The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design
Abstract
1. Introduction
2. Interest of Fluorine and Methyl-Containing Groups in Drug Design
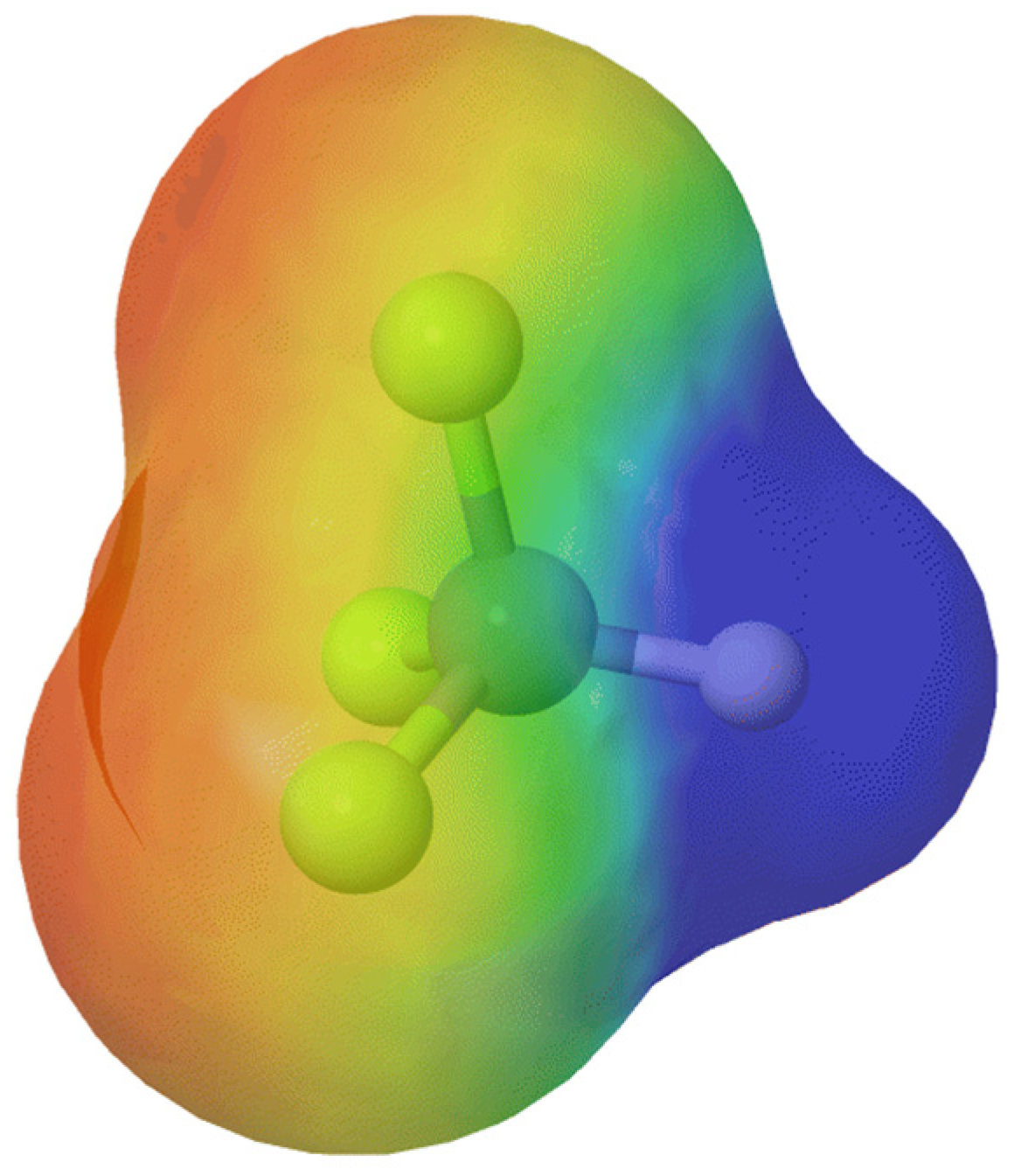
3. Trifluoromethyl Group
4. Trifluoromethoxy Group
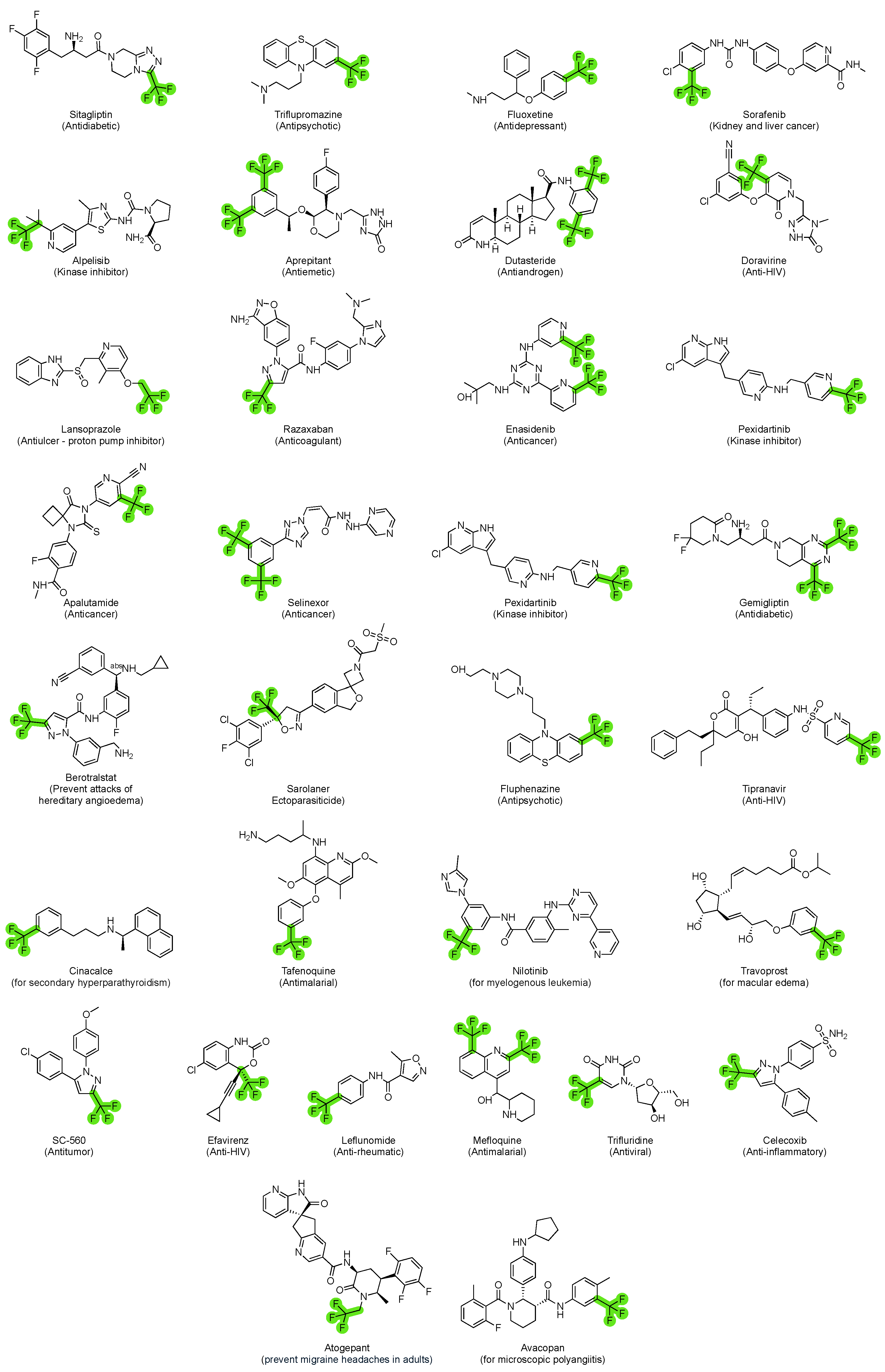
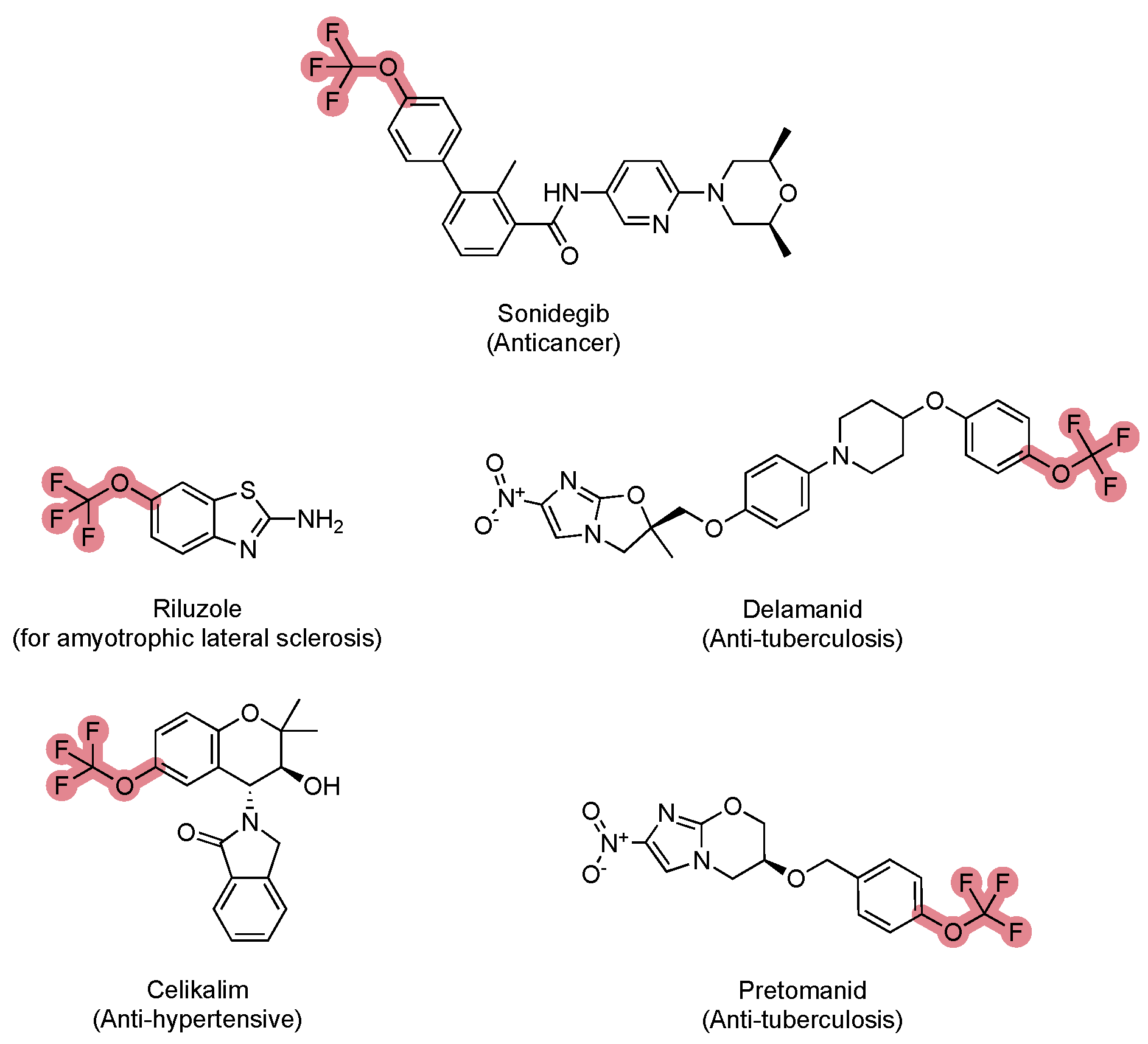
5. FDA-Approved Drugs Containing Trifluoromethyl and Trifluoromethoxy Groups
5.1. FDA-Approved Drugs Containing the Trifluoromethyl Group
5.2. FDA-Approved Drugs Containing the Trifluoromethoxy Group
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Y.; Stumpfe, D.; Bajorath, J. Computational Exploration of Molecular Scaffolds in Medicinal Chemistry. J. Med. Chem. 2016, 59, 4062–4076. [Google Scholar] [CrossRef] [PubMed]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Zdrazil, B.; Guha, R. The Rise and Fall of a Scaffold: A Trend Analysis of Scaffolds in the Medicinal Chemistry Literature. J. Med. Chem. 2018, 61, 4688–4703. [Google Scholar] [CrossRef]
- Ertl, P. Craig plot 2.0: An interactive navigation in the substituent bioisosteric space. J. Cheminform. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Shu, Y.Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bian, Y.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Makarem, A.; Soloshonok, V.A.; Han, J. FDA approved fluorine-containing drugs in 2023. Chin. Chem. Lett. 2024, 35, 109780. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. Engl. 2013, 52, 8214–8264. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.C.; Wang, L.J.; Jiang, B.; Wang, S.Y.; Wu, N.; Li, X.Q.; Shi, D.Y. Application of Fluorine in Drug Design During 2010–2015 Years: A Mini-Review. Mini Rev. Med. Chem. 2017, 17, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Singh, D.V.; Mahato, G.K.; Patel, S. Fluorine-a small magic bullet atom in the drug development: Perspective to FDA approved and COVID-19 recommended drugs. Chem. Zvesti. 2023, 77, 4085–4106. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.S.M.; Franco, L.S.; Fraga, C.A.M. The Magic Methyl and Its Tricks in Drug Discovery and Development. Pharmaceuticals 2023, 16, 1157. [Google Scholar] [CrossRef] [PubMed]
- Henary, E.; Casa, S.; Dost, T.L.; Sloop, J.C.; Henary, M. The Role of Small Molecules Containing Fluorine Atoms in Medicine and Imaging Applications. Pharmaceuticals 2024, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J.; Boblak, K.N.; Topinka, M.J.; Kindelin, P.J.; Briski, J.M.; Zheng, C.; Klumpp, D.A. Superelectrophiles and the effects of trifluoromethyl substituents. J. Am. Chem. Soc. 2010, 132, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acenã, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.S.; Danos, A.; Stachelek, P.; Fox, M.A.; Batsanov, A.S.; Monkman, A.P.; Bryce, M.R. Exploiting trifluoromethyl substituents for tuning orbital character of singlet and triplet states to increase the rate of thermally activated delayed fluorescence. Mater. Chem. Front. 2020, 4, 3602. [Google Scholar] [CrossRef]
- Sroor, F.M.; Mahrous, K.F.; El-Kader, H.A.M.A.; Othman, A.M.; Ibrahim, N.S. Impact of trifluoromethyl and sulfonyl groups on the biological activity of novel aryl-urea derivatives: Synthesis, in-vitro, in-silico and SAR studies. Sci. Rep. 2023, 13, 17560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J.L.; Izawa, K.; Liu, H.; Soloshonok, V.A. Recent advances in the trifluoromethylation methodology and new CF3-containing drugs. J. Fluor. Chem. 2014, 167, 37–54. [Google Scholar] [CrossRef]
- Oishi, M.; Kondo, H.; Amii, H. Aromatic trifluoromethylation catalytic in copper. Chem. Commun. 2009, 86, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Chatterjee, T.J. Synthesis of (4-trifluoromethyl)isoxazoles through a tandem trifluoromethyloximation/cyclization/elimination reaction of α, β-unsaturated carbonyls. Org. Chem. 2023, 88, 5420–5430. [Google Scholar] [CrossRef] [PubMed]
- Gonda, Z.; Kovács, S.; Wéber, C.; Gáti, T.; Mészáros, A.; Kotschy, A.; Novák, Z. Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and Heteroaromatic Iodides: The Beneficial Anchoring Effect of Borates. Org. Lett. 2014, 16, 4268–4271. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Senecal, T.D.; Kinzel, T.; Zhang, Y.; Watson, D.A.; Buchwald, S.L. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 2010, 328, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; MacMillan, D.W.C. Metallaphotoredox Perfluoroalkylation of Organobromides. J. Am. Chem. Soc. 2020, 142, 19480–19486. [Google Scholar] [CrossRef] [PubMed]
- Firuz, M.E.; Raijai-Daryasarei, S.; Rominger, F.; Biglari, A.; Balalaie, S. Mn-Mediated Direct Regioselective C–H Trifluoromethylation of Imidazopyridines and Quinoxalines. J. Org. Chem. 2023, 88, 10599–10608. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, W.; Sorochinsky, A.E.; Butler, G.; Landa, A.; Han, J.; Soloshonok, V.A. Successful trifluoromethoxy-containing pharmaceuticals and agrochemicals. J. Fluor. Chem. 2022, 257–258, 109978. [Google Scholar] [CrossRef]
- Kyzer, J.L.; Martens, M. Metabolism and Toxicity of Fluorine Compounds. Chem. Res. Toxicol. 2021, 34, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Singh, A.K.; Kumar, A.; Kumar, S.; Sukumaran, S.; Koyiparambath, V.P.; Pappachen, L.K.; Rangarajan, T.M.; Kim, H.; Mathew, B. FDA-Approved Trifluoromethyl Group-Containing Drugs: A Review of 20 Years. Processes 2022, 10, 2054. [Google Scholar] [CrossRef]
- Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W.; Aisa, H.A. Substitution Effect of the Trifluoromethyl Group on the Bioactivity in Medicinal Chemistry: Statistical Analysis and Energy Calculations. J. Chem. Inf. Model 2020, 60, 6242–6250. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.L. Substituent Constants for Correlation Analysis in Chemistry and Biology. J. Pharm. Sci. 1980, 69, 1109. [Google Scholar] [CrossRef]
- Si, Y.; Tang, P. Development and Application of Trifluoromethoxylating Reagents. Chin. J. Chem. 2023, 41, 2179–2196. [Google Scholar] [CrossRef]
- Leroux, F.R.; Manteau, B.; Vors, J.P.; Pazenok, S. Trifluoromethyl ethers–synthesis and properties of an unusual substituent. J. Org. Chem. 2008, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, X.; Sorochinsky, A.E.; Butler, G.; Han, J.; Soloshonok, V.A. Advances in the Development of Trifluoromethoxylation Reagents. Symmetry 2021, 13, 2380. [Google Scholar] [CrossRef]
- Iashin, V.; Wirtanen, T.; Perea-Buceta, J.E. Tetramethylammonium Fluoride: Fundamental Properties and Applications in C-F Bond-Forming Reactions and as a Base. Catalysts 2022, 12, 233. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yao, J.F.; Yan, W.; Luo, Z.; Tang, Z.Y. Silver-Mediated Trifluoromethoxylation of (Hetero)aryldiazonium Tetrafluoroborates. Org Lett. 2019, 21, 8003–8007. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Adachi, K.; Ishihara, S. CF3 Oxonium Salts, O-(Trifluoromethyl)dibenzofuranium Salts: In Situ Synthesis, Properties, and Application as a Real CF3+ Species Reagent. J. Org. Chem. 2007, 72, 6905–6917. [Google Scholar] [CrossRef] [PubMed]
- Duhail, T.; Bortolato, T.; Mateos, J.; Anselmi, E.; Jelier, B.; Togni, A.; Magnier, E.; Dagousset, G.; Dell’Amico, L. Radical α-Trifluoromethoxylation of Ketones under Batch and Flow Conditions by Means of Organic Photoredox Catalysis. Org. Lett. 2021, 23, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, T.D.; Bagnoli, L.; Rosati, O.; Marini, F.; Sancineto, L.; Santi, C. New Halogen-Containing Drugs Approved by FDA in 2021: An Overview on Their Syntheses and Pharmaceutical Use. Molecules 2022, 27, 1643. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2023, 34, 107578. [Google Scholar] [CrossRef]
- Baraldi, C.; Beier, D.; Martelletti, P.; Pellesi, L. The preclinical discovery and development of atogepant for migraine prophylaxis. Expert Opin. Drug Discov. 2024, 19, 783–788. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novás, M.; Matos, M.J. The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design. Molecules 2025, 30, 3009. https://doi.org/10.3390/molecules30143009
Novás M, Matos MJ. The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design. Molecules. 2025; 30(14):3009. https://doi.org/10.3390/molecules30143009
Chicago/Turabian StyleNovás, Manuel, and Maria J. Matos. 2025. "The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design" Molecules 30, no. 14: 3009. https://doi.org/10.3390/molecules30143009
APA StyleNovás, M., & Matos, M. J. (2025). The Role of Trifluoromethyl and Trifluoromethoxy Groups in Medicinal Chemistry: Implications for Drug Design. Molecules, 30(14), 3009. https://doi.org/10.3390/molecules30143009







