Catalyst-Driven Improvements in Conventional Methods for Imine-Linked Covalent Organic Frameworks
Abstract
1. Introduction
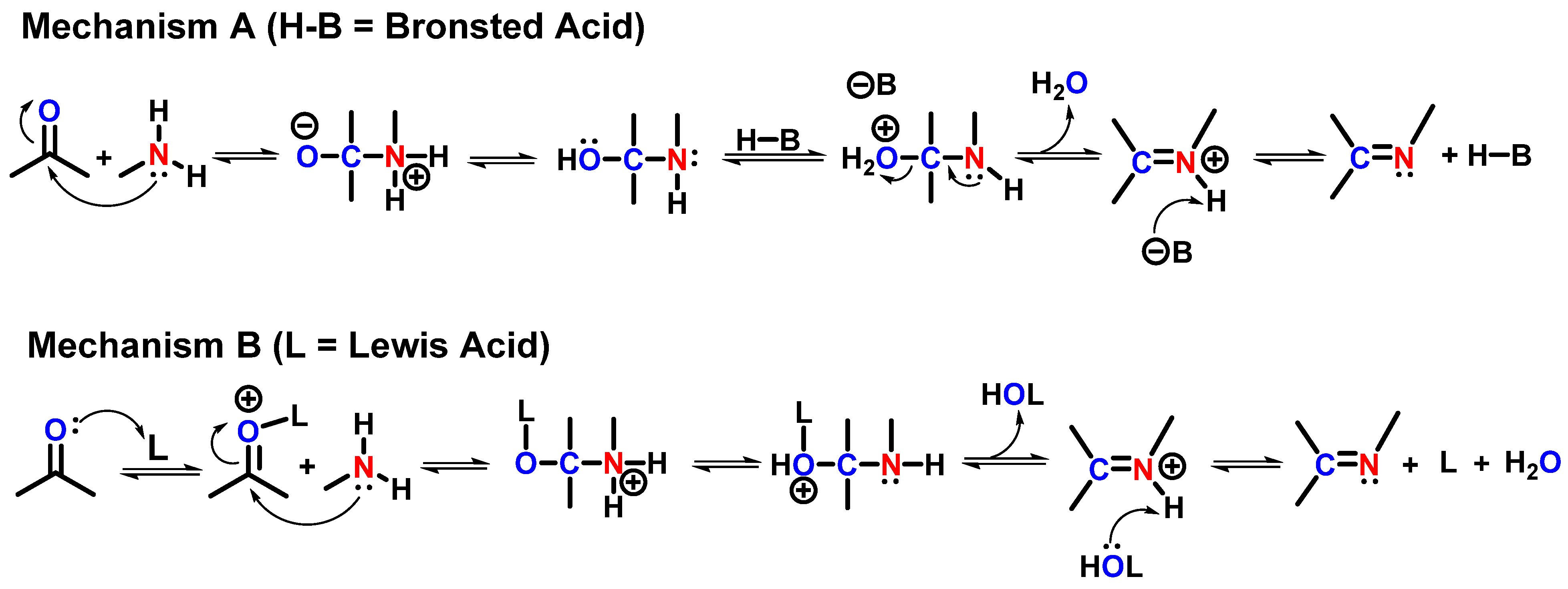
2. Catalytic Synthesis of Imine-Linked COFs
2.1. Metal Triflates
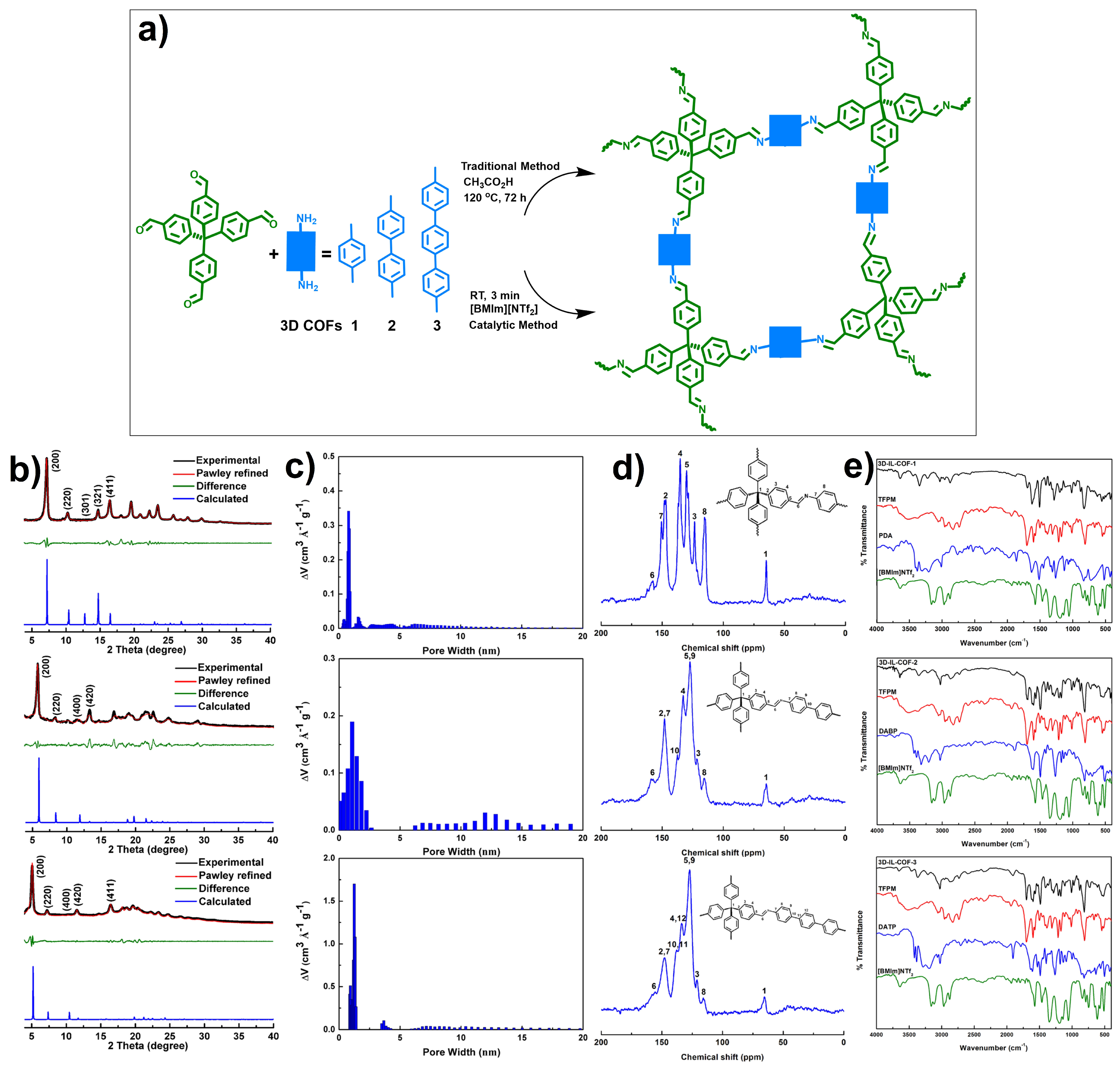
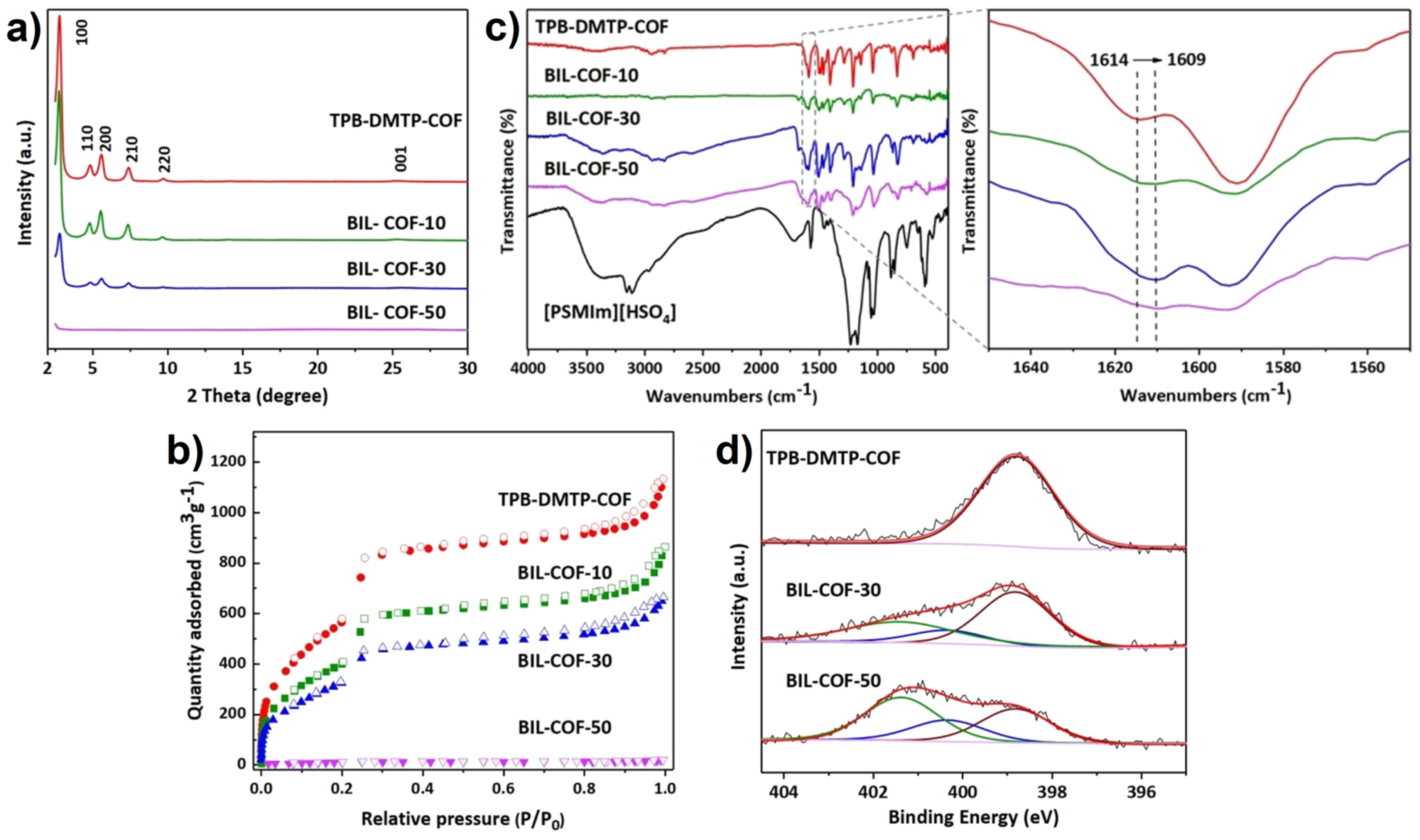
2.2. Ionic Liquids
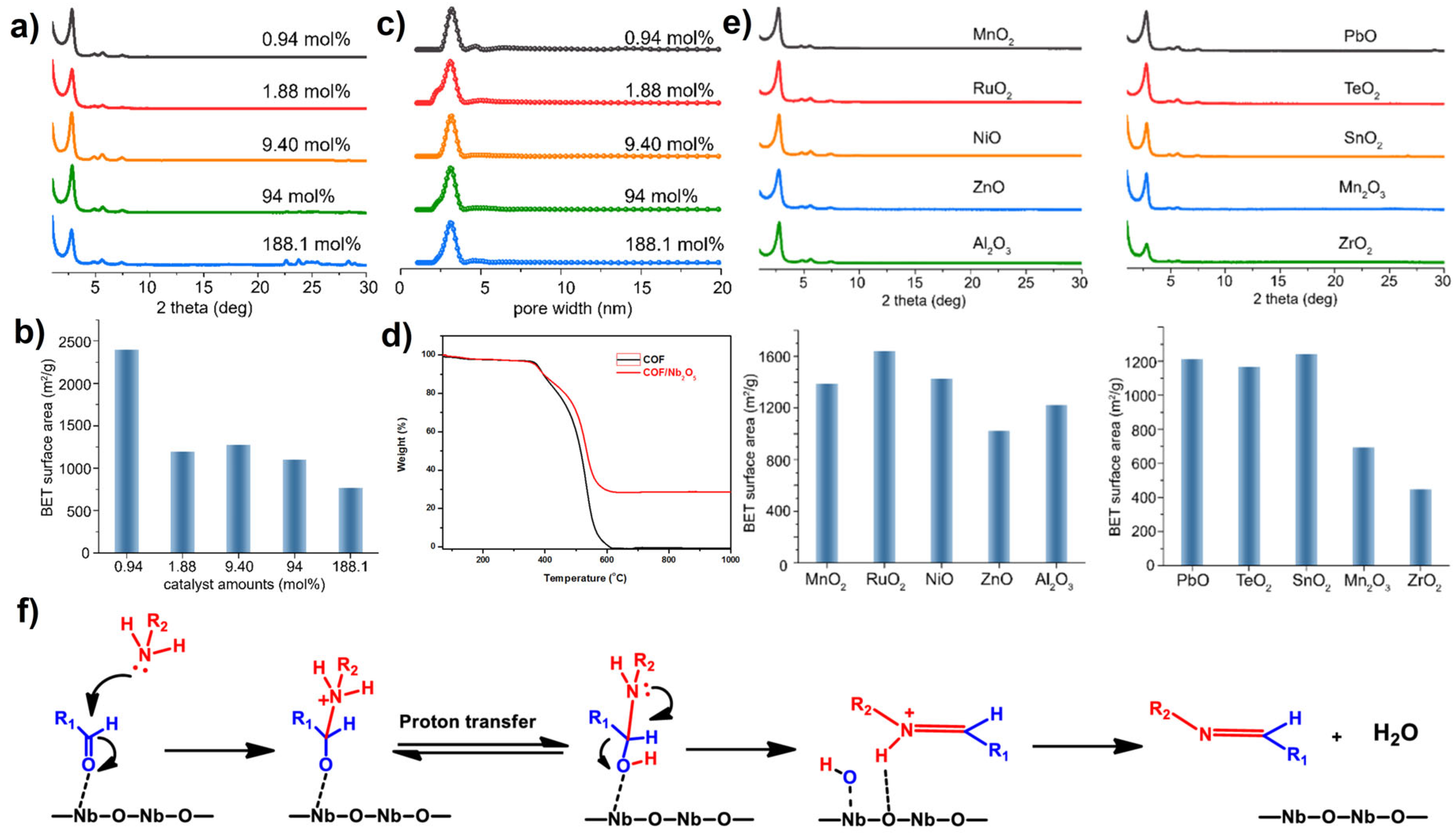
2.3. Metal Oxides
2.4. Metal Halides
2.5. Metal Nitrates
2.6. Heteropoly Acids
3. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Almarza, A.; López-Magano, A.; Marzo, L.; Cabrera, S.; Mas-Ballesté, R.; Alemán, J. Imine-Based Covalent Organic Frameworks as Photocatalysts for Metal Free Oxidation Processes under Visible Light Conditions. ChemCatChem 2019, 11, 4916–4922. [Google Scholar] [CrossRef]
- Romero-Muñiz, I.; Mavrandonakis, A.; Albacete, P.; Vega, A.; Briois, V.; Zamora, F.; Platero-Prats, A.E. Unveiling the Local Structure of Palladium Loaded into Imine-Linked Layered Covalent Organic Frameworks for Cross-Coupling Catalysis. Angew. Chem. Int. Ed. 2020, 59, 13013–13020. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Yang, Z.; Chen, L. Novel imine-linked covalent organic frameworks: Preparation, characterization and application. J. Mater. Chem. A 2019, 7, 5650–5655. [Google Scholar] [CrossRef]
- Gamraoui, H.; Khojastehnezhad, A.; Bélanger-Bouliga, M.; Nazemi, A.; Siaj, M. Covalent Organic Framework-Templated N-Heterocyclic Carbene-Functionalized Gold Nanoparticles for the Catalytic Reduction of Nitrophenol. ACS Appl. Nano Mater. 2024, 7, 22570–22580. [Google Scholar] [CrossRef]
- Nejabat, M.; Samie, A.; Khojastehnezhad, A.; Hadizadeh, F.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M.; Siaj, M. Stimuli-Responsive Covalent Organic Frameworks for Cancer Therapy. ACS Appl. Mater. Interfaces 2024, 16, 51837–51859. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-W.; Li, J.-J.; Wang, Y.-H.; Gao, F.-H.; Su, J.-L.; Liu, Y.; Wang, H.-S.; Ding, Y. Metal/covalent-organic framework-based biosensors for nucleic acid detection. Coord. Chem. Rev. 2023, 491, 215249. [Google Scholar] [CrossRef]
- Yuan, R.; Li, H.-K.; He, H.J. Recent advances in metal/covalent organic framework-based electrochemical aptasensors for biosensing applications. Dalton Trans. 2021, 50, 14091–14104. [Google Scholar] [CrossRef]
- Carvalho, S.; Pires, J.; Moiteiro, C.; Pinto, M.L. Evaluation of an Imine-Linked Polymer Organic Framework for Storage and Release of H2S and NO. Materials 2023, 16, 1655. [Google Scholar] [CrossRef]
- Li, Z.; Zhi, Y.; Feng, X.; Ding, X.; Zou, Y.; Liu, X.; Mu, Y. An Azine-Linked Covalent Organic Framework: Synthesis, Characterization and Efficient Gas Storage. Chem. Eur. J. 2015, 21, 12079–12084. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Sekizkardes, A.K.; Kahveci, Z.; Reich, T.E.; Ding, R.; El-Kaderi, H.M. A 2D Mesoporous Imine-Linked Covalent Organic Framework for High Pressure Gas Storage Applications. Chem.Eur. J. 2013, 19, 3324–3328. [Google Scholar] [CrossRef]
- Saleh, T.; Yousif, E.; Al-Tikrity, E.; Ahmed, D.; Bufaroosha, M.; Al-Mashhadani, M.; Yaseen, A. Design, synthesis, structure, and gas (CO2, CH4, and H2) storage properties of porous imine-linkage organic compounds. Mater. Sci. Energy Technol. 2022, 5, 344–352. [Google Scholar] [CrossRef]
- Lebar, G.; Khojastehnezhad, A.; Peng, Z.; Moeinpour, F.; Siaj, M. Impact of imine-linked covalent organic framework structures on their adsorption performance. Sep. Purif. Technol. 2025, 363, 132305. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Z.; Hu, J.; Chen, Z. In situ growth of imine-based covalent organic framework as stationary phase for high-efficiency electrochromatographic separation. J. Chromatogr. A 2023, 1694, 463905. [Google Scholar] [CrossRef] [PubMed]
- Natraj, A.; Ji, W.; Xin, J.; Castano, I.; Burke, D.W.; Evans, A.M.; Strauss, M.J.; Ateia, M.; Hamachi, L.S.; Gianneschi, N.C.; et al. Single-Crystalline Imine-Linked Two-Dimensional Covalent Organic Frameworks Separate Benzene and Cyclohexane Efficiently. J. Am. Chem. Soc. 2022, 144, 19813–19824. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Z.; Shen, S.; Liu, D.; Zhang, G. Preparation of PVDF mixed matrix membrane based on hydrophilic imine type covalent organic framework(COF) for dye and salt separation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133431. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Liu, Y.; Wang, Z.; Li, C.; Sun, S.; Lyu, Q.; Hu, S. Potential and design of imine-linked two-dimensional covalent organic framework membranes for Ethane/Methane separation. Appl. Surf. Sci. 2022, 585, 152601. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Moeinpour, F.; Jafari, M.; Shehab, M.K.; Samih ElDouhaibi, A.; El-Kaderi, H.M.; Siaj, M. Postsynthetic modification of core–shell magnetic covalent organic frameworks for the selective removal of mercury. ACS Appl. Mater. Interfaces 2023, 15, 28476–28490. [Google Scholar] [CrossRef]
- Chen, X.; Geng, K.; Liu, R.; Tan, K.T.; Gong, Y.; Li, Z.; Tao, S.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Chemical Approaches to Designer Structures and Built-In Functions. Angew. Chem. Int. Ed. 2020, 59, 5050–5091. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Z.; Liu, Y. Beyond Solvothermal: Alternative Synthetic Methods for Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202306999. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Khojastehnezhad, A.; Samie, A.; Bisio, A.; El-Kaderi, H.M.; Siaj, M. Impact of Postsynthetic Modification on the Covalent Organic Framework (COF) Structures. ACS Appl. Mater. Interfaces 2024, 17, 11415–11442. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiang, Y.; Li, X.; Li, X.; Wang, J.; Wu, Q.; Liu, X. Solvothermal Synthesis of Microporous, Crystalline Covalent Organic Framework Nanofibers and Their Colorimetric Nanohybrid Structures. ACS Appl. Mater. Interfaces 2013, 5, 8845–8849. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Addicoat, M.; Jin, S.; Sakurai, T.; Gao, J.; Xu, H.; Irle, S.; Seki, S.; Jiang, D. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat. Commun. 2015, 6, 7786. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, G.-B.; Zhou, L.-L.; Li, W.-Y.; Dong, Y.-B. Nanoscale covalent organic frameworks as theranostic platforms for oncotherapy: Synthesis, functionalization, and applications. Nanoscale Adv. 2020, 2, 3656–3733. [Google Scholar] [CrossRef]
- Smith, B.J.; Overholts, A.C.; Hwang, N.; Dichtel, W.R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 2016, 52, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klöck, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Guo, X.; Zhang, M.; Chen, B.; Wei, G.; Li, X.; Li, X.; Li, S.; Ma, L. Laminated self-standing covalent organic framework membrane with uniformly distributed subnanopores for ionic and molecular sieving. Nat. Commun. 2020, 11, 599. [Google Scholar] [CrossRef]
- Martín-Illán, J.Á.; Rodríguez-San-Miguel, D.; Franco, C.; Imaz, I.; Maspoch, D.; Puigmartí-Luis, J.; Zamora, F. Green synthesis of imine-based covalent organic frameworks in water. Chem. Commun. 2020, 56, 6704–6707. [Google Scholar] [CrossRef]
- Zhang, B.; Mao, H.; Matheu, R.; Reimer, J.A.; Alshmimri, S.A.; Alshihri, S.; Yaghi, O.M. Reticular Synthesis of Multinary Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 11420–11424. [Google Scholar] [CrossRef] [PubMed]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.C. Light-promoted synthesis of highly-conjugated crystalline covalent organic framework. Commun. Chem. 2019, 2, 60. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kim, J.; Cho, H.-Y.; Kim, S.; Ahn, W.-S. Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv. 2012, 2, 10179–10181. [Google Scholar] [CrossRef]
- Wei, H.; Chai, S.; Hu, N.; Yang, Z.; Wei, L.; Wang, L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 2015, 51, 12178–12181. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Zhang, S.; Zhou, X.; He, L.; Sheridan, M.V.; Yuan, M.; Zhang, M.; Chen, L.; Dai, X.; et al. Electron Beam Irradiation as a General Approach for the Rapid Synthesis of Covalent Organic Frameworks under Ambient Conditions. J. Am. Chem. Soc. 2020, 142, 9169–9174. [Google Scholar] [CrossRef]
- Acharjya, A.; Pachfule, P.; Roeser, J.; Schmitt, F.-J.; Thomas, A. Vinylene-Linked Covalent Organic Frameworks by Base-Catalyzed Aldol Condensation. Angew. Chem. Int. Ed. 2019, 58, 14865–14870. [Google Scholar] [CrossRef]
- Spitler, E.L.; Giovino, M.R.; White, S.L.; Dichtel, W.R. A mechanistic study of Lewis acid-catalyzed covalent organic framework formation. Chem. Sci. 2011, 2, 1588–1593. [Google Scholar] [CrossRef]
- Wang, J.-C.; Kan, X.; Shang, J.-Y.; Qiao, H.; Dong, Y.-B. Catalytic Asymmetric Synthesis of Chiral Covalent Organic Frameworks from Prochiral Monomers for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2020, 142, 16915–16920. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Q.; Wu, X.; Zhao, D. The development of catalysts and auxiliaries for the synthesis of covalent organic frameworks. Chem. Soc. Rev. 2024, 53, 7531–7565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.; Sun, B.; Cai, S.; Chen, Z.; Lv, Y.; Zhang, J.; Liu, Y. Expeditious synthesis of covalent organic frameworks: A review. J. Mater. Chem. A 2020, 8, 16045–16060. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; McCandless, G.T.; Karunathilake, A.A.K.; Thompson, C.M.; Smaldone, R.A. Enhanced Structural Organization in Covalent Organic Frameworks Through Fluorination. Chem. Eur. J. 2017, 23, 4255–4259. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, L.; Sick, T.; Margraf, J.T.; Lapidus, S.H.; Calik, M.; Hettstedt, C.; Karaghiosoff, K.; Döblinger, M.; Clark, T.; Chapman, K.W.; et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 2016, 8, 310–316. [Google Scholar] [CrossRef]
- Auras, F.; Ascherl, L.; Hakimioun, A.H.; Margraf, J.T.; Hanusch, F.C.; Reuter, S.; Bessinger, D.; Döblinger, M.; Hettstedt, C.; Karaghiosoff, K.; et al. Synchronized Offset Stacking: A Concept for Growing Large-Domain and Highly Crystalline 2D Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 16703–16710. [Google Scholar] [CrossRef]
- Chen, X.; Huang, N.; Gao, J.; Xu, H.; Xu, F.; Jiang, D. Towards covalent organic frameworks with predesignable and aligned open docking sites. Chem. Commun. 2014, 50, 6161–6163. [Google Scholar] [CrossRef]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef]
- DeBlase, C.R.; Dichtel, W.R. Moving Beyond Boron: The Emergence of New Linkage Chemistries in Covalent Organic Frameworks. Macromolecules 2016, 49, 5297–5305. [Google Scholar] [CrossRef]
- Halder, A.; Kandambeth, S.; Biswal, B.P.; Kaur, G.; Roy, N.C.; Addicoat, M.; Salunke, J.K.; Banerjee, S.; Vanka, K.; Heine, T.; et al. Decoding the Morphological Diversity in Two Dimensional Crystalline Porous Polymers by Core Planarity Modulation. Angew. Chem. Int. Ed. 2016, 55, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhu, Y.; Zhang, W. Development of organic porous materials through Schiff-base chemistry. CrystEngComm 2013, 15, 1484–1499. [Google Scholar] [CrossRef]
- Schwab, M.G.; Hamburger, M.; Feng, X.; Shu, J.; Spiess, H.W.; Wang, X.; Antonietti, M.; Müllen, K. Photocatalytic hydrogen evolution through fully conjugated poly(azomethine) networks. Chem. Commun. 2010, 46, 8932–8934. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent Organic Frameworks with High Charge Carrier Mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.L.; Clowes, R.; Ritchie, L.K.; Cooper, A.I. Rapid Microwave Synthesis and Purification of Porous Covalent Organic Frameworks. Chem. Mater. 2009, 21, 204–206. [Google Scholar] [CrossRef]
- Feriante, C.H.; Jhulki, S.; Evans, A.M.; Dasari, R.R.; Slicker, K.; Dichtel, W.R.; Marder, S.R. Rapid Synthesis of High Surface Area Imine-Linked 2D Covalent Organic Frameworks by Avoiding Pore Collapse During Isolation. Adv. Mater. 2020, 32, 1905776. [Google Scholar] [CrossRef]
- Neti, V.S.P.K.; Wu, X.; Deng, S.; Echegoyen, L. Selective CO2 capture in an imine linked porphyrin porous polymer. Polym. Chem. 2013, 4, 4566–4569. [Google Scholar] [CrossRef]
- Ciaccia, M.; Di Stefano, S. Mechanisms of imine exchange reactions in organic solvents. Org. Biomol. Chem. 2015, 13, 646–654. [Google Scholar] [CrossRef]
- Giuseppone, N.; Schmitt, J.-L.; Lehn, J.-M. Generation of Dynamic Constitutional Diversity and Driven Evolution in Helical Molecular Strands under Lewis Acid Catalyzed Component Exchange. Angew. Chem. Int. Ed. 2004, 43, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Giuseppone, N.; Schmitt, J.-L.; Schwartz, E.; Lehn, J.-M. Scandium(III) Catalysis of Transimination Reactions. Independent and Constitutionally Coupled Reversible Processes. J. Am. Chem. Soc. 2005, 127, 5528–5539. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Boeck, F.; Hintermann, L. Hidden Brønsted Acid Catalysis: Pathways of Accidental or Deliberate Generation of Triflic Acid from Metal Triflates. J. Org. Chem. 2011, 76, 9353–9361. [Google Scholar] [CrossRef] [PubMed]
- Deuss, P.J.; Lahive, C.W.; Lancefield, C.S.; Westwood, N.J.; Kamer, P.C.J.; Barta, K.; de Vries, J.G. Metal Triflates for the Production of Aromatics from Lignin. ChemSusChem 2016, 9, 2974–2981. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Le Roux, C. Bismuth(III) Triflate in Organic Synthesis. Eur. J. Org. Chem. 2004, 2004, 2517–2532. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S. Advances in indium triflate catalyzed organic syntheses. J. Mol. Catal. A Chem. 2007, 264, 1–8. [Google Scholar] [CrossRef]
- Hajra, S.; Sinha, D.; Bhowmick, M. Metal Triflate Catalyzed Reactions of Alkenes, NBS, Nitriles, and TMSN3: Synthesis of 1,5-Disubstituted Tetrazoles. J. Org. Chem. 2007, 72, 1852–1855. [Google Scholar] [CrossRef]
- Hartley, C.S.; Moore, J.S. Programmed Dynamic Covalent Assembly of Unsymmetrical Macrocycles. J. Am. Chem. Soc. 2007, 129, 11682–11683. [Google Scholar] [CrossRef]
- Jin, Y.; Voss, B.A.; Jin, A.; Long, H.; Noble, R.D.; Zhang, W. Highly CO2-Selective Organic Molecular Cages: What Determines the CO2 Selectivity. J. Am. Chem. Soc. 2011, 133, 6650–6658. [Google Scholar] [CrossRef]
- Kobayashi, S. Scandium Triflate in Organic Synthesis. Eur. J. Org. Chem. 1999, 1999, 15–27. [Google Scholar] [CrossRef]
- Kobayashi, S.; Moriwaki, M.; Hachiya, I. The Catalytic Fries Rearrangement and o-Acylation Reactions Using Group 3 and 4 Metal Triflates as Catalysts. Bull. Chem. Soc. Jpn. 1997, 70, 267–273. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.L. Rare-Earth Metal Triflates in Organic Synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef]
- Li, C.-J. Organic Reactions in Aqueous Media with a Focus on Carbon−Carbon Bond Formations: A Decade Update. Chem. Rev. 2005, 105, 3095–3166. [Google Scholar] [CrossRef]
- Massi, L.; Gal, J.-F.; Duñach, E. Metal Triflates as Catalysts in Organic Synthesis: Determination of Their Lewis Acidity by Mass Spectrometry. ChemPlusChem 2022, 87, e202200037. [Google Scholar] [CrossRef] [PubMed]
- Noji, M.; Konno, Y.; Ishii, K. Metal Triflate-Catalyzed Cationic Benzylation and Allylation of 1,3-Dicarbonyl Compounds. J. Org. Chem. 2007, 72, 5161–5167. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Mathew, T.; Olah, G.A. Gallium(III) Triflate: An Efficient and a Sustainable Lewis Acid Catalyst for Organic Synthetic Transformations. Acc. Chem. Res. 2012, 45, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Lei-Yang, Z.; Nai-Xing, W.; Xing, Y. Recent advances in the rare-earth metal triflates-catalyzed organic reactions. Catal. Rev. 2022, 64, 679–715. [Google Scholar] [CrossRef]
- Matsumoto, M.; Dasari, R.R.; Ji, W.; Feriante, C.H.; Parker, T.C.; Marder, S.R.; Dichtel, W.R. Rapid, Low Temperature Formation of Imine-Linked Covalent Organic Frameworks Catalyzed by Metal Triflates. J. Am. Chem. Soc. 2017, 139, 4999–5002. [Google Scholar] [CrossRef]
- Li, R.L.; Flanders, N.C.; Evans, A.M.; Ji, W.; Castano, I.; Chen, L.X.; Gianneschi, N.C.; Dichtel, W.R. Controlled growth of imine-linked two-dimensional covalent organic framework nanoparticles. Chem. Sci. 2019, 10, 3796–3801. [Google Scholar] [CrossRef]
- Jiang, D.; Hill, J.P.; Henzie, J.; Nam, H.N.; Phung, Q.M.; Zhu, L.; Wang, J.; Xia, W.; Zhao, Y.; Kang, Y. Selective Electrochemical Capture of Monovalent Cations Using Crown Ether-Functionalized COFs. J. Am. Chem. Soc. 2025, 147, 12460–12468. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Zhang, G.; Sun, J.; Tian, N.; Sun, Q.; Wu, Z. Controlled Sensor Derived from COF Materials for the Effective Detection of N-Methylpyrrolidone. ACS Sensors 2024, 9, 5866–5874. [Google Scholar] [CrossRef]
- Yang, T.; Hu, H.; Wang, Y.; Chen, X.; Fan, J.; Li, D.; Liu, S.; Li, J.; He, T.; Lu, S.; et al. Constructing Pillar-Layered Covalent Organic Frameworks via Metal–Ligand Coordination for Electrochemical CO2 Reduction. Adv. Mater. 2025, 37, 2419547. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Shin, B.; Wang, X.; Jiang, S.-Y.; Alazmi, A.; Zhang, R.; Han, Y.; Wu, X.; Verduzco, R. Gram-Scale Synthesis of Imine-Linked Covalent Organic Frameworks at Ambient Conditions Using Metal Triflimides. Chem. Mater. 2025, 37, 2258–2267. [Google Scholar] [CrossRef]
- Li, M.; Qiao, S.; Zheng, Y.; Andaloussi, Y.H.; Li, X.; Zhang, Z.; Li, A.; Cheng, P.; Ma, S.; Chen, Y. Fabricating Covalent Organic Framework Capsules with Commodious Microenvironment for Enzymes. J. Am. Chem. Soc. 2020, 142, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.L.; Meng, F.L.; Yang, C.X.; Yan, X.P. Irreversible amide-linked covalent organic framework for selective and ultrafast gold recovery. Angew. Chem. Int. Ed. 2020, 59, 17607–17613. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Y.-J.; Ma, S.-H.; Yang, C.; Wang, Z.-P.; Ding, S.-Y.; Li, Y.; Wang, W. Fused-Ring-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2022, 144, 6594–6603. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Miao, W.; Chan, T.H.J. Ionic-liquid-supported synthesis: A novel liquid-phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X.J. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Guan, X.; Ma, Y.; Li, H.; Yusran, Y.; Xue, M.; Fang, Q.; Yan, Y.; Valtchev, V.; Qiu, S. Fast, Ambient Temperature and Pressure Ionothermal Synthesis of Three-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 4494–4498. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Valtchev, V.; Majano, G.; Mintova, S.; Pérez-Ramírez, J. Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev. 2013, 42, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Kubisa, P. Ionic liquids in the synthesis and modification of polymers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4675–4683. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, C.Y.; Patel, Y.H.; Wu, J.; Malhotra, S. Synthesis of Porous Polyurea with Room-Temperature Ionic Liquids via Interfacial Polymerization. Macromol. Rapid Commun. 2006, 27, 1306–1311. [Google Scholar] [CrossRef]
- Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Three-periodic nets and tilings: Edge-transitive binodal structures. Acta Crystallogr. Sect. A 2006, 62, 350–355. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal Synthesis of Zeolites, Metal–Organic Frameworks, and Inorganic–Organic Hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, C.; Wang, Y.; Sun, J.; Gao, Y. Encapsulation of an ionic liquid into the nanopores of a 3D covalent organic framework. RSC Adv. 2017, 7, 1697–1700. [Google Scholar] [CrossRef]
- Du, Y.-R.; Xu, B.-H.; Pan, J.-S.; Wu, Y.-W.; Peng, X.-M.; Wang, Y.-F.; Zhang, S.-J. Confinement of Brønsted acidic ionic liquids into covalent organic frameworks as a catalyst for dehydrative formation of isosorbide from sorbitol. Green Chem. 2019, 21, 4792–4799. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Hu, H.; Ge, R.; Lu, M.; Zhang, J.; Li, Z.; Shao, P.; Jiang, D. Synthesis of Two-Dimensional Covalent Organic Frameworks in Ionic Liquids. Chem. Eur. J. 2019, 25, 15488–15492. [Google Scholar] [CrossRef]
- Guan, P.; Qiu, J.; Zhao, Y.; Wang, H.; Li, Z.; Shi, Y.; Wang, J. A novel crystalline azine-linked three-dimensional covalent organic framework for CO2 capture and conversion. Chem. Commun. 2019, 55, 12459–12462. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, H.; Zhao, Y.; Guan, P.; Li, Z.; Zhang, H.; Gao, H.; Zhang, S.; Wang, J. Hierarchically porous covalent organic frameworks assembled in ionic liquids for highly effective catalysis of C–C coupling reactions. Green Chem. 2020, 22, 2605–2612. [Google Scholar] [CrossRef]
- Dong, B.; Wang, W.-J.; Pan, W.; Kang, G.-J. Ionic liquid as a green solvent for ionothermal synthesis of 2D keto-enamine-linked covalent organic frameworks. Mater. Chem. Phys. 2019, 226, 244–249. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, M.; Lang, Z.-L.; Liu, J.; Liu, M.; Chang, J.-N.; Li, L.-Y.; Shang, L.-J.; Wang, M.; Li, S.-L.; et al. Semiconductor/Covalent-Organic-Framework Z-Scheme Heterojunctions for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 6500–6506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Alahakoon, S.B.; Egap, E. Synthesis of Imine-Based Covalent Organic Frameworks Catalyzed by Metal Halides and in Situ Growth of Perovskite@COF Composites. ACS Mater. Lett. 2020, 2, 1561–1566. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Teo, W.L.; Wang, K.; Zhang, L.; Zeng, Y.; Zhao, Y. Covalent-Organic-Framework-Based Composite Materials. Chem 2020, 6, 3172–3202. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Wei, H.; Fang, Y.; Mulligan, P.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.R.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, F.; Gao, F.; Huang, J.; Zhao, Y.; Shen, Y.; Zhang, Y.; Qian, X. Facile synthesis of magnetic covalent organic frameworks for the hydrophilic enrichment of N-glycopeptides. J. Mater. Chem. B 2017, 5, 4052–4059. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Zhao, J.; Park, E.; Jin, Y.; Liu, Q.; Zhang, W. Covalent organic framework-supported Fe–TiO2 nanoparticles as ambient-light-active photocatalysts. J. Mater. Chem. A 2019, 7, 16364–16371. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Chen, J.; Ge, R.; Leng, W.; Dong, B.; Huang, J.; Gao, Y. A novel 3D covalent organic framework membrane grown on a porous α-Al2O3 substrate under solvothermal conditions. Chem. Commun. 2015, 51, 15562–15565. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Namuangruk, S.; Kong, W.; Kungwan, N.; Guo, J.; Wang, C. Manipulation of Amorphous-to-Crystalline Transformation: Towards the Construction of Covalent Organic Framework Hybrid Microspheres with NIR Photothermal Conversion Ability. Angew. Chem. Int. Ed. 2016, 55, 13979–13984. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, D.; Yan, Q.; Gao, G.; Xu, J.; Liu, Y.; Alahakoon, S.B.; Rahman, M.M.; Ajayan, P.M.; Egap, E.; et al. Metal Oxide Catalysts for the Synthesis of Covalent Organic Frameworks and One-Step Preparation of Covalent Organic Framework-Based Composites. Chem. Mater. 2021, 33, 6158–6165. [Google Scholar] [CrossRef]
- Zhu, D.; Verduzco, R. Ultralow Surface Tension Solvents Enable Facile COF Activation with Reduced Pore Collapse. ACS Appl. Mater. Interfaces 2020, 12, 33121–33127. [Google Scholar] [CrossRef]
- Feriante, C.; Evans, A.M.; Jhulki, S.; Castano, I.; Strauss, M.J.; Barlow, S.; Dichtel, W.R.; Marder, S.R. New Mechanistic Insights into the Formation of Imine-Linked Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 18637–18644. [Google Scholar] [CrossRef]
- Chandra Shekhar, A.; Ravi Kumar, A.; Sathaiah, G.; Luke Paul, V.; Sridhar, M.; Shanthan Rao, P. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Kobayashi, S.; Busujima, T.; Nagayama, S. A Novel Classification of Lewis Acids on the Basis of Activity and Selectivity. Chem. Eur. J. 2000, 6, 3491–3494. [Google Scholar] [CrossRef]
- Satchell, D.P.N.; Satchell, R.S. Quantitative aspects of Lewis acidity. Q. Rev. Chem. Soc. 1971, 25, 171–199. [Google Scholar] [CrossRef]
- Sick, T.; Rotter, J.M.; Reuter, S.; Kandambeth, S.; Bach, N.N.; Döblinger, M.; Merz, J.; Clark, T.; Marder, T.B.; Bein, T.; et al. Switching on and off Interlayer Correlations and Porosity in 2D Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 12570–12581. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.; Ren, X.-R.; Wang, D.; Wan, L.-J. Lewis Acid Catalyzed Synthesis of Vinylene Linked Two Dimensional Covalent Organic Frameworks. Chin. J. Chem. 2022, 40, 1807–1812. [Google Scholar] [CrossRef]
- Maschita, J.; Banerjee, T.; Savasci, G.; Haase, F.; Ochsenfeld, C.; Lotsch, B.V. Ionothermal Synthesis of Imide-Linked Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 15750–15758. [Google Scholar] [CrossRef] [PubMed]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Je, S.H.; Talapaneni, S.N.; Kim, D.; Coskun, A. Charged Covalent Triazine Frameworks for CO2 Capture and Conversion. ACS Appl. Mater. Interfaces 2017, 9, 7209–7216. [Google Scholar] [CrossRef]
- Kuecken, S.; Schmidt, J.; Zhi, L.; Thomas, A. Conversion of amorphous polymer networks to covalent organic frameworks under ionothermal conditions: A facile synthesis route for covalent triazine frameworks. J. Mater. Chem. A 2015, 3, 24422–24427. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Jiang, G.; Ye, Q.; Wei, B.; Wang, H. Fluorinated, Sulfur-Rich, Covalent Triazine Frameworks for Enhanced Confinement of Polysulfides in Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 37731–37738. [Google Scholar] [CrossRef]
- Yi, J.-D.; Xu, R.; Chai, G.-L.; Zhang, T.; Zang, K.; Nan, B.; Lin, H.; Liang, Y.-L.; Lv, J.; Luo, J.; et al. Cobalt single-atoms anchored on porphyrinic triazine-based frameworks as bifunctional electrocatalysts for oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2019, 7, 1252–1259. [Google Scholar] [CrossRef]
- Harrod, C.; Alsudairy, Z.; Zheng, Q.; Campbell, A.; Brown, N.; Uddin, M.H.; Cai, S.; Li, X. Sustainable Synthesis of Palladium-Immobilized Covalent Organic Frameworks: A One-step Sonochemical Strategy. Adv. Sustain. Syst. 2025, 9, 2400700. [Google Scholar] [CrossRef]
- Jin, L.; Deng, M.; Gao, J.; Wang, L.; Zhou, Q.; Tang, X.; Li, Q.; Du, H.; Hao, D.; Wang, Q. A crystalline triazine covalent organic framework with partial fluorination for efficient hydrogen peroxide production toward water treatment. Chem. Eng. J. 2025, 515, 163722. [Google Scholar] [CrossRef]
- Wei, S.; Pan, W.; Liu, J.; Wu, J.; Xie, Y.; Wu, X.; Lu, C.-Z. High-Performance Perovskite Solar Cells via 3D Covalent Organic Frameworks: Enhanced Efficiency Through Precision Interface Engineering. Angew. Chem. Int. Ed. 2025, 64, e202500163. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Maity, P.; Qian, J.; Xu, G.; Wang, H.; Chen, T.; Zhou, K.; Hu, H.; He, Q.; et al. Synergistic stabilization of perovskite quantum dots via in situ encapsulation in a thiomethyl-functionalized covalent organic framework. Cell Rep. Phys. Sci. 2025, 6, 102398. [Google Scholar] [CrossRef]
- Emsley, J. Unsporting scandium. Nat. Chem. 2014, 6, 1025. [Google Scholar] [CrossRef] [PubMed]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef]
- Shinde, D.B.; Aiyappa, H.B.; Bhadra, M.; Biswal, B.P.; Wadge, P.; Kandambeth, S.; Garai, B.; Kundu, T.; Kurungot, S.; Banerjee, R. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 2016, 4, 2682–2690. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Z.; Alemany, L.B.; Li, Y.; Nnorom, N.; Barnes, M.; Khalil, S.; Rahman, M.M.; Ajayan, P.M.; Verduzco, R. Rapid, Ambient Temperature Synthesis of Imine Covalent Organic Frameworks Catalyzed by Transition-Metal Nitrates. Chem. Mater. 2021, 33, 3394–3400. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Kalhor, M. An efficient synthesis of Schiff bases containing benzimidazole moiety catalyzed by transition metal nitrates. Turk. J. Chem. 2010, 34, 367–374. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Steel, P.J.; Polson, M. Rapid and Efficient Synthesis of Schiff Bases Catalyzed by Copper Nitrate. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2009, 39, 189–192. [Google Scholar] [CrossRef]
- Cifuentes, J.M.C.; Ferreira, B.X.; Esteves, P.M.; Buarque, C.D. Decarboxylative Cross-Coupling of Cinnamic Acids Catalyzed by Iron-Based Covalent Organic Frameworks. Top. Catal. 2018, 61, 689–698. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Cai, S.; Lei, X.; Altoe, V.; Hong, F.; Urban, J.J.; Ciston, J.; Chan, E.M.; Liu, Y. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Commun. 2018, 9, 2998. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, C.-W.; Aguila, B.; Perman, J.; Wang, S.; Huang, H.-Y.; Xiao, F.-S.; Ma, S. Pore Environment Control and Enhanced Performance of Enzymes Infiltrated in Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 984–992. [Google Scholar] [CrossRef]
- Gomes, R.; Bhanja, P.; Bhaumik, A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 2015, 51, 10050–10053. [Google Scholar] [CrossRef]
- Mullangi, D.; Chakraborty, D.; Pradeep, A.; Koshti, V.; Vinod, C.P.; Panja, S.; Nair, S.; Vaidhyanathan, R. Highly Stable COF-Supported Co/Co(OH)2 Nanoparticles Heterogeneous Catalyst for Reduction of Nitrile/Nitro Compounds under Mild Conditions. Small 2018, 14, 1801233. [Google Scholar] [CrossRef]
- Shao, P.; Li, J.; Chen, F.; Ma, L.; Li, Q.; Zhang, M.; Zhou, J.; Yin, A.; Feng, X.; Wang, B. Flexible Films of Covalent Organic Frameworks with Ultralow Dielectric Constants under High Humidity. Angew. Chem. Int. Ed. 2018, 57, 16501–16505. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.A.; Butts, T.; Xiao, F.-S.; Ma, S. Integrating Superwettability within Covalent Organic Frameworks for Functional Coating. Chem 2018, 4, 1726–1739. [Google Scholar] [CrossRef]
- Burgess, J. Metal Ions in Solution. In Chemical Science; Series, E.H., Ed.; ACS Publications: Washington, DC, USA, 1978. [Google Scholar]
- Wulfsberg, G. Principles of Descriptive Inorganic Chemistry; University Science Books: Melville, NY, USA, 1991. [Google Scholar]
- Khojastehnezhad, A.; Rhili, K.; Shehab, M.K.; Gamraoui, H.; Peng, Z.; Samih ElDouhaibi, A.; Touzani, R.; Hammouti, B.; El-Kaderi, H.M.; Siaj, M. Rapid, Mild, and Catalytic Synthesis of 2D and 3D COFs with Promising Supercapacitor Applications. ACS Appl. Energy Mater. 2023, 6, 12216–12225. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, X.; Yang, X.; Wang, X.; Chu, W.; Hu, Y. Heterogenization of Heteropolyacids: A General Discussion on the Preparation of Supported Acid Catalysts. Ind. Eng. Chem. Res. 1996, 35, 2546–2560. [Google Scholar] [CrossRef]
- Allameh, S.; Davoodnia, A.; Khojastehnezhad, A. An efficient and eco-friendly synthesis of 14-aryl-14H-dibenzo [a, j] xanthenes using H4 [SiW12O40] as a heterogeneous and reusable catalyst under solvent-free conditions. Chin. Chem. Lett. 2012, 23, 17–20. [Google Scholar] [CrossRef]
- Ataie, F.; Davoodnia, A.; Khojastehnezhad, A. Graphene oxide functionalized organic-inorganic hybrid (GO–Si–NH2–PMo): An efficient and green catalyst for the synthesis of tetrahydrobenzo [b] pyran derivatives. Polycyclic. Aromat. Compd. 2021, 41, 781–794. [Google Scholar] [CrossRef]
- Javid, A.; Khojastehnezhad, A.; Heravi, M.; Bamoharram, F.F. Silica-supported preyssler nanoparticles catalyzed simple and efficient one-pot synthesis of 1, 8-dioxodecahydroacridines in aqueous media. Synth. React. Inorg. Met.Org. Nano-Met. Chem. 2012, 42, 14–17. [Google Scholar] [CrossRef]
- Hosseininasab, N.; Davoodnia, A.; Rostami-Charati, F.; Khojastehnezhad, A. Preparation, characterization, and first catalytic application of a novel phosphotungstic acid-containing ionic liquid immobilized on CuFe2O4@SiO2 magnetic nanoparticles in the synthesis of 1 H-pyrazolo [1, 2-b] phthalazine-5, 10-diones. Russ. J. Gen. Chem. 2017, 87, 2436–2443. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.-T.; Yuan, Z.-Y. Increasing the utilization of SiBeta support to anchor dual active sites of transition metal and heteropolyacids for efficient oxidative desulfurization of fuel. Appl. Catal. B 2022, 305, 121044. [Google Scholar] [CrossRef]
- Eshghi, H.; Khojastehnezhad, A.; Moeinpour, F.; Rezaeian, S.; Bakavoli, M.; Teymouri, M.; Rostami, A.; Haghbeen, K. Nanomagnetic organic–inorganic hybrid (Fe@Si-Gu-Prs): A novel magnetically green catalyst for the synthesis of tetrahydropyridine derivatives at room temperature under solvent-free conditions. Tetrahedron 2015, 71, 436–444. [Google Scholar] [CrossRef]
- Ishikawa, S.; Noda, N.; Wada, M.; Tsurumi, S.; Ueda, W. Selective Oxidation of Methacrolein over Crystalline Mo3VOx Catalysts and Comparison of Their Catalytic Properties with Heteropoly Acid Catalysts. ACS Catal. 2020, 10, 10535–10545. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Pereira, M.M.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V.; da Silva Rocha, K.A. Heteropoly acid catalysts in upgrading of biorenewables: Synthesis of para-menthenic fragrance compounds from α-pinene oxide. Catal. Today 2020, 344, 166–170. [Google Scholar] [CrossRef]
- Taghavi, F.; Khojastehnezhad, A.; Khalifeh, R.; Rajabzadeh, M.; Rezaei, F.; Abnous, K.; Taghdisi, S.M. Design and synthesis of a new magnetic metal organic framework as a versatile platform for immobilization of acidic catalysts and CO2 fixation reaction. New J. Chem. 2021, 45, 15405–15414. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; Ferreira, S.O.; da Silva, R.C.; Gabriel Filho, J.B.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn(II) doping on the Keggin heteropolyacid-catalyzed etherification of glycerol with tert-butyl alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- Eshghi, H.; Khojastehnezhad, A.; Moeinpour, F.; Bakavoli, M.; Seyedi, S.M.; Abbasi, M. Synthesis, characterization and first application of keggin-type heteropoly acids supported on silica coated NiFe2O4 as novel magnetically catalysts for the synthesis of tetrahydropyridines. RSC Adv. 2014, 4, 39782–39789. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Lukoyanov, I.A.; Panchenko, V.N.; Timofeeva, M.N.; Taran, O.P.; Parmon, V.N. Formic Acid Production via One-Pot Hydrolysis-Oxidation of Starch over Quaternary Ammonium Salts of Vanadium-Containing Keggin-Type Heteropoly Acids. Catalysts 2022, 12, 1252. [Google Scholar] [CrossRef]
- Dutta, T.K.; Patra, A. Post-synthetic Modification of Covalent Organic Frameworks through in situ Polymerization of Aniline for Enhanced Capacitive Energy Storage. Chem. Asian J. 2021, 16, 158–164. [Google Scholar] [CrossRef]
- Khayum, M.A.; Vijayakumar, V.; Karak, S.; Kandambeth, S.; Bhadra, M.; Suresh, K.; Acharambath, N.; Kurungot, S.; Banerjee, R. Convergent Covalent Organic Framework Thin Sheets as Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28139–28146. [Google Scholar] [CrossRef]
- Umezawa, S.; Douura, T.; Yoshikawa, K.; Takashima, Y.; Yoneda, M.; Gotoh, K.; Stolojan, V.; Silva, S.R.P.; Hayashi, Y.; Tanaka, D. Supercapacitor electrode with high charge density based on boron-doped porous carbon derived from covalent organic frameworks. Carbon 2021, 184, 418–425. [Google Scholar] [CrossRef]


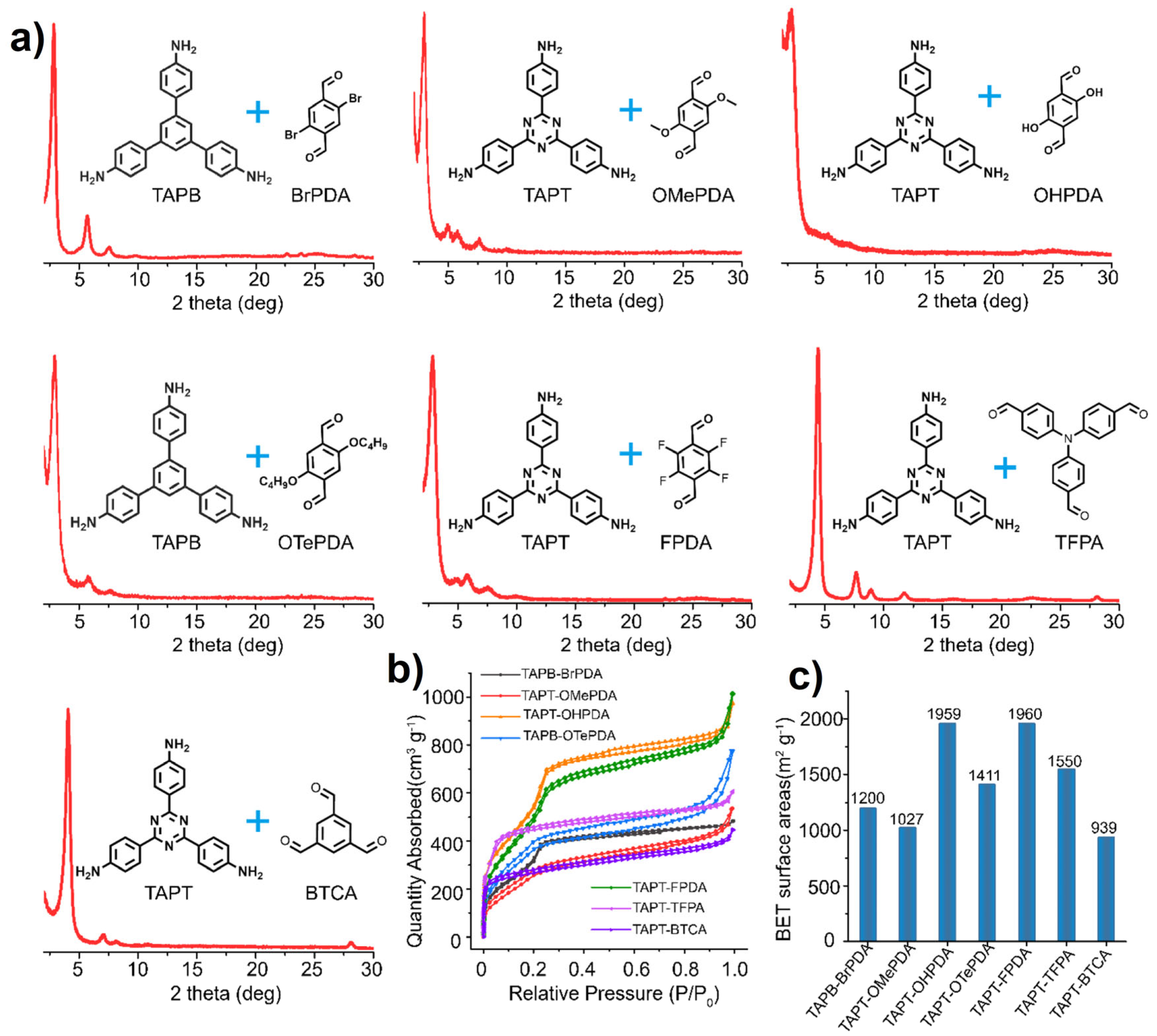
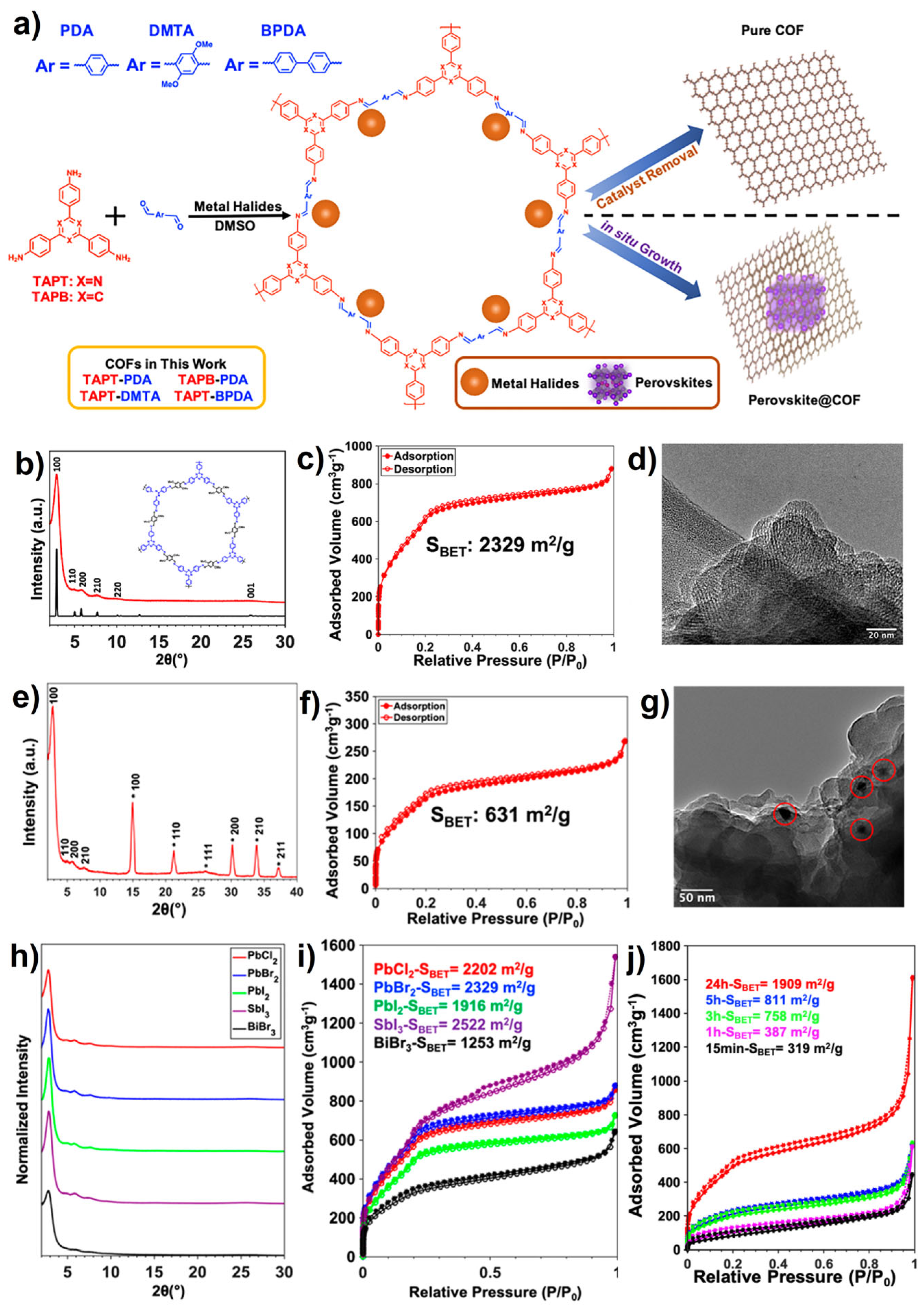

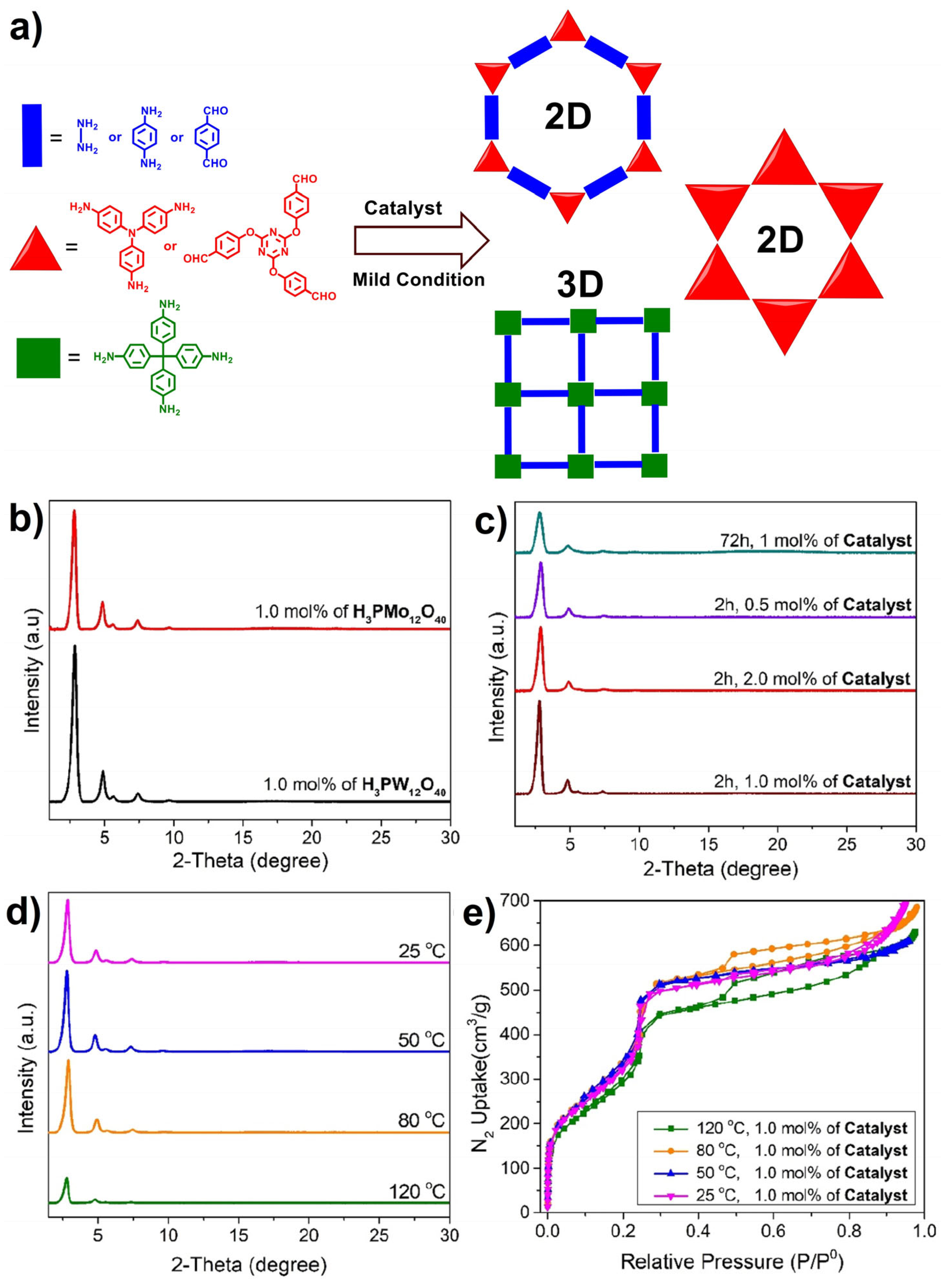
| Catalyst | Amount | Temperature/Pressure | Reaction Time | Yield | Crystallinity | Surface Area | Reference |
|---|---|---|---|---|---|---|---|
| Acetic Acid | 6 M | 80–120 °C/Autogeneous pressure | 24–96 h | 50–85% | Highly Crystalline | 300–1500 m2 g−1 | [32] |
| Metal Triflate | 0.002 eq. | 20 °C/Ambient pressure | 1 min | 95% | 2175 m2 g−1 | [79] | |
| Ionic Liquids | 100 μL | Ambient temperature and pressure | 3 min | 65% | 517 m2 g−1 | [91] | |
| Metal Oxides | 94 mol% | 120 °C/Ambient pressure | 3 days | 65–95% | 2405 m2 g−1 | [116] | |
| Metal Halides | 0.056 eq. | 90 °C/Ambient pressure | 24 h | 79% | 2522 m2 g−1 | [107] | |
| Metal Nitrates | 10 mol% | Ambient temperature and pressure | 120 min | 93.1% | 1674 m2 g−1 | [140] | |
| Heteropoly Acids | 1.0 mol% | 50 °C/Ambient pressure | 120 min | 92% | 1277 m2 g−1 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, M.; Peng, Z.; Samie, A.; Taghavi, F.; Khojastehnezhad, A.; Siaj, M. Catalyst-Driven Improvements in Conventional Methods for Imine-Linked Covalent Organic Frameworks. Molecules 2025, 30, 2969. https://doi.org/10.3390/molecules30142969
Jafari M, Peng Z, Samie A, Taghavi F, Khojastehnezhad A, Siaj M. Catalyst-Driven Improvements in Conventional Methods for Imine-Linked Covalent Organic Frameworks. Molecules. 2025; 30(14):2969. https://doi.org/10.3390/molecules30142969
Chicago/Turabian StyleJafari, Maziar, Zhiyuan Peng, Ali Samie, Faezeh Taghavi, Amir Khojastehnezhad, and Mohamed Siaj. 2025. "Catalyst-Driven Improvements in Conventional Methods for Imine-Linked Covalent Organic Frameworks" Molecules 30, no. 14: 2969. https://doi.org/10.3390/molecules30142969
APA StyleJafari, M., Peng, Z., Samie, A., Taghavi, F., Khojastehnezhad, A., & Siaj, M. (2025). Catalyst-Driven Improvements in Conventional Methods for Imine-Linked Covalent Organic Frameworks. Molecules, 30(14), 2969. https://doi.org/10.3390/molecules30142969








