The Capacitive Property Enhancement of CoFeP-Ni(OH)2/Nickel Foam Electrodes via an Interfacial Integration Strategy for Asymmetric Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
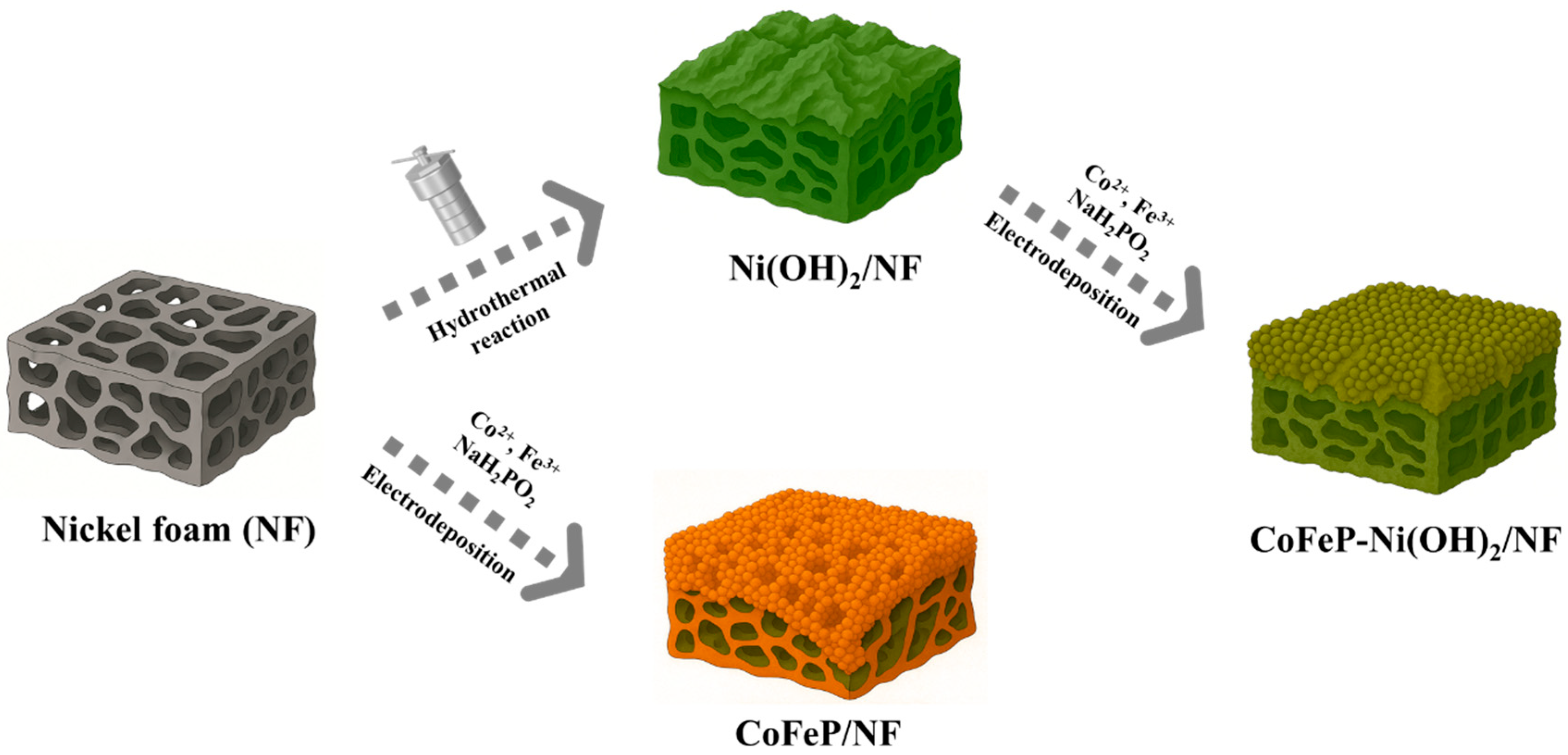
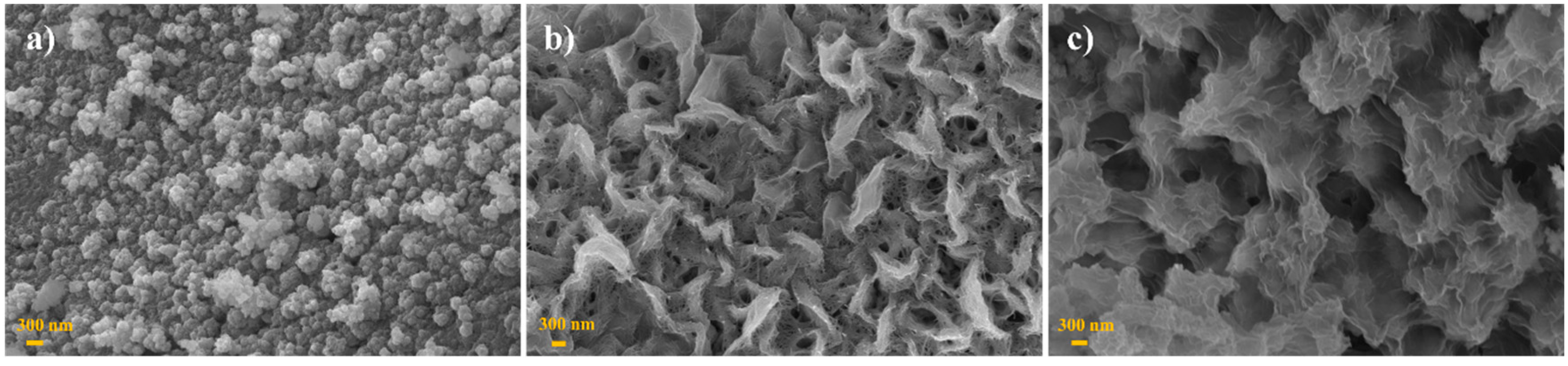
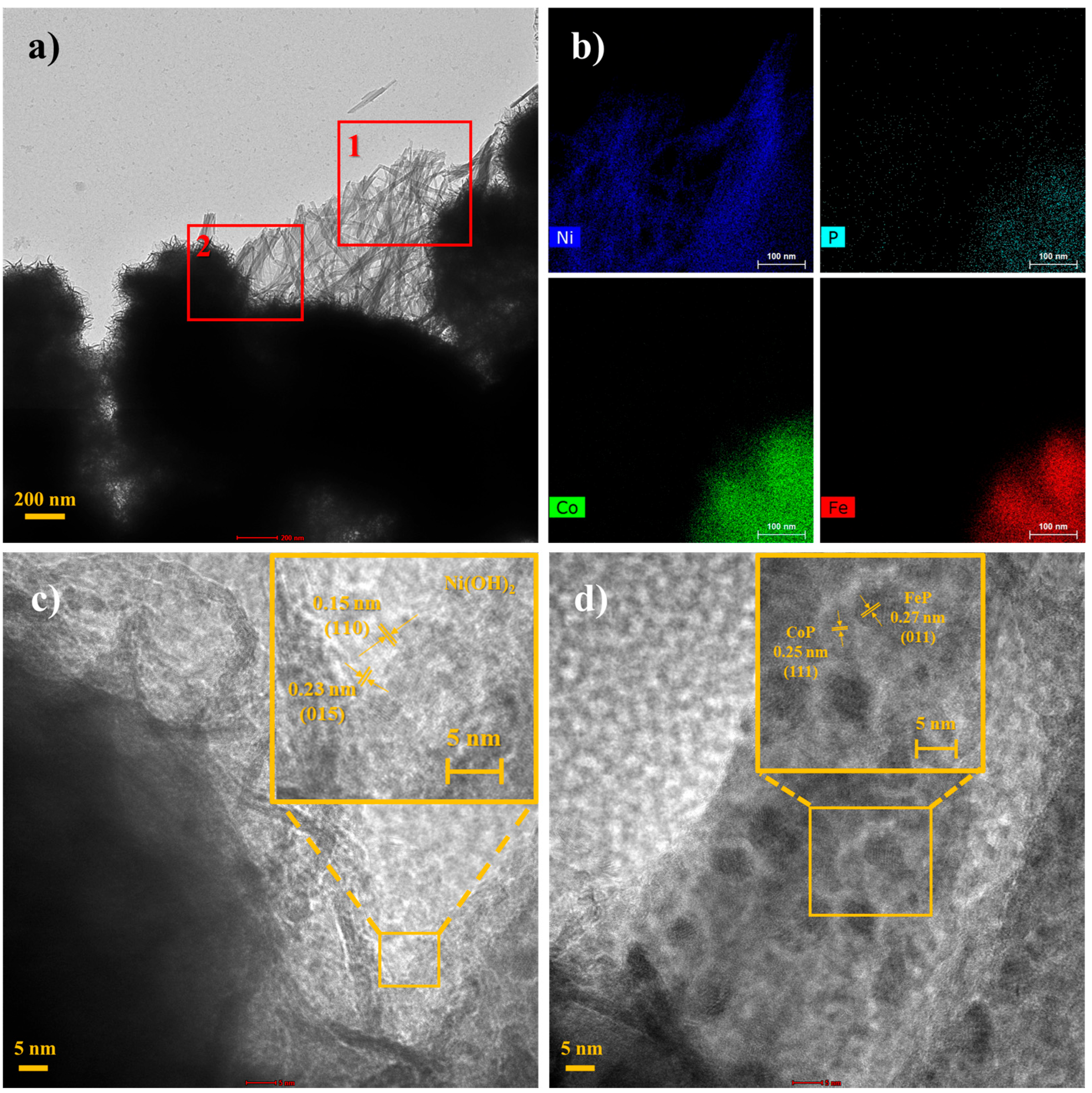
2.1. Material Characterization
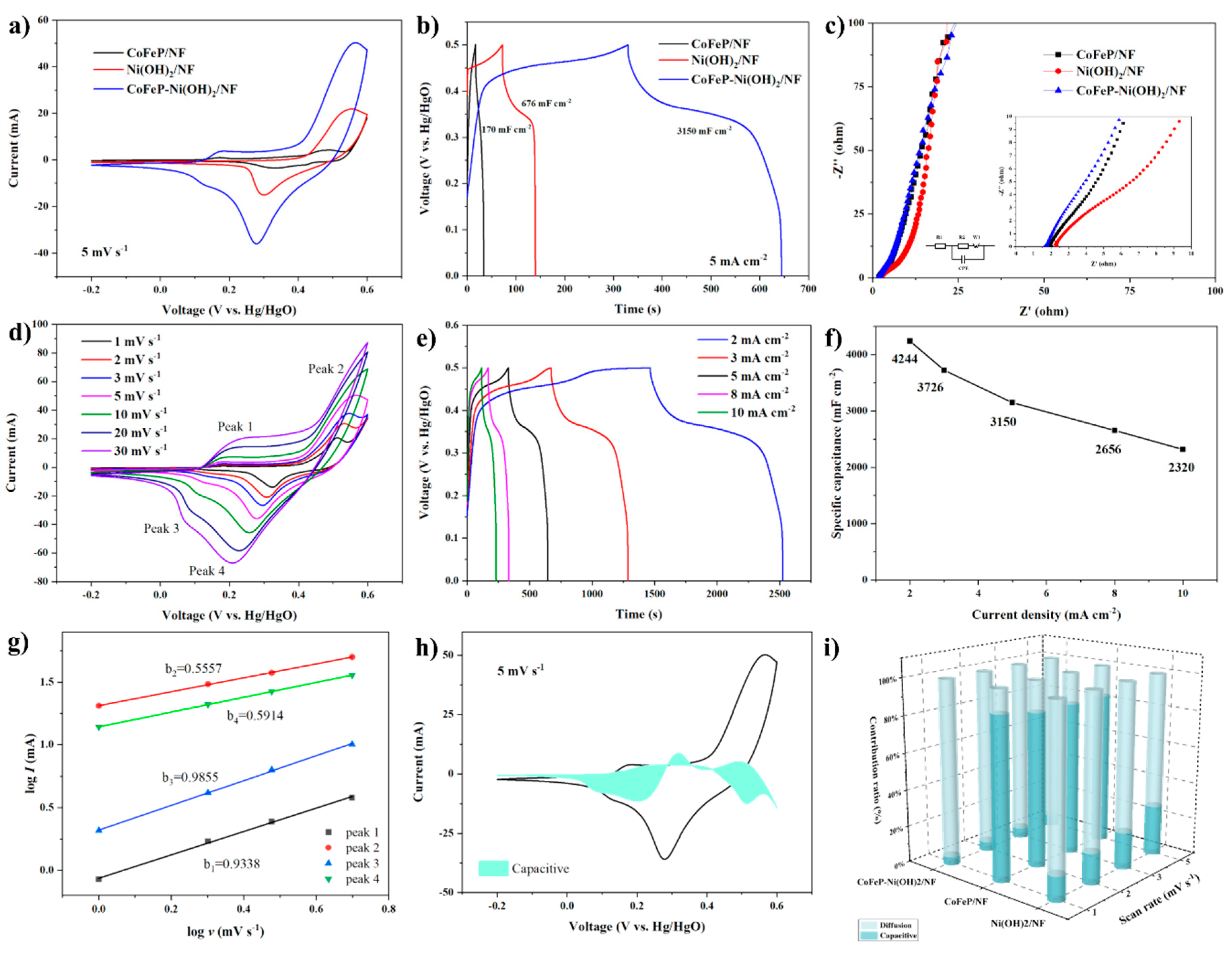
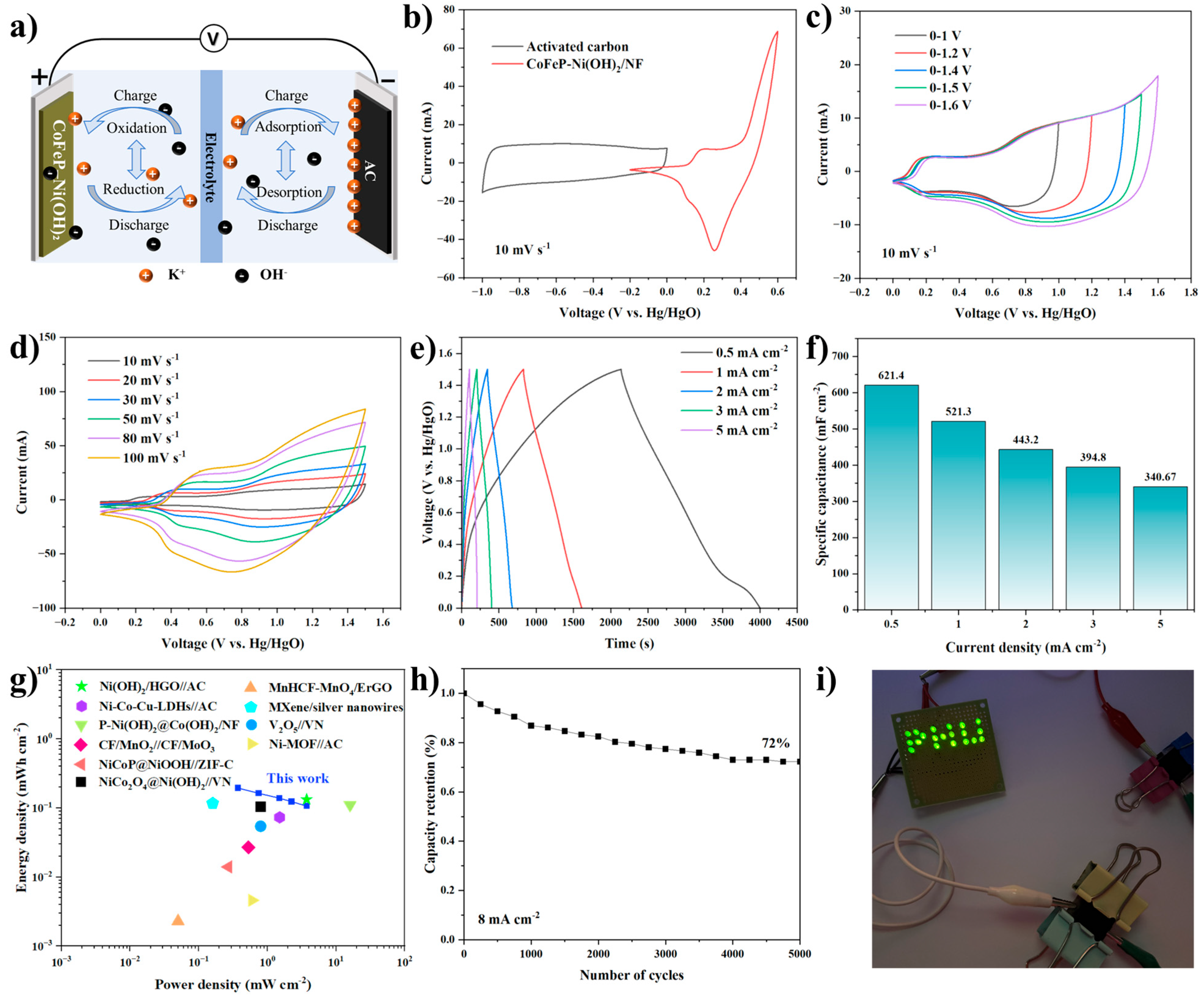
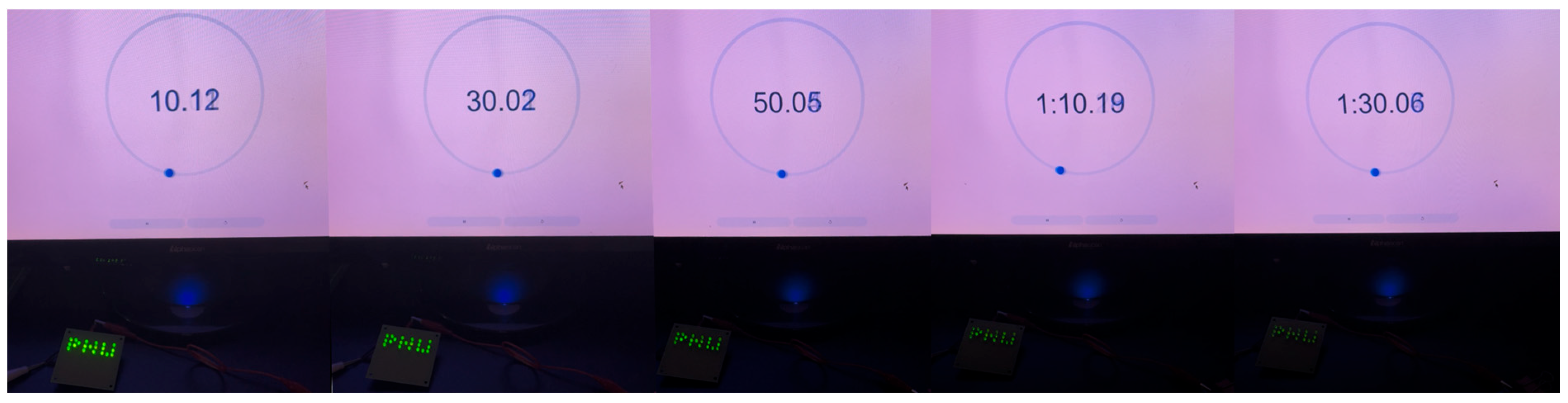
2.2. Electrochemical Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ni(OH)2/NF
3.3. Preparation of CoFeP/NF and CoFeP-Ni(OH)2/NF
3.4. Preparation of PVA/KOH Electrolyte
3.5. Preparation of Activated Carbon (AC) Electrode
3.6. Materials Characterization
3.7. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, W.; Yuan, M.; Wang, K.; Lian, X.; Niu, H. Flexible N, S-co-doped hollow porous carbon film for high-rate and long-cycle supercapacitors. Chem. Eng. J. 2025, 516, 163970. [Google Scholar] [CrossRef]
- Xiang, G.-T.; Chen, N.; Lu, B.; Xu, J.-L.; Rodriguez, R.D.; Sheremet, E.; Hu, Y.-D.; Chen, J.-J. Flexible solid-state Zn-Co MOFs@ MXene supercapacitors and organic ion hydrogel sensors for self-powered smart sensing applications. Nano Energy 2023, 118, 108936. [Google Scholar] [CrossRef]
- Chamberland, J.P.; Parent, J.S.; Barz, D.P. High-performance symmetric battery-supercapacitor hybrid using three oxidation states of TEMPO. Chem. Eng. J. 2025, 512, 162051. [Google Scholar] [CrossRef]
- Myrthong, B.; Ansari, S.; Choudhary, R.B. Bimetallic oxide-conducting polymer composites for robust supercapacitor performance and applications: A review. J. Energy Storage 2025, 117, 116182. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Li, L.; Lai, C.; Tang, Z.; Lou, X.; Ju, L.; Fu, J. Self-assembled microflower-like NiCo2X4 (X= O, S, Se) as electrodes for asymmetric supercapacitors. J. Alloys Compd. 2024, 973, 172913. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Li, D.; Dong, Y.; Hu, J.; Zhao, S.; Zhao, J.; Fu, Y.; He, D.; Li, J. High-performance aqueous asymmetric supercapacitor based on a composite cathode composed of MnCo2O4 and CoMnP4. J. Energy Storage 2025, 111, 115410. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Xie, Y.; Jiang, F.; Gao, X.; Bai, H.; Yao, F.; Yue, H. Vertically aligned graphene-MXene nanosheets based electrodes for high electrochemical performance asymmetric supercapacitor. Chem. Eng. J. 2024, 482, 149063. [Google Scholar] [CrossRef]
- Khan, A.J.; Ding, H.; Zhang, Y.; Zhang, D.; Gao, L.; Zhao, G. NiCo2O4@ MXene composite electrodes: Unveiling high-performance asymmetric supercapacitor capabilities through enhanced redox activity. Chem. Eng. J. 2025, 506, 160287. [Google Scholar] [CrossRef]
- Manikandan, E.; Muthulakshmi, M.; Abuthahir, S.; Papanasam, E.; Dubal, D.; Ansari, S.A. Synthesis and fabrication of MXene/copper selenide composite for high-performance asymmetric supercapacitor. J. Energy Storage 2025, 126, 117038. [Google Scholar] [CrossRef]
- Xu, X.; Lin, H.; Ding, J.; Zhou, P.; Ying, Y.; Jia, H.; Li, L.; Liu, Y. NiCoMn-LDH with core-shell heterostructures based on CoS nanotube arrays containing multiple ion diffusion channels for boosted supercapacitor applications. Green Energy Environ. 2024, 10, 780–792. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, Q.; Shu, X.; Hu, J.; Han, S. Photo-assisted charging of heterostructured NiCo2S4@ NiCo-LDH composite electrode with remarkable photoelectronic memory effect for high-performance asymmetric supercapacitor. Energy Conv. Manag. 2024, 315, 118769. [Google Scholar] [CrossRef]
- Chen, W.; Fu, M.; Yang, S.; Ning, L.; Yu, H.; Gao, M.; Wang, K.; Guan, M.; Lv, R.; Li, N. Cobalt phthalocyanine modified holey Graphene/Cobalt phosphide for supercapacitor electrodes. Chem. Eng. J. 2025, 514, 163341. [Google Scholar] [CrossRef]
- Torabi, M.; Shervedani, R.K.; Shahrokhi, S.M.; Khosravi, M.; Mohagheghnia, M.; Shalamzari, Y.H. Porous coral-like nickel-cobalt-phosphide composited with graphene nanosheets: A supercapacitive behavior. J. Energy Storage 2024, 102, 114083. [Google Scholar] [CrossRef]
- Natarajan, S.; Ulaganathan, M.; Aravindan, V. Building next-generation supercapacitors with battery type Ni(OH)2. J. Mater. Chem. A 2021, 9, 15542–15585. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Wang, F.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C.; Tong, Y.; Yang, G. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chu, X.; Zhang, H.; Zhang, B.; Su, H.; Jin, L.; Wang, Z.; Huang, H.; Yang, W. Synthesis of self-assembly 3D porous Ni(OH)2 with high capacitance for hybrid supercapacitors. Electrochim. Acta 2018, 269, 102–110. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.; Ali, M.A.; Chun, D.-M. Electrodeposition of CoSx-graphene nanoplatelets on Ni(OH)2 nanosheets for improving the pseudocapacitance performance. Appl. Surf. Sci. 2024, 673, 160903. [Google Scholar] [CrossRef]
- Li, G.; Ren, H.; Geng, C.; Wu, X. PPy-decorated ZnCo2O4@Ni(OH)2 electrode materials for flexible supercapacitor. J. Solid State Electrochem. 2024, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Gu, S.; Lu, Y.; Zhang, G.; Li, B.; Dou, F.; Cao, S.; Li, Q.; Sun, Y.; Shakouri, M. Stabilizing Ni2+ in hollow nano MOF/polymetallic phosphides composites for enhanced electrochemical performance in 3D-printed micro-supercapacitors. Adv. Mater. 2024, 36, 2401856. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Chen, H.; Zhu, S.; Zhao, W.; Chen, S.; Wen, Y.; Chen, J.; Ha, R.; Hao, Y.; Wang, J. Rational design of cobalt phosphide nanorods via hydrothermal-phosphorization for high-performance asymmetric supercapacitors. J. Alloys Compd. 2025, 1029, 180758. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, M.; Chen, D.; Wang, R. Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.S.; Numan, A.; Duraisamy, N.; Bashir, S.; Ramesh, K.; Ramesh, S. Ultrahigh capacitance of amorphous nickel phosphate for asymmetric supercapacitor applications. RSC Adv. 2016, 6, 76298–76306. [Google Scholar] [CrossRef]
- Deng, C.; He, J.; Wang, G.; Wang, K.; Dong, W.; Hong, X. Cyclic voltammetry activation for boosting the supercapacitance of trimetallic Ni-Co-Mn phosphides. Appl. Surf. Sci. 2023, 616, 156526. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Guo, W.; Chen, T.; Qiao, Y.; Mu, S.; Zhao, Y.; Gao, F. Honeycomb-like mesoporous cobalt nickel phosphate nanospheres as novel materials for high performance supercapacitor. Electrochim. Acta 2016, 190, 118–125. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, F.; Dang, Y.; Luan, J.; Li, M.; Shen, Z.; Han, D. Conformal phosphating hierarchical interface of CC/CoNiMn–P for hybrid supercapacitors with high cycling stability. Inorg. Chem. Front. 2025, 12, 3098–3109. [Google Scholar] [CrossRef]
- Anuratha, K.S.; Su, Y.-Z.; Huang, M.-K.; Hsieh, C.-K.; Xiao, Y.; Lin, J.-Y. High-performance hybrid supercapacitors based on electrodeposited amorphous bimetallic nickel cobalt phosphide nanosheets. J. Alloys Compd. 2022, 897, 163031. [Google Scholar] [CrossRef]
- Ye, R.; Sheng, Z.; Yang, P.; Xu, L.; Tao, Y.; Wu, X.; Cui, X. Ni-doped CoFeP as high-efficeint electrocatalysts for water-splitting. Electrochim. Acta 2024, 507, 145152. [Google Scholar] [CrossRef]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.-X.; Liu, T. A review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Lett. 2020, 12, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wei, F.; Lv, H.; Zhao, D.; Wang, N.; Li, L.; Li, N.; Wang, X. Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting. Materials 2021, 14, 1473. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Dou, M.; Liu, L.; Zhang, L.; Bai, X.; Yang, R.; Wang, H.; Dou, J. Facile Synthesis of Sandwich-Type Porous Structured Ni(OH)2/NCNWs/rGO Composite for High Performance Supercapacitor. Molecules 2025, 30, 1119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, J.; Song, S.; Dai, M.; Li, Y.; Zhang, D.; Zhao, D. Development of Zn-CoS@Ni(OH)2 Heterostructured Nanosheets for High-Performance Supercapacitors. Molecules 2024, 29, 6022. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wei, L.; Park, S.-J.; Kim, S. Electrochemical behaviors of binder-free CoFe-layered double-hydroxide-decorated hexagonal-flower-like NiCo2O4 as electrode for supercapacitors. J. Alloys Compd. 2023, 940, 168875. [Google Scholar] [CrossRef]
- Cui, M.; Park, S.-J.; Kim, S. Carboxylated group effect of graphene oxide on capacitance performance of Zr-based metal organic framework electrodes. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1939–1945. [Google Scholar] [CrossRef]
- Luo, C.-W.; Zhang, K.; Tang, Z.-H.; Zhang, M.-L.; Feng, B.; Zeng, H.-Y. Constructing a NiCoO/NiCoP Heterostructure with a Built-In Electric Field for High-Performance Supercapacitors. Inorg. Chem. 2025, 64, 9044–9052. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Cui, M.; Lim, J.Y.; Jung, Y.; Kim, S. Modulation of capacitive response for prussian blue analogue-based NiFeS2@ polydopamine@ NiCo2S4 core-shell composite electrodes of capacitors by a surface modification. Appl. Surf. Sci. 2025, 690, 162615. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, F.; Song, X.; Sha, X.; Zhou, H.; Zhang, X.; Liu, Z.; Yu, M.; Jiang, C. In-situ growth of Ni(OH)2 nanoplates on highly oxidized graphene for all-solid-state flexible supercapacitors. Chem. Eng. J. 2023, 456, 140947. [Google Scholar] [CrossRef]
- Liang, J.; Tian, B.; Li, S.; Jiang, C.; Wu, W. All-printed MnHCF-MnOx-based high-performance flexible supercapacitors. Adv. Energy Mater. 2020, 10, 2000022. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Nagaraju, G.; Ramulu, B.; Arbaz, S.J.; Narsimulu, D.; Hussain, S.K.; Yu, J.S. An eco-friendly hot-water therapy towards ternary layered double hydroxides laminated flexible fabrics for wearable supercapatteries. Nano Energy 2020, 76, 105016. [Google Scholar] [CrossRef]
- Cao, Z.; Liang, G.; Ho, D.; Zhi, C.; Hu, H. Interlayer Injection of Low-Valence Zn Atoms to Activate MXene-Based Micro-Redox Capacitors with Battery-Type Voltage Plateaus. Adv. Funct. Mater. 2023, 33, 2303060. [Google Scholar] [CrossRef]
- Li, K.; Zhao, B.; Bai, J.; Ma, H.; Fang, Z.; Zhu, X.; Sun, Y. A High-Energy-Density Hybrid Supercapacitor with P-Ni(OH)2@ Co(OH)2 Core–Shell Heterostructure and Fe2O3 Nanoneedle Arrays as Advanced Integrated Electrodes. Small 2020, 16, 2001974. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Park, S.-J.; Kim, S. Flexible N-doped heterostructure of cobalt sulfide and cobalt oxide composite electrodes derived from metal-organic frameworks and their electrochemical behaviors. Inorg. Chem. Commun. 2024, 159, 111805. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, H.; Zhang, Y.; Yu, S.; Malyi, O.I.; Zhao, X.; Wang, L.; Wang, H.; Peng, J.; Li, X. Direct coherent multi-ink printing of fabric supercapacitors. Sci. Adv. 2021, 7, eabd6978. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Sun, P.; Cui, G.; Tong, Y.; Mai, W. A flexible microsupercapacitor with integral photocatalytic fuel cell for self-charging. ACS Nano 2019, 13, 8246–8255. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Yoon, C.-M.; Kim, Y.K.; Jang, J. High performance asymmetric supercapacitor twisted from carbon fiber/MnO2 and carbon fiber/MoO3. Carbon 2017, 116, 470–478. [Google Scholar] [CrossRef]
- Yan, Y.; Gu, P.; Zheng, S.; Zheng, M.; Pang, H.; Xue, H. Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 19078–19085. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Liang, J.; Li, S.; Tian, B.; Yao, W.; Wu, W. Structure-designed fabrication of all-printed flexible in-plane solid-state supercapacitors for wearable electronics. J. Power Sources 2019, 425, 195–203. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Pei, M.; Kim, S. The Capacitive Property Enhancement of CoFeP-Ni(OH)2/Nickel Foam Electrodes via an Interfacial Integration Strategy for Asymmetric Supercapacitors. Molecules 2025, 30, 2986. https://doi.org/10.3390/molecules30142986
Cui M, Pei M, Kim S. The Capacitive Property Enhancement of CoFeP-Ni(OH)2/Nickel Foam Electrodes via an Interfacial Integration Strategy for Asymmetric Supercapacitors. Molecules. 2025; 30(14):2986. https://doi.org/10.3390/molecules30142986
Chicago/Turabian StyleCui, Meiying, Meiying Pei, and Seok Kim. 2025. "The Capacitive Property Enhancement of CoFeP-Ni(OH)2/Nickel Foam Electrodes via an Interfacial Integration Strategy for Asymmetric Supercapacitors" Molecules 30, no. 14: 2986. https://doi.org/10.3390/molecules30142986
APA StyleCui, M., Pei, M., & Kim, S. (2025). The Capacitive Property Enhancement of CoFeP-Ni(OH)2/Nickel Foam Electrodes via an Interfacial Integration Strategy for Asymmetric Supercapacitors. Molecules, 30(14), 2986. https://doi.org/10.3390/molecules30142986






