | 10, 50, 100 µmol/L | [123] | |||
| LXRα, CYP7A1↑ | Promotion of Cholesterol Uptake | human aortic endothelial cell ApoE–/–mice | 0.5, 5, 50 μM food (0.06% w/w) | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, J.; Zhou, Y.; Wang, Q.; Xue, S.; Zhang, Y.; Niu, W. Research Progress and Prospects of Flavonoids in the Treatment of Hyperlipidemia: A Narrative Review. Molecules 2025, 30, 3103. https://doi.org/10.3390/molecules30153103
Chen X, Yang J, Zhou Y, Wang Q, Xue S, Zhang Y, Niu W. Research Progress and Prospects of Flavonoids in the Treatment of Hyperlipidemia: A Narrative Review. Molecules. 2025; 30(15):3103. https://doi.org/10.3390/molecules30153103
Chicago/Turabian StyleChen, Xingtong, Jinbiao Yang, Yunyue Zhou, Qiao Wang, Shuang Xue, Yukun Zhang, and Wenying Niu. 2025. "Research Progress and Prospects of Flavonoids in the Treatment of Hyperlipidemia: A Narrative Review" Molecules 30, no. 15: 3103. https://doi.org/10.3390/molecules30153103
APA StyleChen, X., Yang, J., Zhou, Y., Wang, Q., Xue, S., Zhang, Y., & Niu, W. (2025). Research Progress and Prospects of Flavonoids in the Treatment of Hyperlipidemia: A Narrative Review. Molecules, 30(15), 3103. https://doi.org/10.3390/molecules30153103





