Aquathermolytic Upgrading of Zarafshanian Extra Heavy Oil Using Ammonium Alum
Abstract
1. Introduction
2. Results and Discussions
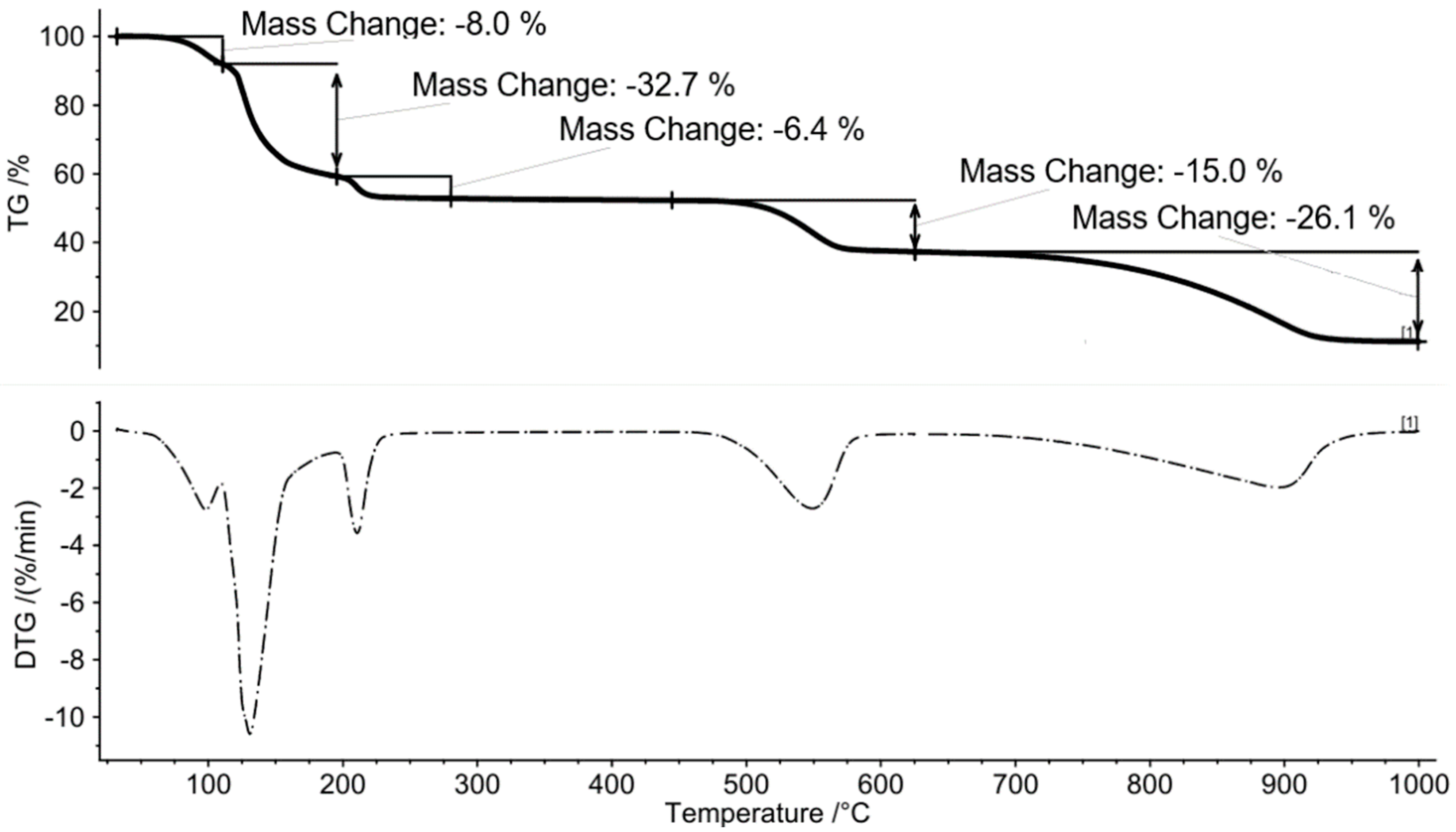
2.1. Thermal Decomposition Pathway of Ammonium Alum
2.2. Upgrading Performance of Ammonium Alum
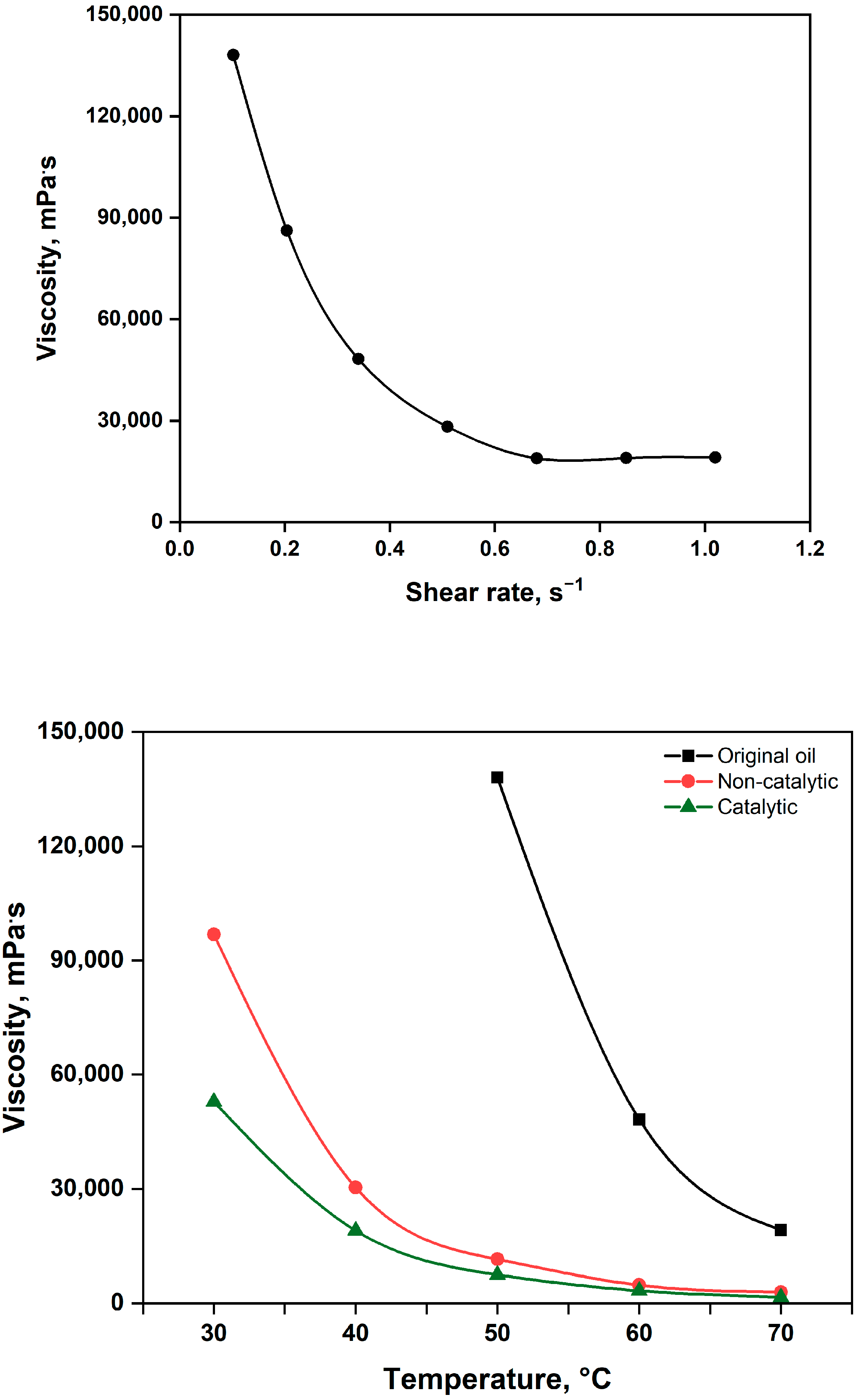
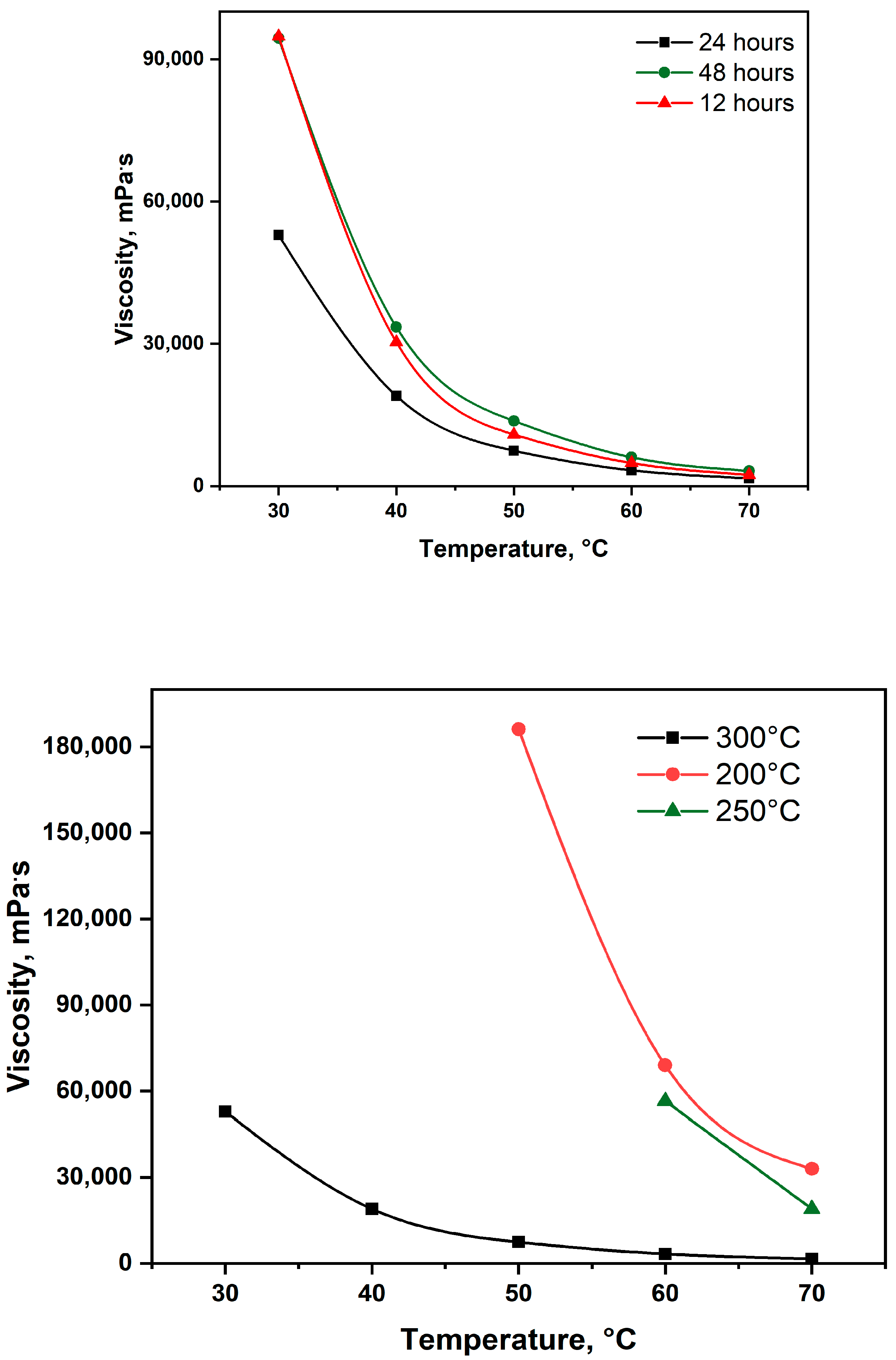
2.2.1. Viscosity of Upgraded Oil
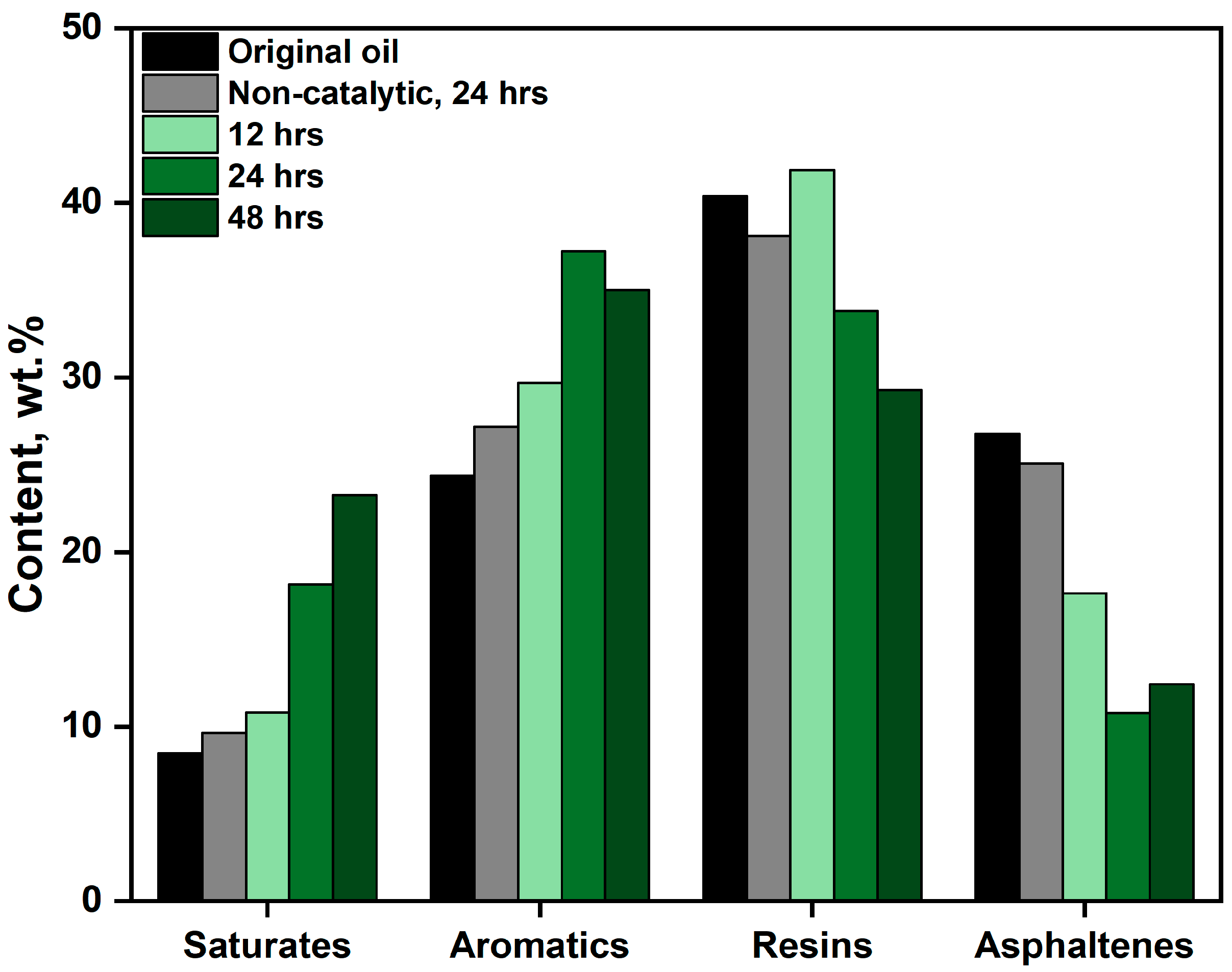
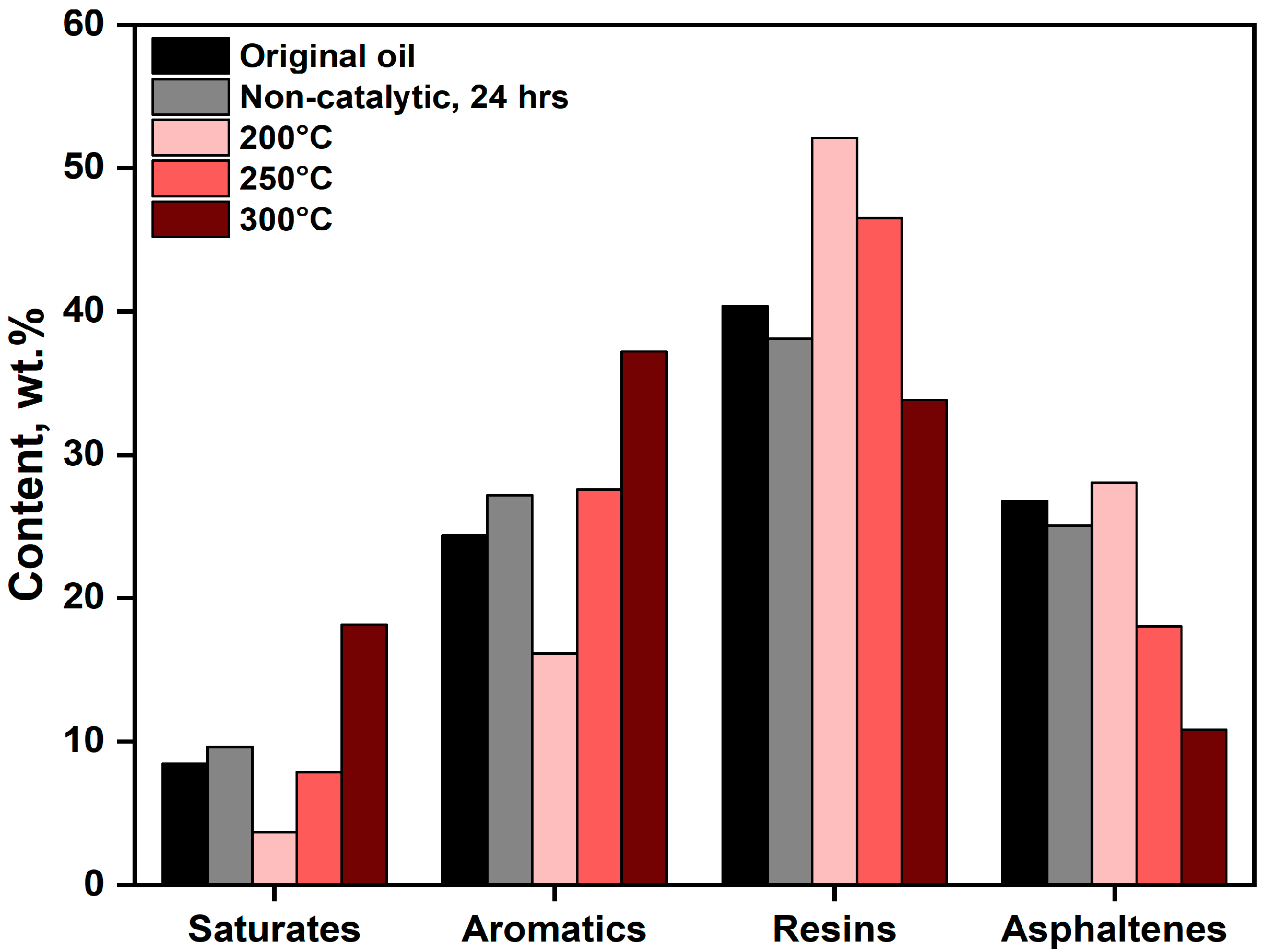
2.2.2. Group-Chemical Composition of Upgraded Oil
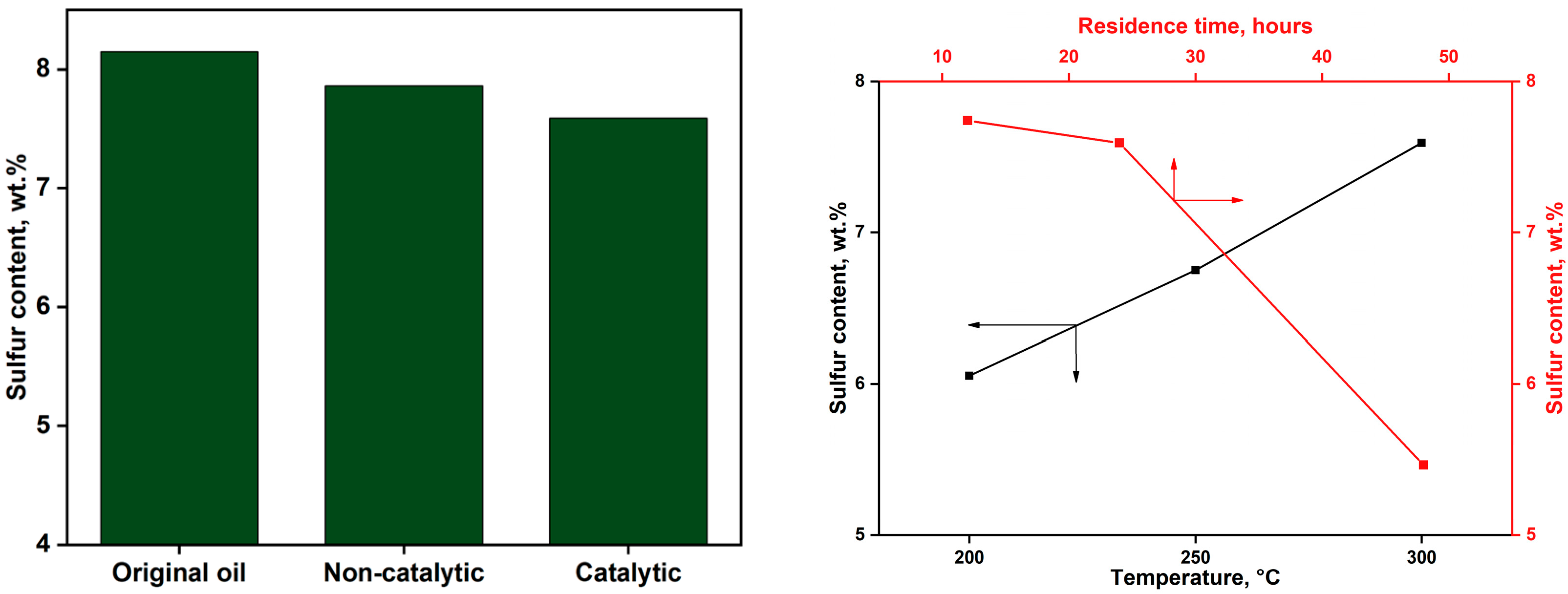
2.2.3. Sulfur Content of Upgraded Oil
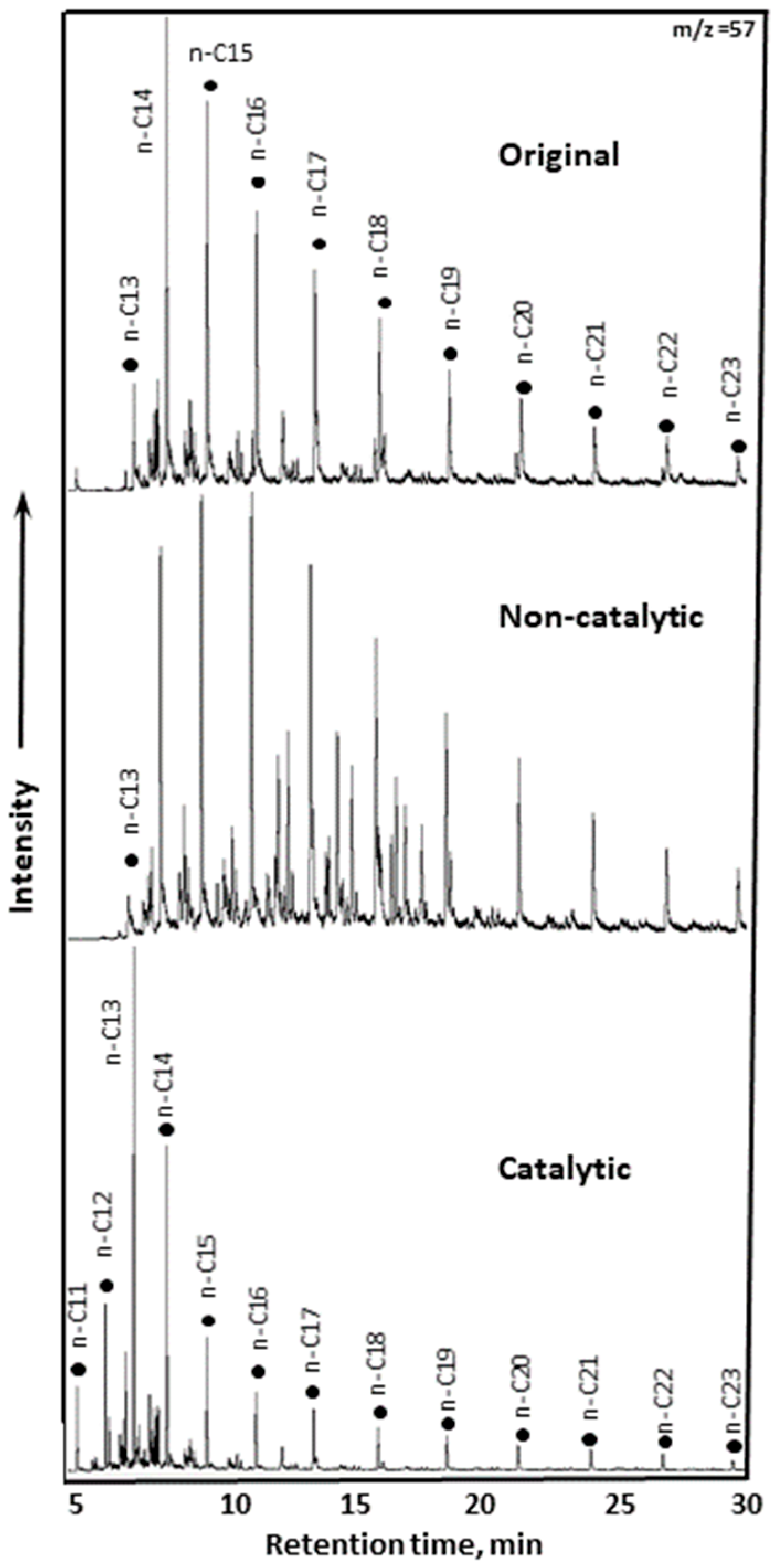
2.2.4. GC-MS of Saturates and Aromatics of Upgraded Oil
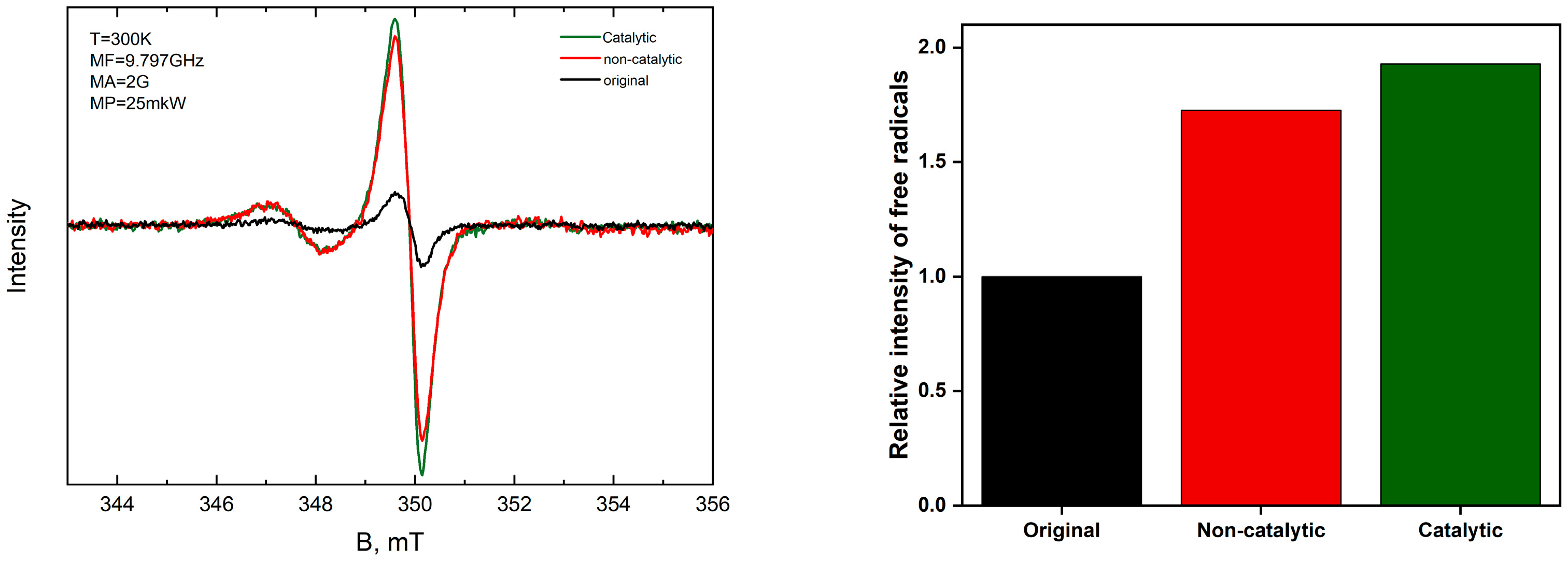
2.2.5. Electron Paramagnetic Resonance (EPR) Spectroscopy of Upgraded Extra-Heavy Oil
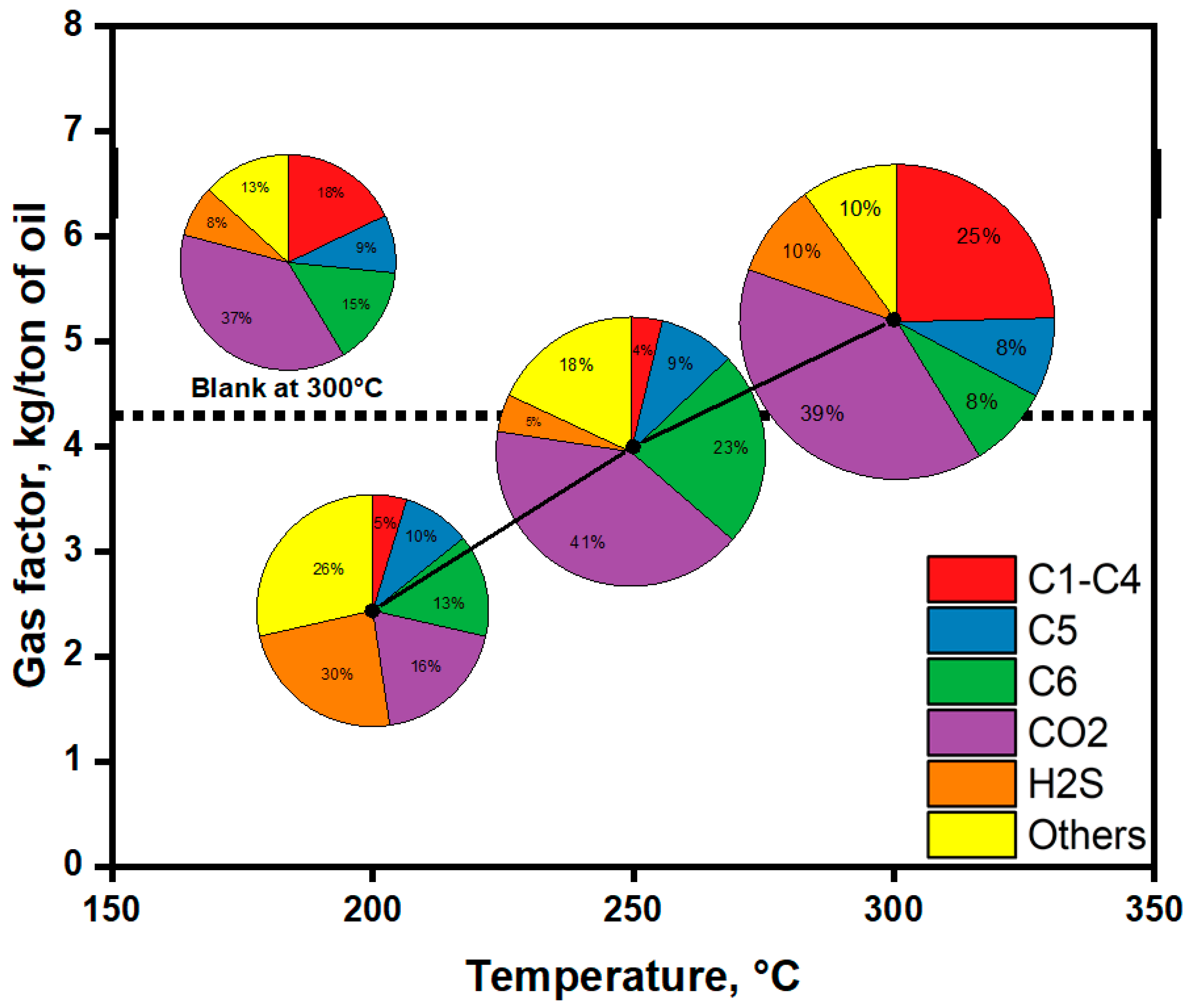
2.2.6. Gaseous Products Evolved After Upgrading of Extra-Heavy Oil
3. Materials and Methods
3.1. Materials
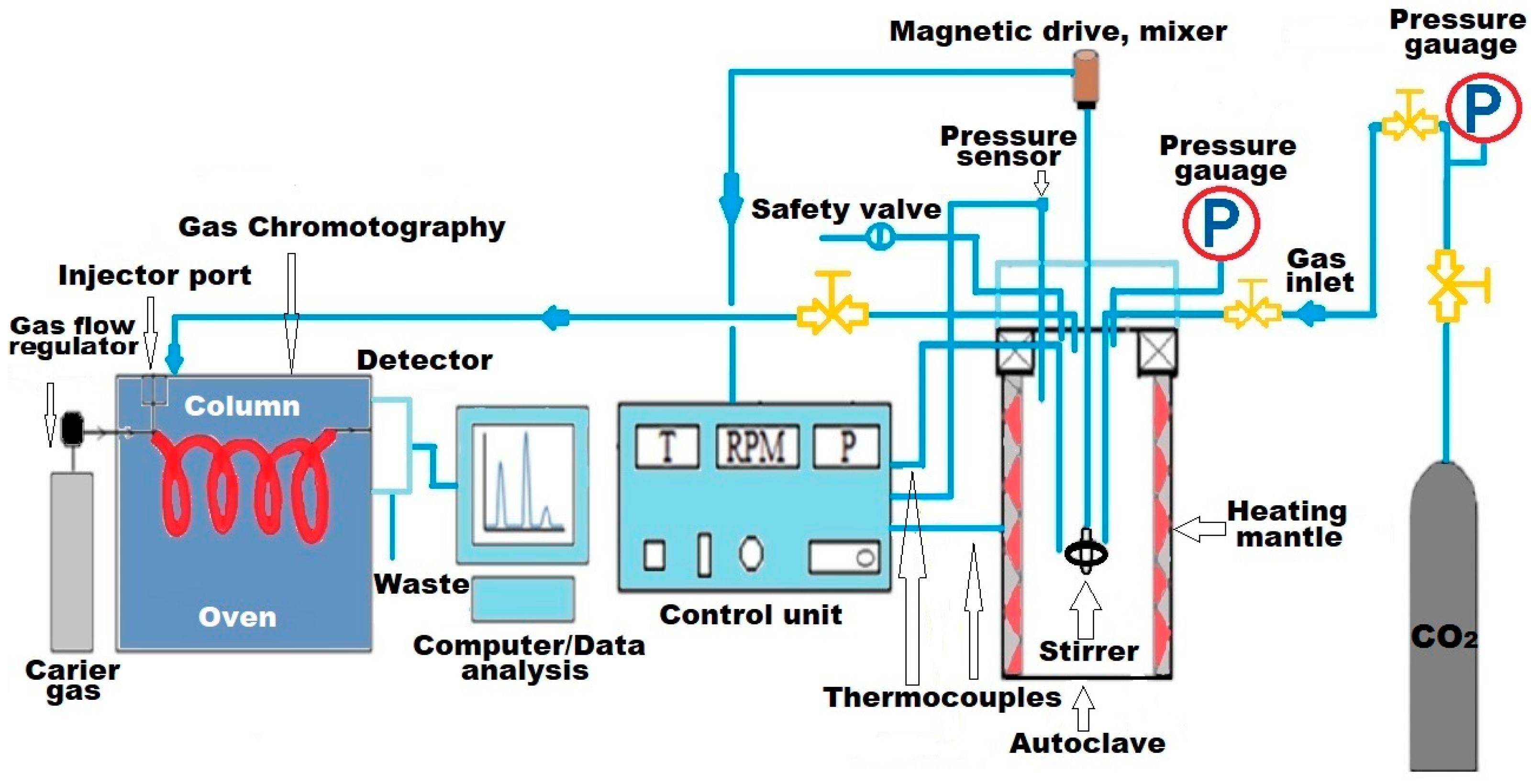
3.2. Catalytic Performance of Ammonium Alum
3.3. Analytical Methods
3.3.1. Gas Chromatography Analysis
3.3.2. Viscosity Measurement
3.3.3. SARA Fractionation Analysis
3.3.4. Total Sulfur Analysis
3.3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.3.6. Electron Paramagnetic Resonance (EPR) Spectroscopy
3.3.7. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.3.8. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hascakir, B. Accelerating the Energy Transition with Carbon Capture, Utilization, and Interdisciplinary Innovation. Pet. Sci. Technol. 2025, 43, 1–2. [Google Scholar] [CrossRef]
- Pang, X. Evaluation of the Global Oil and Gas Resources. In Quantitative Evaluation of the Whole Petroleum System: Hydrocarbon Thresholds and Their Application; Springer: Berlin/Heidelberg, Germany, 2023; pp. 369–411. [Google Scholar]
- Seidy-Esfahlan, M.; Tabatabaei-Nezhad, S.A.; Khodapanah, E. Comprehensive Review of Enhanced Oil Recovery Strategies for Heavy Oil and Bitumen Reservoirs in Various Countries: Global Perspectives, Challenges, and Solutions. Heliyon 2024, 10, e37826. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Chai, L.; Nourozieh, H.; Chen, Z.; Yang, M. A Technical, Economic, and Environmental Assessment on Dimethyl Ether (DME) as a Renewable Solvent from Carbon Dioxide Utilization (CCU) for Heavy Oil Recovery: A Real Field in Surmont, Canada as Case Study. Chem. Eng. J. 2024, 482, 148936. [Google Scholar] [CrossRef]
- Rodríguez, J.R.; Naranjo, P.A.L.; Molina, J.W.; Molina, J.D.; Molina, F.; Matute, A.K. Unveiling Success From Cyclic Steam Injections for Heavy Oil Recovery in India and the Orinoco Oil Belt. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 2 April 2014; p. D031S035R003. [Google Scholar]
- Bursell, C.G.; Pittman, G.M. Performance of Steam Displacement in the Kern River Field. J. Pet. Technol. 1975, 27, 997–1004. [Google Scholar] [CrossRef]
- Gates, C.F.; Sklar, I. Combustion as a Primary Recovery Process-Midway Sunset Field. J. Pet. Technol. 1971, 23, 981–986. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Wu, K.; Chen, Z. EOR Potential in the Post Steam Injection Era: Current and Future Trends. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 21–23 April 2026; p. D041S018R004. [Google Scholar]
- Hyne, J.B. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands During Simulated Steam Stimulation. 1986. Available online: https://www.osti.gov/etdeweb/biblio/220464 (accessed on 13 July 2025).
- Wang, J.; Liu, L.; Zhang, L.; Li, Z. Aquathermolysis of Heavy Crude Oil with Amphiphilic Nickel and Iron Catalysts. Energy Fuels 2014, 28, 7440–7447. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.; Li, C.; Xiong, R.; Chen, X.; Zhang, X. Advancements and Future Prospects in In-Situ Catalytic Technology for Heavy Oil Reservoirs in China: A Review. Fuel 2024, 374, 132376. [Google Scholar] [CrossRef]
- Kobayashi, S.; Manabe, K. Development of Novel Lewis Acid Catalysts for Selective Organic Reactions in Aqueous Media. Acc. Chem. Res. 2002, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.F.; Sapawe, N. A Review on the Current Techniques and Technologies of Organic Pollutants Removal from Water/Wastewater. Mater. Today Proc. 2020, 31, A158–A165. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-Situ Catalytic Upgrading of Heavy Oil Using Oil-Soluble Transition Metal-Based Catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of Heavy Oil: A Review and Perspective on Catalyst Development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Chen, X.; Ni, J.; Du, J.; Li, Y.; Xin, X.; Zhao, B.; Chen, G. Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil. Molecules 2024, 29, 3761. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.D.; Lesage, K.L.; Tsang, G.T.; Hyne, J.B. Reactions of Benzo [b] Thiophene with Aqueous Metal Species: Their Influence on the Production and Processing of Heavy Oils. Energy Fuels 1988, 2, 578–581. [Google Scholar] [CrossRef]
- Clark, P.D.; Kirk, M.J. Studies on the Upgrading of Bituminous Oils with Water and Transition Metal Catalysts. Energy Fuels 1994, 8, 380–387. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Hyne, J.B.; Lesage, K.L. The Chemistry of Organosulphur Compound Types Occurring in Heavy Oils: 4. the High-Temperature Reaction of Thiophene and Tetrahydrothiophene with Aqueous Solutions of Aluminium and First-Row Transition-Metal Cations. Fuel 1987, 66, 1353–1357. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Feng, J.; Long, X.; Hu, T.; Chen, G. Water-Soluble Fe (III) Complex Catalyzed Coupling Aquathermolysis of Water-Heavy Oil-Methanol. Catalysts 2024, 14, 353. [Google Scholar] [CrossRef]
- Aliev, F.; Kholmurodov, T.; Mirzayev, O.; Tajik, A.; Mukhamadiev, N.; Slavkina, O.; Nourgalieva, N.; Vakhin, A. Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4. Catalysts 2023, 13, 153. [Google Scholar] [CrossRef]
- Khafizov, N.R.; Madzhidov, T.I.; Yuan, C.; Varfolomeev, M.A.; Kadkin, O.N. Theoretical Insight into the Catalytic Effect of Transition Metal Ions on the Aquathermal Degradation of Heavy Oil: A DFT Study of Cyclohexyl Phenyl Amine Cleavage. Fuel 2022, 312, 123002. [Google Scholar] [CrossRef]
- Medina, O.E.; Céspedes, S.; Zabala, R.D.; Franco, C.A.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. A Theoretical and Experimental Approach to the Analysis of Hydrogen Generation and Thermodynamic Behavior in an in Situ Heavy Oil Upgrading Process Using Oil-Based Nanofluids. Catalysts 2022, 12, 1349. [Google Scholar] [CrossRef]
- Lakhova, A.; Petrov, S.; Ibragimova, D.; Kayukova, G.; Safiulina, A.; Shinkarev, A.; Okekwe, R. Aquathermolysis of Heavy Oil Using Nano Oxides of Metals. J. Pet. Sci. Eng. 2017, 153, 385–390. [Google Scholar] [CrossRef]
- Husein, M.M.; Alkhaldi, S.J. In Situ Preparation of Alumina Nanoparticles in Heavy Oil and Their Thermal Cracking Performance. Energy Fuels 2014, 28, 6563–6569. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Effect of Surface Acidity and Basicity of Aluminas on Asphaltene Adsorption and Oxidation. J. Colloid Interface Sci. 2011, 360, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.; Domínguez-Álvarez, E.; Yuan, C.; Suwaid, M.A.; Varfolomeev, M.A.; Ancheyta, J.; Al-mishaal, O.F.; Kudryashov, S.I.; Afanasiev, I.S.; Antonenko, D.A. On the Use of Metallic Nanoparticulated Catalysts for In-Situ Oil Upgrading. Fuel 2022, 313, 122677. [Google Scholar] [CrossRef]
- Rana, M.S.; Ancheyta, J.; Rayo, P.; Maity, S.K. Effect of Alumina Preparation on Hydrodemetallization and Hydrodesulfurization of Maya Crude. Catal. Today 2004, 98, 151–160. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Chuklina, S.G.; Maslenkova, S.A. Study of Cu Modified Zr and Al Mixed Oxides in Ethanol Conversion: The Structure-Catalytic Activity Relationship. Catal. Today 2021, 379, 159–165. [Google Scholar] [CrossRef]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid Catalytic Cracking Technology: Current Status and Recent Discoveries on Catalyst Contamination. Catal. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]
- Cardona, L.; Medina, O.E.; Cespedes, S.; Lopera, S.H.; Cortes, F.B.; Franco, C.A. Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes 2021, 9, 1009. [Google Scholar] [CrossRef]
- Mukhamed’yarova, A.N.; Gareev, B.I.; Nurgaliev, D.K.; Aliev, F.A.; Vakhin, A.V. A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining. Processes 2021, 9, 1811. [Google Scholar] [CrossRef]
- Abdelsalam, Y.I.I.; Galiakhmetova, L.K.; Sharifullin, A.V.; Tajik, A.; Mukhamatdinova, R.E.; Davletshin, R.R.; Vakhin, A.V. Comparative Study of the Catalytic Effects of Al (CH3COO)3 and Al2 (SO4)3 on Heavy Oil Aquathermolysis in CO2 and N2 Atmospheres. Appl. Catal. A Gen. 2025, 699, 120247. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Li, Y.; Dong, S.; Peng, S.; Chen, G. Viscosity Reduction and Mechanism of Aquathermolysis of Heavy Oil Co-Catalyzed by Bentonite and Transition Metal Complexes. Catalysts 2022, 12, 1383. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, M.; Chen, X.; Zhang, L. The Effect of Free Radical Initiator in Promoting Aquathermolysis of Heavy Oil under Mild Conditions. Fuel 2024, 375, 132576. [Google Scholar] [CrossRef]
- Avbenake, O.P.; Al-Hajri, R.S.; Jibril, B.Y. Catalytic Upgrading of Heavy Oil Using NiCo/γ-Al2O3 Catalyst: Effect of Initial Atmosphere and Water-Gas Shift Reaction. Fuel 2019, 235, 736–743. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, T.; Zhu, G.; Wang, K.; Xu, X.; Liu, L. A Review on the Role of Hydrogen Donors in Upgrading Heavy Oil and Bitumen. Sustain. Energy Fuels 2022, 6, 1866–1890. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D. Oil Dispersed Nickel-Based Catalyst for Catalytic Upgrading of Heavy Oil Using Supercritical Water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Shen, Z.; Fang, X.; He, W.; Zhang, L.; Li, Y.; Qi, G.; Xin, X.; Zhao, B.; Chen, G. Enhanced Aquathermolysis of Water–Heavy Oil–Ethanol Catalyzed by B@ Zn (II) L at Low Temperature. Molecules 2024, 29, 2057. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Alidrisi, H.; Balubaid, M.A. API Gravity, Sulfur Content, and Desulfurization of Crude Oil. Pet. Sci. Technol. 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Agarwal, P.; Sharma, D.K. Comparative Studies on the Bio-Desulfurization of Crude Oil with Other Desulfurization Techniques and Deep Desulfurization through Integrated Processes. Energy Fuels 2010, 24, 518–524. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Volodin, M.A.; Rodionov, A.A.; Sorokina, A.T.; Dolomatov, M.Y.; Petrov, A.V.; Vakhin, A.V.; Mamin, G.V.; Orlinskii, S.B. EPR Study of Spectra Transformations of the Intrinsic Vanadyl-Porphyrin Complexes in Heavy Crude Oils with Temperature to Probe the Asphaltenes’ Aggregation. J. Pet. Sci. Eng. 2018, 166, 363–368. [Google Scholar] [CrossRef]
- Shabalin, K.V.; Foss, L.E.; Borisova, Y.Y.; Borisov, D.N.; Yakubova, S.G.; Yakubov, M.R. Study of the Heavy Oil Asphaltenes Oxidation Products Composition Using EPR and IR Spectroscopy. Pet. Sci. Technol. 2020, 38, 992–997. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hsieh, T.-C.; Jiang, T.L. An Experimental Study on Carbon Monoxide Conversion and Hydrogen Generation from Water Gas Shift Reaction. Energy Convers. Manag. 2008, 49, 2801–2808. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Mustakimova, E.A.; Baigildin, I.G.; Talanova, M.Y.; Cherednichenko, K.A.; Maximov, A.L.; Karakhanov, E.A.; Vutolkina, A.V. Simultaneous Hydrotransformation of Aromatics and Sulfur Compounds over Unsupported NiMoS Catalysts under Water Gas Shift Reaction Conditions. Fuel 2025, 384, 134034. [Google Scholar] [CrossRef]
- Aliev, F.; Abdelsalam, Y.; Lapuk, S.; Khelkhal, M.A.; Suwaid, M.; Vakhin, A. Efficient Heavy Oil Upgrading with Water-Soluble Nickel and Copper Acetate Catalysts. Ind. Eng. Chem. Res. 2024, 63, 6546–6561. [Google Scholar] [CrossRef]


| Parameters | Values |
|---|---|
| Viscosity (at 50 °C), mPa·s | 138,059 |
| Elemental composition, % wt. | |
| C | 75.92 |
| H | 11.22 |
| S | 8.15 |
| N | 2.54 |
| O * | 2.17 |
| H/Catomic | 1.77 |
| Group composition, % wt. | |
| Saturates | 9 |
| Aromatics | 24 |
| Resins | 40 |
| Asphaltenes | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Akhunov, A.; Aliev, F.; Mukhamadiev, N.; Kahwir, O.F.; Dengaev, A.; Majeed, M.Y.; Esmaeel, M.; Al-Qaz, A.; Mirzaev, O.; Vakhin, A. Aquathermolytic Upgrading of Zarafshanian Extra Heavy Oil Using Ammonium Alum. Molecules 2025, 30, 3013. https://doi.org/10.3390/molecules30143013
Ali Akhunov A, Aliev F, Mukhamadiev N, Kahwir OF, Dengaev A, Majeed MY, Esmaeel M, Al-Qaz A, Mirzaev O, Vakhin A. Aquathermolytic Upgrading of Zarafshanian Extra Heavy Oil Using Ammonium Alum. Molecules. 2025; 30(14):3013. https://doi.org/10.3390/molecules30143013
Chicago/Turabian StyleAli Akhunov, Amirjon, Firdavs Aliev, Nurali Mukhamadiev, Oscar Facknwie Kahwir, Alexey Dengaev, Mohammed Yasin Majeed, Mustafa Esmaeel, Abdulvahhab Al-Qaz, Oybek Mirzaev, and Alexey Vakhin. 2025. "Aquathermolytic Upgrading of Zarafshanian Extra Heavy Oil Using Ammonium Alum" Molecules 30, no. 14: 3013. https://doi.org/10.3390/molecules30143013
APA StyleAli Akhunov, A., Aliev, F., Mukhamadiev, N., Kahwir, O. F., Dengaev, A., Majeed, M. Y., Esmaeel, M., Al-Qaz, A., Mirzaev, O., & Vakhin, A. (2025). Aquathermolytic Upgrading of Zarafshanian Extra Heavy Oil Using Ammonium Alum. Molecules, 30(14), 3013. https://doi.org/10.3390/molecules30143013









