Nickel-Rich Cathodes for Solid-State Lithium Batteries: Comparative Study Between PVA and PIB Binders
Abstract
1. Introduction
2. Results and Discussion
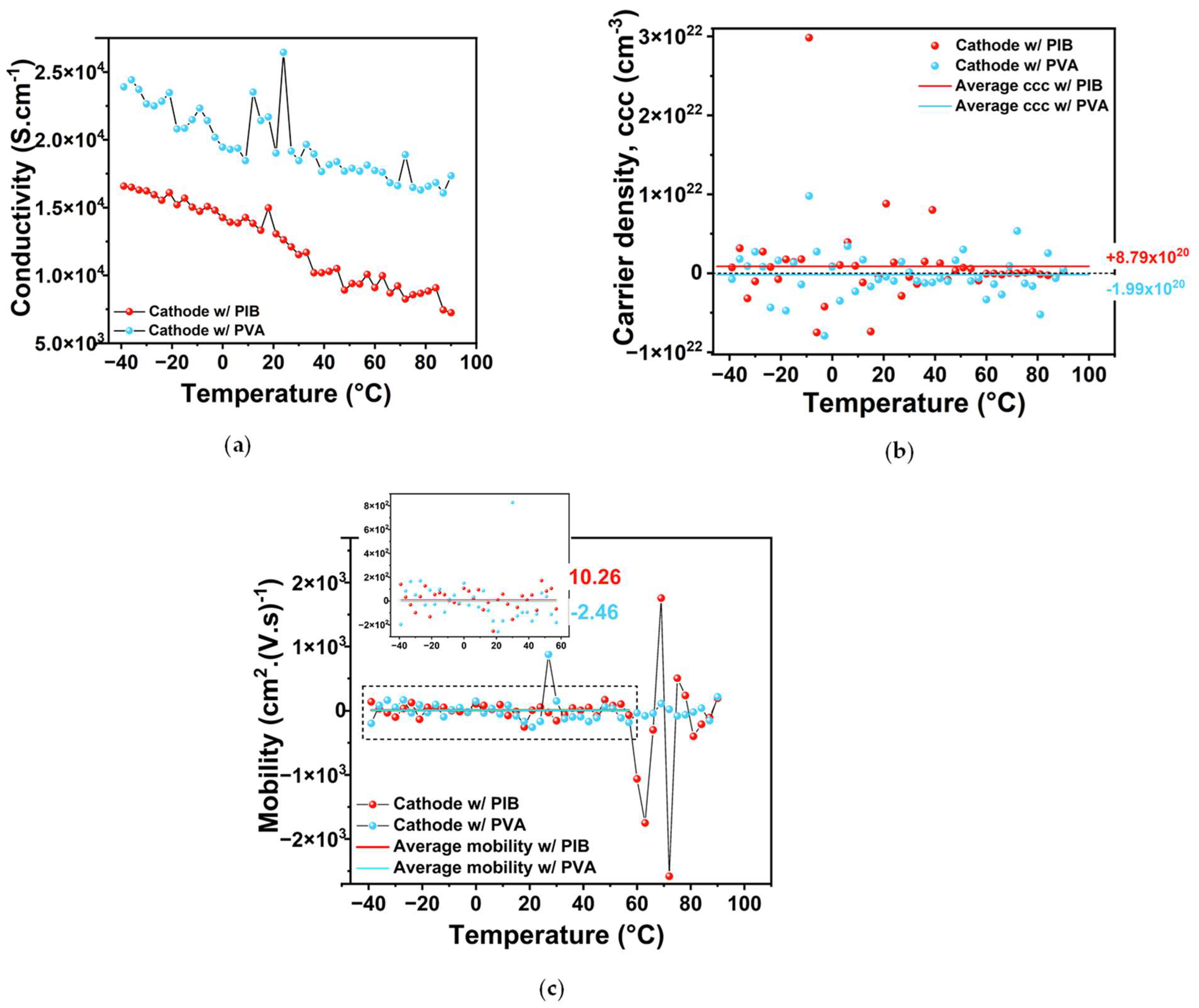
2.1. Cathode Characterization (SEM/EDX and Sheet Resistance/Hall Effect)
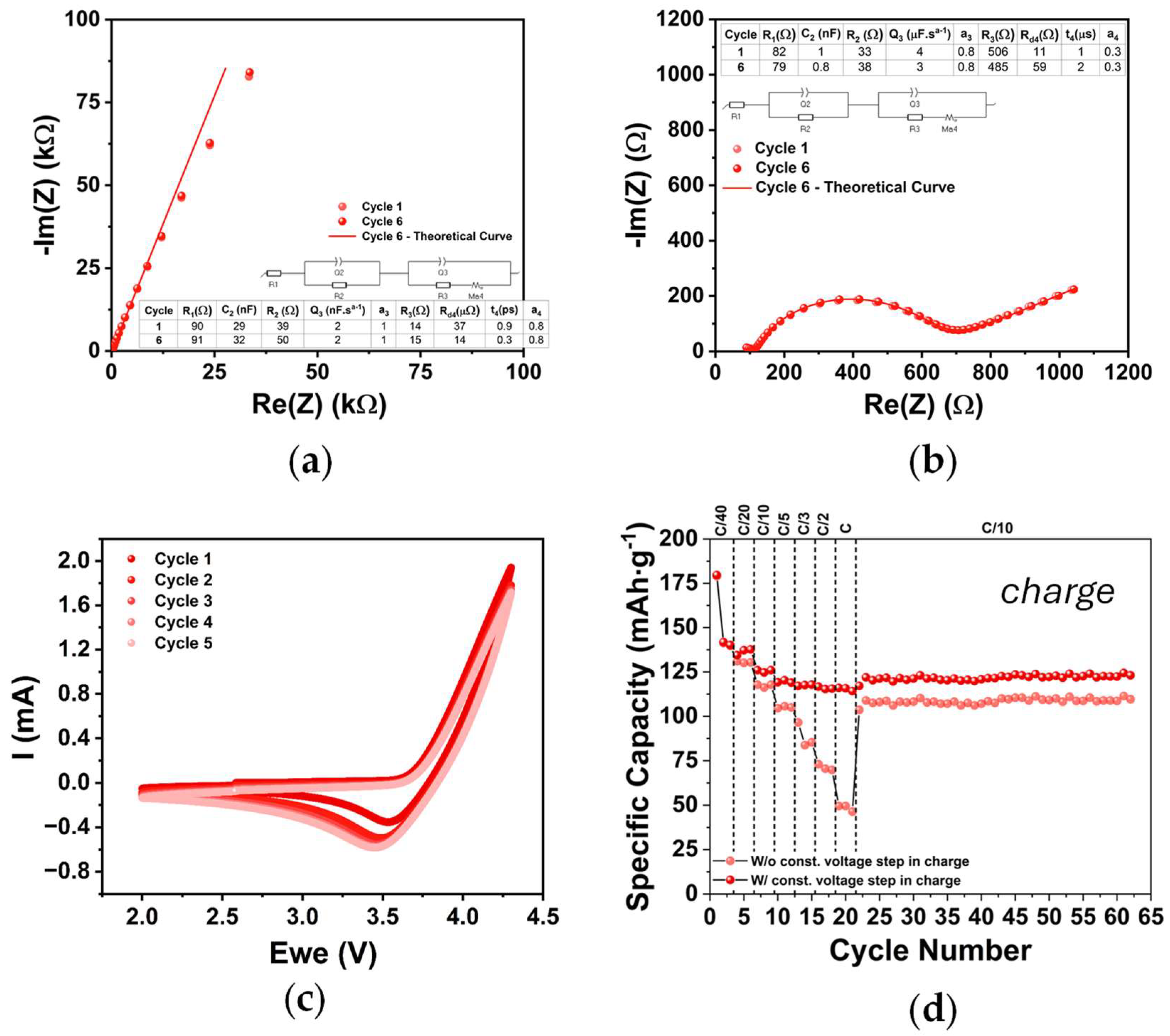
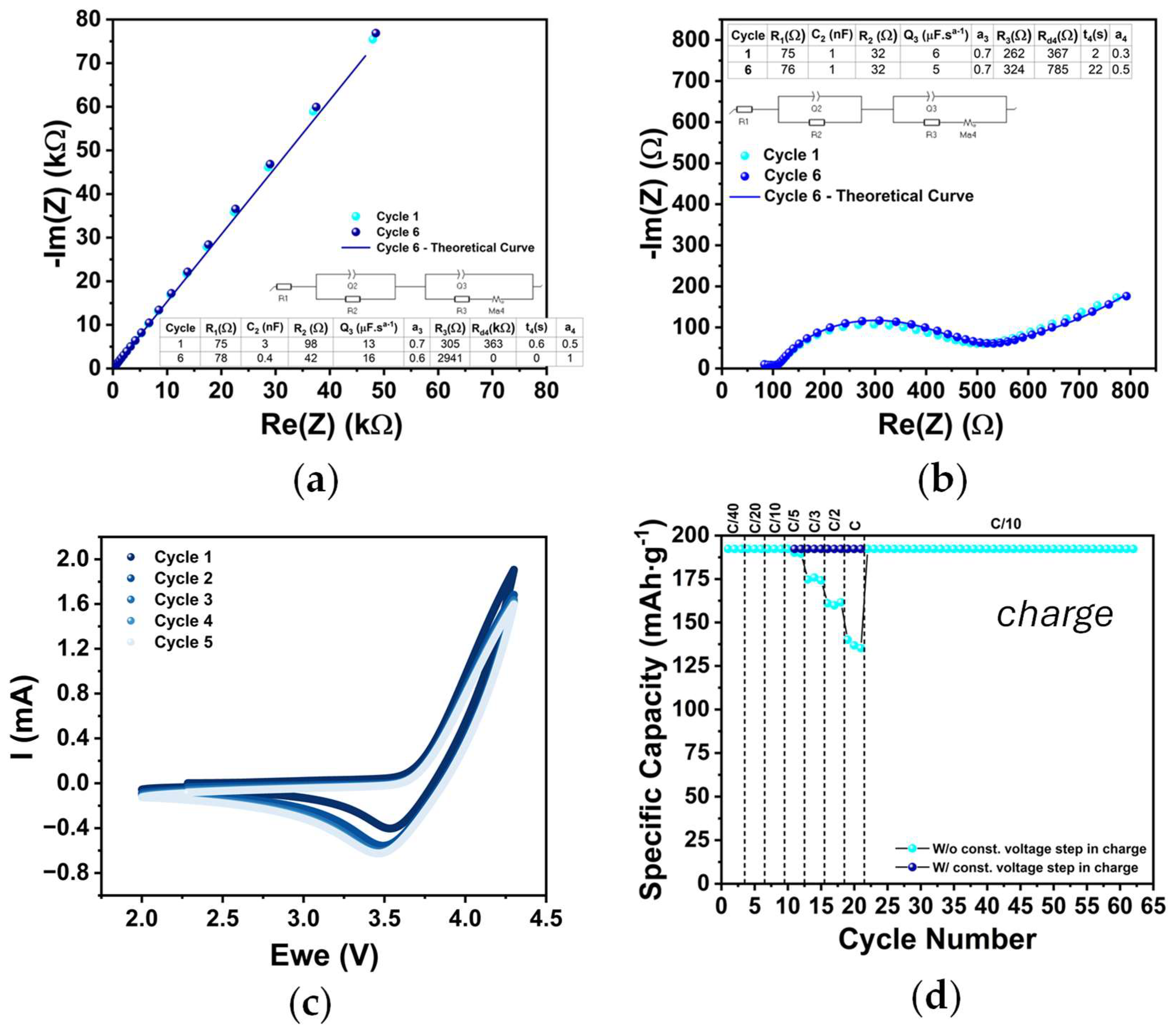
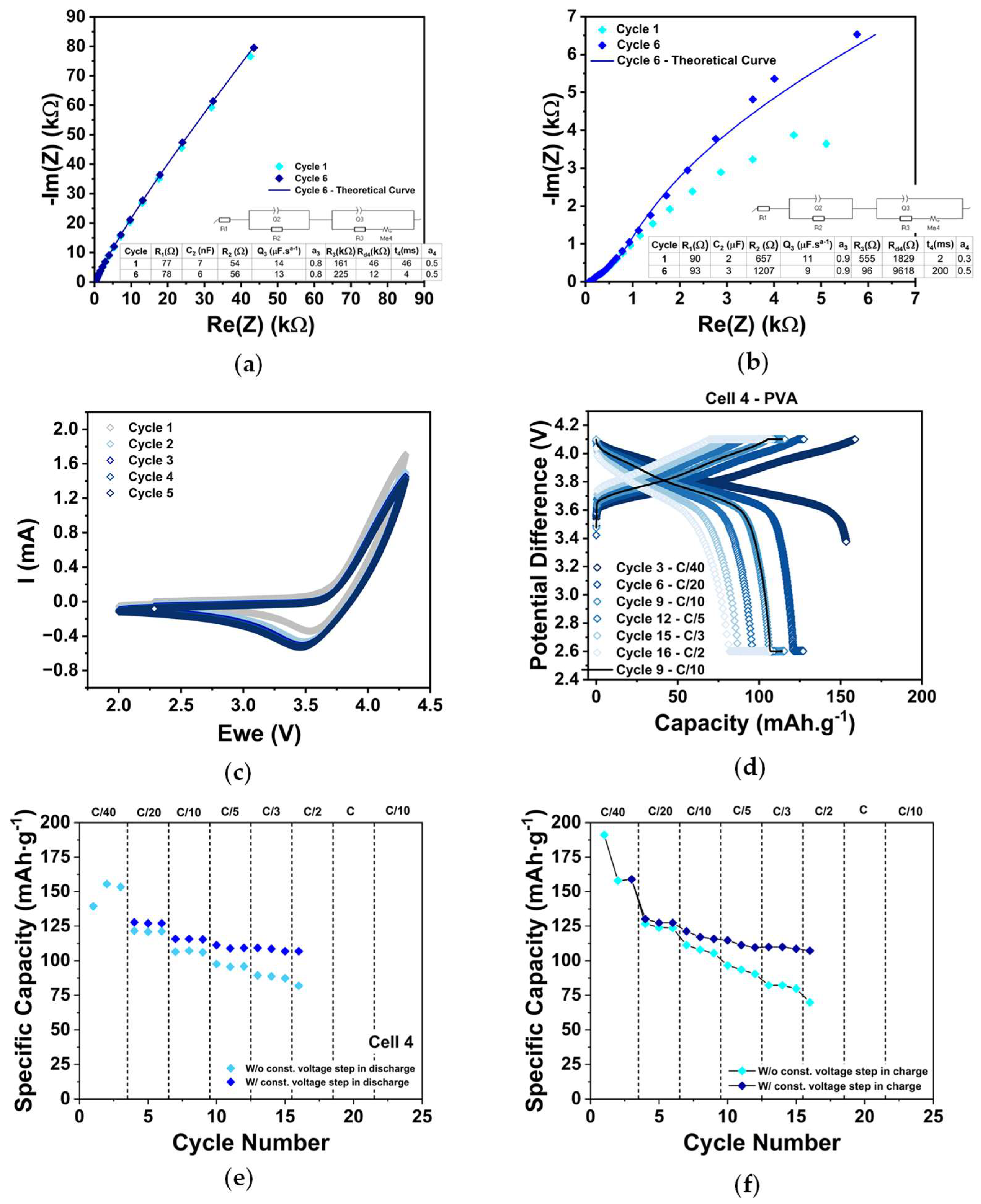
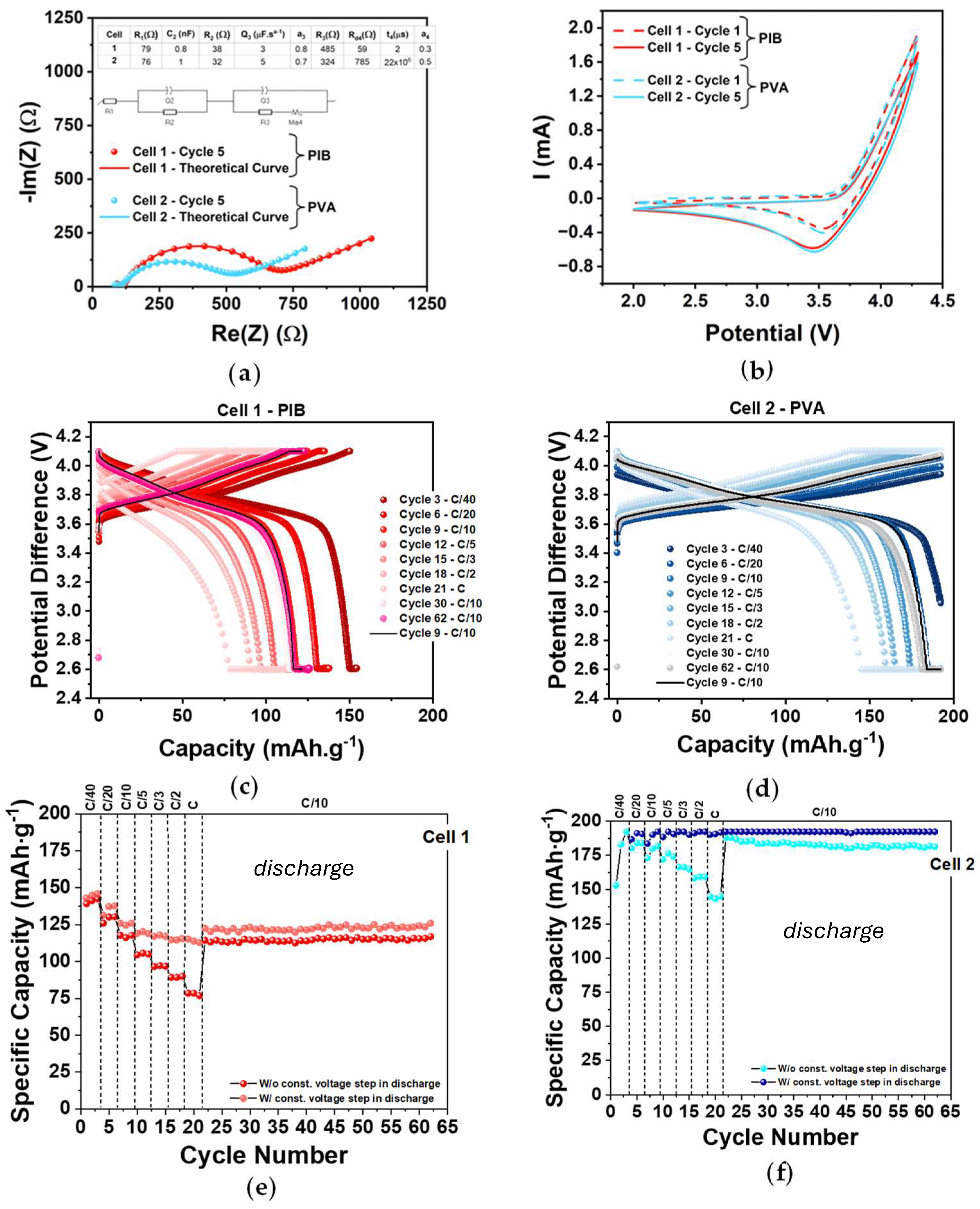
2.2. Cathode Electrochemical Characterization (PEIS, CV, and GCD)
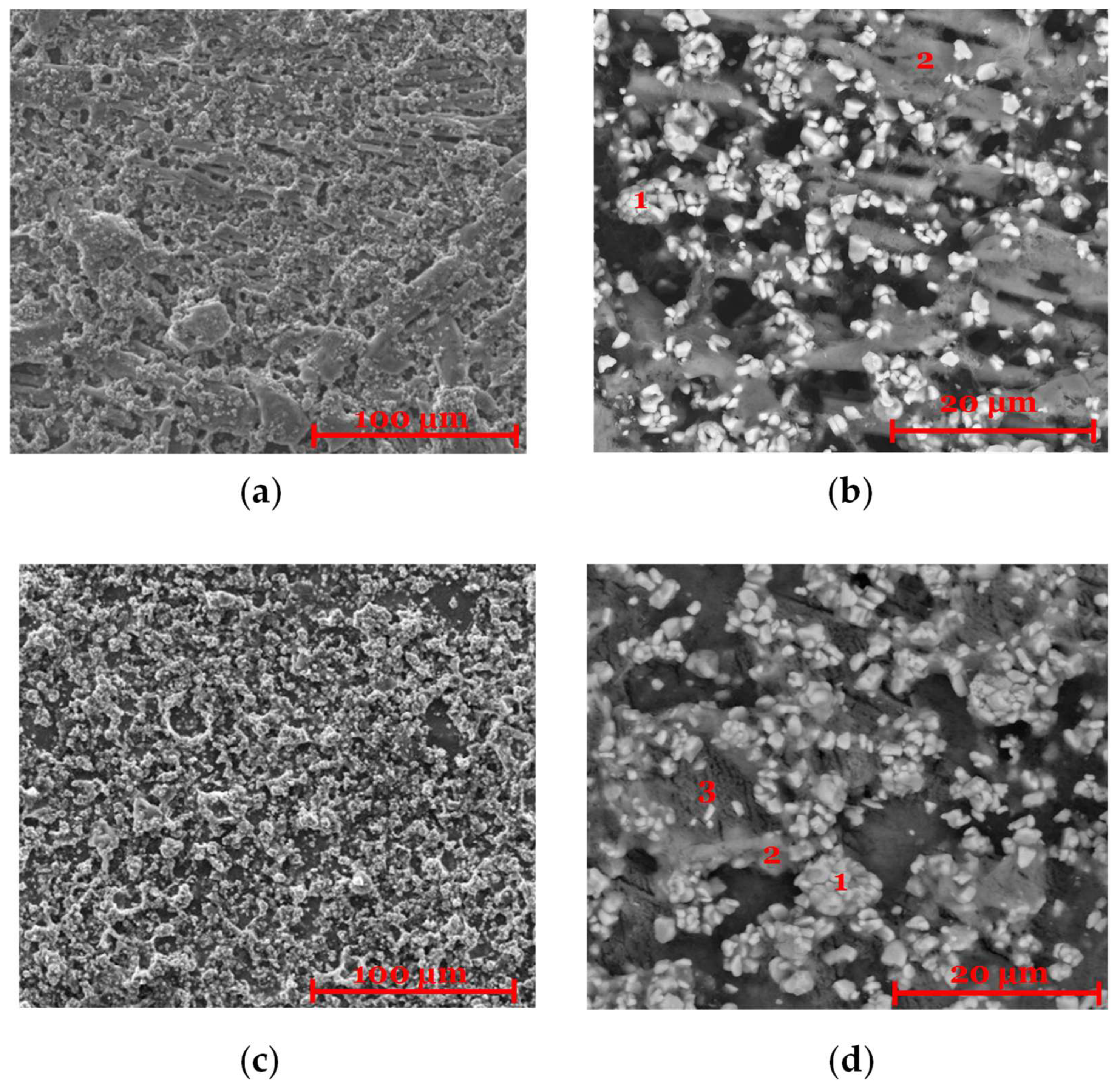
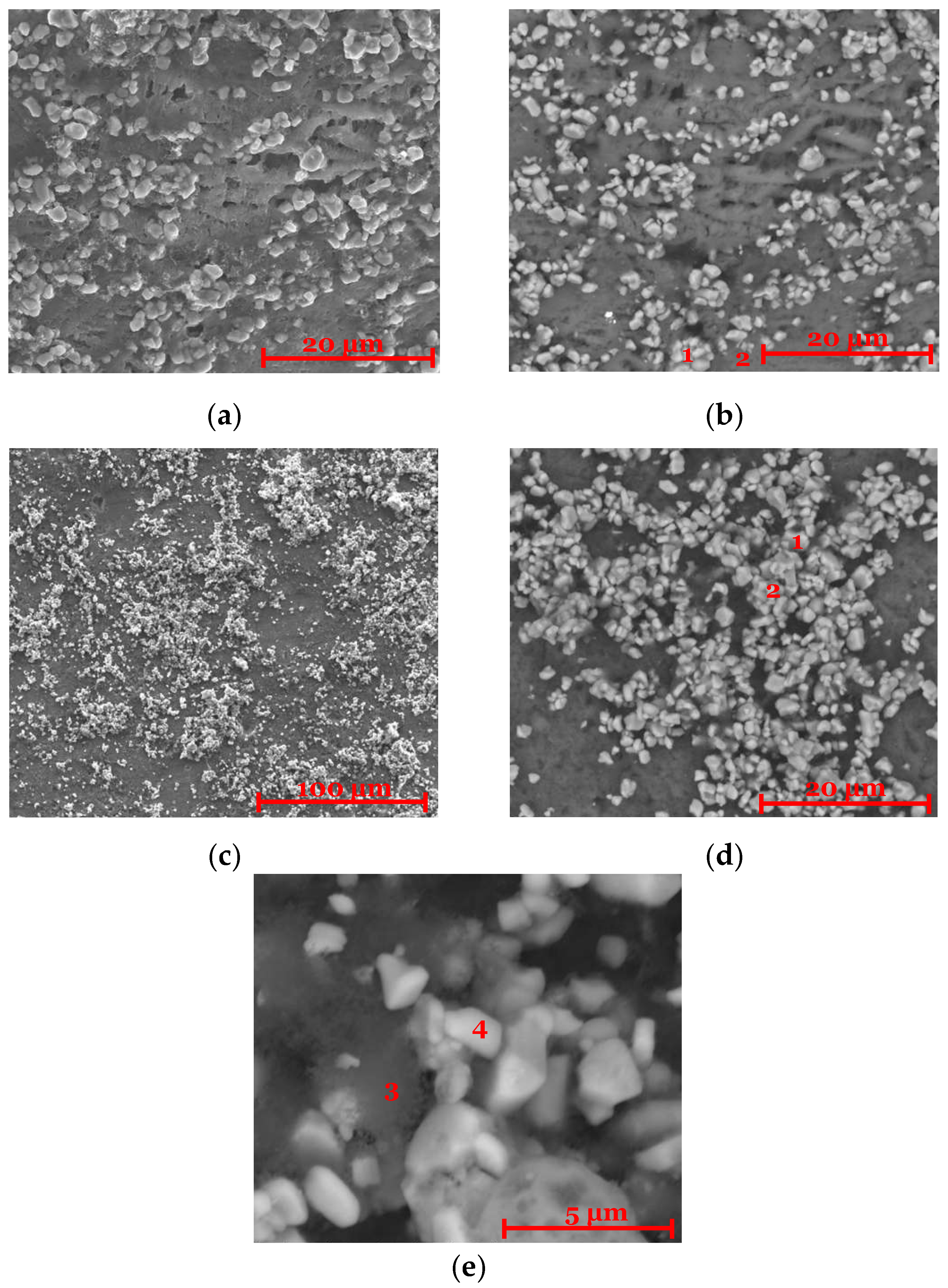
2.3. Post-Mortem Characterization (SEM/EDX)
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASSB | all-solid-state battery |
| CAM | cathode active material |
| CV | cyclic voltammetry |
| EDX | energy-dispersive X-ray spectroscopy |
| EV | electric vehicle |
| GCD | galvanostatic charge and discharge |
| LE | liquid electrolyte |
| LIB | lithium-ion battery |
| LPSCl | Li6PS5Cl |
| NMC | LiNixMnyCo1-x-yO2 |
| PEIS | potentiostatic electrochemical impedance spectroscopy |
| PIB | polyisobutene |
| PVA | polyvinyl acetate |
| PVDF | polyvinylidene fluoride |
| SEM | scanning electron microscope |
| SSE | solid-state electrolyte |
Appendix A
| Cell ID | Binder | Mass of NMC 955 (mg) | Areal Capacity (mAhcm−2) | Correspondent Figures |
|---|---|---|---|---|
| Cell 1 | PIB | 438 | 0.168 | Figure 1a,b, Figure 2, Figure 3a–c,e and Figure 4a,b |
| Cell 2 | PVA | 182 | 0.07 | Figure 1c,d, Figure 2, Figure 3a,b,d,f and Figure 4c–e |
| Cell 3 | PIB | 438 | 0.168 | Figure A3 |
| Cell 4 | PVA | 292 | 0.112 | Figure A4 |
| At % | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ni | Mn | Co | P | S | Cl | |
| 1 | 4.29 | 54.24 | 34.54 | 1.70 | 1.49 | 0.29 | 2.61 | 0.84 |
| 2 | 12.15 | 28.47 | 2.15 | 0.14 | 0.28 | 8.45 | 41.99 | 6.37 |
| At % | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ni | Mn | Co | P | S | Cl | |
| 1 | 5.00 | 44.67 | 38.52 | 1.93 | 2.01 | 0.90 | 6.22 | 0.75 |
| 2 | 8.78 | 3.00 | 66.91 | 1.33 | 2.20 | 2.61 | 13.23 | 1.94 |
| 3 | 13.19 | 19.15 | 2.65 | 0.44 | 0.50 | 7.66 | 47.40 | 9.01 |
| At % | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ni | Mn | Co | P | S | Cl | |
| 1 | 9.01 | 47.69 | 37.68 | 1.69 | 1.87 | 0.33 | 1.40 | 0.33 |
| 2 | 24.28 | 25.87 | 1.12 | 0.22 | 0.26 | 8.10 | 33.82 | 6.33 |
| At % | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ni | Mn | Co | P | S | Cl | |
| 1 | 51.29 | 25.60 | 17.37 | 0.88 | 1.48 | 0.23 | 2.90 | 0.25 |
| 2 | 7.22 | 52.53 | 34.18 | 1.55 | 1.81 | 0.12 | 2.43 | 0.16 |
| 3 | 71.14 | 11.98 | 11.56 | 0.67 | 0.79 | 0.43 | 3.00 | 0.43 |
| 4 | 13.68 | 50.34 | 30.15 | 1.47 | 1.51 | 0.38 | 2.17 | 0.30 |

References
- Markets and Markets. Lithium-Ion Battery Market Size, Share and Growth Analysis. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/lithium-ion-battery-market-49714593.html?gad_source=1&gclid=Cj0KCQjw1um-BhDtARIsABjU5x4h8ruL5crN1XqYbete5aC-1FNAa1kVcqh6W4N1yiFgMxRgJaleLmQaAuEDEALw_wcB (accessed on 24 March 2025).
- Li, W.; Song, B.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yu, Y.; Chen, C. A review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Global EV Outlook 2024 Moving Towards Increased Affordability. 2024. Available online: https://www.iea.org (accessed on 14 July 2025).
- Celadon, A.; Sun, H.; Sun, S.; Zhang, G. Batteries for electric vehicles: Technical advancements, environmental challenges, and market perspectives. SusMat 2024, 4, e234. [Google Scholar] [CrossRef]
- Nkulu, C.B.L.; Casas, L.; Haufroid, V.; De Putter, T.; Saenen, N.D.; Kayembe-Kitenge, T.; Obadia, P.M.; Mukoma, D.K.W.; Ilunga, J.-M.L.; Nawrot, T.S.; et al. Sustainability of artisanal mining of cobalt in DR Congo. Nat. Sustain. 2018, 1, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, W.; Zhu, X.; Ling, M.; Liang, C. Understanding the synthesis of inorganic solid-state electrolytes for Li ion batteries: Features and progress. Sustain. Mater. Technol. 2020, 33, e40091. [Google Scholar] [CrossRef]
- Huo, S.; Sheng, L.; Xue, W.; Wang, L.; Xu, H.; Zhang, H.; He, X. Challenges of polymer electrolyte with wide electrochemical window for high energy solid-state lithium batteries. InfoMat 2023, 5, e12394. [Google Scholar] [CrossRef]
- Phillip, N.D.; Westover, A.S.; Daniel, C.; Veith, G.M. Structural Degradation of High Voltage Lithium Nickel Manganese Cobalt Oxide (NMC) Cathodes in Solid-State Batteries and Implications for Next Generation Energy Storage. ACS Appl. Energy Mater. 2020, 3, 1768–1774. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Janek, J. Microstructural Modeling of Composite Cathodes for All-Solid-State Batteries. J. Phys. Chem. C 2019, 123, 1626–1634. [Google Scholar] [CrossRef]
- Han, S.C.; Ali, M.; Kim, Y.J.; Park, J.; Lee, Y.-J.; Park, J.-W.; Park, H.; Park, G.; Lee, E.; Kim, B.G.; et al. Unraveling electrochemo-mechanical aspects of core–shell composite cathode for sulfide based all-solid-state batteries. J. Mater. Chem. A 2024, 12, 24896–24905. [Google Scholar] [CrossRef]
- Singer, C.; Schmalzbauer, S.; Daub, R. Influence of the slurry composition on thin-film components for the wet coating process of sulfide-based all-solid-state batteries. J. Energy Storage 2023, 68, 107703. [Google Scholar] [CrossRef]
- PFAS Restriction Proposal RECHARGE Statement for 2 nd Call for Evidence-October 2021, n.d. Available online: https://rechargebatteries.org/wp-content/uploads/2022/09/Call-for-Evidence_RECHARGE-_-PFAS-restriction-V1.pdf (accessed on 24 March 2025).
- Rolandi, A.C.; de Meatza, I.; Casado, N.; Forsyth, M.; Mecerreyes, D.; Pozo-Gonzalo, C. Unlocking sustainable power: Advances in aqueous processing and water-soluble binders for NMC cathodes in high-voltage Li-ion batteries. RSC Sustain. 2024, 2, 2125–2149. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Huang, C.J.; Taklu, B.W.; Su, W.N.; Hwang, B.J. Chemical stability of sulfide solid-state electrolytes: Stability toward humid air and compatibility with solvents and binders. Energy Environ. Sci. 2022, 15, 991–1033. [Google Scholar] [CrossRef]
- Gomes, B.M.; Baptista, M.C.; Orue, A.; Dhrubajyoti, B.; Terlicka, S.; Sjövall, P.; Zamperlin, N.; Fonseca, C.; Smajic, J.; Kekkonen, V.; et al. All-solid-state lithium batteries with NMC955 cathodes: PVDF-free formulation with SBR and capacity recovery insights. Energy Mater. 2025, 5, 500091. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, J.M.; Gomes, B.M.; Baptista, M.C.; Braga, M.H. Nickel-Rich Cathodes for Solid-State Lithium Batteries: Comparative Study Between PVA and PIB Binders. Molecules 2025, 30, 2974. https://doi.org/10.3390/molecules30142974
Pinheiro JM, Gomes BM, Baptista MC, Braga MH. Nickel-Rich Cathodes for Solid-State Lithium Batteries: Comparative Study Between PVA and PIB Binders. Molecules. 2025; 30(14):2974. https://doi.org/10.3390/molecules30142974
Chicago/Turabian StylePinheiro, José M., Beatriz Moura Gomes, Manuela C. Baptista, and M. Helena Braga. 2025. "Nickel-Rich Cathodes for Solid-State Lithium Batteries: Comparative Study Between PVA and PIB Binders" Molecules 30, no. 14: 2974. https://doi.org/10.3390/molecules30142974
APA StylePinheiro, J. M., Gomes, B. M., Baptista, M. C., & Braga, M. H. (2025). Nickel-Rich Cathodes for Solid-State Lithium Batteries: Comparative Study Between PVA and PIB Binders. Molecules, 30(14), 2974. https://doi.org/10.3390/molecules30142974







