Characterization of Carboxylated Cellulose Nanocrystals Isolated Through Oxalic Acid Hydrolysis from Solid Residues of Softwood-Derived Glycol Lignin Production †
Abstract
1. Introduction
2. Results and Discussion
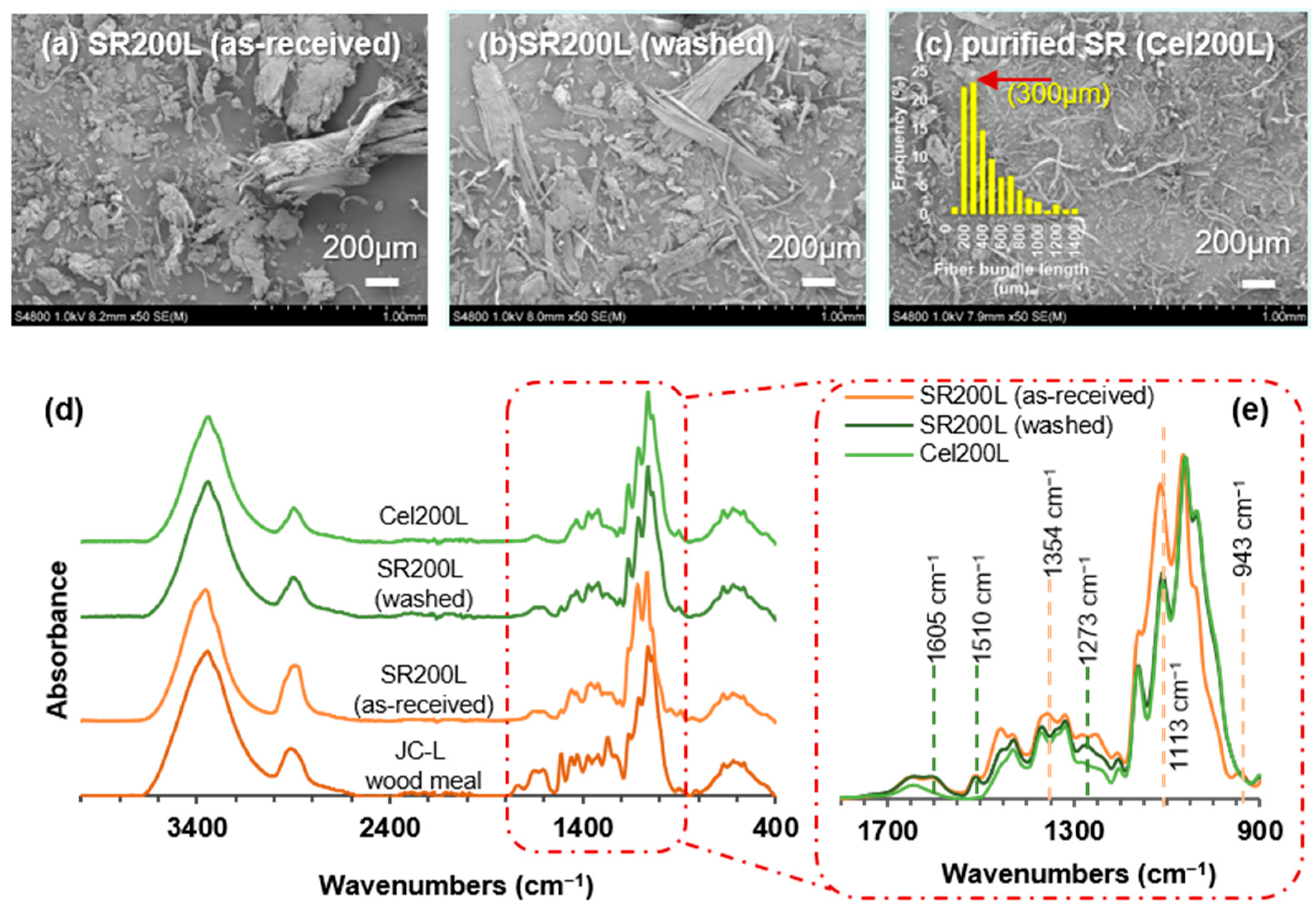
2.1. Pretreatment of As-Received Solid Residues (SRs)
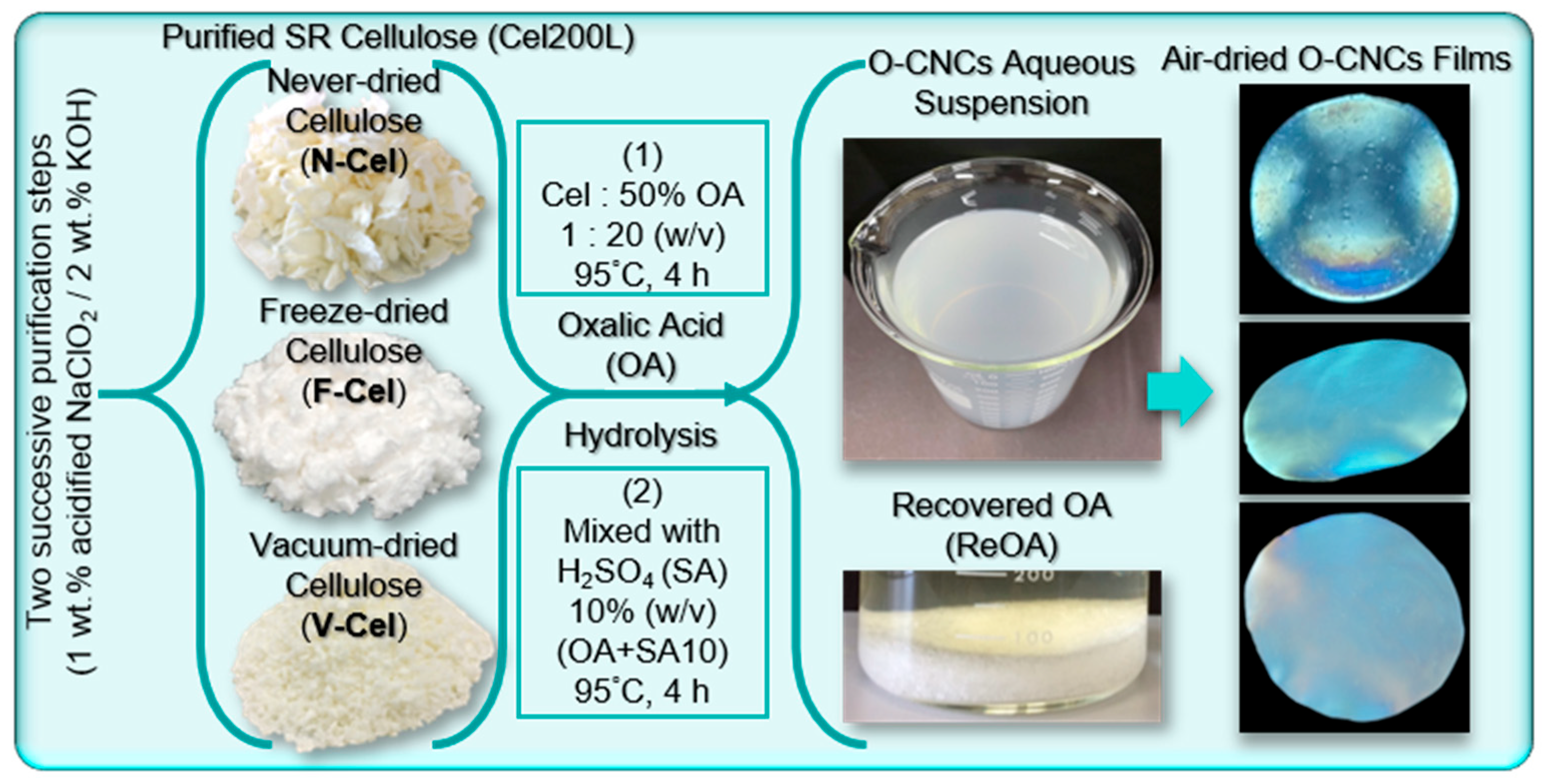
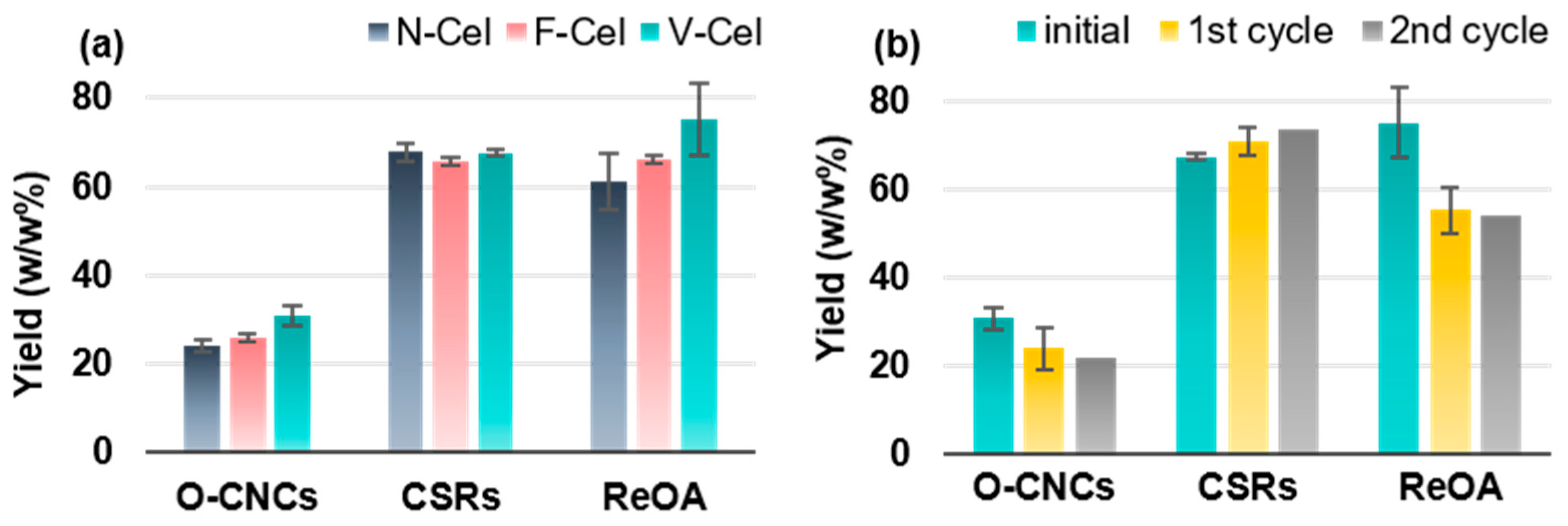
2.2. Preparation Strategy and CNC Yields
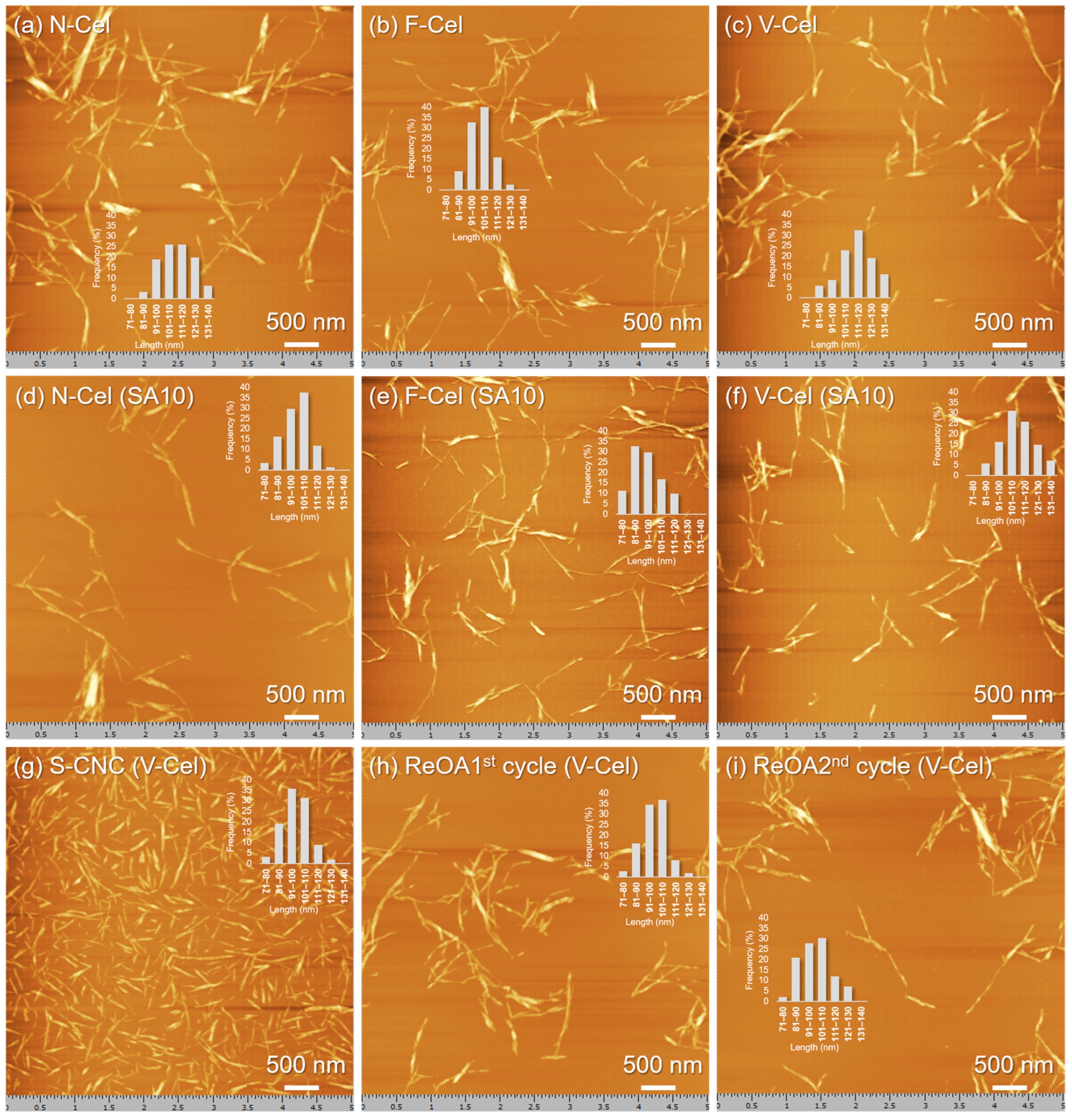
2.3. Morphologies and Surface Charges
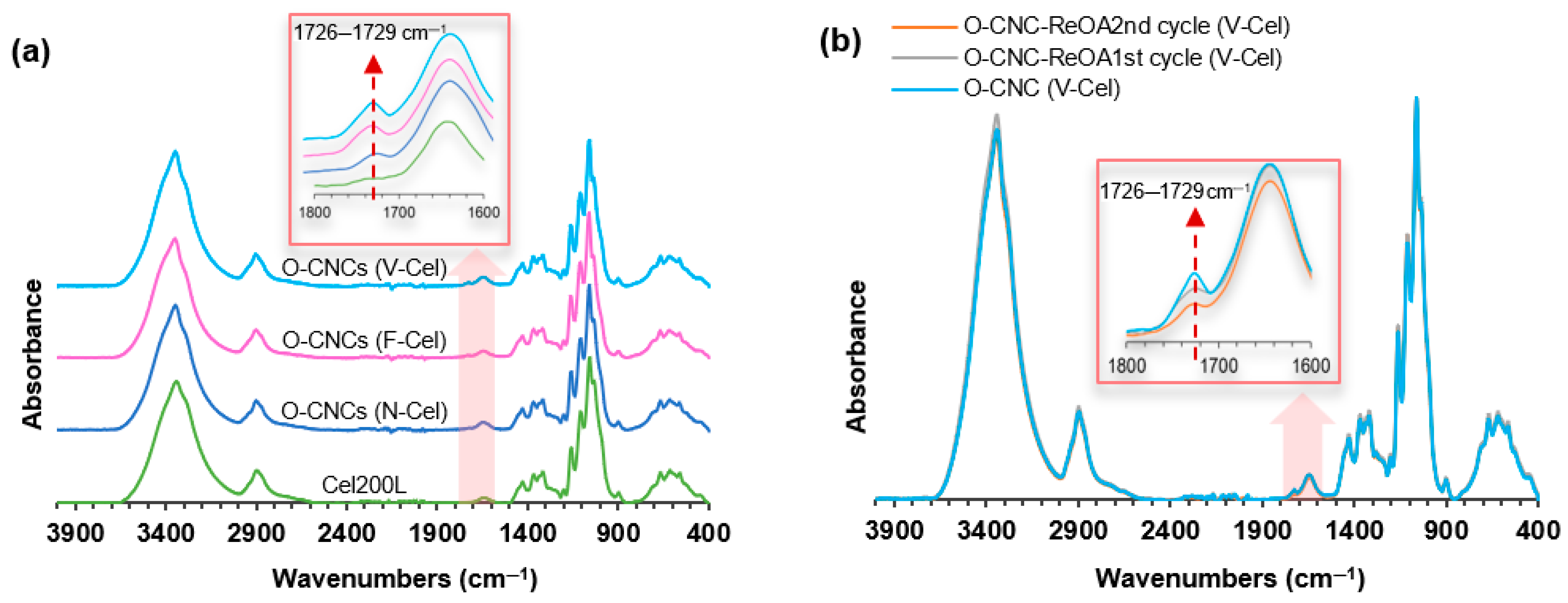
2.4. Esterification and Carboxylation
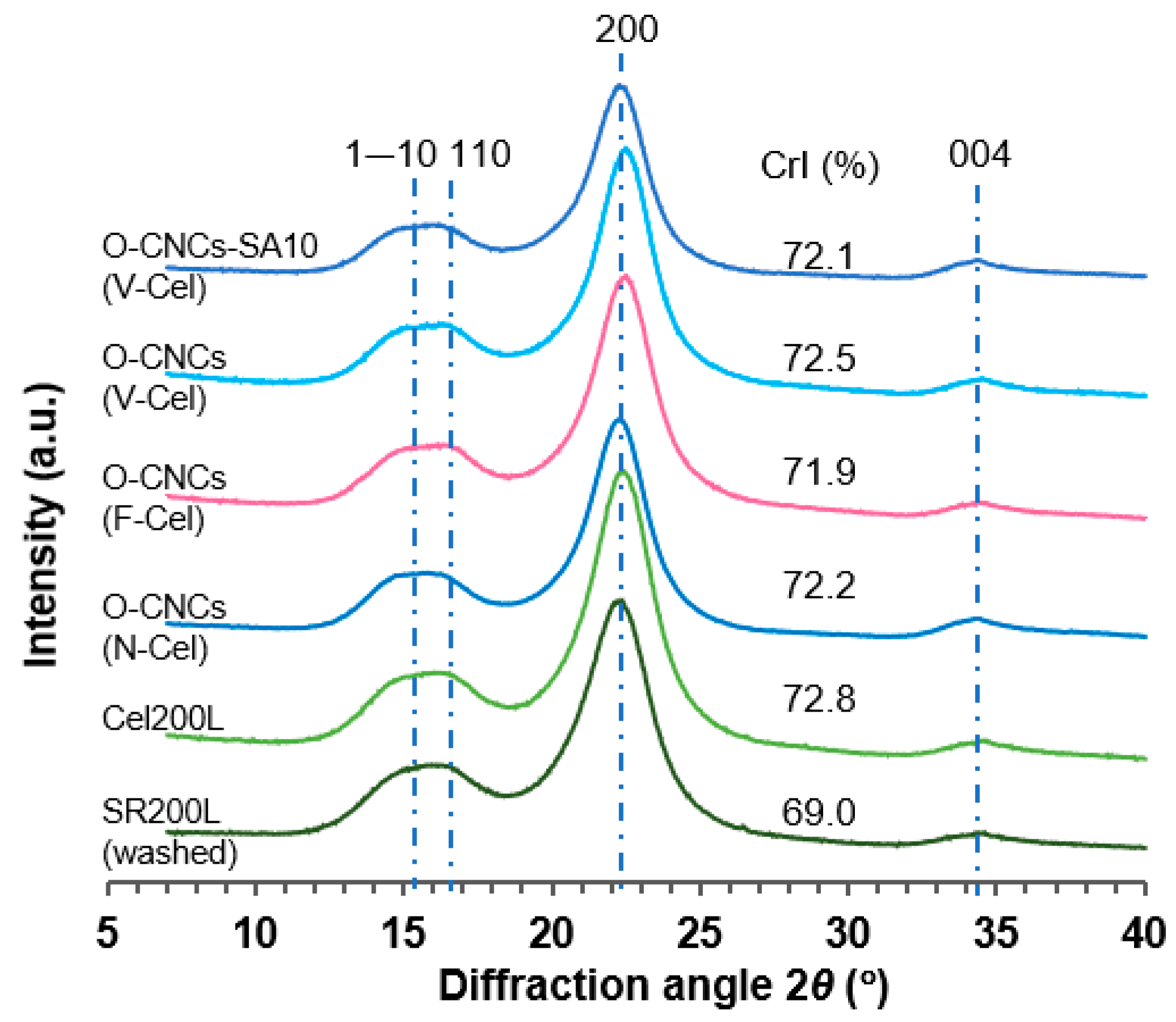
2.5. Crystallinity
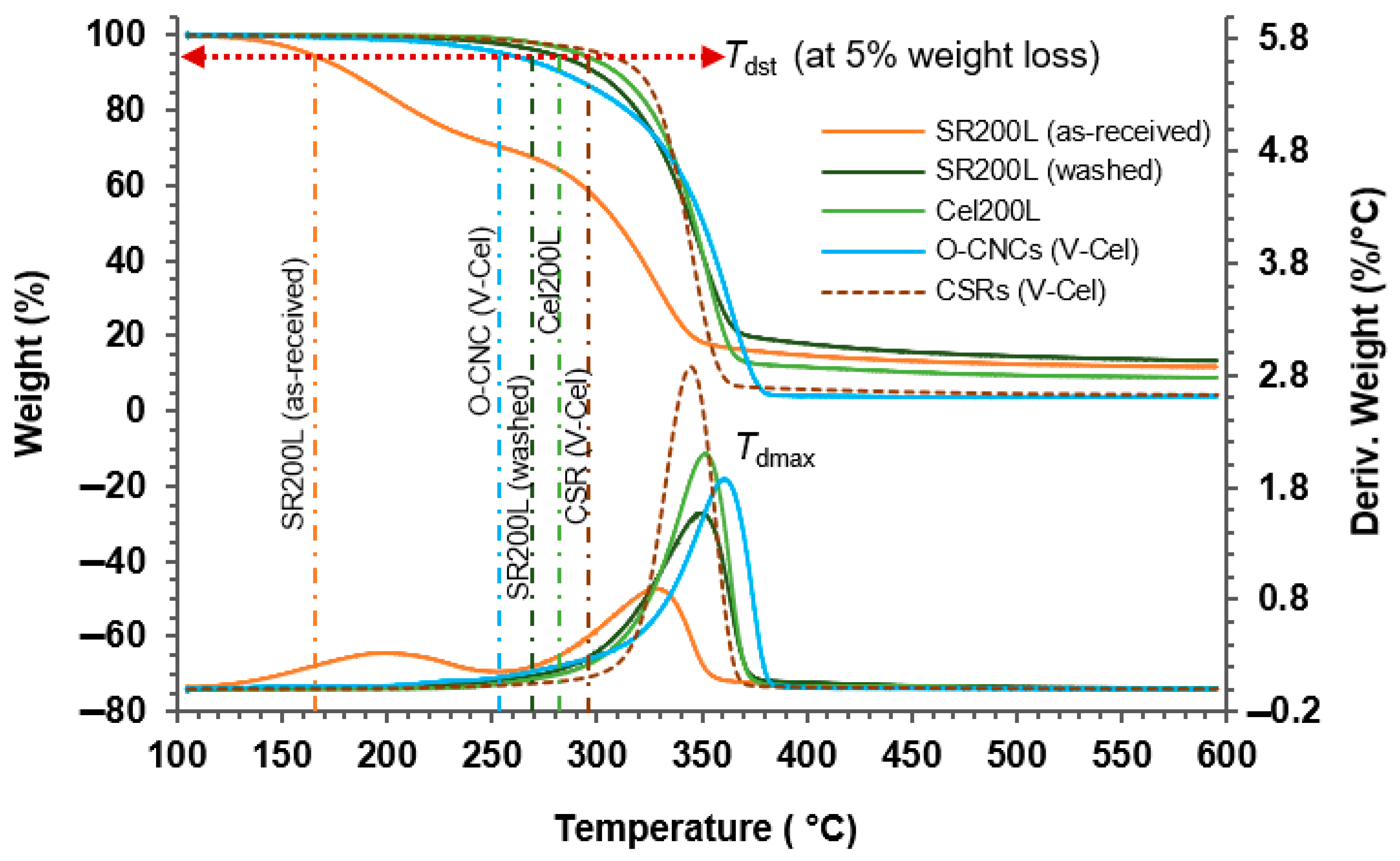
2.6. Thermal Stability
3. Materials and Methods
3.1. Materials
3.2. Purification of Cellulose from Solid Residue
3.3. Isolation of Cellulose Nanocrystals
3.4. Characterization of O-CNCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulis, D.B.; Lavoine, N.; Sederoff, H.; Jiang, X.; Marques, B.M.; Lan, K.; Cofre-Vega, C.; Barrangou, R.; Wang, J.P. Advances in Lignocellulosic Feedstocks for Bioenergy and Bioproducts. Nat. Commun. 2025, 16, 1244. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Sun, P.; Peng, C. A Review on Renewable Energy: Conversion and Utilization of Biomass. Smart Mol. 2024, 2, e20240019. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, L.C.; Larson, D.W. Cellulose and the Evolution of Plant Life. Bioscience 1989, 39, 238–241. [Google Scholar] [CrossRef]
- Henriksson, G. Lignin. In Pulp and Paper Chemistry and Technology, Wood Chemistry and Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; de Gruyter: Berlin, Germany, 2009; Volume 1, pp. 121–146. [Google Scholar]
- Nge, T.T.; Tobimatsu, Y.; Takahashi, S.; Takata, E.; Yamamura, M.; Miyagawa, Y.; Ikeda, T.; Umezawa, T.; Yamada, T. Isolation and Characterization of Polyethylene Glycol (PEG)-Modified Glycol Lignin via PEG Solvolysis of Softwood Biomass in a Large-Scale Batch Reactor. ACS Sustain. Chem. Eng. 2018, 6, 7841–7848. [Google Scholar] [CrossRef]
- Yamada, T.; Takata, E.; Takahashi, S.; Nge, T.T.; Ikeda, T. Glycol Lignin Production and Method and Its System. JP. Patent No. 6890821B2, 28 May 2021. [Google Scholar]
- Nge, T.T.; Yamada, T.; Tobimatsu, Y.; Yamamura, M.; Ishii, R.; Tanaike, O.; Ebina, T. Fractionation and Characterization of Glycol Lignins by Stepwise-pH Precipitation of Japanese Cedar/Poly(ethylene glycol) Solvolysis Liquor. ACS Sustain. Chem. Eng. 2021, 9, 756–764. [Google Scholar] [CrossRef]
- Tanks, J.; Tamura, K.; Naito, K.; Nge, T.T.; Yamada, T. Glycol Lignin/MAH-g-PP Blends and Composites with Exceptional Mechanical Properties for Automotive Applications. Compos. Sci. Technol. 2023, 238, 110030. [Google Scholar] [CrossRef]
- Nge, T.T.; Hayashi, N.; Yamada, T. Isolation and Characterization of Cellulose Nanocrystals from Residual Softwood Pulp of the Glycol Lignin Production. In Proceedings of the “The 5th International Cellulose Conference,” International Conference Center Hiroshima, Hiroshima, Japan, 26–28 September 2023. [Google Scholar]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.M.; Matharu, G.K.; Hamad, W.Y.; Lizundia, E.; MacLachlan, M.J. Chiral Nematic Cellulose Nanocrystal/Germania and Carbon/Germania Composite Aerogels as Supercapacitor Materials. Chem. Mater. 2021, 33, 5197–5209. [Google Scholar] [CrossRef]
- Chang, T.; Wang, B.; Yuan, D.; Wang, Y.; Smalyukh, I.; Zhou, G.; Zhang, Z. Cellulose Nanocrystal Chiral Photonic Micro-flakes for Multilevel Anti-Counterfeiting and Identification. Chem. Eng. J. 2022, 446, 136630. [Google Scholar] [CrossRef]
- Fu, Q.; Zheng, T.; Wan, W.; Niu, C.; Chen, Y.; Xu, Y.; Long, T.; Lu, L.; Jiang, H. Liquid Crystal Phase Behavior of Oxalated Cellulose Nanocrystal and Optical Films with Controllable Structural Color Induced by Centripetal Force. Int. J. Biol. Macromol. 2024, 280, 135883. [Google Scholar] [CrossRef]
- An, B.; Xu, M.; Sun, J.; Sun, W.; Miao, Y.; Ma, C.; Luo, S.; Li, J.; Li, W.; Liu, S. Cellulose Nanocrystals-Based Bio-Composite Optical Materials for Reversible Colorimetric Responsive Films and Coatings. Int. J. Biol. Macromol. 2023, 233, 123600. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Nizamuddin, S.; Xu, J.; Vancov, T.; Chen, C. Cellulose Nanocrystals from Agriculture and Forestry Biomass: Synthesis Methods, Characterization and Industrial Applications. Environ. Sci. Pollut. Res. 2024, 31, 58745–58778. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the Yield and Characteristics of Wood Cellulose nanocrystals (CNC) using Concentrated Acid Hydrolysis. Cellulose 2015, 22, 1753–1762. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching Zero Cellulose Loss in Cellulose Nanocrystal (CNC) Production: Recovery and Characterization of Cellulosic Solid Residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Abitbol, T.; Kubat, M.; Brännvall, E.; Kotov, N.; Johnson, C.M.; Nizamov, R.; Nyberg, M.; Miettunen, K.; Nordgren, N.; Stevanic, J.S.; et al. Isolation of Mixed Compositions of Cellulose Nanocrystals, Microcrystalline Cellulose, and Lignin Nanoparticles from Wood Pulps. ACS Omega 2023, 8, 21474–21484. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile Extraction of Thermally Stable Cellulose Nanocrystals with a High Yield of 93% through Hydrochloric Acid Hydrolysis under Hydrothermal Conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Espinosa, S.C.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of Thermally Stable Cellulose Nanocrystals by Phosphoric Acid Hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.R.; Cranston, E.D. Insight into Thermal Stability of Cellulose Nanocrystals from New Hydrolysis Methods with Acid Blends. Cellulose 2019, 26, 507–528. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A Novel Approach for the Preparation of Nanocrystalline Cellulose by Using Phosphotungstic Acid. Carbohydr. Polym. 2014, 110, 415–422. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly Thermal-Stable and Functional Cellulose Nanocrystals and Nanofibrils Produced using Fully Recyclable Organic Acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-Free Preparation of Cellulose Nanocrystals by TEMPO Oxidation and Subsequent Cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.C.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; He, Y.; Qian, Y.; Wang, C.; Chen, G. Large-Scale Preparation of Carboxylated Cellulose Nanocrystals and Their Application for Stabilizing Pickering Emulsions. ACS. Omega 2023, 8, 15114–15123. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirvio, J.A.; Hormi, O.E.O.; Niinimaki, J. Enhancement of the Nanofibrillation of Wood Cellulose through Sequential Periodate-Chlorite Oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef]
- Zhou, L.; Li, N.; Shu, J.; Liu, Y.; Wang, K.; Cui, X.; Yuan, Y.; Ding, B.; Geng, Y.; Wang, Z.; et al. One-Pot Preparation of Carboxylated Cellulose Nanocrystals and Their Liquid Crystalline Behaviors. ACS Sustain. Chem. Eng. 2018, 6, 12403–12410. [Google Scholar] [CrossRef]
- Jia, C.; Chen, L.; Shao, Z.; Agarwal, U.P.; Hu, L.; Zhu, J.Y. Using a Fully Recyclable Dicarboxylic Acid for Producing Dispersible and Thermally Stable Cellulose Nanomaterials from Different Cellulosic Sources. Cellulose 2017, 24, 2483–2498. [Google Scholar] [CrossRef]
- Wang, W.; Sun, N.; Cai, Z.; Sun, K.; Gu, F.; Jin, Y.; Xiao, H. Sustainable High Yield Production of Cellulose Nanomaterials for Easy-Cleaning Surfaces of Cellulose-Based Materials. BioResources 2020, 15, 1014–1025. [Google Scholar] [CrossRef]
- Lin, C.; Wang, P.; Liu, Y.; Lv, Y.; Ye, X.; Liu, M.; Zhu, J.Y. Chiral Self-Assembly Behavior of Carboxylated Cellulose Nanocrystals Isolated by Recyclable Oxalic Acid from Degreasing Cotton. ACS Sustain. Chem. Eng. 2023, 11, 8035–8043. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Effect of Fiber Drying on Properties of Lignin Containing Cellulose Nanocrystals and Nanofibrils Produced through Maleic Acid Hydrolysis. Cellulose 2017, 24, 4205–4216. [Google Scholar] [CrossRef]
- Xu, W.; Grénman, H.; Liu, J.; Kronlund, D.; Li, B.; Backman, P.; Peltonen, J.; Willför, S.; Sundberg, A.; Xu, C. Mild Oxalic-Acid-Catalyzed-Hydrolysis as a Novel Approach to Prepare Cellulose Nanocrystals. ChemNanoMat 2016, 3, 109–119. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.; Ek, M. Esterification and Hydrolysis of Cellulose using Oxalic Acid Dihydrate in a Solvent-Free Reaction Suitable for Preparation of Surface-Functionalised Cellulose Nanocrystals with High Yield. Green Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Saini, A.; Yadav, C.; Xue, B.-L.; Wang, N.; Dai, L.; Li, X. Mixed-Acid-Assisted Hydrothermal Process for Simultaneous Preparation and Carboxylation of Needle-Shaped Cellulose Nanocrystals. ACS. Appl. Polym. Mater. 2020, 2, 548–562. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-Mediated Surface Oxidation of Cellulose Whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]


| Cellulose Source | Sample Condition | Oxalic Acid Concentration (wt%) | Solid: Liquid Ratio | Temp. (°C) | Time (h) | Yield (%) | Carboxyl Group Content (mmol/g) | Reference |
|---|---|---|---|---|---|---|---|---|
| Bleached SRs- softwood Japanese cedar (200–500 μm) | (1) N-Cel (2) F-Cel (3) V-Cel | 50 | 1:20 (w/v) | 95 | 4 | (1) 24.3 (2) 25.9 (3) 30.9 | (1) 0.13 (2) 0.22 (3) 0.15 | this study |
| Bleached kraft eucalyptus pulp (BEP) | soaked in water disintegration 2% consistency air-dried | 30–70 50 50 70 | 1:8 (w/v) | 80–120 100 100 100 | 0.5–4 4 1.5 1 | 1.4–24.7 15.8 11.0 24.7 | 0.11–0.39 NA 0.17 0.23 | [25] |
| (1) BEP (2) Macerated spruce dissolving pulp (SDP) (3) Native cotton fiber (QFP) | soaked in water disintegration 2% consistency air-dried | (1) 10–50 50 (2) 10–50 50 (3) 10–50 50 | 1:10 (w/v) | 100 | 1 | (1) 0–4.4 4.4 (2) 0–5.0 5.0 (3) 0–5.8 5.8 | 0.20 0.24 0.07 | [31] |
| BEP | not mentioned | 40 40 + SA10 a 40 + HA10 b | 1:20 (w/v) | 90 | 4 | 9.2 33.9 25.2 | 0.11 0.42 0.32 | [32] |
| Degreased cotton (20–50 μm) | dried fiber | 60–80 60 80 | 1:33 (w/w) | 100–110 100 105 | 2–4 3 4 | 14.7–33.4 14.7 33.4 | 0.11–0.17 0.12 0.11 | [33] |
| CNCs | Length a | Height b | Zeta Potential | Carboxyl Group |
|---|---|---|---|---|
| (nm) | (nm) | (mV) | mmol/g CNCs | |
| O-CNC (N-Cel) | 111 ± 12 | 5 ± 2 | −47.8 ± 0.7 | 0.13 |
| O-CNC-SA10 (N-Cel) | 101 ± 10 | 6 ± 2 | −43.8 ± 0.4 | 0.13 |
| O-CNC (F-Cel) | 103 ± 9 | 4 ± 2 | −54.0 ± 0.8 | 0.22 |
| O-CNC-SA10 (F-Cel) | 93 ± 12 | 6 ± 2 | −54.5 ± 0.5 | 0.23 |
| O-CNC (V-Cel) | 114 ± 13 | 5 ± 2 | −49.5 ± 0.4 | 0.15 |
| O-CNC-SA10 (V-Cel) | 110 ± 10 | 5 ± 2 | −51.2 ± 0.6 | 0.23 |
| O-CNC-ReOA1st cycle (V-Cel) | 100 ± 10 | 6 ± 2 | −47.6 ± 0.6 | 0.15 |
| O-CNC-ReOA2nd cycle (V-Cel) | 100 ± 12 | 5 ± 2 | −47.0 ± 0.5 | 0.11 |
| S-CNCs (V-Cel) | 99 ± 10 | 4 ± 2 | −48.8 ± 0.7 | - |
| Tdst | Tdmax | Char Residues | Tdst | Tdmax | Char Residues | |
|---|---|---|---|---|---|---|
| (°C) | (°C) | (%) | (°C) | (°C) | (%) | |
| SR200L (as-received) | 163 | 328 | 11.7 | - | - | - |
| SR200L (washed) | 278 | 350 | 13.4 | - | - | - |
| Cel200L | 292 | 352 | 8.9 | - | - | - |
| O-CNCs | CSRs | |||||
| N-Cel | 271 | 360 | 3.4 | 279 | 337 | 2.3 |
| F-Cel | 253 | 360 | 3.2 | 306 | 352 | 5.3 |
| V-Cel | 257 | 364 | 3.9 | 304 | 345 | 4.3 |
| O-CNCs-SA10 | CSRs (SA10) | |||||
| N-Cel | 282 | 362 | 3.3 | 278 | 358 | 2.8 |
| F-Cel | 258 | 360 | 4.2 | 306 | 343 | 4.3 |
| V-Cel | 266 | 364 | 2.7 | 304 | 338 | 3.7 |
| O-CNCs-ReOA | CSRs (ReOA) | |||||
| V-Cel-ReOA1st cycle | 258 | 361 | 3.1 | 304 | 343 | 4.2 |
| V-Cel-ReOA2nd cycle | 264 | 360 | 3.5 | - | - | - |
| S-CNCs | 231 | 262 | 18.6 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nge, T.T.; Yamada, T. Characterization of Carboxylated Cellulose Nanocrystals Isolated Through Oxalic Acid Hydrolysis from Solid Residues of Softwood-Derived Glycol Lignin Production. Molecules 2025, 30, 2922. https://doi.org/10.3390/molecules30142922
Nge TT, Yamada T. Characterization of Carboxylated Cellulose Nanocrystals Isolated Through Oxalic Acid Hydrolysis from Solid Residues of Softwood-Derived Glycol Lignin Production. Molecules. 2025; 30(14):2922. https://doi.org/10.3390/molecules30142922
Chicago/Turabian StyleNge, Thi Thi, and Tatsuhiko Yamada. 2025. "Characterization of Carboxylated Cellulose Nanocrystals Isolated Through Oxalic Acid Hydrolysis from Solid Residues of Softwood-Derived Glycol Lignin Production" Molecules 30, no. 14: 2922. https://doi.org/10.3390/molecules30142922
APA StyleNge, T. T., & Yamada, T. (2025). Characterization of Carboxylated Cellulose Nanocrystals Isolated Through Oxalic Acid Hydrolysis from Solid Residues of Softwood-Derived Glycol Lignin Production. Molecules, 30(14), 2922. https://doi.org/10.3390/molecules30142922








