1. Introduction
Plants belonging to the genus
Artemisia (Asteraceae family) are widely distributed throughout the temperate zone of Europe and Asia. These perennials were also introduced to other parts of the world, especially North America and Australia. Several
Artemisia species have been recognized as important medicinal plants, such as
A. annua,
A.
dracunculus,
A. vulgaris, and
A. absinthium [
1,
2]. Among these,
Artemisia absinthium, commonly known as wormwood, is the most widely investigated. Its aerial parts (herb;
Absinthi herba), described in a European Pharmacopoeia (EP) monograph, are traditionally used to stimulate appetite and improve digestion [
3,
4]. The herb is particularly rich in EO, the content of which, according to EP requirements, should not be lower than 2 mL per kg [
3]. The EO, chemically quite variable, is composed mainly of the following: α-thujone, β-thujone, β-pinene, sabinene,
cis-epoxyocimene, sabinyl acetate, chrysanthenol, chrysanthenyl acetate, nerolidol, and chamazulene [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. The raw material also contains significant amounts of non-volatile sesquiterpene derivatives, specifically sesquiterpene lactones of the guaianolide type (absinthin, anabsinthin, artabsinthin, matricin) and the pelenolide type (arabsin, artabin). Among these, absinthin, present at a level of 0.20–0.28%, is responsible for the herb’s characteristic bitter taste [
4,
10]. Other important constituents are phenolics, including phenolic acids, flavonoids, tannins, and coumarins. The most abundant phenolic acids are caffeoylquinic, dicaffeoylquinic, vanillic, coumaric, syringic, and chlorogenic acids. Flavonoids are mainly represented by artemetin, homorientin, quercetin, isorhamnetin, and their derivatives, while tannins are mainly represented by epigallocatechin gallate. Among coumarins, isofraxidine and scopolin have been identified here [
1,
4,
25,
26,
27].
Wormwood exhibits digestive, choleretic, antispasmodic, carminative, hepatoprotective, and gastroprotective activity. The herb, considered as
aromatica amara, is traditionally used to prepare tea infusions and alcoholic tinctures, applied mainly in the case of gastrointestinal disorders (flatulence, cramps) as a bitter aromatic medicine for temporary loss of appetite and to stimulate bile secretion in liver diseases [
1,
4,
28]. Furthermore, wormwood herb exhibits antimicrobial, antioxidant, antipyretic, analgesic, anti-atherosclerotic, and anti-inflammatory activity [
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40]. It also demonstrates antiparasitic, especially anthelmintic, activity, resulting from the presence of α- and β-thujone [
41,
42]. However, due to the neurotoxic properties of these compounds, wormwood herb should be used only for a short period and in doses not exceeding the recommended limits. Toxicological studies of thujone are summarized, i.a., in the Public Statement on the use of herbal medicinal products containing thujone (EMA/HMPC/732886/2012) [
43,
44,
45].
Given its broad spectrum of biological activity, the use of
Absinthi herba is also recommended in veterinary practice, both internally, as a feed additive to prevent and treat gastrointestinal disorders, and externally against skin parasites such as lice and scabies [
35,
38,
40,
41,
42]. Due to its strong antimicrobial activity, it is considered as a natural fungicide, effective mainly against
Fusarium,
Alternaria, and
Botrytis strains [
46,
47]. Moreover, extracts from this plant have been shown to have insecticidal and repellent activity against
Sitophilus granarius and
Rhyzopertha dominica [
48]. Due to its versatile applications, the plant has recently become the object of interest of various industries, especially the phytopharmaceutical, food, and agricultural sectors [
1,
2,
28,
29].
In Poland, wormwood herb is collected both from cultivation and, more commonly, from wild-growing plants [
49,
50]. The species grows naturally in various habitats, in a wide range of ecological conditions, mainly in grasslands, meadows, and ruderal and segetal communities. From a phytosociological perspective, wormwood is a characteristic species of the
Onopordetalia acanthii order within the
Artemisietea vulgaris class [
51,
52]. As mentioned earlier, the species is highly polymorphic. Raw materials collected from wild-growing plants are chemically heterogeneous, especially in terms of EO composition what may reduce its commercial quality. On the other hand, wild-growing populations can be a valuable source of genotypes useful for future breeding programs, particularly when searching for non-thujone forms [
53].
The aim of the present work was to determine intraspecific variability of Artemisia absinthium growing wild in Poland in terms of developmental traits as well as the content and composition of biologically active compounds (essential oil, phenolic acids, polyphenols, and tannins) in the herb.
2. Results and Discussion
The phytosociological analysis indicates the heterogeneous character of wormwood plant communities (
Table 1). Based on the species composition, two main types of communities may be distinguished here: ruderal/synanthropic communities, as well as sandy grasslands. In the frame of the first group, the presence of species such as
Oenothera biennis,
Echium vulgare,
Melilotus officinalis,
Verbascum thapsus, and
Artemisia absinthium allow us to classify populations 2, 4, 5, 7, 8, 9, 10, and 11 as comprising the
Oenopordetalia acanthi order within the
Artemisietea vulgaris class. This type of plant community, characteristic for a wormwood occurrence, usually develops on sites disturbed by human activity [
51,
52]. In turn, populations 1 and 6 represent more natural or semi-natural dry habitats (sandy grasslands), with typical species such as
Thymus serpyllum,
Helichrysum arenarium, and
Sedum acre. Populations 3 and 11 show species typical for meadow and ruderal communities, including nitrophilous plants such as
Urtica dioica (
Table 1).
The investigated populations varied in terms of developmental traits (
Table 2). In general, the greatest variation was observed in the number of shoots per plant and internode length, whereas plant height and inflorescence length showed more uniformity across populations. The average plant height was at a level of 173.7 cm, with the highest plants observed in population 11 (196.0 ± 10.6 cm), which differed visibly from population 8 (165.2 ± 4.6 cm). Regarding the number of shoots per plant, a wide range (from 38 to 51) was noticed, followed by a high coefficient of variation (35.02%), indicating considerable variability within populations. Internode length varied from 21 mm in population 8 to 33 mm in population 1. Inflorescence length was relatively uniform across populations, with no significant differences observed. Here, the mean inflorescence length was 91.2 cm, with the longest recorded in populations 4 (112.7 ± 19.2 cm) and 11 (108.8 ± 13.6 cm) (
Table 2). Such variability in morphological and developmental traits is typical for wild-growing plants, where phenotypic variation reflects their adaptation to the environment [
49]. Moreover, features such as height, shoot number, and inflorescence length can be important from a practical viewpoint, since they affect the yield of herb and its mechanical harvest.
Both the mass of dry herb and grated herb exhibited relatively high variability within populations; however, these results were not statistically important (
Table 3). The average dry herb mass per plant was at a level of 1.15 kg, with individual population values ranging from 0.83 kg (population 3) to 1.60 kg (population 6). Mass of grated herb, being a commercial raw material, was also variable: population 6 was characterized by the highest mass of grated herb (0.51 kg), and populations 1 and 2 by the lowest (0.26 kg) (
Table 3).
Among examined wormwood populations, the content of EO varied from 0.94 to 1.69 g/100 g. Populations 7, 10, and 11 showed the highest content of this substance (1.47; 1.41; 1.69 g/100 g, respectively), visibly exceeding that in populations 1, 2, and 3 (0.94; 0.75 g/100 g) (
Table 4). It is worth noting that according to EP requirements, EO content in wormwood herb should be not lower than 0.2 g/100 g [
3]. Lachenmeier et al. (2006) claim that the amount of this substance ranges from 0.2% to 1.5% [
45]. According to Juteau et al. (2003), the main factors influencing its accumulation are geographical origin of the population, stage of plant development, and the type of raw material. The authors noticed that the content of EO in wormwood herb decreases from the full blooming (0.68%) to the post-flowering stage (0.56%) of plant vegetation. Moreover, the upper part of flowering shoots contains 2–3 more EOs in comparison to lower parts [
8].
In the present work, 41 compounds were detected in the EOs, comprising 81.02% to 97.18% of the whole fraction, depending on the population (
Table 5). Identified compounds were classified as monoterpenes and sesquiterpenes, both in the form of hydrocarbons and oxygenated derivatives, as well as other group such as esters, alcohols, and aldehydes, present in minor concentrations. The oxygenated monoterpenes were a fundamental part, accounting for 41.10% (population 4) to 90.34% (population 8) of the total EOs. The main oxygenated monoterpenes included 1,8-cineole, sabinyl acetate,
cis-chrysanthenol, and chrysantenyl acetate as well as α- and β-thujone. Their content exhibited remarkable variations depending on the population. The content of 1,8-cineole ranged from 0.47 to 40.64%, and was predominant in both populations 3 (40.64%) and 5 (28.93%). Sabinyl acetate was found as highly abundant in populations 11 (76.02%), 7 (56.80%), 8 (41.91%), 6 (39.02%), 10 (30.77%), and 1 (17.37%), being nearly absent in the others.
Cis-chrysanthenol was present in significant amount in populations 1 (32.09%) and 2 (49.48%) while its derivative chrysanthenol acetate was present only in population 8 (19.83%). Regarding the two isomers of thujone, α-thujone and β-thujone, both were present in remarkable amounts only in population 9 (19.31% and 19.26%, respectively). Monoterpene hydrocarbons varied among populations, ranging from 1.26% in population 8 to 44.04% in population 4. Major compounds in this group included β-myrcene, with the highest concentrations recorded in populations 4 (25.76%) and 5 (16.33%). Sesquiterpene hydrocarbons and oxygenated sesquiterpenes were present in smaller quantities, usually below 4%, with slight variability between populations. Among the more significant compounds in this group were caryophyllene, α-humulene, and β-copaene (
Table 5).
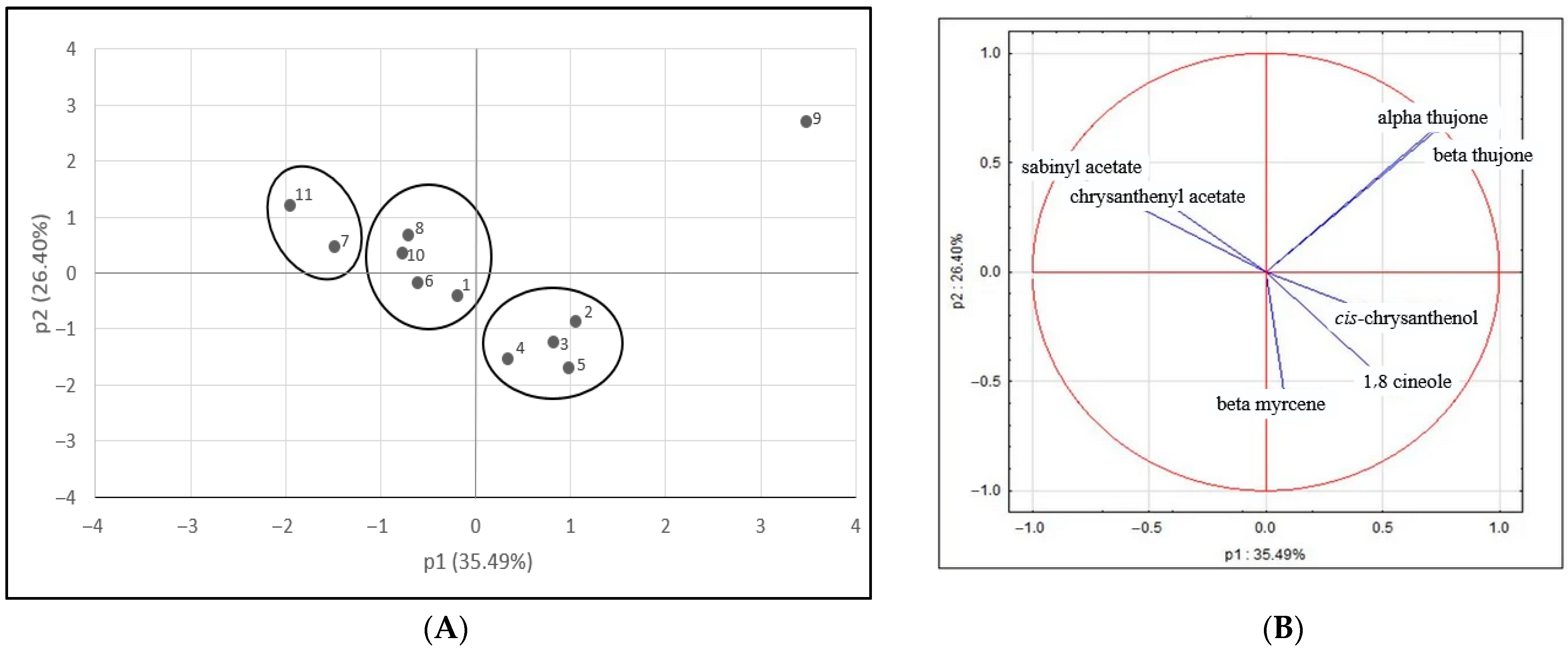
Taking into account such a variable composition of the EOs, several chemotypes may be distinguished within the examined wormwood populations. Here, populations 11 and 7 may be qualified as a pure sabinyl acetate chemotype. Populations 1, 6, 8, and 10 constitute mixed chemotypes where sabinyl acetate is accompanied by β-myrcene,
cis-chrysanthenol, chrysanthenyl acetete, and 1,8-cineole. Population 9 alone represents a mixed chemotype dominated by thujones followed by
cis-chrysanthenol. Finally, populations 2, 3, 4, and 5 represent mixed chemotypes with β-myrcene, 1,8-cineole,
cis-chrysanthenol, and
cis-epoxyocymene as dominants (
Figure 1).
The obtained results are in good agreement with the literature data and confirm the highly variable character of wormwood. The chemical diversity of wormwood essential oil is well established, with numerous studies confirming that both genetic and environmental factors, as well as plant developmental stage and organ specificity, contribute to its pronounced polymorphism [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24].
Globally, α-thujone and β-thujone are often regarded as the most frequent dominant compounds in wormwood EO, yet this is only one aspect of a highly diverse species. In Central Europe, at least four main chemotypes have been identified: α- and β-thujone, sabinene and β-myrcene,
cis-epoxyocimene, and sabinyl acetate [
11,
12,
26]. Regional differences are worth noting; for example, in Lithuania, thujones and
trans-sabinyl acetate predominate, whereas in Poland, sabinyl acetate, chrysanthenyl acetate, and sabinene chemotypes are most common, with β-thujone-dominant types occurring only rarely [
11,
26]. In France, EO profiles can be dominated by
cis-chrysanthenol or
cis-epoxyocimene, while in the Spanish Pyrenees,
cis-epoxyocimene (up to 49.7%) or its combination with
cis-chrysanthenyl acetate (up to 36.7%) is typical. Italian and Spanish populations may also contain significant levels of 1,8-cineole (18.0%), carvone (18.5%), thymol (10.8%), and carvacrol (9.7%) [
13,
14]. Recent comprehensive analyses confirm that the chemotype spectrum in
A. absinthium is even broader. For instance, Raal et al. (2024) examined commercial EO samples from 14 European countries, identifying 41 compounds and revealing that while monoterpenes and their derivatives dominate, the quantitative composition varies widely. Pure chemotypes such as E-sabinyl acetate and E-epoxyocymene were observed, as well as numerous mixed chemotypes, with major constituents differing even within a single country. This variability is not strictly associated with geographic or altitudinal gradients, indicating a complex interplay of factors shaping EO profiles [
15].
Outside Europe, Egyptian wormwood is characterized by high α-phellandrene and terpinen-4-ol content, and Iranian populations may be rich in β-pinene [
16,
17]. A particularly instructive example of environmental impact comes from Tunisia, where Riahi et al. (2015) investigated
A. absinthium from four bioclimatic zones ranging from humid to arid. They found high qualitative and quantitative variation in EO composition depending on locality. For example, plants from the Inferior Arid region (Gafsa) had chamazulene, α-thujone, and camphor as main constituents, while those from the humid zone (Ghar Dimaou) were characterized by high camphor, Z-sabinene hydrate, and 1-terpinen-4-ol. The antioxidant activity of the essential oils also increased significantly from humid to arid zones, correlating with higher thujone and chamazulene content in arid environments. These results highlight that both environmental and genetic factors shape the chemotype and biological activity of wormwood EO, and that adaptation to local stressors (such as drought) may drive the synthesis of specific bioactive compounds [
18].
Environmental conditions and developmental stages significantly influence EO composition as well. Malaspina et al. (2025) demonstrated that both geographical location and phenological stage (vegetative versus flowering) can shift the dominant chemical classes in the essential oil. For example, in Northern Italy, oxygenated monoterpenes prevailed, while in Southern Italy, oxygenated sesquiterpenes or monoterpene hydrocarbons dominated depending on the developmental stage. The main EO constituents also changed: myroxide and 3,6-dihydrochamazulene were abundant in Cogne, whereas camphor, davanone, β-myrcene, and β-phellandrene were prominent in Ogliastro [
19].
Organ- and stage-specific differences are also significant. Nguyen et al. (2018) showed that EO content is generally higher in flowers than in leaves, and the proportions of key compounds such as thujones and sabinyl acetate change during plant development. In thujone chemotypes, α- and β-thujone are most abundant at early stages and decrease after flowering, while in
trans-sabinyl acetate chemotypes, this ester declines over time with a concomitant increase in thujones post flowering. These changes are primarily quantitative, but qualitative differences between organs and stages were also observed [
20].
Investigated populations were differentiated by the content of phenolic compounds, including polyphenols, tannins, and phenolic acids (
Table 4). Average polyphenols content reached 0.66 g/100 g (CV = 11.77%), with populations 5 and 6 characterized by their highest level (0.79 g/100 g), and populations 2, 9, and 11 characterized by the lowest (around 0.58–0.60 g/100 g). The content of polyphenols in wormwood was investigated earlier by other authors [
25,
26,
28]. In our work, phenolic acids content was at a level of 0.42 g/100 g with moderate variability (CV = 13.04%), with populations 1 and 9 distinguished by the highest amount of these compounds (0.50 g/100 g). Tannin content was more variable (CV = 28.04%), ranging from 0.10 g/100 g in population 2 to 0.26 g/100 g in population 5 (
Table 4).
In general, such chemical variability within the content of biologically active compounds is often driven by genetic differences and environmental influences, which affect their biosynthesis and accumulation in plant tissues. From a physiological perspective, both EOs and phenolics play crucial roles in plant stress responses [
54,
55,
56]. These compounds contribute to antioxidative protection by scavenging reactive oxygen species generated under abiotic stresses like drought, UV radiation, and temperature extremes, as well as biotic stresses such as pathogen attack. Tannins and EO, in particular, may inhibit microbial (including bacteria and fungi) growth, enhancing plant survival in wild habitats [
54,
55,
56,
57,
58,
59]. The reported coefficients of variation indicate that both tannin and EO content in the examined populations is particularly variable (CV = 28.04 and 23.89%, respectively), suggesting that these classes of compounds are responsive to environmental factors. In turn, polyphenols and phenolic acids showed moderate but statistically significant variation (CV = 11.77% and 13.04%, respectively).
The observed variability provides opportunities for selecting wormwood populations with desirable traits for cultivation and commercial use, both medicinal and non-medicinal. The medicinal properties of the abovementioned phenolic compounds present in wormwood herb are well-documented. They are considered mainly as strong antioxidants, exhibiting also antimicrobial, anti-inflammatory, and astringent activities [
25,
26,
27,
28,
29,
30,
31]. Therefore, populations 5 and 6 with an elevated content of polyphenols and tannins appeared to be valuable from a medicinal viewpoint. Given the EO content, all populations meet or even exceed European Pharmacopoeia standards [
3]. Due to the biological activity of sabinyl acetate, both pure and mixed chemotypes with this as the dominant compound may contribute to medicinal, e.g., antimicrobial and anti-inflammatory, applications [
60,
61]. However, the use of the thujone-rich chemotype (population 9), due to its neurotoxic activity, would be limited for direct human consumption. Nevertheless, thujone’s strong repellent activity against pests and bactericidal effects on phytopathogens make it valuable biopesticide in organic crop protection [
62,
63,
64].
To sum up, our results indicate that population 6 stands out as the most promising candidate for practical applications. This population achieved the highest average dry herb mass per plant (1.60 kg) and the highest mass of grated herb (0.51 kg). The essential oil content in this population, reaching 1.21 g/100 g, exceeds the minimum requirements set by the European Pharmacopoeia. From a quality perspective, population 6 represents a mixed sabinyl acetate chemotype, characterized by a high proportion of sabinyl acetate (39.02%) and a favorable profile of oxygenated monoterpenes, while maintaining a low content of thujones. Additionally, population 6 is distinguished by a high content of polyphenols (0.79 g/100 g) and an elevated tannin level (0.22 g/100 g), both of which are associated with antioxidant and antimicrobial activities, enhancing the therapeutic and functional value of the raw material. All these makes population 6 particularly attractive for cultivation and further breeding.