Molecular Modification Strategies for Enhancing CO2 Electroreduction
Abstract
1. Introduction
2. Major Strategies of Molecular Modification
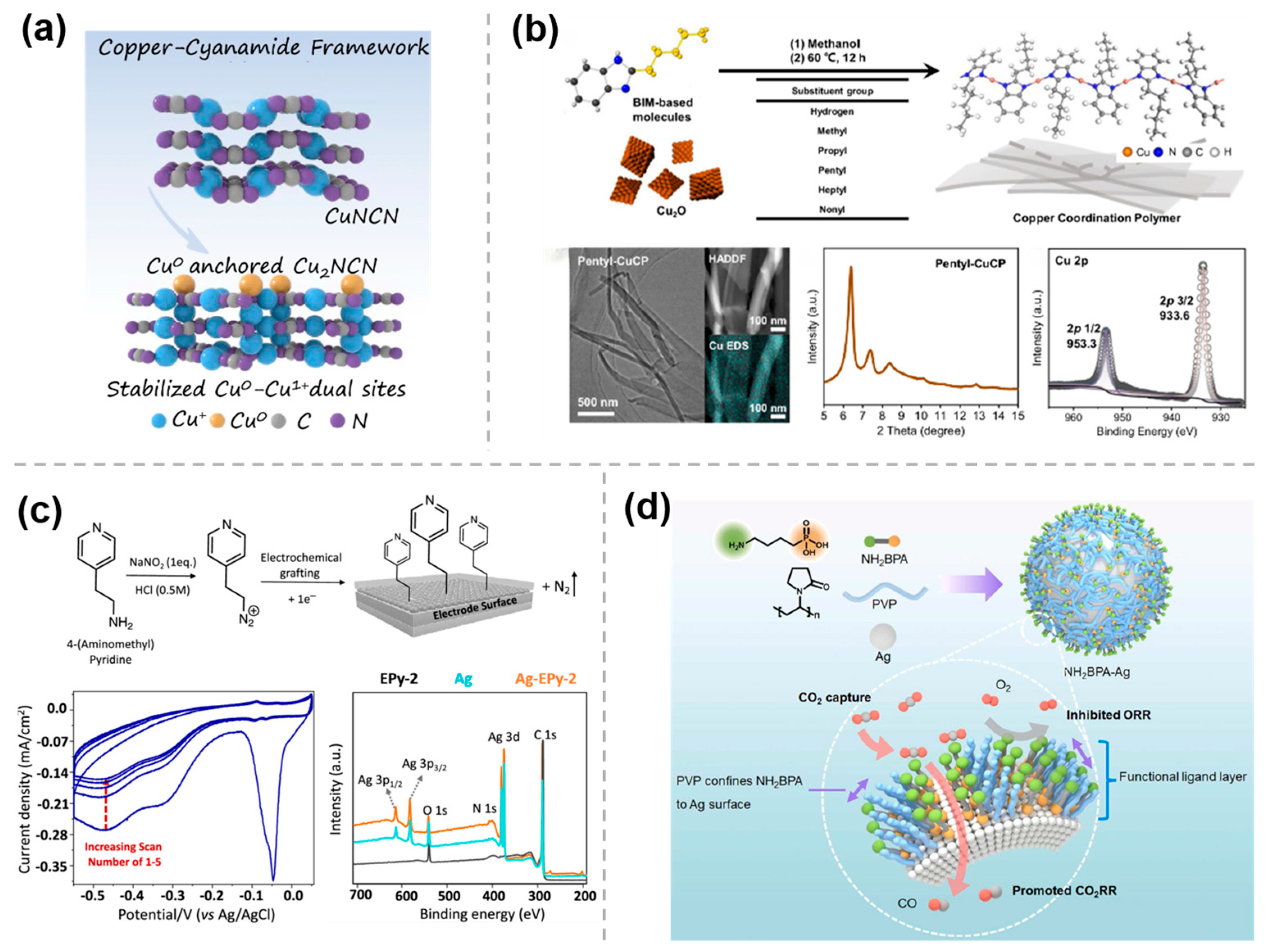
2.1. Metal–Organic Molecular Bonding Modification
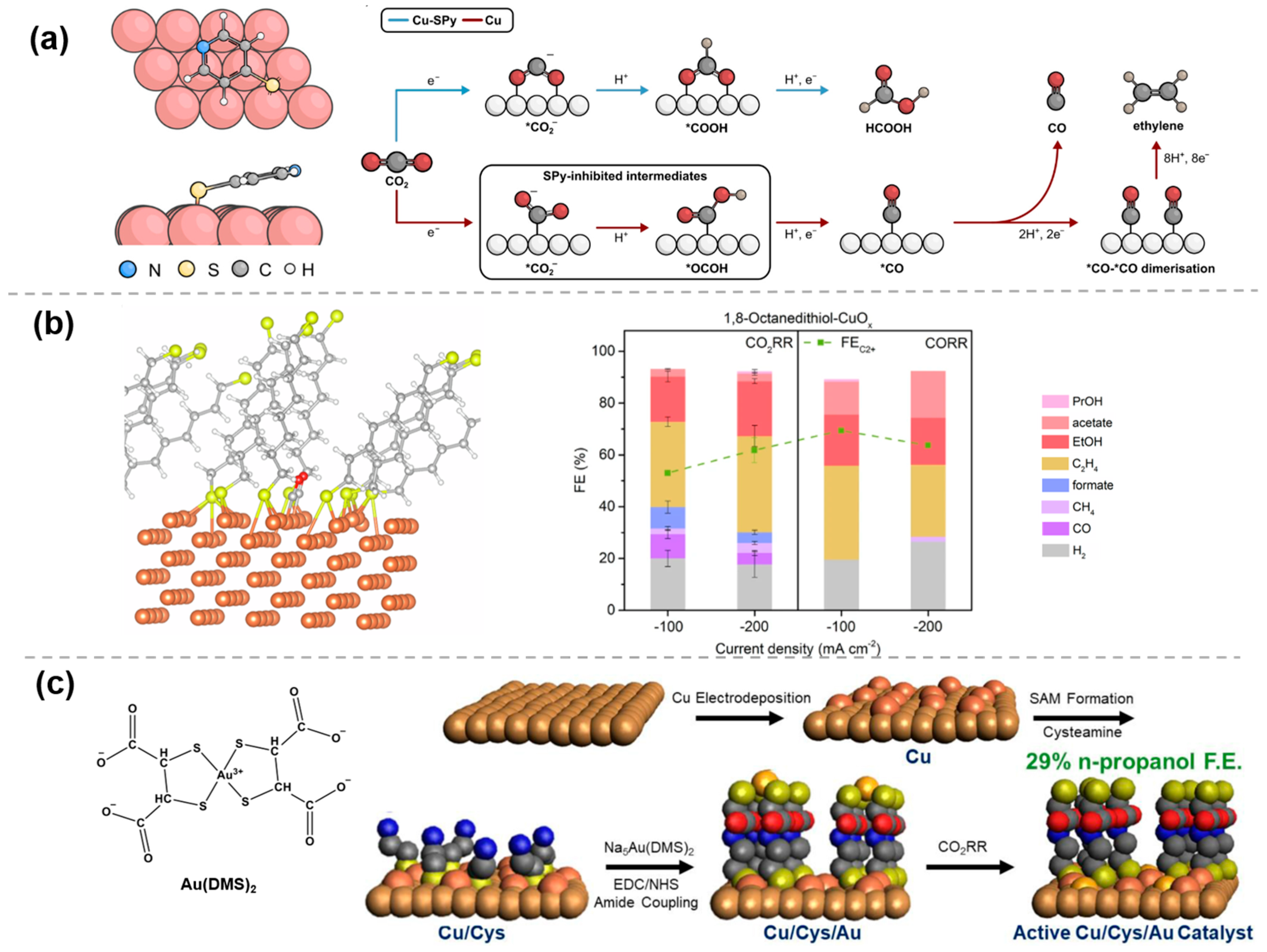
2.2. Weak Interaction Modification
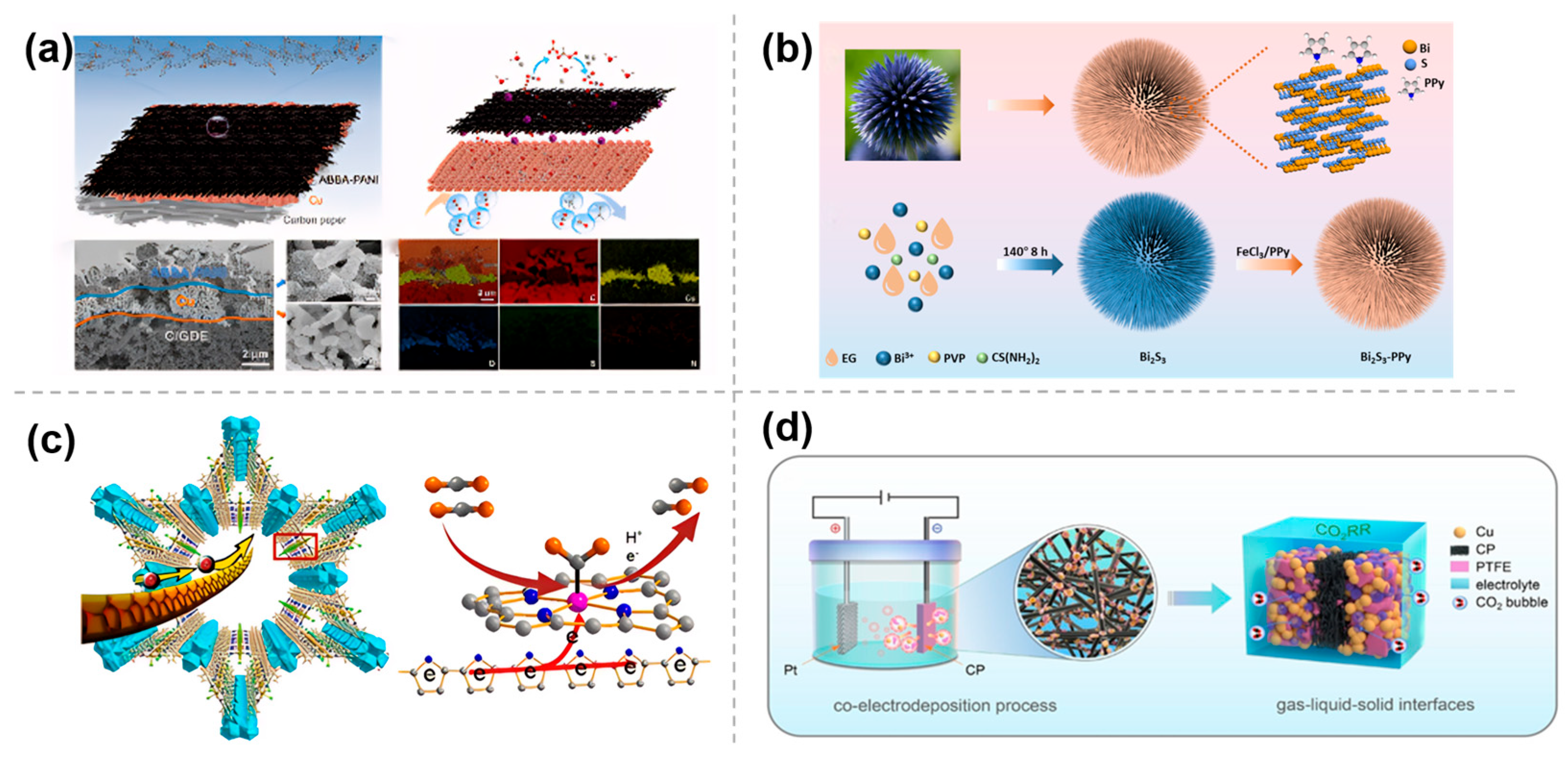
2.3. Polymer-Based Material Modification
3. Effects of Molecularly Modified Materials in CO2RR
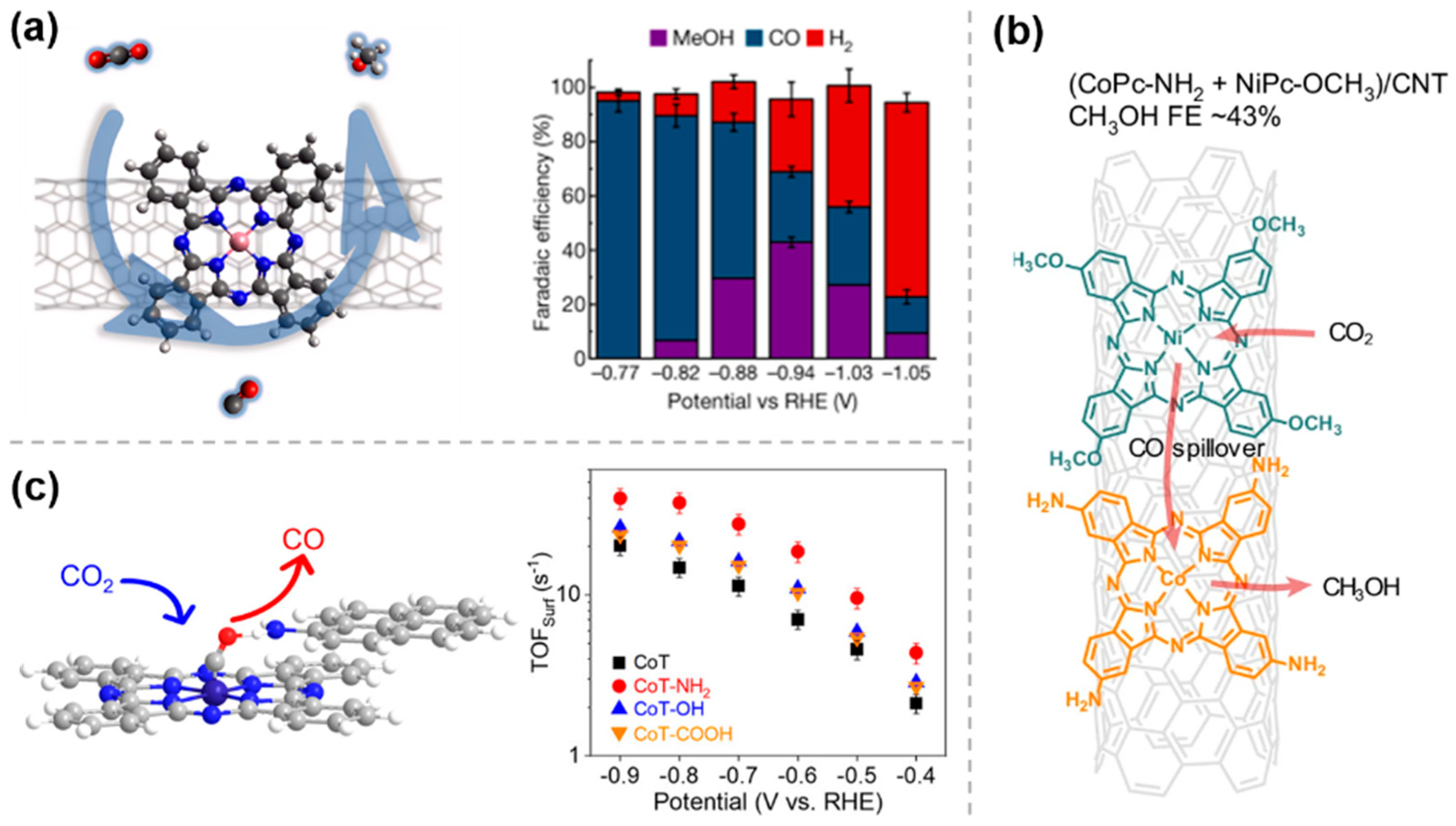
3.1. Electronic Effects
3.2. Steric Hindrance Effects
3.3. Promoting Reactant Adsorption and Stabilizing Intermediates
3.4. Hydrophobic Environment
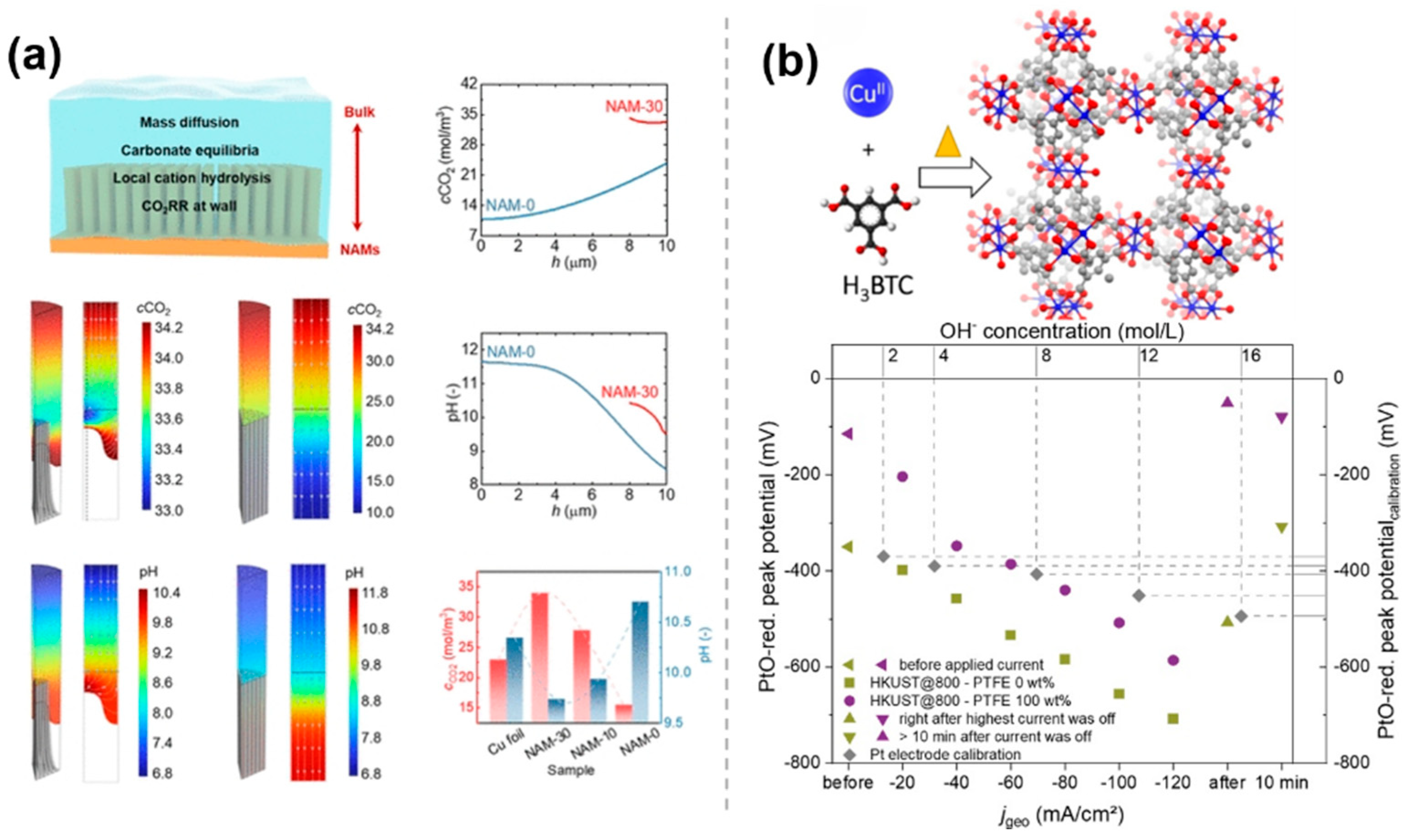
3.5. Requlation of Interfacial PH
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Han, J.; Bai, X.; Xu, X.; Husile, A.; Zhang, S.; Qi, L.; Guan, J. Advances and challenges in the electrochemical reduction of carbon dioxide. Chem. Sci. 2024, 15, 7870. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.A.; Javed, M.S.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.J. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coordin. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.H.; Zhang, X.; Wang, H.; Zhao, Q. Metal-organic frameworks and their derivatives for electrochemical CO2 reduction reaction: Insights from molecular engineering. J. Mater. Chem. A 2024, 12, 20578. [Google Scholar] [CrossRef]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J. Highly efficient CO2 electroreduction to methanol through atomically dispersed Sn coupled with defective CuO catalysts. Angew. Chem. Int. Ed. 2021, 60, 21979. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, J.; Yang, Y.; Hung, S.-F.; Li, J.; Zhang, S.; Zhao, Y.; Zhang, A.; Wang, C.; Appadoo, D. Stabilizing copper sites in coordination polymers toward efficient electrochemical CC coupling. Nat. Commun. 2023, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Fang, Q.; Du, R.; Jin, Y.; Peng, C.; Cheng, D.; Li, T.; Zhao, T.; Zhang, S.; Zheng, Y. Selective electrochemical CO2 reduction to ethylene or ethanol via tuning* OH adsorption. Angew. Chem. Int. Ed. 2025, e202501773. [Google Scholar] [CrossRef]
- Bondue, C.J.; Graf, M.; Goyal, A.; Koper, M.T. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 2020, 143, 279. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Barile, C.J. Electrochemical CO2 reduction to methane with remarkably high Faradaic efficiency in the presence of a proton permeable membrane. Energ. Environ. Sci. 2020, 13, 3567. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 electrochemical reduction to methane and methanol on copper-based alloys: Theoretical insight. J. Phy. Chem. C 2015, 119, 8238. [Google Scholar] [CrossRef]
- Zhou, X.; Shan, J.; Chen, L.; Xia, B.Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Stabilizing Cu2+ ions by solid solutions to promote CO2 electroreduction to methane. J. Am. Chem. Soc. 2022, 144, 2079. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, J.; Liu, Y.; Su, C.; Yang, H.; Huang, Y.; Liu, B. Molecular tuning for electrochemical CO2 reduction. Joule 2023, 7, 1700. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Li, S.-B.; Liang, G.-H.; Xie, Y.-M.; Zhang, Y.; Zhang, H.; Tian, J.-H.; Zheng, S.; Zheng, Q.-N.; Chen, Z. Promoting water activation via molecular engineering enables efficient asymmetric C–C coupling during CO2 electroreduction. J. Am. Chem. Soc. 2024, 146, 32870. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Yang, Z.; Morgan, H.; Shi, J.; Shi, F.; Liu, M.; Wong, H.-W.; Gu, Z.; Che, F. Enhanced CO2 reactive capture and conversion using aminothiolate ligand–metal interface. J. Am. Chem. Soc. 2023, 145, 26038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.D.; Huang, J.R.; Shi, W.; Liao, P.Q.; Chen, X.M. Self-accelerating effect in a covalent-organic framework with Imidazole groups boosts electroreduction of CO2 to CO. Angew. Chem. 2023, 135, e202308195. [Google Scholar] [CrossRef]
- Yu, H.; Han, X.; Hua, Z.; Yang, W.; Wu, X.; Wu, Y.; Chen, S.; Hong, W.; Deng, S.; Zhang, J. Modulating electronic properties of carbon for selective electrochemical reduction of CO2 to methanol on Cu3P@C. ACS Catal. 2024, 14, 12783. [Google Scholar] [CrossRef]
- Yang, H.; Cai, H.; Li, D.; Kong, Y.; Feng, S.; Jiang, X.; Hu, Q.; He, C. Molecular modification enables CO2 electroreduction to methane on platinum surface in acidic media. Nat. Sci. Rev. 2024, 11, nwae361. [Google Scholar] [CrossRef] [PubMed]
- Creissen, C.E.; Rivera de la Cruz, J.G.; Karapinar, D.; Taverna, D.; Schreiber, M.W.; Fontecave, M. Molecular Inhibition for Selective CO2 Conversion. Angew. Chem. Int. Ed. 2022, 61, e202206279. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Flake, J.C. Electrochemical reduction of CO2 at functionalized Au electrodes. J. Am. Chem. Soc. 2017, 139, 3399. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Xi, S.; Zhang, T.; Liu, Z.; Chen, J.; Shen, L.; Kawi, S.; Wang, L. Functionalized Ag with thiol ligand to promote effective CO2 electroreduction. ACS Nano 2022, 16, 13982. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.L.; Lima, F.H.B. Dibenzyldithiocarbamate-functionalized small gold nanoparticles as selective catalysts for the electrochemical r eduction of CO2 to CO. ACS Catal. 2021, 11, 12208. [Google Scholar] [CrossRef]
- Li, J.-K.; Dong, J.-P.; Liu, S.-S.; Hua, Y.; Zhao, X.-L.; Li, Z.; Zhao, S.-N.; Zang, S.-Q.; Wang, R. Promoting CO2 electroreduction to hydrocarbon products via sulfur-enhanced proton feeding in atomically precise thiolate-protected Cu clusters. Angew. Chem. Int. Ed. 2024, 63, e202412144. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fang, N.; Liu, X.; Ling, Y.; Su, Y.; Tan, T.; Chen, F.; Lin, H.; Zhao, B.; Wang, J.; et al. Observation of metal-organic interphase in Cu-based electrochemical CO2-to-ethanol conversion. Nat. Commun. 2025, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, F.; Ren, X.; Liu, Y.; Li, Y.; Shen, Z.; Wang, T.; Wang, W.; Wang, Y.-G.; Cui, Y.; et al. Molecular tuning boosts asymmetric C-C coupling for CO conversion to acetate. Nat. Commun. 2024, 15, 3641. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, E.; Jin, Q.; Zeraati, A.S.; Dorakhan, R.; Goncalves, T.J.; Abed, J.; Lee, B.-H.; Rasouli, A.S.; Wicks, J.; Zhang, J.; et al. Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity. Nat. Commun. 2024, 15, 2995. [Google Scholar] [CrossRef] [PubMed]
- Bhoumik, N.C.; Padovan, Q.A.; Akter, T.; Stem, D.K.; Barile, C.J. Gold self-assembly on copper electrodes promotes n-propanol formation in electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2025, 64, e202423882. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Qin, Y.; Huang, H.; Lv, Z.; Cai, M.; Su, Y.; Huang, F.; Yan, Y. Stabilized Cu0-Cu1+ dual sites in a cyanamide framework for selective CO2 electroreduction to ethylene. Nat. Commun. 2024, 15, 7820. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, T.; Zhao, W.; Wang, J.; Zhang, Y.; Zhang, S.; Yang, Y.; Yang, C.; Teng, W.; Chen, Z.; et al. Substituent tuning of Cu coordination polymers enables carbon-efficient CO2 electroreduction to multi-carbon products. Nat. Commun. 2024, 15, 9706. [Google Scholar] [CrossRef] [PubMed]
- Abdinejad, M.; Irtem, E.; Farzi, A.; Sassenburg, M.; Subramanian, S.; van Montfort, H.-P.I.; Ripepi, D.; Li, M.; Middelkoop, J.; Seifitokaldani, A.; et al. CO2 electrolysis via surface-engineering electrografted pyridines on silver catalysts. ACS Catal. 2022, 12, 7862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, T.; Shi, H.; Pan, H.; Kang, P. Grafting amine-functionalized ligand layer on catalyst for electrochemical CO2 capture and utilization. Appl. Catal. B Environ. 2024, 343, 123456. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.; Chang, A.; Cheon, S.; Gao, Y.; Shang, B.; Li, H.; Rooney, C.L.; Ren, L.; Jiang, Z.; et al. Molecular-scale CO spillover on a dual-site electrocatalyst enhances methanol production from CO2 reduction. Nat. Nanotechnol. 2025, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, X.; Xu, X.; Win, P.E.P.; Ren, S.-B.; Wang, J. Noncovalent construction of hangman cobalt phthalocyanine for enhanced electrochemical carbon dioxide reduction. Chem. Mater. 2025, 37, 360. [Google Scholar] [CrossRef]
- Su, L.; Hua, Q.; Feng, G.; Yang, Y.; Mei, H.; Yu, Y.; Chang, X.; Huang, Z. Multifunctional conductive polymer modification for efficient CO2 electroreduction in acidic electrolyte. Adv. Funct. Mater. 2025, 2425636. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Zhou, X.; Zhang, L.; Fu, Z.; Wu, Y.; Lv, X.; Zheng, G.; Chen, H. Bio-inspired engineering of Bi2S3-PPy composite for the efficient electrocatalytic reduction of carbon dioxide. Energ. Environ. Sci. 2023, 16, 3885. [Google Scholar] [CrossRef]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting polypyrrole in metal-porphyrin MOFs: Enhanced electrocatalytic performance for CO2RR. ACS Appl. Mater. Interf. 2021, 13, 54959. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Jia, S.; Chen, C.; Jiao, J.; Chen, X.; Xue, C.; Xia, W.; Xing, X.; Zhu, Q.; Wu, H.; et al. Polymer modification strategy to modulate reaction microenvironment for enhanced CO2 electroreduction to ethylene. Angew. Chem. Int. Ed. 2024, 63, e202313796. [Google Scholar] [CrossRef] [PubMed]
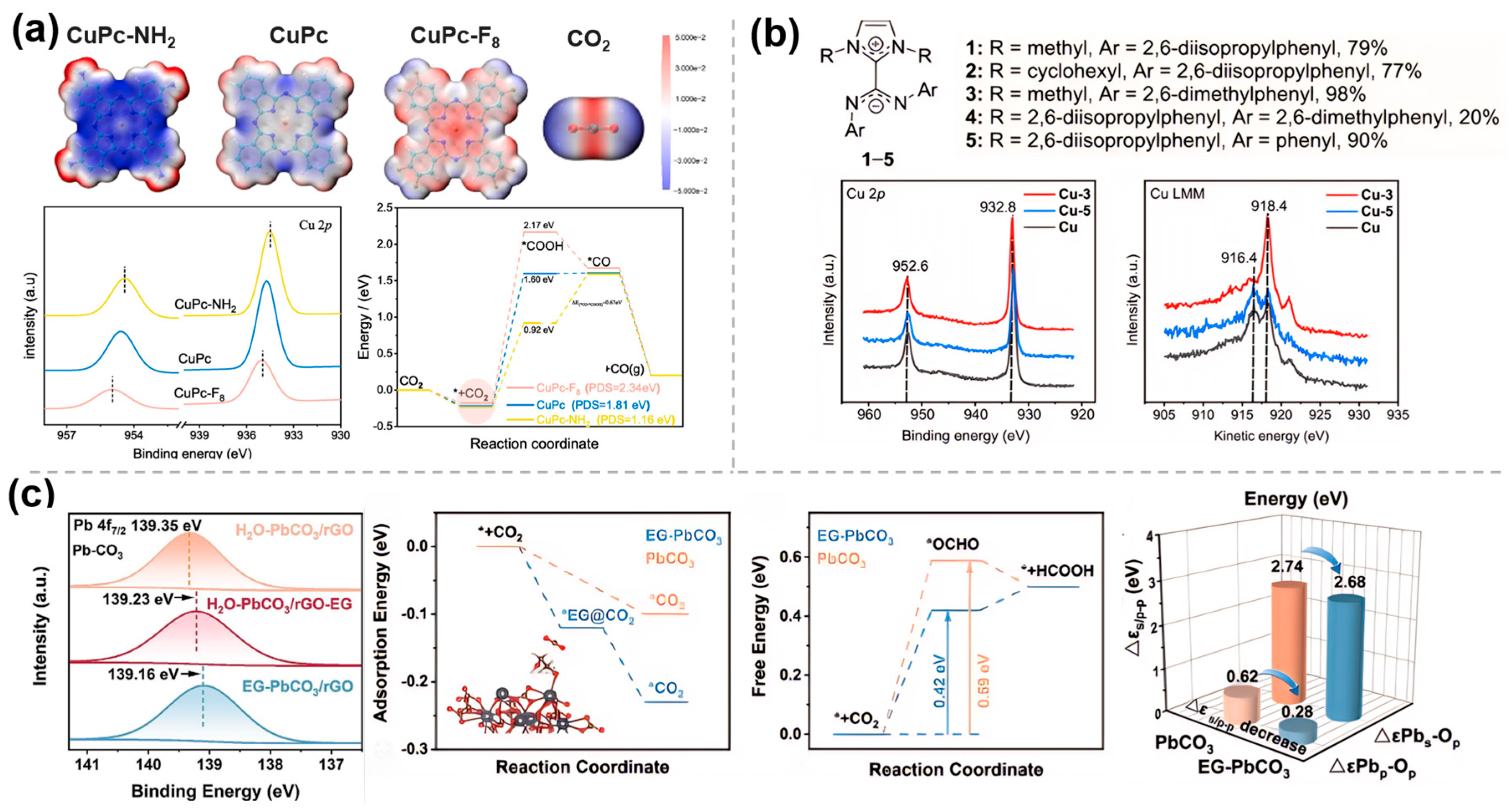
- Fang, J.; Qin, B.; Zhang, Q.; Yang, G.; Du, S.; Liu, Z.; Peng, F. Influence of electron-inducted effect of ligand on electrocatalytic reduction of CO2 by copper phthalocyanine. Chem. Eng. J. 2025, 506, 160154. [Google Scholar] [CrossRef]
- Wang, K.; Huang, K.; Wang, Z.; An, G.; Zhang, M.; Liu, W.; Fu, S.; Guo, H.; Zhang, B.; Lian, C.; et al. Functional group engineering of single-walled carbon nanotubes for anchoring copper nanoparticles toward selective CO2 electroreduction to C2 products. Small 2025, 21, 2502733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, G.; Dong, Y.; Ji, J.; Li, L.; Xue, M.; Zhang, X.; Cheng, H.-M. Controlling the polarity of metal-organic frameworks to promote electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2024, 64, e202416367. [Google Scholar] [CrossRef] [PubMed]
- Kolding, K.N.; Bretlau, M.; Zhao, S.; Ceccato, M.; Torbensen, K.; Daasbjerg, K.; Rosas-Hernández, A. NHC-CDI ligands boost multicarbon production in electrocatalytic CO2 reduction by increasing accumulated charged intermediates and promoting *CO dimerization on Cu. J. Am. Chem. Soc. 2024, 146, 13034. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wang, Z.; Song, Z.; Wang, W.; Wang, X.; Chen, Z.; Ren, Z. Microenvironment modulation induced by ethylene-glycol modification enables high activity in selective CO2 electroreduction over lead-based catalysts. Chem. Eng. J. 2025, 512, 161963. [Google Scholar] [CrossRef]
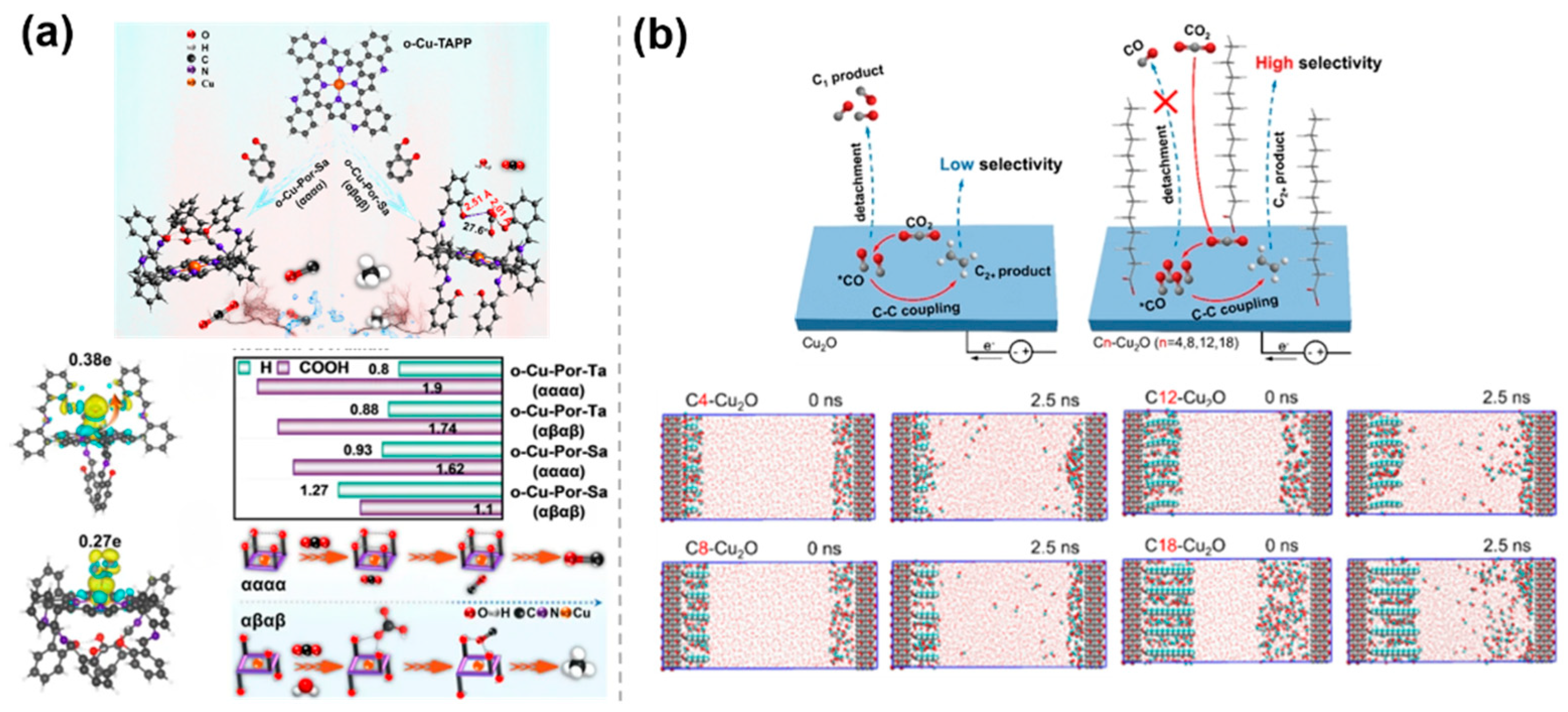
- Wang, H.; Ma, C.; Lu, Q.; Gu, M.; Jiang, L.; Hao, Y.; Hu, F.; Li, L.; Wang, G.; Peng, S.; et al. Precise tuning of functional group spatial distribution on porphyrin rings for enhanced CO2 electroreduction selectivity. Angew. Chem. Int. Ed. 2025, 64, e202501091. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Guo, C.; Jia, S.; Zhou, Y.; Liu, Z.; Jiang, E.; Chen, X.; Zou, Y.; Huo, P.; et al. Confined intermediates boost C2+ selectivity in CO2 electroreduction. ACS Catal. 2024, 14, 13400. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, C.; Liu, W.; Liu, R.; Liu, Y.; Feng, X.; Yang, W.; Wang, B. Enhanced CO2 adsorption and conversion in diethanolamine-Cu interfaces achieving stable neutral ethylene electrosynthesis. Adv. Energy Mater. 2024, 14, 2402551. [Google Scholar] [CrossRef]
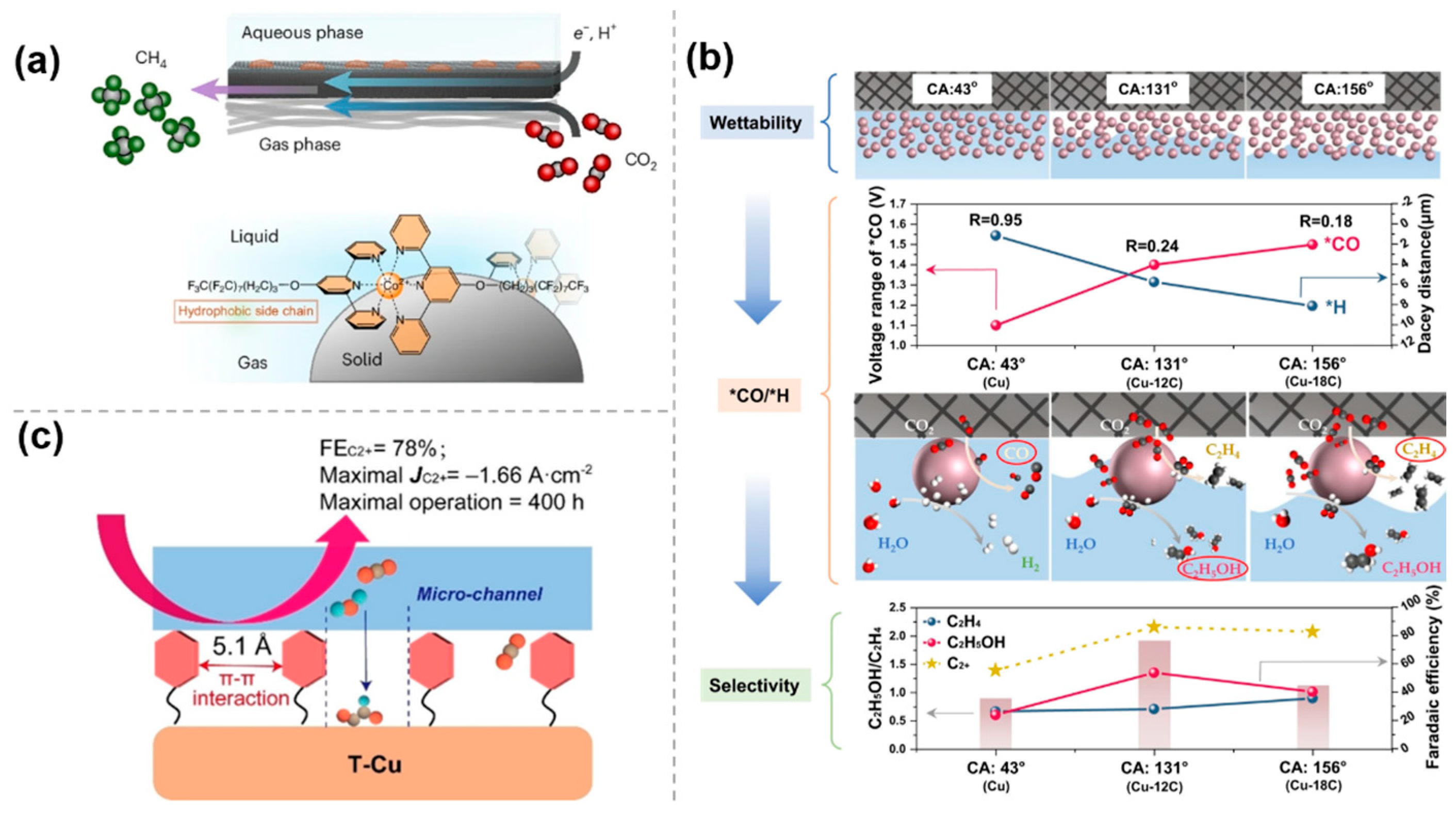
- McKee, M.; Kutter, M.; Wu, Y.; Williams, H.; Vaudreuil, M.-A.; Carta, M.; Yadav, A.K.; Singh, H.; Masson, J.-F.; Lentz, D.; et al. Hydrophobic assembly of molecular catalysts at the gas-liquid-solid interface drives highly selective CO2 electromethanation. Nat. Chem. 2025, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, T.; Zhang, L.; Zhang, G.; Li, L.; Chang, Q.; Pang, Z.; Gao, H.; Huang, K.; Zhang, P.; et al. Tunable CO2 electroreduction to ethanol and ethylene with controllable interfacial wettability. Nat. Commun. 2023, 14, 3575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, X.; Kong, S.; Liu, M.; Liu, K.; Zhang, J.; Wu, B.; Zhang, Q.; Tang, Y.; Qian, L.; et al. Interfacial water tuning by intermolecular spacing for stable CO2 electroreduction to C2+ products. Angew. Chem. Int. Ed. 2023, 62, e202309319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Q.; Salaman, M.I.B.; Wei, C.; Wang, Q.; Ma, X.; Liu, B.; Wong, A.B. Microenvironment tailoring for electrocatalytic CO2 reduction: Effects of interfacial structure on controlling activity and selectivity. J. Am. Chem. Soc. 2025, 147, 12438. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, N.; Junqueira, J.R.C.; Dieckhöfer, S.; Quast, T.; Braun, M.; Song, Y.; Aiyappa, H.B.; Seisel, S.; Weidner, J.; Öhl, D.; et al. A metal-organic framework derived CuxOyCz catalyst for electrochemical CO2 reduction and impact of local pH change. Angew. Chem. Int. Ed. 2021, 60, 23427. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, L.; Li, G.; Mei, J.; Zhang, F.; Lu, J.; Wang, H. Molecular Modification Strategies for Enhancing CO2 Electroreduction. Molecules 2025, 30, 3038. https://doi.org/10.3390/molecules30143038
Wang Y, Chen L, Li G, Mei J, Zhang F, Lu J, Wang H. Molecular Modification Strategies for Enhancing CO2 Electroreduction. Molecules. 2025; 30(14):3038. https://doi.org/10.3390/molecules30143038
Chicago/Turabian StyleWang, Yali, Leibing Chen, Guoying Li, Jing Mei, Feng Zhang, Jiaxing Lu, and Huan Wang. 2025. "Molecular Modification Strategies for Enhancing CO2 Electroreduction" Molecules 30, no. 14: 3038. https://doi.org/10.3390/molecules30143038
APA StyleWang, Y., Chen, L., Li, G., Mei, J., Zhang, F., Lu, J., & Wang, H. (2025). Molecular Modification Strategies for Enhancing CO2 Electroreduction. Molecules, 30(14), 3038. https://doi.org/10.3390/molecules30143038