Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles
Abstract
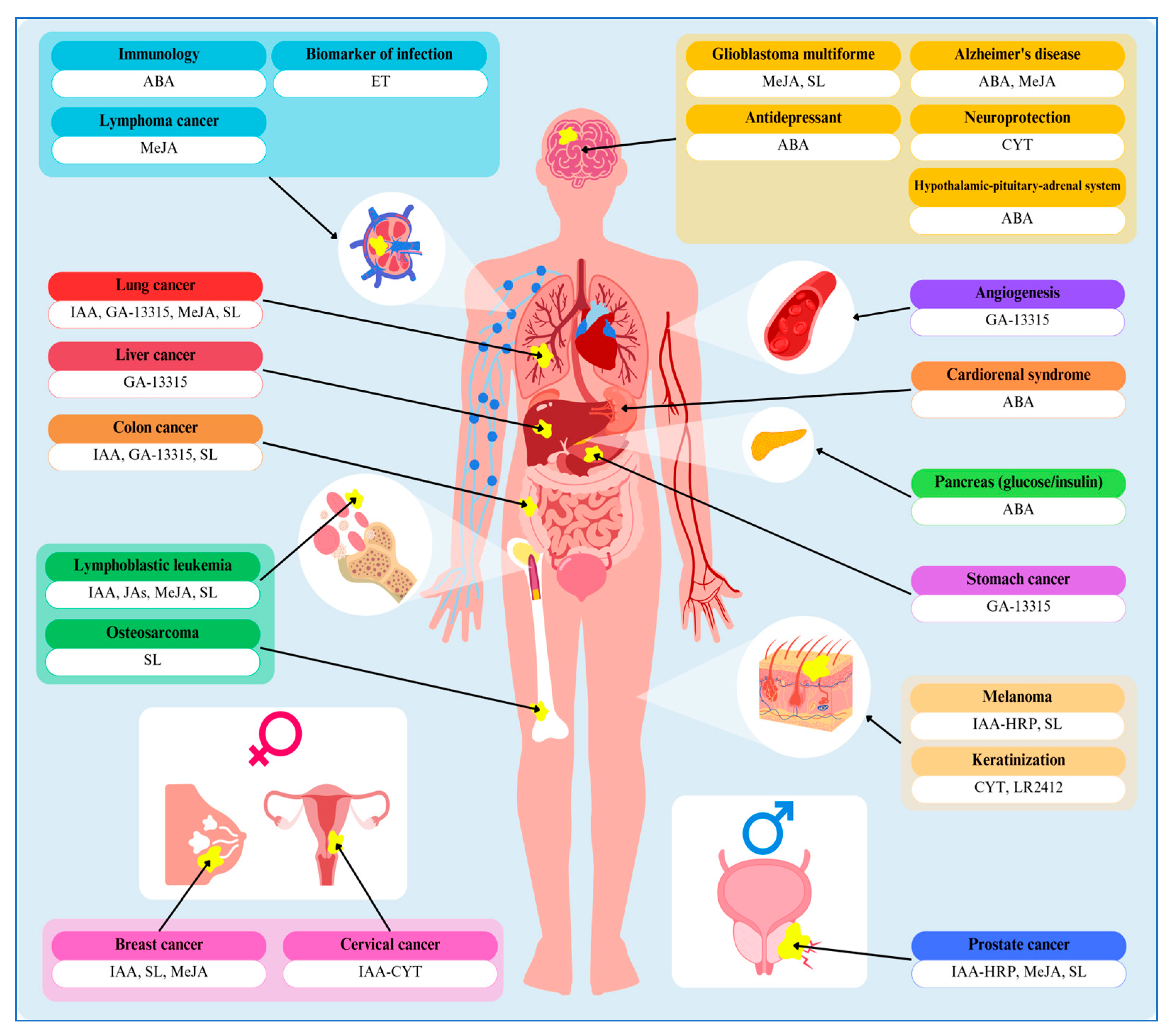
1. Introduction
2. Phytohormones Action
2.1. Auxin-Related Approaches for Cancer Therapies
2.2. Abscisic Acid as a Therapeutic Agent
2.3. Promising Effects of Cytokinins
2.4. Jasmonates—Battle Against Cancer
2.5. Ethylene—An Animal Stress Hormone
2.6. Strigolactones—Antiproliferative Agents
2.7. Dual Face of Gibberellins
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehin, M.; Bagchi, R.; Estelle, M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 2015, 27, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gazaryan, I.G.; Lagrimini, L.M.; Ashby, G.A.; Thorneley, R.N. Mechanism of indole-3-acetic acid oxidation by plant peroxidases: Anaerobic stopped-flow spectrophotometric studies on horseradish and tobacco peroxidases. Biochem. J. 1996, 313, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, H.; King, W.; Sjoerdsma, A.; Udenfriend, S. Formation of indole-3-acetic acid and tryptamine in animals: A method for estimation of indole-3-acetic acid in tissues. J. Biol. Chem. 1959, 234, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Fry, R.J.; Barr, S. Origin or urinary auxin in the germfree and conventional mouse. Am. J. Physiol. 1972, 222, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gan, M.; Li, J.; Li, H.; Su, M.; Tan, D.; Wang, S.; Jia, M.; Zhang, L.; Chen, G. Endogenous Indole Pyruvate Pathway for Tryptophan Metabolism Mediated by IL4I1. J Agric. Food Chem. 2020, 68, 10678–10684. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Dai, W. Aryl hydrocarbon receptor: Its roles in physiology. Biochem. Pharmacol. 2021, 185, 114428. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Medici, M.A.; Leonello, G.; Buemi, A.; Kohnke, F.H.; Villari, A.; Santoro, D.; Buemi, M. Auxin induces cell proliferation in an experimental model of mammalian renal tubular epithelial cells. Ren. Fail. 2015, 37, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Khirallah, M.G.; Ghafar, M.T.A. Diagnosis of acute appendicitis in children using urinary 5-hydroxy indol acetic acid and pediatric appendicitis score: A randomized control trial. Ann. Med. Surg. 2021, 65, 102274. [Google Scholar] [CrossRef] [PubMed]
- Katja, E.; Ćurković-Perica, M.; Kralj, M. The phytohormone auxin induces G1 cell-cycle arrest of human tumor cells. Planta Med. 2009, 75, 1423–1426. [Google Scholar] [CrossRef]
- Spadiut, O.; Herwig, C. Production and purification of the multifunctional enzyme horseradish peroxidase. Pharm. Bioprocess. 2013, 1, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Candeias, L.P.; Wardman, P. Toward targeted “oxidation therapy” of cancer: Peroxidase-catalyzed cytotoxicity of indole-3-acetic acids. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Candeias, L.P.; Folkes, L.K.; Porssa, M.; Parrick, J.; Wardman, P. Enhancement of lipid peroxidation by indole-3-acetic acid and derivatives: Substitutent effects. Free Radic. Res. 1995, 23, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Dennis, M.F.; Stratford, M.R.; Candeias, L.P.; Wardman, P. Peroxidase-catalyzed effects of indole-3-acetic acid and analogues on lipid membranes, DNA, and mammalian cells in vitro. Biochem. Pharmacol. 1999, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Kawano, N.; Hosoya, H.; Lapeyrie, F. Fungal auxin antagonist hypaphorine competitively inhibits indole-3-acetic acid-dependent superoxide generation by horseradish peroxidase. Biochem. Biophys. Res. Commun. 2001, 288, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Indole-3-acetic acids and horseradish peroxidase: A new prodrug/enzyme combination for targeted cancer therapy. Curr. Pharm. Des. 2002, 8, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jeon, S.E.; Park, K.C. Oxidation of indole-3-acetic acid by horseradish peroxidase induces apoptosis in G361 human melanoma cells. Cell. Signal. 2003, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Jeon, S.E.; Jeong, Y.M.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Hydrogen peroxide is a mediator of indole-3-acetic acid/horseradish peroxidase-induced apoptosis. FEBS Lett. 2006, 580, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, S.Y.; Jeong, Y.M.; Jeon, S.E.; Kim, M.K.; Kwon, S.B.; Na, J.I.; Park, K.C. Light-activated indole-3-acetic acid induces apoptosis in G361 human melanoma cells. Biol. Pharm. Bull. 2006, 29, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Ryu, J.S.; Li, H.; Park, W.J.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Sohn, U.D.; Kim, D.S. UVB-activated indole-3-acetic acid induces apoptosis of PC-3 prostate cancer cells. Anticancer Res. 2010, 30, 4607–4612. [Google Scholar] [PubMed]
- Huang, P.; Oliff, A. Signaling pathways in apoptosis as potential targets for cancer therapy. Trends Cell Biol. 2001, 11, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species- a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 2001, 61, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.M.; Oh, M.K.; Kim, S.Y.; Li, H.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, W.Y.; Kim, D.S. Indole-3-acetic acid/horseradish peroxidase induces apoptosis in TCCSUP human urinary bladder carcinoma cells. Pharmazie 2010, 65, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tupper, J.; Stratford, M.R.; Hill, S.; Tozer, G.M.; Dachs, G.U. In vivo characterization of horseradish peroxidase with indole-3-acetic acid and 5-bromoindole-3-acetic acid for gene therapy of cancer. Cancer Gene Ther. 2010, 17, 420–428. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.P.; de Lima, T.M.; Pithon-Curi, T.C.; Curi, R. The mechanism of indole acetic acid cytotoxicity. Toxicol. Lett. 2004, 148, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, M.; Papeš, D.; Nagy, B. 4-Dichlorophenoxyacetic acid causes chromatin and chromosome abnormalities in plant cells and mutation in cultured mammalian cells. Mutat. Res. 1991, 263, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, E.; Yamaguchi, S.; Murofushi, N. Increase of indole-3-acetic acid in human esophageal cancer tissue. Proc. Jpn. Acad. 1997, 73, 182–185. [Google Scholar] [CrossRef]
- Yamaki, T.; Shimojo, E.; Ichimura, H.; Nishinari, N.; Namekawa, K.; Yoshizawa, K. Possible role of indoleacetic acid in animal cell division and cancer growth. J. UOEH 1979, 1, 471–485. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Bueno, I.; González-Rodríguez, V.E.; Simon, A.; Dalmais, B.; Pradier, J.M.; Le Pêcheur, P.; Mercier, A.; Walker, A.S.; Garrido, C.; Collado, I.G.; et al. Biosynthesis of abscisic acid in fungi: Identification of a sesquiterpene cyclase as the key enzyme in Botrytis cinerea. Environ. Microbiol. 2018, 20, 2469–2482. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.M.; Levy, A. “What I cannot create, I do not understand”: Elucidating microbe-microbe interactions to facilitate plant microbiome engineering. Curr. Opin. Microbiol. 2023, 72, 102283. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Carpaneto, A.; Cerrano, C.; Bavestrello, G.; Giovine, M.; Bruzzone, S.; Guida, L.; Franco, L.; Usai, C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 2001, 98, 14859–14864. [Google Scholar] [CrossRef] [PubMed]
- Puce, S.; Basile, G.; Bavestrello, G.; Bruzzone, S.; Cerrano, C.; Giovine, M.; Arillo, A.; Zocchi, E. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J. Biol. Chem. 2004, 279, 39783–39788. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Moreschi, I.; Usai, C.; Guida, L.; Damonte, G.; Salis, A.; Scarfi, S.; Millo, E.; De Flora, A.; Zocchi, E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. USA 2007, 104, 5759–5764. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfi, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Battaglia, F.; Mannino, E.; Parodi, A.; Fruscione, F.; Basile, G.; Salis, A.; Sturla, L.; Negrini, S.; Kalli, F.; et al. Abscisic acid ameliorates the systemic sclerosis fibroblast phenotype in vitro. Biochem. Biophys. Res. Commun. 2012, 422, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.J.; Rogers, L.J. Compartmentation of terpenoid biosynthesis in green plants: A proposed route of acetyl-coenzyme A synthesis in maize chloroplasts. Biochem. J. 1969, 114, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Ferrer, G.; Casagran, O.; Wankhade, I.; Noble, A.M.; Eizirik, D.L.; Ortis, F.; Cnop, M.; Liu, D.; et al. Loss of PPARg in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J. Nutr. Biochem. 2008, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.; Fresia, C.; Guida, L.; Bruzzone, S.; Scarfì, S.; Usai, C.; Fruscione, F.; Magnone, M.; Millo, E.; Basile, G.; et al. LANCL2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J. Biol. Chem. 2009, 284, 28045–28057. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hontecillas, R.; Philipson, C.W.; Bassaganya-Riera, J. Lanthionine synthetase component C-like protein 2: A new drug target for inflammatory diseases and diabetes. Curr. Drug Targets 2014, 15, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Elmasry, A.; El-Nablaway, M.; Othman, G.; Hamed, S.; Khater, Y.; Ashour, R.H.; Hendawy, M.; Rabei, M.R. Cardioprotective effect of abscisic acid in a rat model of type 3 cardio-renal syndrome: Role of NOX-4, P-53, and HSP-70. Biomed. Pharmacother. 2022, 157, 114038. [Google Scholar] [CrossRef] [PubMed]
- Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin. Nutr. 2010, 29, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Le Page-Degivry, M.T.; Bidard, J.N.; Rouvier, E.; Bulard, C.; Lazdunski, M. Presence of abscisic acid, a phytohormone, in the mammalian brain. Proc. Natl. Acad. Sci. USA 1986, 83, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.C.; Ge, J.F.; Zhou, J.N. Preliminary evidence that abscisic acid improves spatial memory in rats. Physiol. Behav. 2015, 139, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.C.; Zhang, Z.; Fang, H.; Liu, J.; Zhou, N.; Ge, J.F.; Chen, F.H.; Xiang, C.B.; Zhou, J.N. Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu006. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Fernández, V.; Mañas-Ojeda, A.; Pacheco-Herrero, M.; Castro-Salazar, E.; Ros-Bernal, F.; Sánchez-Pérez, A. Early intervention with ABA prevents neuroinflammation and memory impairment in a triple transgenic mice model of Alzheimer’s disease. Behav. Brain Res. 2019, 374, 112106. [Google Scholar] [CrossRef] [PubMed]
- Naderi, R.; Esmaeili-Mahani, S.; Abbasnejad, M. Phosphatidylinositol-3-kinase and protein kinase C are involved in the pro-cognitive and anti-anxiety effects of phytohormone abscisic acid in rats. Biomed. Pharmacother. 2017, 96, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Green, C.L.; Lyons, L.C. PKA and PKC are required for long-term but not short-term in vivo operant memory in Aplysia. Learn Mem 2010, 18, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Q.; Yan, J.Z.; Zhang, X.Y.; Bu, Y.F.; Pan, W.W.; Yao, W.; Tian, T.; Lu, W. PKCλ is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 2013, 32, 1365–1380. [Google Scholar] [CrossRef] [PubMed]
- Scarfì, S.; Ferraris, C.; Fruscione, F.; Fresia, C.; Guida, L.; Bruzzone, S.; Usai, C.; Parodi, A.; Millo, E.; Salis, A.; et al. Cyclic ADP-ribose-mediated expansion and stimulation of human mesenchymal stem cells by the plant hormone abscisic acid. Stem Cells 2008, 26, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Gibb, M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. The Origins and Roles of Methylthiolated Cytokinins: Evidence From Among Life Kingdoms. Front. Cell Dev. Biol. 2020, 8, 605672. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Seegobin, M.; Kisiala, A.; Noble, A.; Kaplan, D.; Brunetti, C.; Emery, R.J.N. Canis familiaris tissues are characterized by different profiles of cytokinins typical of the tRNA degradation pathway. FASEB J. 2018, 32, 6575–6581. [Google Scholar] [CrossRef] [PubMed]
- Andreas, P.; Kisiala, A.; Emery, R.J.N.; De Clerck-Floate, R.; Tooker, J.F.; Price, P.W.; Miller, D.G., III; Chen, M.S.; Connor, E.F. Cytokinins Are Abundant and Widespread Among Insect Species. Plants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, J.; Vrabka, J.; Oeser, B.; Novak, O.; Galuszka, P.; Tudzynski, P. De novo biosynthesis of cytokinins in the biotrophic fungus Claviceps purpurea. Environ. Microbiol. 2015, 17, 2935–2951. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Radakovic, Z.S.; De La Torre, C.M.; Chronis, D.; Novák, O.; Ramireddy, E.; Holbein, J.; Matera, C.; Hütten, M.; Gutbrod, P.; et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Reiter, V.; Matschkal, D.M.; Wagner, M.; Globisch, D.; Kneuttinger, A.C.; Müller, M.; Carell, T. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012, 40, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.M.; Seegobin, M.; Kisiala, A.; Noble, A.; Brunetti, C.; Emery, R.J.N. Phytohormone metabolism in human cells: Cytokinins are taken up and interconverted in HeLa cell culture. FASEB Bioadv. 2019, 1, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, E.; Abate, M.; Laezza, C.; Pisanti, S.; Vitale, M.; Seneca, V.; Torelli, G.; Franceschelli, S.; Catapano, G.; Gazzerro, P.; et al. Antiglioma effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int. J. Cancer 2017, 140, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Pastorino, O.; Della Monica, R.; Buonaiuto, M.; Torelli, G.; Caiazzo, P.; Bifulco, M.; et al. N6-isopentenyladenosine induces cell death through necroptosis in human glioblastoma cells. Cell Death Discov. 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Mittelman; Evans, J.T.; Chheda, G.B. Cytokinins as chemotherapeutic agents. Ann. N. Y. Acad. Sci. 1975, 255, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Hori, Y.; Sakai, S.; Honma, Y. Control of differentiation and apoptosis of human myeloid leukemia cells by cytokinins and cytokinin nucleosides, plant redifferentiation-inducing hormones. Cell Growth Differ. 2002, 13, 19–26. [Google Scholar] [PubMed]
- Zhao, L.; Liu, P.; Guo, G.; Wang, L. Combination of cytokinin and auxin induces apoptosis, cell cycle progression arrest, and blockage of the Akt pathway in HeLa cells. Mol. Med. Rep. 2012, 12, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Grúz, J.; D’Acunto, C.W.; Kaňovský, P.; Strnad, M. Cytokinin plant hormones have neuroprotective activity in in vitro models of Parkinson’s disease. Molecules 2021, 26, 361. [Google Scholar] [CrossRef] [PubMed]
- Berge, U.; Kristensen, P.; Rattan, S.I. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann. N. Y. Acad. Sci. 2006, 1067, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P.; Doležel, P. Apoptosis induced by N6-substituted derivatives of adenosine is related to intracellular accumulation of corresponding mononucleotides in HL-60 cells. Toxicol. Vitro 2005, 19, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Voller, J.; Béres, T.; Zatloukal, M.; Kaminski, P.A.; Niemann, P.; Doležal, K.; Džubák, P.; Hajdúch, M.; Strnad, M. The natural cytokinin 2OH3MeOBAR induces cell death by a mechanism that is different from that of the “classical” cytokinin ribosides. Phytochemistry 2017, 136, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.M.; Naseem, M.; Awad, E.; Dandekar, T.; Stopper, H. The plant hormone cytokinin confers protection against oxidative stress in mammalian cells. PLoS ONE 2016, 11, e0168386. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.; Sodagam, L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuvenation Res. 2005, 8, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Liechti, R.; Farmer, E.E. The Jasmonate pathway. Science 2002, 296, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Stumpe, M.; Goebel, C.; Demchenko, K.; Hoffmann, M.; Kloesgen, R.B.; Pawlowski, K. Identification of an allene oxide synthase (CYP74C) that leads to formation of alpha-ketols from 9-hydroperoxides of linoleic and linolenic acid in below-ground organs of potato. Plant J. 2006, 47, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Brodhun, F.; Cristobal-Sarramian, A.; Zabel, S.; Newie, J.; Hamberg, M.; Feussner, I. An iron 13S-lipoxygenase with an α-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum. PLoS ONE 2013, 8, e64919. [Google Scholar] [CrossRef] [PubMed]
- Oliw, E.H.; Hamberg, M. An allene oxide and 12-oxophytodienoic acid are key intermediates in jasmonic acid biosynthesis by Fusarium oxysporum. J. Lipid Res. 2017, 58, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pagac, M.; Yang, F.; Patkar, R.N.; Naqvi, N.I. Fungal Jasmonate as a Novel Morphogenetic Signal for Pathogenesis. J. Fungi 2021, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Monte, I.; Zamarreño, A.M.; García-Mina, J.M.; Solano, R. Evolution of the jasmonate ligands and their biosynthetic pathways. New Phytol. 2023, 238, 2236–2246. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Marin, J.E.; Zienkiewicz, K.; Gutiérrez-Rojas, M.; Favela-Torres, E.; Feussner, I. Jasmonic acid biosynthesis by fungi: Derivatives, first evidence on biochemical pathways and culture conditions for production. PeerJ 2021, 5, e10873. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism; signaling, and transport of jasmonates. Plant Commun. 2021, 11, 100231. [Google Scholar] [CrossRef] [PubMed]
- Sá-Nakanishi, A.B.; Soni-Neto, J.; Moreira, L.S.; Gonçalves, G.A.; Silva, F.M.S.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-inflammatory and antioxidant actions of methyl jasmonate are associated with metabolic modifications in the liver of arthritic rats. Oxid. Med. Cell. Longev. 2018, 2018, 2056250. [Google Scholar] [CrossRef] [PubMed]
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The anticancer activities of jasmonates. Cancer Chemother. Pharmacol. 2013, 71, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Fingrut, O.; Flescher, E. Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells. Leukemia 2002, 16, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Cesari, I.M.; Carvalho, E.; Rodrigues, M.F.; Mendonça, B.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl jasmonate: Putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef] [PubMed]
- Bömer, M.; Pérez-Salamó, I.; Florance, H.V.; Salmon, D.; Dudenhoffer, J.H.; Finch, P.; Cinar, A.; Smirnoff, N.; Harvey, A.; Devoto, A. Jasmonates induce Arabidopsis bioactivities selectively inhibiting the growth of breast cancer cells through CDC6 and mTOR. New Phytol. 2021, 229, 2120–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, L.; Xiao, Z.; Liu, X.; Liu, X. Methyl jasmonate induces apoptosis and pro-apoptotic autophagy via the ROS pathway in human non-small cell lung cancer. Am. J. Cancer Res. 2016, 6, 187–199. [Google Scholar] [PubMed]
- Rotem, R.; Fingrut, O.; Moskovitz, J.; Flescher, E. The anticancer plant stress-protein methyl jasmonate induces activation of stress-regulated c-Jun N-Terminal kinase and p38 protein kinase in human lymphoid cells. Leukemia 2003, 17, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.Y.; Oh, S.Y.; Han, S.I.; Park, H.G.; Yoo, M.A.; Kang, H. Methyl jasmonate induces apoptosis through induction of Bax/Bcl-XS and activation of caspase-3 via ROS production in A549 cells. Oncol. Rep. 2004, 12, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005, 65, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, E.; Pedersen, P.L. High aerobic glycolysis of rat hepatoma cells in culture: Role of mitochondrial hexokinase. Proc. Natl. Acad. Sci. USA 1977, 74, 3735–3739. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Flescher, E. Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl jasmonate binds to and detaches mitochondria-bound hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; McMillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, C.K.; Mitsiades, C.S. Methyl jasmonate displays in vitro and in vivo activity against multiple myeloma cells. Br. J. Haematol. 2012, 159, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.; Wang, F.; Dai, W.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Xu, S.; Xia, Y.; et al. Methyl jasmonate leads to necrosis and apoptosis in hepatocellular carcinoma cells via inhibition of glycolysis and represses tumor growth in mice. Oncotarget 2017, 8, 45965–45980. [Google Scholar] [CrossRef] [PubMed]
- Uludağ, D.; Bay, S.; Sucu, B.O.; Şavluğ, İ.Ö.; Mohr, T.; Güzel, M.; Karakaş, N. Potential of novel methyl jasmonate analogs as anticancer agents to metabolically target hk-2 activity in glioblastoma cells. Front. Pharmacol. 2022, 13, 828400. [Google Scholar] [CrossRef] [PubMed]
- Sucu, B.O.; İpek, Ö.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of novel methyl jasmonate derivatives and evaluation of their biological activity in various cancer cell lines. Bioorg. Chem. 2019, 91, 103146. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Lee, N.H.; Hyun, C.G. Methyl jasmonate inhibits lipopolysaccharide-induced inflammatory cytokine production via mitogen-activated protein kinase and nuclear factor-κB pathways in RAW 264.7 cells. Pharmazie 2016, 71, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Solomon, U.; Taghogho, E.A. Methyl jasmonate attenuates memory dysfunction and decreases brain levels of biomarkers of neuroinflammation induced by lipopolysaccharide in mice. Brain Res. Bull. 2017, 131, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New jasmonate analogues as potential anti-inflammatory agents. Bioorg. Med. Chem. 2008, 16, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Maeng, K.; Dang, H.T.; Kang, G.J.; Ryou, C.; Jung, J.H.; Kang, H.K.; Prchal, J.T.; Yoo, E.S.; Yoon, D. Anti-inflammatory effect of methyl dehydrojasmonate (J2) is mediated by the NF-κB pathway. J. Mol. Med. 2011, 89, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Michelet, J.F.; Simonetti, L.; Fiat, F.; Garrigues, A.; Potter, A.; Segot, E.; Watson, R.E.; Griffiths, C.E.; de Lacharrière, O. In vitro and in vivo studies with tetra-hydro-jasmonic acid (LR2412) reveal its potential to correct signs of skin ageing. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Michelet, J.F.; Olive, C.; Rieux, E.; Fagot, D.; Simonetti, L.; Galey, J.B.; Dalko-Csiba, M.; Bernard, B.A.; Pereira, R. The anti-ageing potential of a new jasmonic acid derivative (LR2412): In Vitro evaluation using reconstructed epidermis Episkin™. Exp. Dermatol. 2012, 21, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Kapuscinska, A.; Olejnik, A.; Nowak, I. The conjugate of jasmonic acid and tetrapeptide as a novel promising biologically active compound. New J. Chem. 2016, 40, 9007–9011. [Google Scholar] [CrossRef]
- Gilles, P.; Moussou, P.; Contet-Audonneau, J.L.; Danoux, L.; Freis, O.; Sabadotto, M.; Benoit, I.; Misery, L.; Rathjens, A. A new peptidic active ingredient to reduce discomfort and painful sensations in sensitive skin. Int. J. Cosmet. Sci. 2009, 12, 25–30. [Google Scholar] [CrossRef]
- Luckhardt, A.B. Ethylene. J. Am. Med. Assoc. 1924, 83, 2060. [Google Scholar] [CrossRef]
- Dillard, M.M. Ethylene—The New General Anesthetic. J. Natl. Med. Assoc. 1930, 22, 10–11. [Google Scholar] [PubMed]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Chagué, V.; Elad, Y.; Barakat, R.; Tudzynski, P.; Sharon, A.A. Ethylene biosynthesis in Botrytis cinerea. FEMS Microbiol. Ecol. 2002, 40, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, F.; Girelli, G.; Aor, B.; Marchioretti, C.; Pedrotti, M.; Perli, T.; Tonon, E.; Valentini, V.; Avi, D.; Ferrentino, G.; et al. Ethylene-producing bacteria that ripen fruit. ACS Synth. Biol. 2014, 3, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Hochstein, P. Ethylene formation in rat liver microsomes. Science 1966, 152, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A.; Hopfer, S.M.; Reid, M.C.; Wong, S.H.; Sunderman, F.W., Jr. Ethene (ethylene) and ethane exhalation in Ni[II]-treated rats, using an improved rebreathing apparatus. Ann. Clin. Lab. Sci. 1986, 16, 386–394. [Google Scholar] [PubMed]
- Stolik, S.; Ramon-Gallegos, E.; Pacheco, M. Photoacoustic measurement of ethylene as a real-time biomarker of lipid peroxidation processes in mice. Anal. Sci. 2001, 17, 365–367. [Google Scholar] [CrossRef]
- Lieberman, M.; Kunishi, A.; Mapson, L.; Wardale, D. Ethylene production from methionine. Biochem. Pharmacol. 1964, 97, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Kessler, W.; Remmer, H. Generation of volatile hydrocarbons from amino acids and proteins by an iron/ascorbate/GSH system. Biochem. Pharmacol. 1990, 39, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Harren, F.J.M.; Berkelmans, R.; Kuiper, K.; Hakkert, S.T.L.; Scheepers, P.; Dekhuijzen, R.; Hollander, P.; Parker, D.H. On-line laser photoacoustic detection of ethene in exhaled air as a biomarker of ultraviolet radiation damage of the human skin. Appl. Phys. Lett. 1999, 74, 1761. [Google Scholar] [CrossRef]
- Laurent, M.P.; Bogaart, G.V.D.; Kox, M.; Dingjan, I.; Neerincx, A.H.; Bendix, M.B.; Beest, M.T.; Harren, F.J.M.; Risby, T.; Pickkers, P.; et al. Ethylene, an early marker of systemic inflammation in humans. Sci. Rep. 2017, 7, 6889. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, S.M.; Kiss, R.; Hekkert, S.T.L.; Dalby, M.; Harren, F.J.M.; Risby, T.H.; Marczin, N. Real-time monitoring of endogenous lipid peroxidation by exhaled ethylene in patients undergoing cardiac surgery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Krasko, A.; Schröder, H.C.; Perovic, S.; Steffen, R.; Kruse, M.; Reichert, W.; Müller, I.M.; Müller, W.E.G. Ethylene modulates gene expression in cells of the marine sponge Suberites domuncula and reduces the degree of apoptosis. J. Biol. Chem. 1999, 274, 31524–31530. [Google Scholar] [CrossRef] [PubMed]
- Perovic, S.; Seack, J.; Gamulin, V.; Müller, W.; Schröder, H. Modulation of intracellular calcium and proliferative activity of invertebrate and vertebrate cells by ethylene. BMC Cell Biol. 2001, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Delaux, P.M.; Xie, X.; Timme, R.E.; Puech-Pages, V.; Dunand, C.; Lecompte, E.; Delwiche, C.F.; Yoneyama, K.; Bécard, G.; Séjalon-Delmas, N. Origin of strigolactones in the green lineage. New Phytol. 2012, 195, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Delaux, P.M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Sejalon-Delmas, N.; Combier, J.P.; Becard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.B.; Koltai, H.; Kapulnik, Y.; Prandi, C.; Yarden, R.I. Strigolactones: A novel class of phytohormones that inhibit the growth and survival of breast cancer cells and breast cancer stem-like enriched mammosphere cells. Breast Cancer Res. Treat. 2012, 134, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Antika, G.; Cinar, Z.; Seçen, E.; Özbil, M.; Tokay, E.; Köçkar, F.; Prandi, C.; Tumer, T.B. Strigolactone analogs: Two new potential bioactiphores for glioblastoma. ACS Chem. Neurosci. 2022, 13, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.B.; McDonough, S.; Wang, V.S.; Lee, H.; Ringer, L.; Li, X.; Prandi, C.; Lee, R.J.; Feldman, A.S.; Koltai, H.; et al. Strigolactone analogues induce apoptosis through activation of p38 and the stress response pathway in cancer cell lines and in conditionally reprogrammed primary prostate cancer cells. Oncotarget 2014, 5, 1683–1698. [Google Scholar] [CrossRef] [PubMed]
- Croglio, M.P.; Haake, J.M.; Ryan, C.P.; Wang, V.S.; Lapier, J.; Schlarbaum, J.P.; Dayani, Y.; Artuso, E.; Prandi, C.; Koltai, H.; et al. Analogs of the novel phytohormone, strigolactone, trigger apoptosis and synergize with PARP inhibitors by inducing DNA damage and inhibiting DNA repair. Oncotarget 2016, 7, 13984–14001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayzlish-Gati, E.; Laufer, D.; Grivas, C.F.; Shaknof, C.; Sananes, A.; Bier, A.; Ben-Harosh, S.; Belausov, E.; Johnson, M.D.; Artuso, E.; et al. Strigolactone analogs act as new anti-cancer agents in inhibition of breast cancer in xenograft model. Cancer Biol. Ther. 2015, 16, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.X.; Han, Y.S.; Wang, J.C.; Yang, H.; Kong, H.; Liu, K.J.; Chen, S.Y.; Chen, Y.R.; Chang, Y.Q.; Chen, W.M.; et al. Strigolactones: A plant phytohormone as novel anti-inflammatory agents. MedChemComm 2018, 9, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kurt, B.; Ozleyen, A.; Antika, G.; Yilmaz, Y.B.; Tumer, T.B. Multitarget profiling of a strigolactone analogue for early events of Alzheimer’s disease: In vitro therapeutic activities against neuroinflammation. ACS Chem. Neurosci. 2020, 11, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Yaluri, N.; Kokkola, T.; Laakso, M. Plant-derived compounds strigolactone GR24 and pinosylvin activate SIRT1 and enhance glucose uptake in rat skeletal muscle cells. Sci. Rep. 2017, 7, 17606. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C. Gibberellin signaling in plants—The extended version. Front. Plant Sci. 2012, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.E.; Mantawy, M.M.; Hassan, H.M. Biochemical and molecular profiles of gibberellic acid exposed albino rats. J. Am. Sci. 2010, 6, 224–229. [Google Scholar]
- Muthu, S.; Muthuraman, P.; Muthuviveganandavel, V.; Srikumar, K. Acute effect of gibberellic acid on serum enzymes and blood markers in male albino wistar rats. Int. J. Drug Deliv. 2011, 3, 340–347. [Google Scholar]
- Hosseinchi, M.; Soltanalinejad, F.; Najafi, G.; Roshangar, L. Effect of gibberellic acid on the quality of sperm and in vitro fertilization outcome in adult male rats. Vet. Res. Forum 2013, 4, 259–264. [Google Scholar] [PubMed]
- Kamel, K.I.; Elkomy, A.E.; El-Sbeiy, M.E. The androgenic action of gibberellic acid (GA3) on the reproductive performance of New Zealand white rabbit bucks. World J. Agr. Sci. 2009, 5, 40–48. [Google Scholar]
- Celik, I.; Tuluce, Y.; Isik, I. Evaluation of toxicity of abscisic acid and gibberellic acid in rats: 50 days drinking water study. J. Enzym. Inhib. Med. Chem. 2007, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.F.; Farahat, F.Y.; Abass, M.A.; Shehata, A.S. Hepatotoxic potential of gibberellic acid (GA3) in adult male albino rats. Life Sci. J. 2011, 8, 373–383. [Google Scholar]
- Hussein, N.; Farag, A.I.; Mohammed, H.O. Histological and immunohistochemical study of the toxic effect of gibberellic acid postnatally on the renal cortex of albino rats. Egypt. J. Histol. 2020, 43, 1070–1086. [Google Scholar] [CrossRef]
- El-Mofty, M.M.; Sakr, S.A. Induction of neoplasms in the egyptian toad Bufo regularis by gibberellin A3. Oncology 1988, 45, 61–64. [Google Scholar] [CrossRef] [PubMed]
- El-Mofty, M.M.; Sakr, S.A.; Rizk, A.M.; Moussa, E.A. Carcinogenic effect of gibberellin A3 in Swiss Albino Mice. Nutr. Cancer 1994, 21, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Zalinian, G.G.; Arutiumian, R.M.; Sarkisian, G.G. The cytogenetic effect of natural mutagenesis modifiers in a human lymphocyte culture. The action of amino benzamide during the gibberellic acid induction of chromosome aberrations. Tsitol Genet 1990, 24, 31–34. [Google Scholar] [PubMed]
- Sakr, S.A.; Hassab-Elnabi, S.E.; El-Ghonaimy, D.A. Effect of green tea on cytogenetic changes induced by gibberellin A3 in human lymphocyte culture. Can. J. Appl. Sci. 2009, 3, 917–924. [Google Scholar]
- Chen, J.; Sun, Z.; Zhang, Y.; Zeng, X.; Qing, C.; Liu, J.; Li, L.; Zhang, H. Synthesis of gibberellin derivatives with anti-tumor bioactivities. Bioorg. Med. Chem. Lett. 2009, 19, 5496–5499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Chen, J.; Zhao, H.; Zeng, X.; Zhang, H.; Qing, C. Antitumor and antiangiogenic effects of GA-13315, a gibberellin derivative. Investig. New Drugs 2012, 30, 8–16. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kućko, A.; Walczak, A.; Wilmowicz, E.; Wolski, B.; Wiktorska, K. Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles. Molecules 2025, 30, 2984. https://doi.org/10.3390/molecules30142984
Kućko A, Walczak A, Wilmowicz E, Wolski B, Wiktorska K. Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles. Molecules. 2025; 30(14):2984. https://doi.org/10.3390/molecules30142984
Chicago/Turabian StyleKućko, Agata, Agata Walczak, Emilia Wilmowicz, Bartłomiej Wolski, and Katarzyna Wiktorska. 2025. "Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles" Molecules 30, no. 14: 2984. https://doi.org/10.3390/molecules30142984
APA StyleKućko, A., Walczak, A., Wilmowicz, E., Wolski, B., & Wiktorska, K. (2025). Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles. Molecules, 30(14), 2984. https://doi.org/10.3390/molecules30142984









