Volatile Compounds Profiling of Fresh R. alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenylethanoids and Benzenoids
2.2. Terpenoids
2.2.1. Monoterpenes
2.2.2. Sesquiterpenoids
2.3. Esters
2.4. Stearopten
2.5. Key Aroma Compounds in R. alba Fresh Blossom
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. SPME
3.2.2. Gas Chromatography-Mass Spectrometry (GC/MS)
3.2.3. Gas Chromatography with Flame-Ionization Detector (GC-FID)
3.2.4. Identification and Quantitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC/MS | Gas chromatography-Mass spectrometry |
| GC-FID | Gas chromatography with flame ionization detection |
| HS | Headspace |
| SPME | Solid-phase microextraction |
| TIC | Total-ion current |
| VOCs | Volatile organic compounds |
| DVB/CAR/PDMS | Divinylbenzene/Carboxen/Polydimethylsiloxane |
| LOQ | Limit of quantitation |
| SD | Standard deviation |
| RI | Retention Index |
| n.d. | not detected |
| W | whole blossom |
| Wmixn (n = 30, 45, 60 °C (SPME temperature)) | Whole blossom, mixed sample |
| Wwhiten (n = 30, 45, 60 °C (SPME temperature)) | Whole blossom, white |
| Wpinkn (n = 30, 45, 60 °C (SPME temperature)) | Whole blossom, pink |
| P | petals |
| Pmixn (n = 30, 45, 60 °C (SPME temperature)) | Petals, mixed sample |
| Ppinkn (n = 30, 45, 60 °C (SPME temperature)) | Petals, pink |
| Pwhiten (n = 30, 45, 60 °C (SPME temperature)) | Petals, white-coloured sample |
References
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian Rose Oil of White Oil-Bearing Rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Verma, A.; Srivastava, R.; Sonar, P.K.; Yadav, R. Traditional, Phytochemical, and Biological Aspects of Rosa alba L.: A Systematic Review. Future J. Pharm. Sci. 2020, 6, 114. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Flower Phenotype Variation, Essential Oil Variation and Genetic Diversity among Rosa alba L. Accessions Used for Rose Oil Production in Bulgaria. Sci. Hortic. 2013, 161, 76–80. [Google Scholar] [CrossRef]
- Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Bakalova, R.; Aoki, I.; Mileva, M. Cytotoxic and Genotoxic Potential of Bulgarian Rosa alba L. Essential Oil – in Vitro Model Study. Biotechnol. Biotechnol. Equip. 2018, 32, 513–519. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Dobreva, A.; Doynovska, R.; Krastev, D.; Mileva, M. Antiviral Activity of Rosa damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir. Biology 2021, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H. Fragrance and Pigments | Odoriferous Substances and Pigments. In Encyclopedia of Rose Science; Elsevier: Amsterdam, The Netherlands, 2003; pp. 231–240. ISBN 978-0-12-227620-0. [Google Scholar]
- Lo, M.-M.; Benfodda, Z.; Molinié, R.; Meffre, P. Volatile Organic Compounds Emitted by Flowers: Ecological Roles, Production by Plants, Extraction, and Identification. Plants 2024, 13, 417. [Google Scholar] [CrossRef]
- Louw, S. Recent Trends in the Chromatographic Analysis of Volatile Flavor and Fragrance Compounds: Annual Review 2020. Anal. Sci. Adv. 2021, 2, 157–170. [Google Scholar] [CrossRef]
- Psillakis, E. Vacuum-Assisted Headspace Solid-Phase Microextraction: A Tutorial Review. Anal. Chim. Acta 2017, 986, 12–24. [Google Scholar] [CrossRef]
- Risticevic, S.; Niri, V.H.; Vuckovic, D.; Pawliszyn, J. Recent Developments in Solid-Phase Microextraction. Anal. Bioanal. Chem. 2009, 393, 781–795. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Combined Extraction and Microextraction Techniques: Recent Trends and Future Perspectives. TrAC Trends Anal. Chem. 2018, 103, 74–86. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction Techniques in the Analysis of Food Flavor Compounds: A Review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Karami, A.; Khosh-Khui, M.; Salehi, H.; Saharkhiz, M.J.; Rowshan, V. Headspace Analysis of Floral Scent from Two Distinct Genotypes of Iranian Damask Rose (Rosa damascena Mill.). J. Essent. Oil Bear. Plants 2013, 16, 489–498. [Google Scholar] [CrossRef]
- Yang, L.; Ren, J.; Wang, Y. Chemical Investigation of Volatiles Emitted from Flowers of Three Varieties of Damask Rose Cultivated in Beijing. Hortic. Environ. Biotechnol. 2014, 55, 524–530. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Y.; Li, H.; Chen, H. Volatile Organic Compounds and Their Roles in Bacteriostasis in Five Conifer Species. J. Integr. Plant Biol. 2005, 47, 499–507. [Google Scholar] [CrossRef]
- Koksall, N.; Aslancan, H.; Sadighazadi, S.; Kafkas, E. Chemical Investigation on Rose damascena Mill. Volatiles: Effects of Storage and Drying Conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Baydar, H.; Erbaş, S.; Kazaz, S. Variations in Floral Characteristics and Scent Composition and the Breeding Potential in Seed-Derived Oil-Bearing Roses (Rosa damascena Mill.). Turk. J. Agric. For. 2016, 40, 560–569. [Google Scholar] [CrossRef]
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites Profiling of Egyptian Rosa damascena Mill. Flowers as Analyzed via Ultra-High-Performance Liquid Chromatography-Mass Spectrometry and Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry in Relation to Its Anti-Collagenase Skin Effect. Ind. Crops Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Joichi, A.; Yomogida, K.; Awano, K.; Ueda, Y. Volatile Components of Tea-Scented Modern Roses and Ancient Chinese Roses. Flavour Fragr. J. 2005, 20, 152–157. [Google Scholar] [CrossRef]
- Ibrahim, M.; Agarwal, M.; Hardy, G.; Ren, Y. Optimized Method to Analyze Rose Plant Volatile Organic Compounds by HS-SPME-GC-FID/MSD. J. Biosci. Med. 2017, 5, 13–31. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.; Cheng, B.; Han, Y.; Luo, L.; Pan, H.; Zhang, Q. Studies on the Volatile Compounds in Flower Extracts of Rosa odorata and R. chinensis. Ind. Crops Prod. 2020, 146, 112143. [Google Scholar] [CrossRef]
- Kiralan, M. Use of Headspace Solid-Phase Microextraction in Rose (Rosa damescena Mill) Products for Volatile Compounds. J. Essent. Oil Bear. Plants 2015, 18, 1266–1270. [Google Scholar] [CrossRef]
- Koksal, N.; Saribas, R.; Kafkas, E.; Aslancan, H.; Sadighazadi, S. Determination of Volatile Compounds of the First Rose Oil and the First Rose Water by HS-SPME/GC/MS Techniques. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 145. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Zarifikhosroshahi, M.; Kafkas, N.E. Determination of Fatty Acids and Volatile Compounds in Fruits of Rosehip (Rosa L.) Species by HS-SPME/GC-MS and Im-SPME/GC-MS Techniques. Turk. J. Agric. For. 2016, 40, 269–279. [Google Scholar] [CrossRef]
- Issa, M.Y.; Mohsen, E.; Younis, I.Y.; Nofal, E.S.; Farag, M.A. Volatiles Distribution in Jasmine Flowers Taxa Grown in Egypt and Its Commercial Products as Analyzed via Solid-Phase Microextraction (SPME) Coupled to Chemometrics. Ind. Crops Prod. 2020, 144, 112002. [Google Scholar] [CrossRef]
- Bicchi, C.; Drigo, S.; Rubiolo, P. Influence of Fibre Coating in Headspace Solid-Phase Microextraction–Gas Chromatographic Analysis of Aromatic and Medicinal Plants. J. Chromatogr. A 2000, 892, 469–485. [Google Scholar] [CrossRef]
- Hu, X.; Lu, L.; Guo, Z.; Zhu, Z. Volatile Compounds, Affecting Factors and Evaluation Methods for Rice Aroma: A Review. Trends Food Sci. Technol. 2020, 97, 136–146. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent Developments and Applications of Solid Phase Microextraction (SPME) in Food and Environmental Analysis—A Review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Kobakhidze, T.; Morton, D. Solid-Phase Microextraction Techniques and Application in Food and Horticultural Crops. Molecules 2023, 28, 6880. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z. Genetic and Biochemical Aspects of Floral Scents in Roses. Int. J. Mol. Sci. 2022, 23, 8014. [Google Scholar] [CrossRef]
- Mileva, M.; Kusovski, V.; Krastev, D.; Dobreva, A.; Galabov, A. Chemical Composition, in Vitro Antiradical and Antimicrobial Activities of Bulgarian Rosa alba L. Essential Oil against Some Oral Pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 11–20. [Google Scholar]
- ISO 9842:2024; Essential Oil of Rose (Rosa x damascena Miller). International Organization for Standardization: Geneva, Switzerland, 2024.
- Hadian, Z.; Maleki, M.; Feizollahi, E.; Alibeyk, S.; Saryazdi, M. Health Aspects of Geraniol as a Main Bioactive Compound of Rosa damascena Mill: A Systematic Review. Electron. Physician 2020, 12, 7724–7735. [Google Scholar] [CrossRef]
- Ohloff, G.; Demole, E. Importance of the Odoriferous Principle of Bulgarian Rose Oil in Flavour and Fragrance Chemistry. J. Chromatogr. A 1987, 406, 181–183. [Google Scholar] [CrossRef]


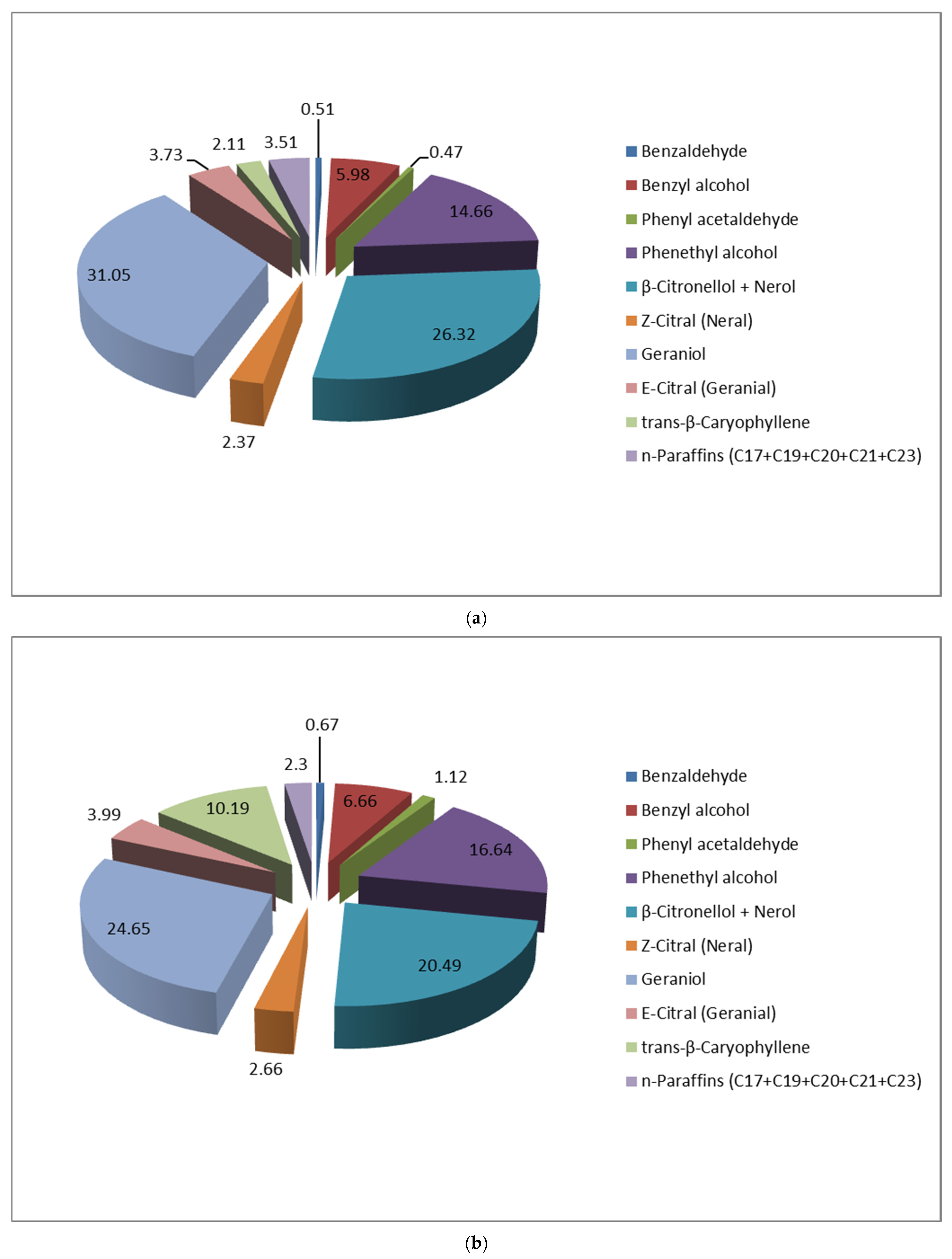

| No | Compound | Aroma Description | R. alba HS-SPME VOCs, in Relative % (Averaged from 3 Replicates), as Measured by GC/FID | Min | Max | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wmix30 | Wmix45 | Wmix60 | Pmix 30 | Pmix 45 | Pmix 60 | Wpink30 | Wpink45 | Wwhite30 | Wwhite45 | Ppink30 | Ppink45 | Pwhite30 | Pwhite45 | |||||
| 1 | α-Pinene | Herbal type | 1.18 | 0.93 | 0.42 | n.d | n.d | n.d | 1.49 | 1.01 | 1.12 | 1.64 | n.d | n.d | n.d | n.d | 0.42 | 1.64 |
| 2 | Benzaldehyde | Sharp sweet bitter-almond cherry | 1.69 | 0.81 | 0.48 | 4.04 | 0.69 | 0.42 | 1.64 | 0.51 | 1.02 | 0.67 | 0.49 | 0.61 | 0.47 | 0.75 | 0.42 | 4.04 |
| 3 | β-Pinene/β-Myrcene | Woody-green pine like/Sweet-balsamic-resinous | 1.51 | 0.98 | 0.72 | 1.49 | 0.97 | 0.92 | 1.23 | 2.01 | 0.95 | 1.27 | 1.65 | 1.18 | 1.16 | 1.27 | 0.72 | 2.01 |
| 4 | Benzyl alcohol | Slightly sweet, floral | 11.08 | 8.18 | 3.71 | 13.78 | 7.95 | 2.62 | 12.73 | 5.98 | 10.15 | 6.66 | 9.84 | 7.54 | 7.37 | 8.49 | 2.62 | 13.8 |
| 5 | Phenyl acetaldehyde | Green, floral, reminiscent of Lilac | 1.51 | 0.88 | 0.74 | 1.30 | 0.85 | 0.79 | 0.85 | 0.47 | 0.95 | 1.12 | 0.73 | 0.95 | 0.77 | 1.19 | 0.47 | 1.51 |
| 6 | Linalool | Floral, spicy wood | 0.81 | 0.54 | 0.48 | 0.53 | 0.56 | 0.49 | 0.64 | 0.65 | 0.5 | 0.77 | 0.52 | 0.61 | 0.39 | 0.65 | 0.39 | 0.81 |
| 7 | cis-Rose oxide | Typical rose, floral-green | 0.05 | 0.03 | 0.11 | 0.02 | 0.03 | 0.09 | 0.02 | 0.04 | 0.02 | 0.05 | 0.12 | 0.03 | 0.06 | 0.02 | 0.02 | 0.12 |
| 8 | 2-Phenyl ethyl alcohol | Rose note, very lasting/mild and warm rose honey | 32.74 | 20.96 | 14.97 | 33.93 | 21.64 | 8.41 | 20.45 | 14.66 | 31.67 | 16.64 | 26.35 | 19.07 | 21.91 | 23.29 | 8.41 | 33.9 |
| 9 | trans-Rose oxide | Floral-green, herbal (minty) and fruity | 0.37 | 0.05 | 0.46 | 0.45 | 0.26 | 0.43 | 0.54 | 0.08 | 0.2 | 0.1 | 0.14 | 0.59 | 0.09 | 0.15 | 0.05 | 0.59 |
| 10 | β-Citronellol + Nerol | Sweet, rose like/Rose like, fresh green note | 17.72 | 21.88 | 21.6 | 18.19 | 21.83 | 26.54 | 20.05 | 26.32 | 21.78 | 20.49 | 24.74 | 25.9 | 24.74 | 21.1 | 17.7 | 26.5 |
| 11 | Z-Citral (Neral) | Citrus, milder, and sweeter | 2.53 | 3.33 | 2.84 | 2.41 | 2.52 | 3.23 | 3.15 | 2.37 | 3.5 | 2.66 | 1.23 | 2.19 | 4.88 | 1.77 | 1.23 | 4.88 |
| 12 | Geraniol | Sweet, floral, rose-like | 15.04 | 27.99 | 22.56 | 12.75 | 31.59 | 29.12 | 19.84 | 31.05 | 18.32 | 24.65 | 20.1 | 31.9 | 24.62 | 32.45 | 12.8 | 32.5 |
| 13 | E-Citral (Geranial) | Strong, lemon like | 3.48 | 4.99 | 4.4 | 3.18 | 3.75 | 4.99 | 5.31 | 3.73 | 5.12 | 3.99 | 1.49 | 3.53 | 6.93 | 2.7 | 1.49 | 6.93 |
| 14 | Geranyl acetate | Floral, fruity, rose like | 0.09 | 0.37 | 0.45 | 0.21 | 0.36 | 0.97 | 0.66 | 1.54 | 0.46 | 1.09 | 3.51 | 0.77 | 2.48 | 0.78 | 0.09 | 3.51 |
| 15 | β-Damascone | Intense rose-like | 0.21 | 0.11 | 0.09 | 0.27 | 0.10 | 0.1 | 0.09 | 0.09 | 0.03 | 0.08 | 0.05 | 0.09 | 0.19 | 0.10 | 0.03 | 0.27 |
| 16 | trans-β-Caryophyllene | Softly spicy, woody | 1.06 | 1.99 | 4.44 | 0.67 | 0.27 | 0.46 | 1.01 | 2.11 | 1.19 | 10.19 | 0.13 | 0.24 | 0.19 | 0.11 | 0.11 | 10.2 |
| 17 | Pentadecane (C15) | Odorless | 0.09 | 0.11 | 0.18 | 0.09 | 0.14 | 0.39 | 0.1 | 0.14 | 0.06 | 0.06 | 0.12 | 0.09 | 0.1 | 0.08 | 0.06 | 0.39 |
| 18 | Heptadecene (C17:1) | Odorless | 0.17 | 0.25 | 0.75 | 0.14 | 0.39 | 1.35 | 0.22 | 0.36 | 0.17 | 0.11 | 0.17 | 0.33 | 0.18 | 0.19 | 0.11 | 1.35 |
| 19 | Heptadecane (C17) | Odorless | 0.28 | 0.29 | 1.4 | 0.19 | 0.41 | 1.83 | 0.18 | 0.32 | 0.21 | 0.22 | 0.21 | 0.28 | 0.18 | 0.23 | 0.18 | 1.83 |
| 20 | Nonadecene (C19:1) | Odorless | 0.37 | 0.71 | 3.2 | 0.23 | 1.09 | 3.21 | 0.45 | 0.91 | 0.4 | 0.43 | 0.63 | 0.84 | 0.45 | 0.68 | 0.23 | 3.21 |
| 21 | Nonadecane (C19) | Odorless | 1.11 | 2.16 | 10.95 | 0.91 | 2.19 | 8.72 | 0.56 | 2.15 | 0.82 | 1.63 | 2.06 | 1.91 | 0.91 | 1.61 | 0.56 | 11.0 |
| TOTAL | ||||||||||||||||||
| Monoterpene Hydrocarbons | 4.19 | 2.79 | 1.88 | 2.78 | 1.82 | 1.71 | 2.72 | 3.49 | 2.07 | 4.03 | 1.65 | 2.13 | 1.93 | 2.46 | 1.65 | 4.03 | ||
| Monoterpene Oxidized | 40.8 | 59.3 | 52.5 | 38.0 | 60.9 | 65.0 | 49.7 | 64.7 | 50.1 | 53.0 | 48.6 | 64.7 | 62.1 | 59.2 | 48.6 | 65.0 | ||
| Sesquiterpene Hydrocarbons | 1.5 | 2.55 | 5.79 | 1.09 | 0.68 | 1.20 | 1.25 | 2.49 | 1.50 | 10.96 | 0.25 | 0.42 | 0.50 | 0.42 | 0.25 | 11.0 | ||
| Esters | 0.36 | 0.84 | 1.01 | 0.15 | 0.79 | 1.71 | 0.99 | 2.26 | 0.85 | 1.73 | 4.79 | 1.20 | 3.09 | 1.14 | 0.85 | 4.79 | ||
| n-Paraffins (C17 + C19 + C20 + C21 + C23) | 1.76 | 3.25 | 17.32 | 1.33 | 3.88 | 14.88 | 1.19 | 3.51 | 1.43 | 2.30 | 3.17 | 3.18 | 1.66 | 2.63 | 1.19 | 14.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova-Nedeltcheva, D.; Dobreva, A.; Gechovska, K.; Antonov, L. Volatile Compounds Profiling of Fresh R. alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography. Molecules 2025, 30, 3102. https://doi.org/10.3390/molecules30153102
Antonova-Nedeltcheva D, Dobreva A, Gechovska K, Antonov L. Volatile Compounds Profiling of Fresh R. alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography. Molecules. 2025; 30(15):3102. https://doi.org/10.3390/molecules30153102
Chicago/Turabian StyleAntonova-Nedeltcheva, Daniela, Ana Dobreva, Kamelia Gechovska, and Liudmil Antonov. 2025. "Volatile Compounds Profiling of Fresh R. alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography" Molecules 30, no. 15: 3102. https://doi.org/10.3390/molecules30153102
APA StyleAntonova-Nedeltcheva, D., Dobreva, A., Gechovska, K., & Antonov, L. (2025). Volatile Compounds Profiling of Fresh R. alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography. Molecules, 30(15), 3102. https://doi.org/10.3390/molecules30153102








