Three New Physalins from Physalis Alkekengi L. var. franchetii (Mast.) Makino
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Description of Spectral Data
3.5. In Vitro Antitumor Cell Proliferation Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAF | Physalis Alkekengi L. var. franchetii (Mast.) Makino |
| PE | petroleum ether |
| EA | ethyl acetate |
| SGC | silica gel column |
References
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services; Press Release No. 345; World Health Organization: Lyon, France; Geneva, Switzerland, 2024. [Google Scholar]
- Oh, G.S.; Kim, H.J.; Shen, A.H.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolytes Blood Press. 2014, 12, 5565. [Google Scholar] [CrossRef]
- Perazella, M.A. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin. J. Am. Soc. Nephrol. 2012, 7, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Ricordel, C.; Friboulet, L.; Facchinetti, F.; Soria, J.C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann. Oncol. 2018, 29 (Suppl. S1), i28i37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Yao, L.D. Strategies to Improve Outcomes of Patients with EGRF-Mutant Non-Small Cell Lung Cancer: Review of the Literature. J. Thorac. Oncol. 2016, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.L.; Huang, Y.P.; Cheng, M.H.; Rau, K.M.; Fang, Y.P. Improvement of Paclitaxel-Associated Adverse Reactions (ADRs) via the Use of Nano-Based Drug Delivery Systems: A Systematic Review and Network Meta Analysis. Int. J. Nanomed. 2020, 15, 1731–1743. [Google Scholar] [CrossRef]
- Markman, M. Managing taxane toxicities. Support Care Cancer 2003, 11, 144–147. [Google Scholar] [CrossRef]
- Liang, L.Y.; Li, C.Q.; Wang, Y.; Yue, Y.M.; Zhang, H.; Yang, M.R.; Cao, X.; Zhao, M.T.; Du, J.Y.; Peng, M.M.; et al. Physalis alkekengi L. var. franchetii (Mast.) Makino: A review of phrmacognosy, chemical constituents, pharmacological effects, quality control, and applications. Phytomedicine 2022, 105, 154328. [Google Scholar] [CrossRef]
- Esmailpoor, A.; Ghasemian, A.; Dehnavi, E.; Peidayesh, H.; Teimouri, M. Physalis alkekengi hydroalcoholic extract enhances the apoptosis in mouse model of breast cancer cells. Gene Rep. 2019, 15, 100366. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, F.; Ma, Z.; Lv, B.; Wang, X.; Dai, C.; Ma, X.; Liu, P.; Lv, H.; Chen, X.; et al. Physalis alkekengi var. franchetii Extracts Exert Antitumor Effects on Non-Small Cell Lung Cancer and Multiple Myeloma by Inhibiting STAT3 Signaling. OncoTargets Ther. 2021, 14, 301. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, L.; Chen, Y.; Wang, C.H.; ShenTu, J.Z.; Zheng, Y.L. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. Anti-Cancer Drugs 2019, 30, 128–137. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, K.M.; Lee, H.J.; Yun, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 101. [Google Scholar] [CrossRef]
- Zhu, F.F.; Dai, C.Y.; Fu, Y.F.; Loo Jacky, F.C.; Xia, D.J.; Gao, S.; Ma, Z.J.; Chen, Z. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget 2016, 7, 9462–9476. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.P.; Yang, M.; Liu, Y.L.; Li, Z.C.; Li, R.Y.; Li, X.R.; Li, N.; Xu, Q.M.; Khan, I.A.; et al. Physalins and withanolides from the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino and the inhibitory activities against human tumor cells. Phytochem. Lett. 2014, 10, 95–100. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, T.; Zhang, F.B.; Wang, Y.N.; Liu, Z.G.; Guo, S.S.; Li, L. Isolation and characterization of cytotoxic withanolides from the calyx of Physalis alkekengi L. var franchetii. Bioorg. Chem. 2020, 96, 103614. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Liang, J.Y.; Yang, Y.; Liu, R. Chemical constituents from Physalis alkekengi and structural revision of physalin G. Chin. J. Nat. Med. 2007, 5, 186–189. [Google Scholar]
- Qiu, L.; Zhao, F.; Jiang, Z.H.; Chen, L.X.; Zhao, Q.; Liu, H.X.; Yao, X.S.; Qiu, F. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2008, 71, 642–646. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, C.L.; Wu, D.C.; Tang, L.; Cao, X.J.; Xin, Y. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. Francheti. Fitoterapia 2012, 83, 1460–1465. [Google Scholar] [CrossRef]
- Sunayama, R.; Kuroyanagi, M.; Umehara, K.; Ueno, A. Physalin and neophysalins from Physalis alkekengi var. francheti and their differentiation inducing activity. Phytochemistry 1993, 34, 529–533. [Google Scholar] [CrossRef]
- Wu, J.P.; Xia, Z.F.; Liu, Y.L.; Li, X.R.; Xu, Q.M.; Yang, S.L. Study on steroidal chemical constituents of Physalis minima. Chin. Tradit. Herb. Drugs 2018, 49, 62–68. [Google Scholar] [CrossRef]
- Li, K.; Diao, Y.P.; Wang, M.D.; Yang, S.S.; Zhang, H.L.; Huang, H. Studies on Chemical Constituents of the Fruits of Physilis Alkekengi. L. var. franchetii (Mast.) Makino. Chin. J. Org. Chem. 2010, 30, 128–131. (In Chinese) [Google Scholar]
- Sun, J.M.; He, J.X.; Huang, M.; Hu, H.X.; Xu, L.T.; Fang, K.L.; Wang, X.N.; Shen, T. Two new physalins from Physalis alkekengi L. var. franchetii (Mast.) Makino. Nat. Prod. Res. 2022, 36, 5206–5212. [Google Scholar] [CrossRef]
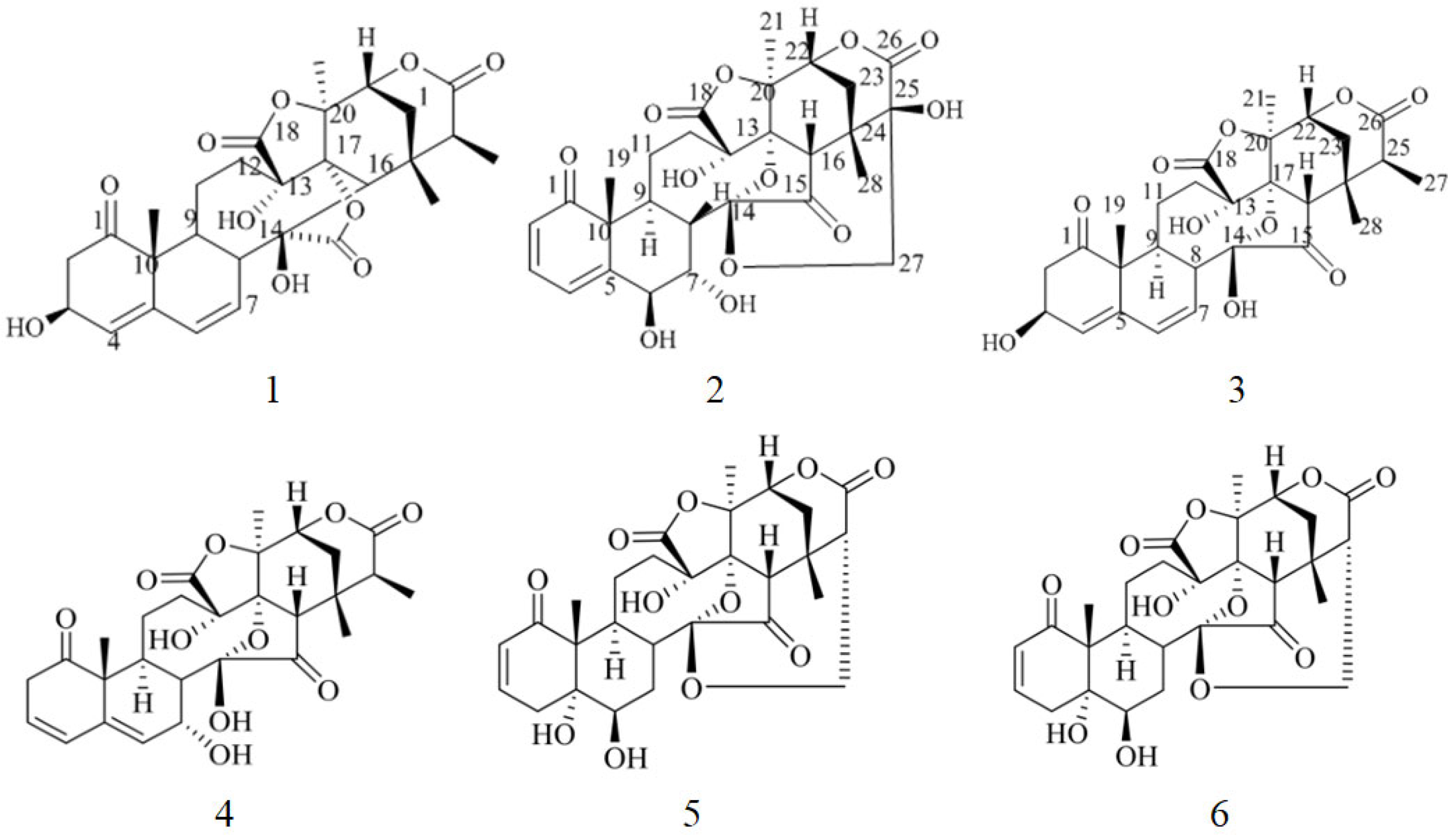
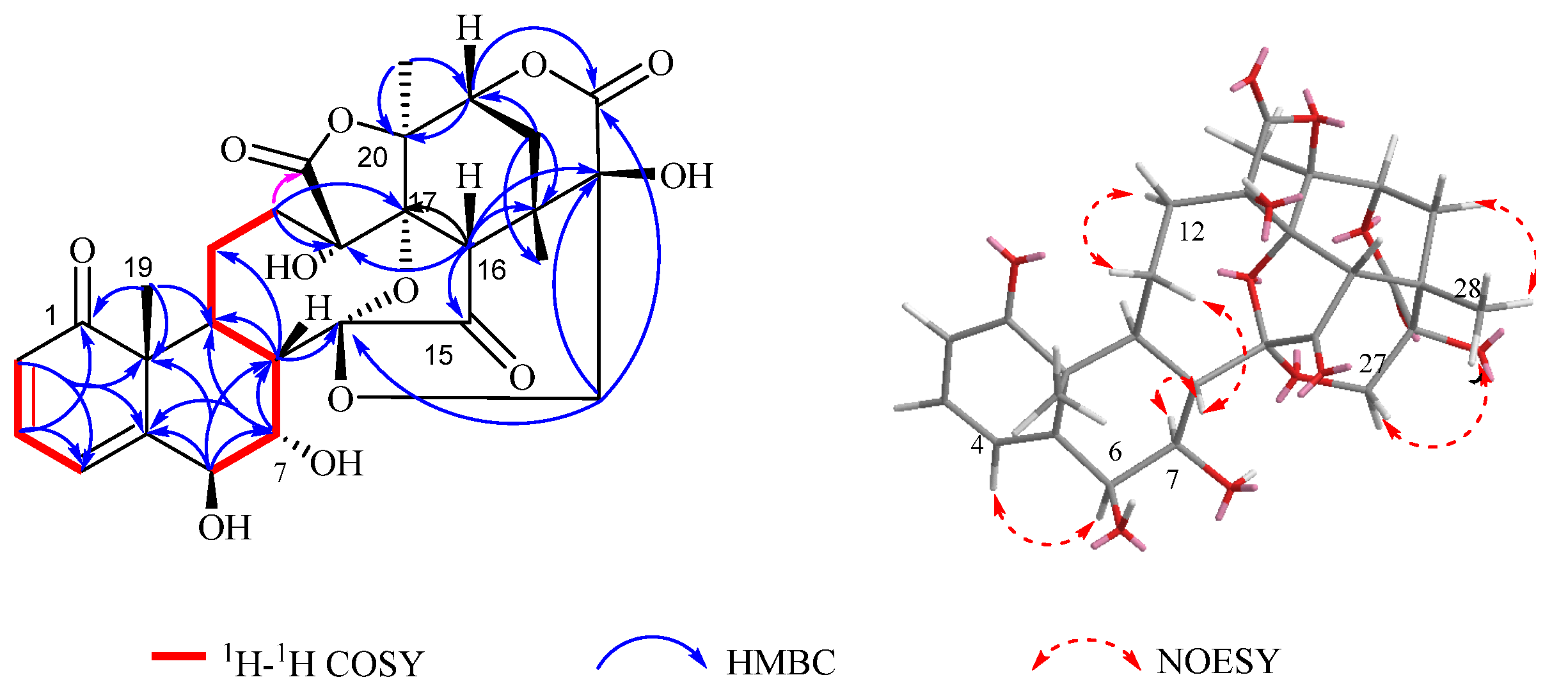
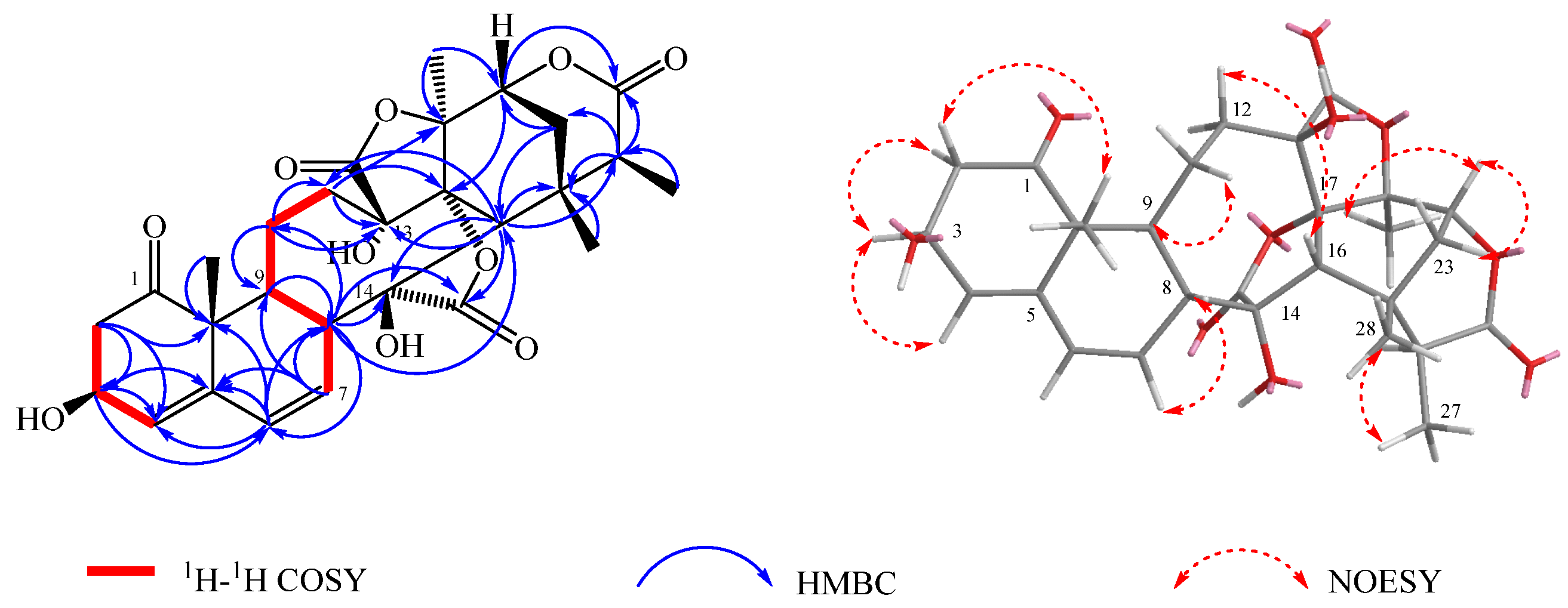
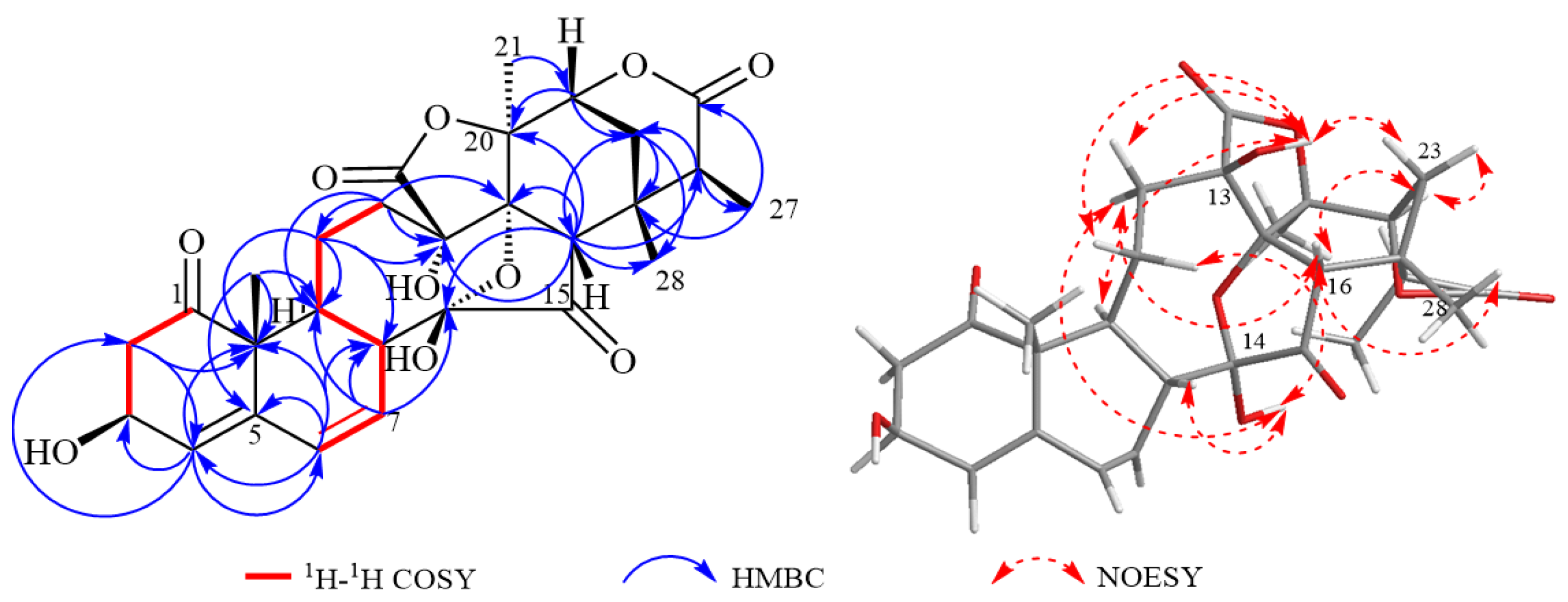
| NO. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 209.6 | 213.0 | 211.7 | |||
| 2 | 126.7 | 5.95 (d, J = 9.7 Hz, 1H) | 47.75 | 2.77 (dd, J = 12.6, 5.2 Hz, 1H) 2.69 (dd, J = 12.6, 8.7 Hz, 1H) | 44.9 | 2.86 (dd, J = 12.5, 8.7 H-z, 1H) 3.10 (dd, J = 12.5, 7.2 H-z, 1H) |
| 3 | 141.7 | 7.01 (dd, J = 9.7, 5.9 Hz, 1H) | 68.5 | 4.47 (t, J = 6.12 Hz, 1H) | 69.6 | 4.67 (s, 1H) |
| 4 | 122.8 | 6.19 (d, J = 5.9 Hz, 1H) | 127.5 | 5.70 (d, J = 1.20 Hz, 1H) | 126.0 | 5.61 (d, J = 3.4 H-z, 1H) |
| 5 | 156.2 | 141.9 | 142.1 | |||
| 6 | 77.3 | 4.21 (d, J = 3.6 Hz, 1H) | 131.0 | 6.45 (d, J = 10.41 Hz, 1H) | 128.0 | 6.17 (dd, J = 10.4, 2.8 H-z, 1H) |
| 7 | 70.4 | 4.43 (dd, J = 3.6, 1.7 Hz, 1H) | 129.6 | 6.19 (dd, J = 10.41, 4.38 Hz, 1H) | 127.9 | 6.33 (d, J = 10.4 H-z, 1H) |
| 8 | 44.3 | 2.67 (dd, J = 11.6, 1.7 Hz, 1H) | 50.1 | 2.90 (t, J = 5.18 Hz, 1H) | 45.2 | 2.74 (d, J = 11.2 H-z, 1H) |
| 9 | 33.7 | 3.26 (ddd, J = 11.6, 9.2 Hz, 1H) | 38.5 | 1.97 (m, 1H) | 31.7 | 3.01 (dd, J = 11.2, 3.6 H-z, 1H) |
| 10 | 54.9 | 51.5 | 51.5 | |||
| 11 | 22.6 | 2.74 (ddd, J = 16.5, 13.0, 1.7 Hz, α-H),1.16 (dddd, J = 16.5, 10.3, 9.2, 6.4 Hz, β-H) | 24.8 | 1.97 (m, 1H), 1.49 (m, 1H) | 24.8 | 1.61 (m, 1H), 1.15 (m, 1H) |
| 12 | 26.5 | 1.49 (ddd, J = 16.8, 10.3, 1.7 Hz, α-H) 2.12 (ddd, J = 16.8, 13.0, 6.4 Hz, β-H) | 29.2 | 2.46 (m, 1H) 2.12 (dd, J = 15.1, 4.2 Hz, 1H) | 29.2 | 1.81 (m, 1H), 2.45 (m, 1H) |
| 13 | 80.5 | 80.5 | 80.7 | |||
| 14 | 108.3 | 82.4 | 101.7 | |||
| 15 | 209.5 | 177.6 | 212.5 | |||
| 16 | 55.8 | 2.70 (s, 3H) | 58.2 | 2.96 (s, 1H) | 54.8 | 2.45 (m, 1H) |
| 17 | 82.6 | 82.5 | 82.5 | |||
| 18 | 173.5 | 171.8 | 171.8 | |||
| 19 | 20.1 | 1.37 (s, 3H) | 20.5 | 1.18 (s, 3H) | 20.5 | 1.39 (s, 3H) |
| 20 | 81.3 | 83.1 | 83.1 | |||
| 21 | 22.7 | 1.88 (s, 3H) | 20.8 | 1.80 (s, 3H) | 20.8 | 1.91 (s, 3H) |
| 22 | 79.4 | 4.58 (dd, J = 3.6, 2.0 Hz, 1H) | 78.7 | 4.52 (dd, J = 4.2, 1.61 Hz, 1H) | 78.7 | 4.57 (d, J = 4.0 Hz, 1H) |
| 23 | 29.8 | 2.50 (dd, J = 14.7, 3.6 Hz, α-H) 1.76 (d, J = 14.7, 2.0 Hz, β-H) | 30.6 | 2.13 (dd, J = 15.1, 4.2 Hz, 1H), 1.66 (d, J = 15.1 Hz, 1H) | 30.6 | 2.19 (dd, J = 15.1, 4.0 Hz, 1H) 1.76 (d, J = 15.1 Hz, 1H) |
| 24 | 37.3 | 35.7 | 35.7 | |||
| 25 | 75.4 | 41.0 | 3.63 (m, 1H) | 42.8 | 2.72 (d, J = 7.2, 1.5 Hz, 2H) | |
| 26 | 172.1 | 176.9 | 176.7 | |||
| 27 | 66.6 | 4.13 (d, J = 12.8 Hz, α-H) 3.60 (d, J = 12.8 Hz, β-H) | 17.4 | 1.32 (d, J = 7.5 Hz, 3H) | 16.3 | 1.32 (d, J = 7.2, 3H) |
| 28 | 20.1 | 1.24 (s, 3H) | 28.7 | 1.37 (s, 3H) | ||
| Compound | HL-60 | A-549 | SMMC-7221 | MDA-MB-231 | SW480 |
|---|---|---|---|---|---|
| 1 | 18.04 ± 1.71 | 2.92 ± 0.84 | 11.68 ± 1.96 | 19.62 ± 1.30 | 15.70 ± 1.50 |
| 2 | 14.38 ± 0.86 | 1.69 ± 1.12 | 0.42 ± 3.12 | 15.20 ± 1.04 | 11.70 ± 1.24 |
| 3 | 4.03 ± 1.93 | 1.84 ± 2.11 | 11.38 ± 1.47 | 7.94 ± 1.61 | 13.18 ± 2.04 |
| 4 | 3.55 ± 0.69 | 12.57 ± 0.47 | 30.37 ± 0.60 | 22.23 ± 1.47 | 20.31 ± 2.22 |
| 5 | 106.29 ± 0.13 | 98.16 ± 0.29 | 99.66 ± 0.11 | 96.14 ± 0.95 | 99.05 ± 0.04 |
| 6 | 12.47 ± 1.75 | 11.10 ± 0.07 | 29.71 ± 0.98 | 9.68 ± 2.19 | 12.94 ± 2.18 |
| Compound | HL-60 | A-549 | SMMC-7221 | MDA-MB-231 | SW480 |
|---|---|---|---|---|---|
| Compound 5 | 0.0520 ± 0.0037 **,## | 2.274 ± 0.090 **,## | 1.080 ± 0.027 **,## | 0.994 ± 0.035 **,## | 0.649 ± 0.027 **,## |
| Cisplatin | 5.865 ± 0.500 **,## | 18.91 ± 0.44 ** | 7.819 ± 0.405 **,## | 21.68 ± 1.56 **,## | 20.35 ± 0.37 **,## |
| Paclitaxel | <0.008 **,## | <0.008 ** | 0.029 ± 0.003 **,## | <0.008 **,## | <0.008 **,## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, X.-R.; Wu, Y.; Liu, Y.-L.; Liang, Y.-F.; Teng, Y. Three New Physalins from Physalis Alkekengi L. var. franchetii (Mast.) Makino. Molecules 2025, 30, 3017. https://doi.org/10.3390/molecules30143017
Zhao J, Zhang X-R, Wu Y, Liu Y-L, Liang Y-F, Teng Y. Three New Physalins from Physalis Alkekengi L. var. franchetii (Mast.) Makino. Molecules. 2025; 30(14):3017. https://doi.org/10.3390/molecules30143017
Chicago/Turabian StyleZhao, Ji, Xiang-Rong Zhang, You Wu, Ying-Li Liu, Yan-Feng Liang, and Yang Teng. 2025. "Three New Physalins from Physalis Alkekengi L. var. franchetii (Mast.) Makino" Molecules 30, no. 14: 3017. https://doi.org/10.3390/molecules30143017
APA StyleZhao, J., Zhang, X.-R., Wu, Y., Liu, Y.-L., Liang, Y.-F., & Teng, Y. (2025). Three New Physalins from Physalis Alkekengi L. var. franchetii (Mast.) Makino. Molecules, 30(14), 3017. https://doi.org/10.3390/molecules30143017





