The Application of Quantitative 1H-NMR for the Determination of Melatonin and Vitamin B6 in Commercial Melatonin Products
Abstract
1. Introduction
2. Results and Discussion
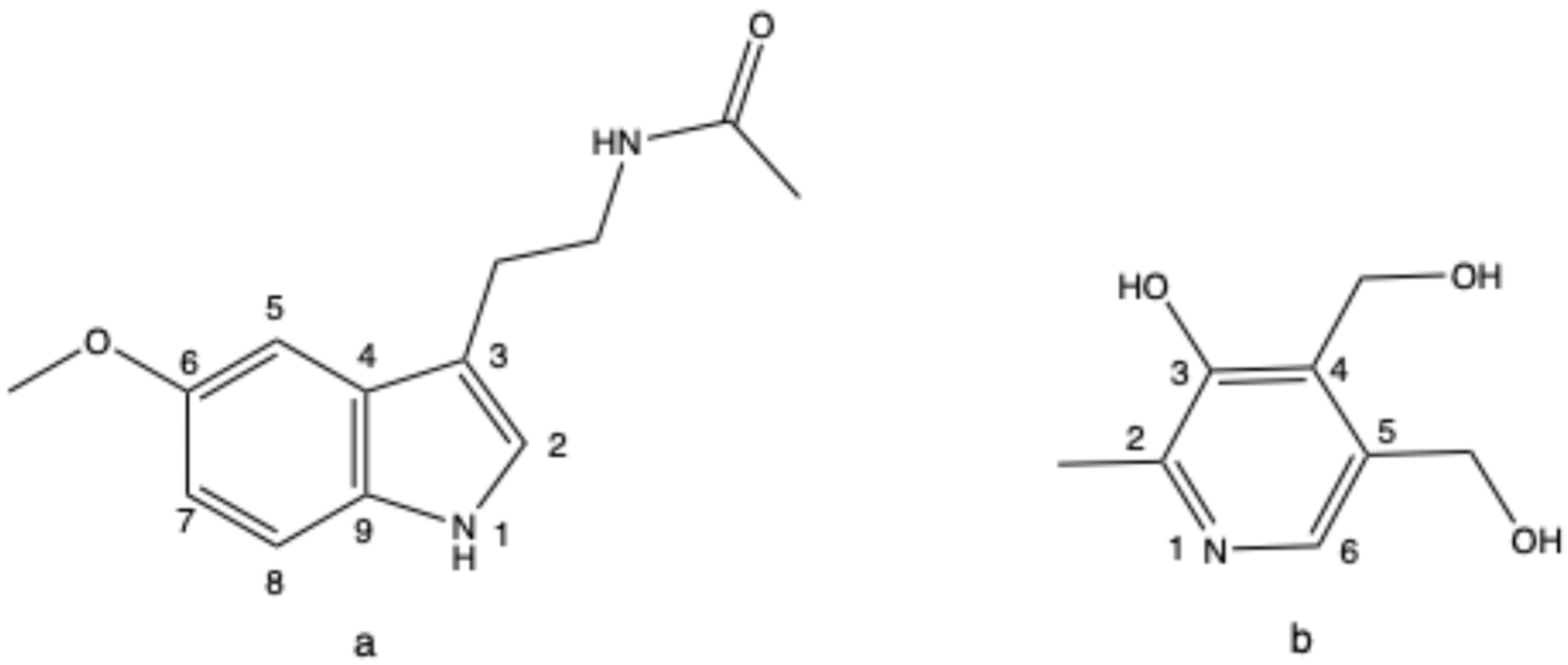
2.1. 1H-NMR Method Development
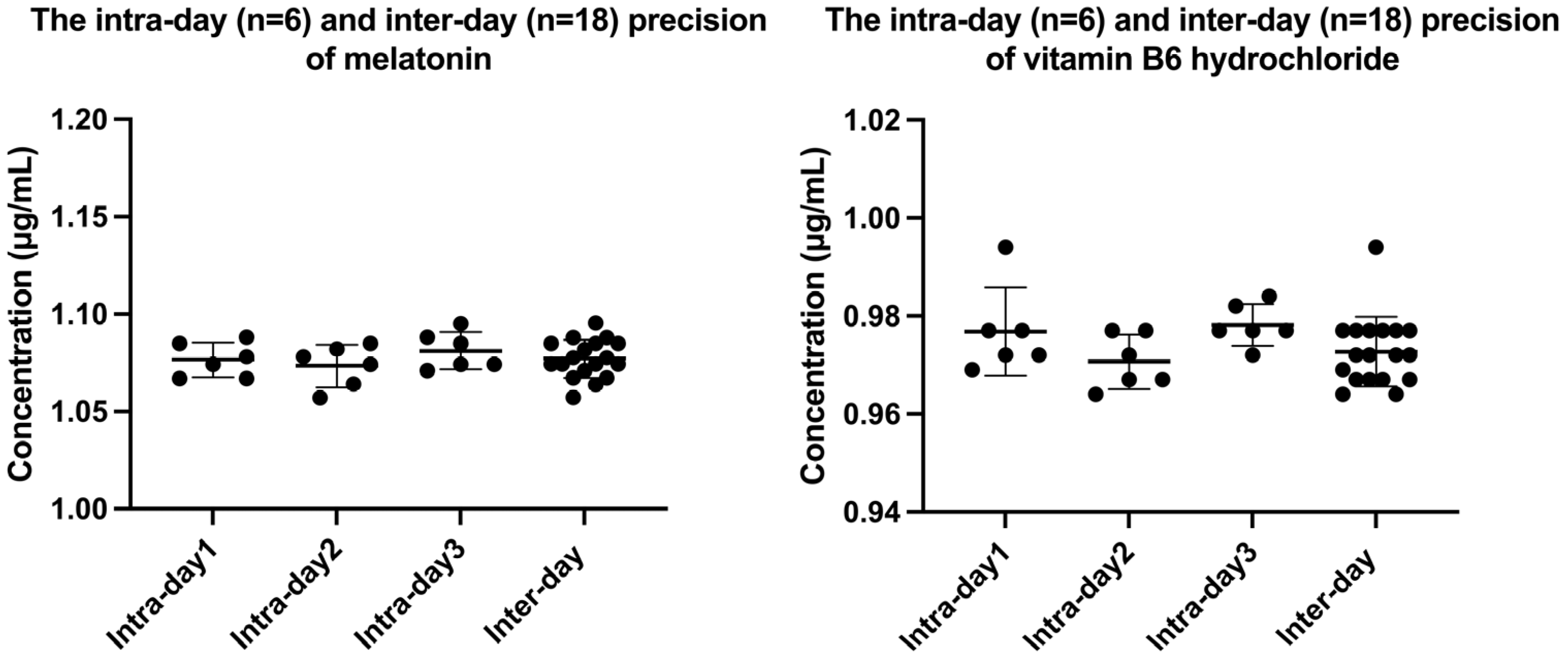
2.2. Method Validation
2.3. Method Application
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample Preparation
3.3. 1H-NMR Measurements
3.4. HPLC-PDA Analysis
3.5. Identification of Contents
3.6. Quantitative 1H-NMR Validation Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grassi, L.; Zachariae, R.; Caruso, R.; Palagini, L.; Campos-Ródenas, R.; Riba, M.B.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; et al. Insomnia in Adult Patients with Cancer: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 102047. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep Disorders and Cancer: State of the Art and Future Perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.K.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef]
- Kim, T.; Slominski, R.M.; Pyza, E.; Kleszczynski, K.; Tuckey, R.C.; Reiter, R.J.; Holick, M.F.; Slominski, A.T. Evolutionary Formation of Melatonin and Vitamin D in Early Life Forms: Insects Take Centre Stage. Biol. Rev. 2024, 99, 1772–1790. [Google Scholar] [CrossRef]
- Geoffriau, M.; Brun, J.; Chazot, G.; Claustrat, B. The Physiology and Pharmacology of Melatonin in Humans. Horm. Res. Paediatr. 1998, 49, 136–141. [Google Scholar] [CrossRef]
- Bliwise, D.L.; Ansari, F.P. Insomnia Associated with Valerian and Melatonin Usage in the 2002 National Health Interview Survey. Sleep 2007, 30, 881–884. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef]
- Matheson, E.; Hainer, B.L. Insomnia: Pharmacologic Therapy. Am. Fam. Physician 2017, 96, 29–35. [Google Scholar] [PubMed][Green Version]
- Arvapally, M.; Asati, A.; Nagendla, N.K.; Mudiam, M.K.R. Development of an Analytical Method for the Quantitative Determination of Multi-Class Nutrients in Different Food Matrices by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry Using Design of Experiments. Food Chem. 2021, 341, 128173. [Google Scholar] [CrossRef]
- Ahmad, I.; Mirza, T.; Qadeer, K.; Nazim, U.; Vaid, F.H. Vitamin B6: Deficiency Diseases and Methods of Analysis. Pak. J. Pharm. Sci. 2013, 26, 1057–1069. [Google Scholar]
- Hellmann, H.; Mooney, S. Vitamin B6: A Molecule for Human Health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.D.; De Lucia, A.; Pirozzi, C.; Pastore, V. Comparative Study between the Use of Melatonin and A Solution with Melatonin, Tryptophan, and Vitamin B6 as an Inducer of Spontaneous Sleep in Children During an Auditory Response Test: An Alternative to Commonly Used Sedative Drugs. J. Int. Adv. Otol. 2017, 13, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Nùñez-Vergara, L.J.; Squella, J.A.; Sturm, J.C.; Baez, H.; Camargo, C. Simultaneous Determination of Melatonin and Pyridoxine in Tablets by Gas Chromatography-Mass Spectrometry. J. Pharm. Biomed. Anal. 2001, 26, 929–938. [Google Scholar] [CrossRef]
- Kamaris, G.; Mitsiou, V.-P.M.; Chachlioutaki, K.; Almpani, S.; Markopoulou, C.K. Development and Validation of an HPLC-FLD Method for the Determination of Pyridoxine and Melatonin in Chocolate Formulations—Digestion Simulation Study. Chemistry 2025, 7, 14. [Google Scholar] [CrossRef]
- Santander, P.; Nuñez-Vergara, L.J.; Sturm, J.C.; Squella, J.A. Voltammetric determination of melatonin and pyridoxine (Vitamin B6) in tablets. Bol. Soc. Chil. Quím. 2001, 46, 1691–1699. [Google Scholar] [CrossRef]
- Cui, C.; Xu, Y.; Jin, G.; Zong, J.; Peng, C.; Cai, H.; Hou, R. Machine Learning Applications for Identify the Geographical Origin, Variety and Processing of Black Tea Using 1H NMR Chemical Fingerprinting. Food Control 2023, 148, 109686. [Google Scholar] [CrossRef]
- Cui, C.; Xia, M.; Wei, Z.; Chen, J.; Peng, C.; Cai, H.; Jin, L.; Hou, R. 1H NMR-Based Metabolomic Approach Combined with Machine Learning Algorithm to Distinguish the Geographic Origin of Huajiao (Zanthoxylum bungeanum Maxim.). Food Control 2023, 145, 109476. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR Applications to Tackle Future Food Fraud Issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef]
- Riswanto, F.D.O.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Fadzilah, N.A.; Rohman, A. Metabolite Fingerprinting Based on 1H-NMR Spectroscopy and Liquid Chromatography for the Authentication of Herbal Products. Molecules 2022, 27, 1198. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Ren, X.; Jiang, M.; Deng, Y. Rapid Authentication and Differentiation of Herbal Medicine Using 1H NMR Fingerprints Coupled with Chemometrics. J. Pharm. Biomed. Anal. 2018, 160, 323–329. [Google Scholar] [CrossRef]
- Dos Santos Ribeiro, H.S.; Dagnino, D.; Schripsema, J. Rapid and Accurate Verification of Drug Identity, Purity and Quality by 1H-NMR Using Similarity Calculations and Differential NMR. J. Pharm. Biomed. Anal. 2021, 199, 114040. [Google Scholar] [CrossRef] [PubMed]
- Holzgrabe, U.; Deubner, R.; Schollmayer, C.; Waibel, B. Quantitative NMR Spectroscopy—Applications in Drug Analysis. J. Pharm. Biomed. Anal. 2005, 38, 806–812. [Google Scholar] [CrossRef]
- Zoppi, A.; Linares, M.; Longhi, M. Quantitative Analysis of Enalapril by 1H NMR Spectroscopy in Tablets. J. Pharm. Biomed. Anal. 2005, 37, 627–630. [Google Scholar] [CrossRef]
- Schripsema, J. Application of NMR in Plant Metabolomics: Techniques, Problems and Prospects. Phytochem. Anal. 2010, 21, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Universal Quantitative NMR Analysis of Complex Natural Samples. Curr. Opin. Biotechnol. 2014, 25, 51–59. [Google Scholar] [CrossRef]
- Vilca-Melendez, S.; Uthaug, M.V.; Griffin, J.L. 1H Nuclear Magnetic Resonance: A Future Approach to the Metabolic Profiling of Psychedelics in Human Biofluids? Front. Psychiatry 2021, 12, 742856. [Google Scholar] [CrossRef]
- Tampieri, A.; Szabó, M.; Medina, F.; Gulyás, H. A Brief Introduction to the Basics of NMR Spectroscopy and Selected Examples of Its Applications to Materials Characterization. Phys. Sci. Rev. 2021, 6, 20190086. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamazaki, M.; Hasada, K.; Nagatsu, A. Application of Quantitative 1H-NMR Method to Determination of Paeoniflorin in Paeoniae Radix. J. Nat. Med. 2013, 67, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chauthe, S.K.; Sharma, R.J.; Aqil, F.; Gupta, R.C.; Singh, I.P. Quantitative NMR: An Applicable Method for Quantitative Analysis of Medicinal Plant Extracts and Herbal Products. Phytochem. Anal. 2012, 23, 689–696. [Google Scholar] [CrossRef]
- Pauli, G.F.; Jaki, B.U.; Lankin, D.C. Quantitative1 H NMR: Development and Potential of a Method for Natural Products Analysis. J. Nat. Prod. 2005, 68, 133–149. [Google Scholar] [CrossRef]
- Tada, A.; Takahashi, K.; Ishizuki, K.; Sugimoto, N.; Suematsu, T.; Arifuku, K.; Tahara, M.; Akiyama, T.; Ito, Y.; Yamazaki, T.; et al. Absolute Quantitation of Stevioside and Rebaudioside A in Commercial Standards by Quantitative NMR. Chem. Pharm. Bull. 2013, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Branch, S.K. Guidelines from the International Conference on Harmonisation (ICH). J. Pharm. Biomed. Anal. 2005, 38, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Yu, M. Rapid Determination of Acarbose in Tablets by 1H NMR Spectroscopy. Curr. Pharm. Anal. 2020, 16, 585–590. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR Spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- CFDA. Pharmacopoeia of the People’s Republic of China; CFDA: Beijing, China, 2020. [Google Scholar]
- Zhang, X.; Wang, C.; Chen, Z.; Zhang, P.; Liu, H. Development and Validation of Quantitative1 H NMR Spectroscopy for the Determination of Total Phytosterols in the Marine Seaweed Sargassum. J. Agric. Food Chem. 2016, 64, 6228–6232. [Google Scholar] [CrossRef]


| Method | Parameters | Melatonin | Vitamin B6 Hydrochloride | |
|---|---|---|---|---|
| 1H-NMR | Linear equation | y = 2.4839x − 0.0666 | y = 2.8872x − 0.1193 | |
| R squared | 0.9985 | 0.9995 | ||
| Linear range (mg/mL) | 0.1–4 | 0.1–5 | ||
| LOD (mg/mL) | 0.007 | 0.006 | ||
| LOQ (mg/mL) | 0.1 | 0.02 | ||
| Precision (RSD, %) | Intra-day | 0.80–1.00 | 0.44–0.93 | |
| Inter-day | 0.90 | 0.73 | ||
| Reproducibility (%) | 1.51 | 0.93 | ||
| Recovery (%) | 107.3 | 97.8 | ||
| HPLC | Linear equation | y = 14,453x + 77,651 | y = 10,469x + 13,686 | |
| R squared | 0.9995 | 1.0000 | ||
| Linear range (mg/mL) | 0.02–1 | 0.02–1 | ||
| Weight (mg) | Melatonin (μg) | Vitamin B6 Hydrochloride (μg) | ||
|---|---|---|---|---|
| 1H-NMR | HPLC | 1H-NMR | HPLC | |
| 10 | 105.8 ± 0.4 | 99.5 ± 0.2 | 368.0 ± 2.0 | 365.2 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Niu, J.; Xiao, Z.; Rong, D.; Yu, M.; Sy, S.K.B.; Wang, C.; Lv, Z. The Application of Quantitative 1H-NMR for the Determination of Melatonin and Vitamin B6 in Commercial Melatonin Products. Molecules 2025, 30, 2942. https://doi.org/10.3390/molecules30142942
Gao X, Niu J, Xiao Z, Rong D, Yu M, Sy SKB, Wang C, Lv Z. The Application of Quantitative 1H-NMR for the Determination of Melatonin and Vitamin B6 in Commercial Melatonin Products. Molecules. 2025; 30(14):2942. https://doi.org/10.3390/molecules30142942
Chicago/Turabian StyleGao, Xinyu, Jiahao Niu, Zhengjian Xiao, Da Rong, Mingming Yu, Sherwin K. B. Sy, Cong Wang, and Zhihua Lv. 2025. "The Application of Quantitative 1H-NMR for the Determination of Melatonin and Vitamin B6 in Commercial Melatonin Products" Molecules 30, no. 14: 2942. https://doi.org/10.3390/molecules30142942
APA StyleGao, X., Niu, J., Xiao, Z., Rong, D., Yu, M., Sy, S. K. B., Wang, C., & Lv, Z. (2025). The Application of Quantitative 1H-NMR for the Determination of Melatonin and Vitamin B6 in Commercial Melatonin Products. Molecules, 30(14), 2942. https://doi.org/10.3390/molecules30142942







