Unveiling the Solvent Effect: DMSO Interaction with Human Nerve Growth Factor and Its Implications for Drug Discovery
Abstract
1. Introduction
2. Results
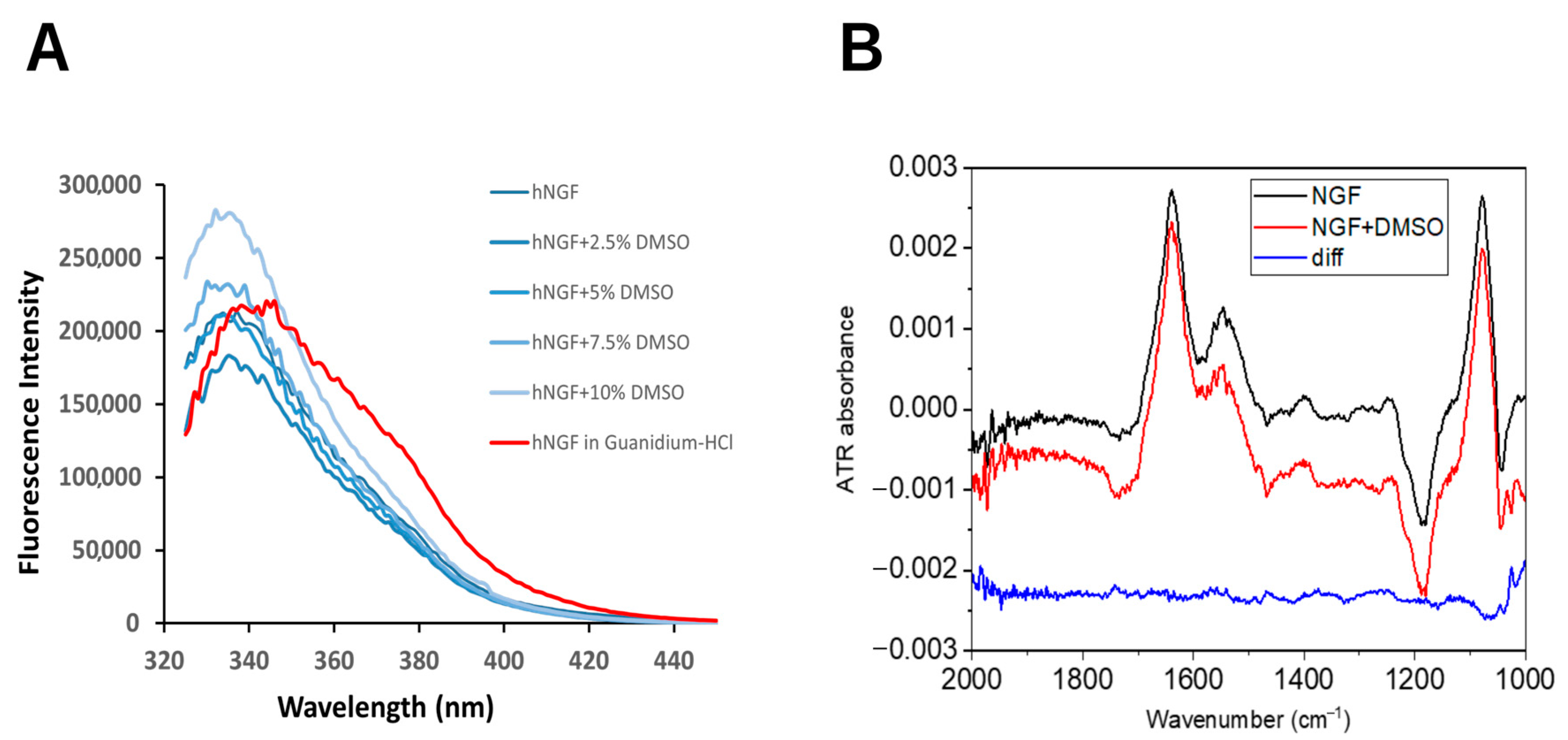
2.1. DMSO Does Not Induce Significant Conformational Changes in hNGF
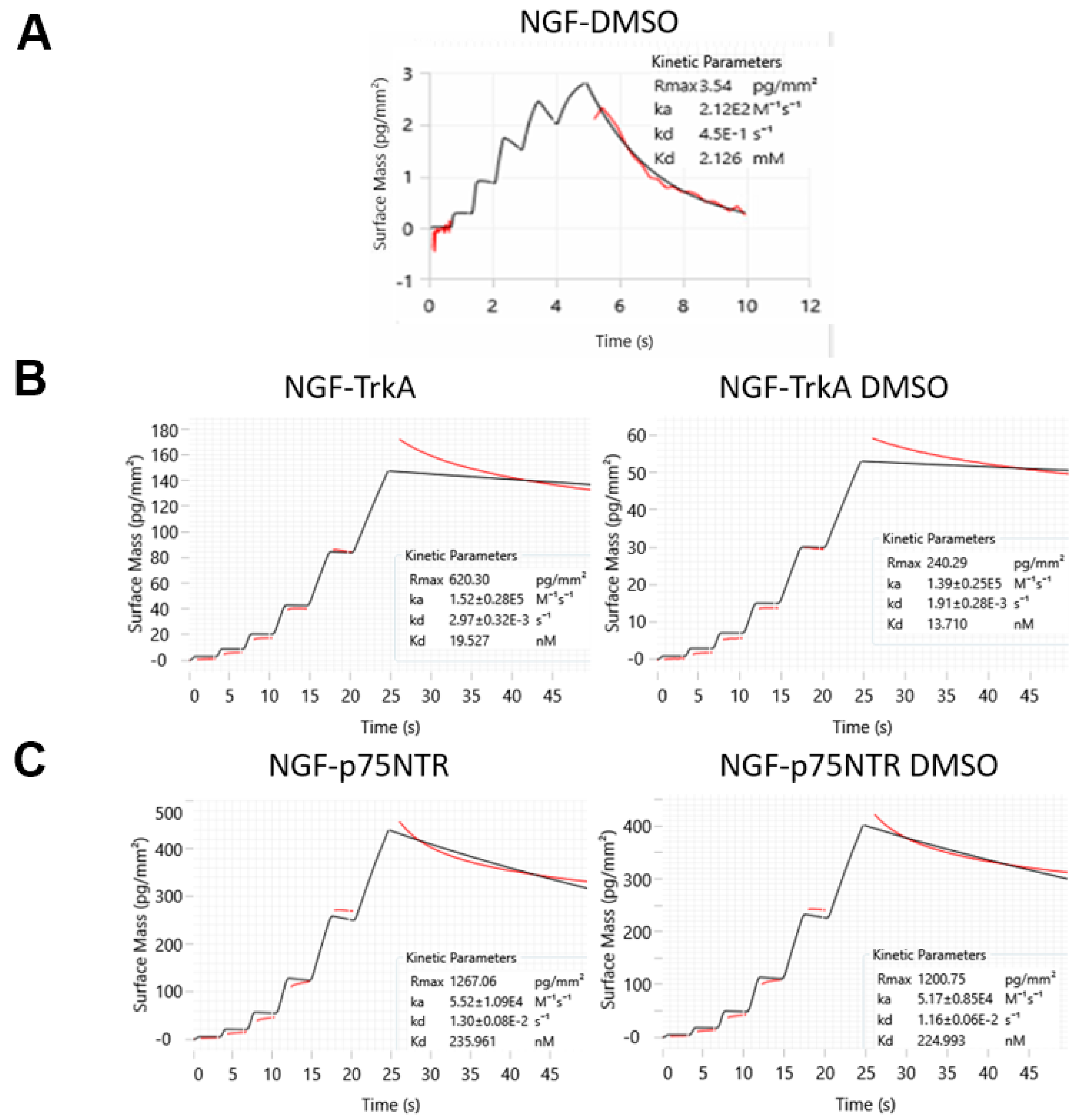
2.2. DMSO Specifically Interacts with hNGF at Low Affinity Without Affecting Its Receptor Binding
2.3. Structural Determinants of hNGF-DMSO Interaction
| DMSO | NGF Antagonists | |||||||
|---|---|---|---|---|---|---|---|---|
| Protenix BP1 (5 Å) | Protenix BP2 (5 Å) | NMR CSP | HADDOCK Site 1 | HADDOCK Site 2 | PD 90780/ALE 0540/Ro 08-2750 [33,34] | Y1036/Y1370 [35,36] | PQC 078/PQC 083 [37] | BVNP-0197 [38] |
| S17 | ||||||||
| V18 | ||||||||
| S19 | ||||||||
| W21 | W21 | |||||||
| V22 | V22 | |||||||
| G23 | ||||||||
| D30 | ||||||||
| I31 | ||||||||
| K32 | K32 | |||||||
| K34 | ||||||||
| V38 | ||||||||
| L39 | L39 | |||||||
| E41 | ||||||||
| V42 | V42 | |||||||
| N43 | N43 | N43 | ||||||
| I44 | ||||||||
| N45 | ||||||||
| N46 | ||||||||
| S47 | ||||||||
| V48 | ||||||||
| F49 | F49 | |||||||
| F53 | ||||||||
| F54 | F54 | F54 | ||||||
| E55 | ||||||||
| T56 | ||||||||
| K57 | ||||||||
| T82 | ||||||||
| F86 | ||||||||
| V87 | ||||||||
| K88 | ||||||||
| A89 | ||||||||
| L90 | ||||||||
| M92 | M92 | |||||||
| G94 | ||||||||
| K95 | K95 | |||||||
| Q96 | Q96 | |||||||
| A97 | A97 | A97 | ||||||
| A98 | A98 | A98 | ||||||
| W99 | W99 | W99 | W99 | |||||
| R100 | R100 | R100 | ||||||
| F101 | F101 | F101 | ||||||
| K115 | ||||||||
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Intrinsic Fluorescence
4.3. Fourier Transform ATR Infrared Spectroscopy (FT-IR)
4.4. Differential Scanning Fluorimetry (DSF)
4.5. Grating Coupled Interferometry (GCI)
4.6. Nuclear Magnetic Resonance (NMR)
4.7. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 1951, 116, 321–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Dechant, G.; Barde, Y.-A. The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002, 5, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.D.; Vasko, M.R. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol. Interv. 2007, 7, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Guo, X. The role of nerve growth factor and its receptors in tumorigenesis and cancer pain. Biosci. Trends 2014, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.S.; Hsu, E.; Hottinger, D.G.; Cohen, S.P. Anti-nerve growth factor in pain management: Current evidence. J. Pain Res. 2016, 9, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, M.; Mantyh, P.; Malfait, A.-M.; Farrar, J.; Yaksh, T.; Tive, L.; Viktrup, L. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: Mechanism of action in the context of efficacy and safety. Pain 2019, 160, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Tarani, L.; Fanfarillo, F.; Tirassa, P.; Ralli, M.; Iannella, G.; Polimeni, A.; Lucarelli, M.; Greco, A.; et al. Nerve Growth Factor and Autoimmune Diseases. Curr. Issues Mol. Biol. 2023, 45, 8950–8973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lai, Y.; Jiao, H.; Huang, J. Nerve growth factor receptor limits inflammation to promote remodeling and repair of osteoarthritic joints. Nat. Commun. 2024, 15, 3225. [Google Scholar] [CrossRef] [PubMed]
- Gondal, F.R.; Bilal, J.; Kent Kwoh, C. Tanezumab for the treatment of osteoarthritis pain. Drugs Today 2022, 58, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, P.; Schnitzer, T.J. Tanezumab in the treatment of chronic musculoskeletal conditions. Expert. Opin. Biol. Ther. 2017, 17, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, P.; West, C.R.; Brown, M.T. Development of pain therapies targeting nerve growth factor signal transduction and the strategies used to resolve safety issues. J. Toxicol. Sci. 2018, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, J.B.; Rizkalla, G.; Tehim, A.; Ross, G.M.; Riopelle, R.J.; Kamboj, R.; Ossipov, M.; Bian, D.; Wegert, S.; Porreca, F.; et al. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J. Pharmacol. Exp. Ther. 1999, 289, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Niederhauser, O.; Mangold, M.; Schubenel, R.; Kusznir, E.A.; Schmidt, D.; Hertel, C. NGF ligand alters NGF signaling via p75(NTR) and trkA. J. Neurosci. Res. 2000, 61, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, M.; Nielsen, J.; Korshunova, I.; Gotfryd, K.; Bock, E.; Pankratova, S.; Michel, T.M. Artemin and an Artemin-Derived Peptide, Artefin, Induce Neuronal Survival, and Differentiation Through Ret and NCAM. Front. Mol. Neurosci. 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tisi, M.A.; Yeo, T.T.; Longo, F.M. Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J. Biol. Chem. 2000, 275, 29868–29874. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, A.; Markova, N.; Griffiths, W.J.; Hallén, D. DMSO-related effects in protein characterization. J. Biomol. Screen. 2006, 11, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cubrilovic, D.; Zenobi, R. Influence of dimehylsulfoxide on protein-ligand binding affinities. Anal. Chem. 2013, 85, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Wallerstein, J.; Akke, M. Minute Additions of DMSO Affect Protein Dynamics Measurements by NMR Relaxation Experiments through Significant Changes in Solvent Viscosity. Chemphyschem 2019, 20, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, S.; Birgersson, S.; Akke, M. Cosolvent Dimethyl Sulfoxide Influences Protein-Ligand Binding Kinetics via Solvent Viscosity Effects: Revealing the Success Rate of Complex Formation Following Diffusive Protein-Ligand Encounter. Biochemistry 2023, 62, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Khodov, I.A.; Belov, K.V.; Krestyaninov, M.A.; Sobornova, V.V.; Dyshin, A.A.; Kiselev, M.G. Does DMSO affect the conformational changes of drug molecules in supercritical CO2 Media? J. Mol. Liq. 2023, 384, 122230. [Google Scholar] [CrossRef]
- Sim, J.; Kim, D.; Kim, B.; Choi, J.; Lee, J. Recent advances in AI-driven protein-ligand interaction predictions. Curr. Opin. Struct. Biol. 2025, 92, 103020. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M.G.; Koumadorakis, D.E.; Lazaros, K.; Ivantsik, O.; Exarchos, T.P.; Vrahatis, A.G.; Kotsiantis, S.; Vlamos, P. AlphaFold3: An Overview of Applications and Performance Insights. Int. J. Mol. Sci. 2025, 26, 3671. [Google Scholar] [CrossRef] [PubMed]
- Team, B.A.A.; Chen, X.; Zhang, Y.; Lu, C.; Ma, W.; Guan, J.; Gong, C.; Yang, J.; Zhang, H.; Zhang, K.; et al. Protenix—Advancing Structure Prediction Through a Comprehensive AlphaFold3 Reproduction. bioRxiv 2025. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015.
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef] [PubMed]
- Honorato, R.V.; Trellet, M.E.; Jiménez-García, B.; Schaarschmidt, J.J.; Giulini, M.; Reys, V.; Koukos, P.I.; Rodrigues, J.P.G.L.M.; Karaca, E.; van Zundert, G.C.P.; et al. The HADDOCK2.4 web server for integrative modeling of biomolecular complexes. Nat. Protoc. 2024, 19, 3219–3241. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A.; Lawrance, G.M.; Shamovsky, I.L.; Riopelle, R.J.; Ross, G.M. Differential activity of the nerve growth factor (NGF) antagonist PD90780 [7-(benzolylamino)-4,9-dihydro-4-methyl-9-oxo-pyrazolo[5,1-b]quinazoline-2-carboxylic acid] suggests altered NGF-p75NTR interactions in the presence of TrkA. J. Pharmacol. Exp. Ther. 2004, 310, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Eibl, J.K.; Strasser, B.C.; Ross, G.M. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem. Int. 2012, 61, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Eibl, J.K.; Chapelsky, S.A.; Ross, G.M. Multipotent neurotrophin antagonist targets brain-derived neurotrophic factor and nerve growth factor. J. Pharmacol. Exp. Ther. 2010, 332, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Eibl, J.K.; Abdallah, Z.; Kennedy, A.E.; Scott, J.A.; Ross, G.M. Affinity Crosslinking of Y1036 to Nerve Growth Factor Identifies Pharmacological Targeting Domain for Small Molecule Neurotrophin Antagonists. Neurosci. Med. 2013, 4, 290–298. [Google Scholar] [CrossRef]
- Eibl, J.K.; Strasser, B.C.; Ross, G.M. Identification of novel pyrazoloquinazolinecarboxilate analogues to inhibit nerve growth factor in vitro. Eur. J. Pharmacol. 2013, 708, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.E.; Laamanen, C.A.; Ross, M.S.; Vohra, R.; Boreham, D.R.; Scott, J.A.; Ross, G.M. Nerve growth factor inhibitor with novel-binding domain demonstrates nanomolar efficacy in both cell-based and cell-free assay systems. Pharmacol. Res. Perspect. 2017, 5, e00339. [Google Scholar] [CrossRef] [PubMed]
- Rattenholl, A.; Lilie, H.; Grossmann, A.; Stern, A.; Schwarz, E.; Rudolph, R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur. J. Biochem. 2001, 268, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; de Chiara, C.; Kelly, G.; Covaceuszach, S.; Malerba, F.; Yan, R.; Lamba, D.; Cattaneo, A.; Pastore, A. Conformational Rigidity within Plasticity Promotes Differential Target Recognition of Nerve Growth Factor. Front. Mol. Biosci. 2016, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; Merzel, F.; Cassetta, A.; Ogris, I.; Covaceuszach, S.; Grdadolnik, J.; Lamba, D.; Golič Grdadolnik, S. Endogenous modulators of neurotrophin signaling: Landscape of the transient ATP-NGF interactions. Comput. Struct. Biotechnol. J. 2021, 19, 2938–2949. [Google Scholar] [CrossRef] [PubMed]
- Kartal, Ö.; Andres, F.; Lai, M.P.; Nehme, R.; Cottier, K. waveRAPID-A Robust Assay for High-Throughput Kinetic Screens with the Creoptix WAVEsystem. SLAS Discov. 2021, 26, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Mureddu, L.; Vuister, G.W. Simple high-resolution NMR spectroscopy as a tool in molecular biology. FEBS J. 2019, 286, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.P.; Fogh, R.H.; Boucher, W.; Ragan, T.J.; Mureddu, L.G.; Vuister, G.W. CcpNmr AnalysisAssign: A flexible platform for integrated NMR analysis. J. Biomol. NMR 2016, 66, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y. How significant is a protein structure similarity with TM-score = 0.5? Bioinformatics 2010, 26, 889–895. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoletti, F.; Goričan, T.; Cassetta, A.; Grdadolnik, J.; Toporash, M.; Lamba, D.; Golič Grdadolnik, S.; Covaceuszach, S. Unveiling the Solvent Effect: DMSO Interaction with Human Nerve Growth Factor and Its Implications for Drug Discovery. Molecules 2025, 30, 3030. https://doi.org/10.3390/molecules30143030
Paoletti F, Goričan T, Cassetta A, Grdadolnik J, Toporash M, Lamba D, Golič Grdadolnik S, Covaceuszach S. Unveiling the Solvent Effect: DMSO Interaction with Human Nerve Growth Factor and Its Implications for Drug Discovery. Molecules. 2025; 30(14):3030. https://doi.org/10.3390/molecules30143030
Chicago/Turabian StylePaoletti, Francesca, Tjaša Goričan, Alberto Cassetta, Jože Grdadolnik, Mykola Toporash, Doriano Lamba, Simona Golič Grdadolnik, and Sonia Covaceuszach. 2025. "Unveiling the Solvent Effect: DMSO Interaction with Human Nerve Growth Factor and Its Implications for Drug Discovery" Molecules 30, no. 14: 3030. https://doi.org/10.3390/molecules30143030
APA StylePaoletti, F., Goričan, T., Cassetta, A., Grdadolnik, J., Toporash, M., Lamba, D., Golič Grdadolnik, S., & Covaceuszach, S. (2025). Unveiling the Solvent Effect: DMSO Interaction with Human Nerve Growth Factor and Its Implications for Drug Discovery. Molecules, 30(14), 3030. https://doi.org/10.3390/molecules30143030








