Chirality Transfer and Oxazolidine Formation in Reaction of L and D Enantiomers of β-Hydroxy Amino Acids with Nitrogenous Carboxaldehydes and Nickel(II)
Abstract
1. Introduction
2. Results and Discussion
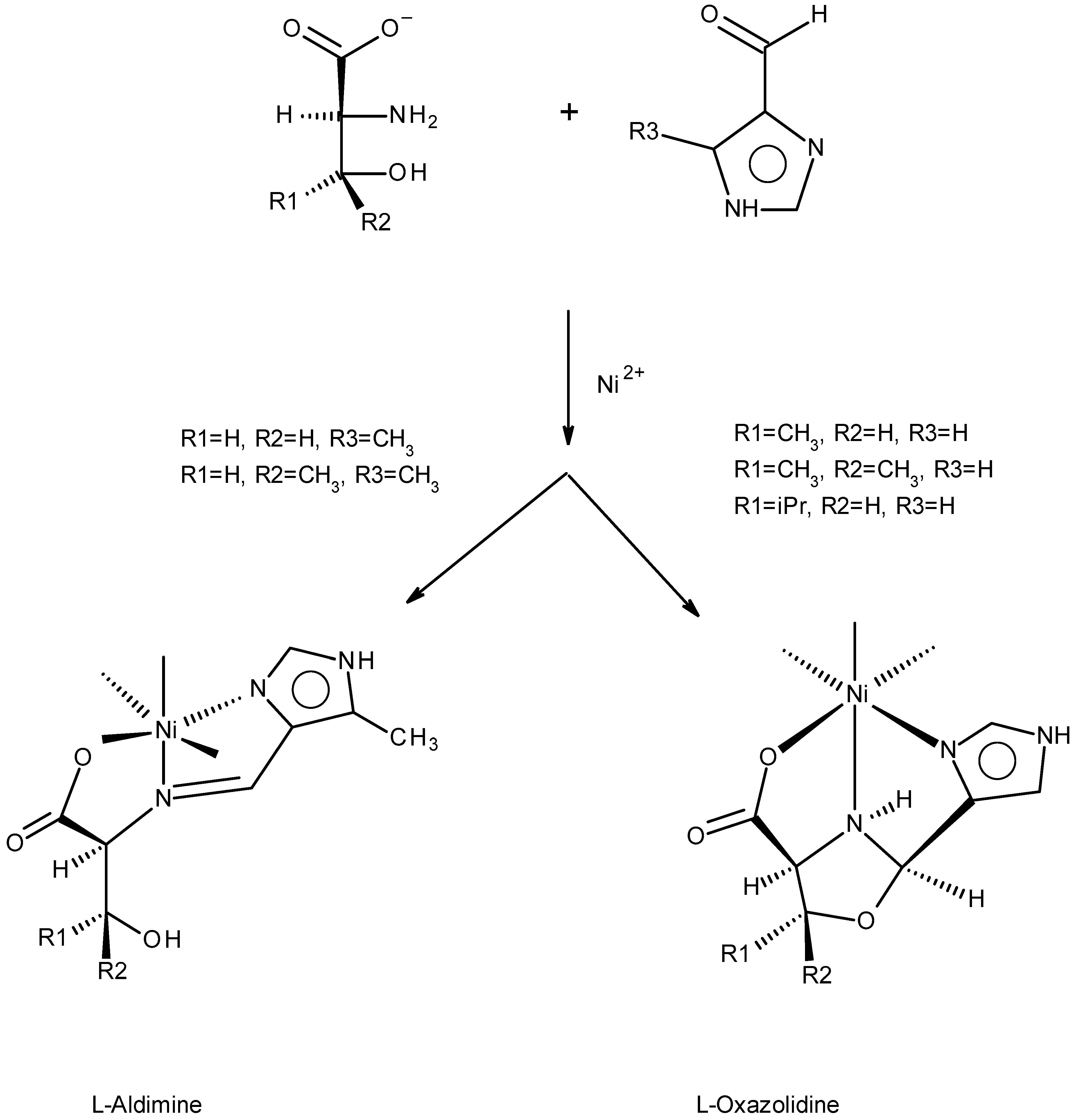
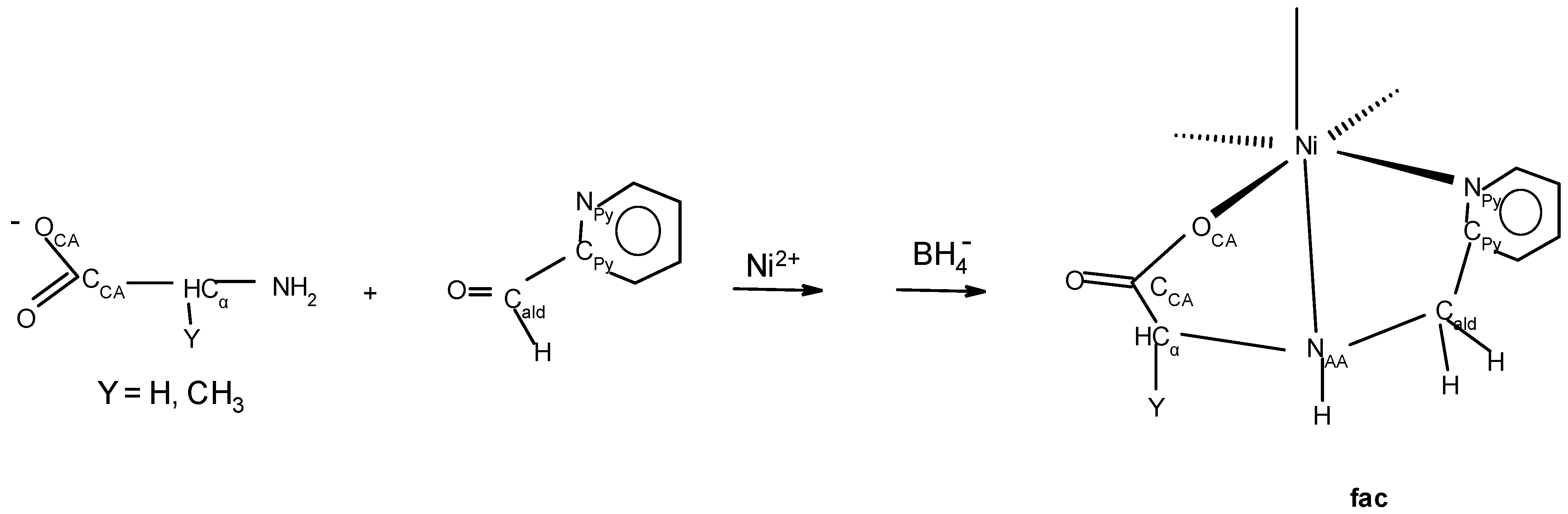
2.1. Approach, Ligands Employed, and Reactions Examined
2.2. Preliminary Characterization
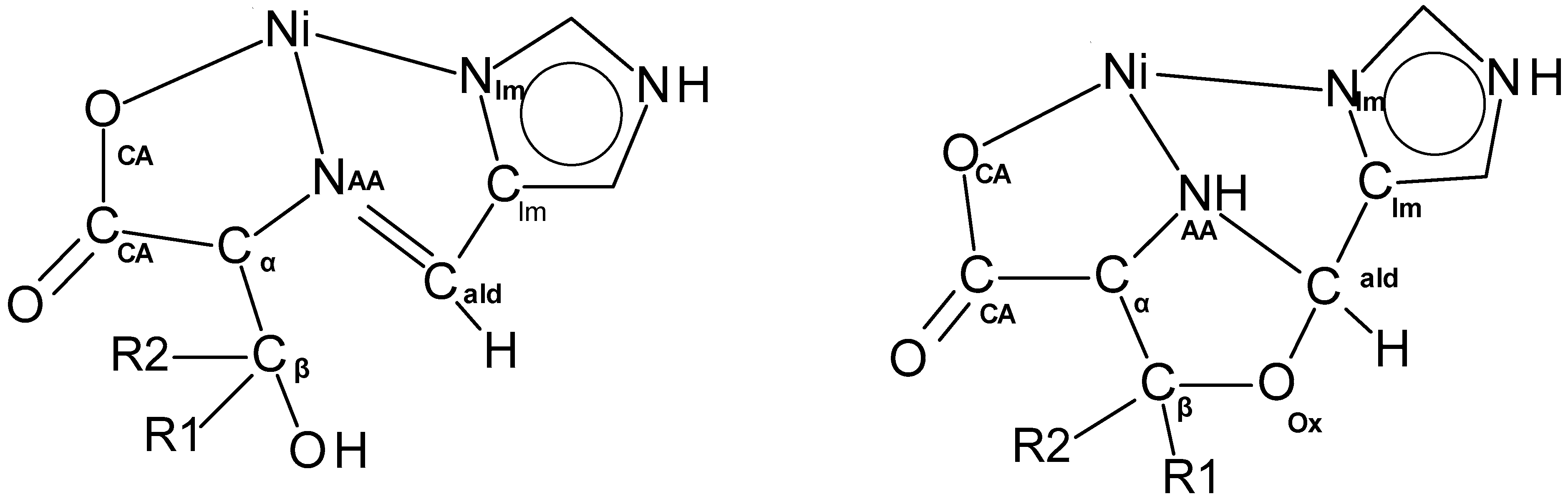
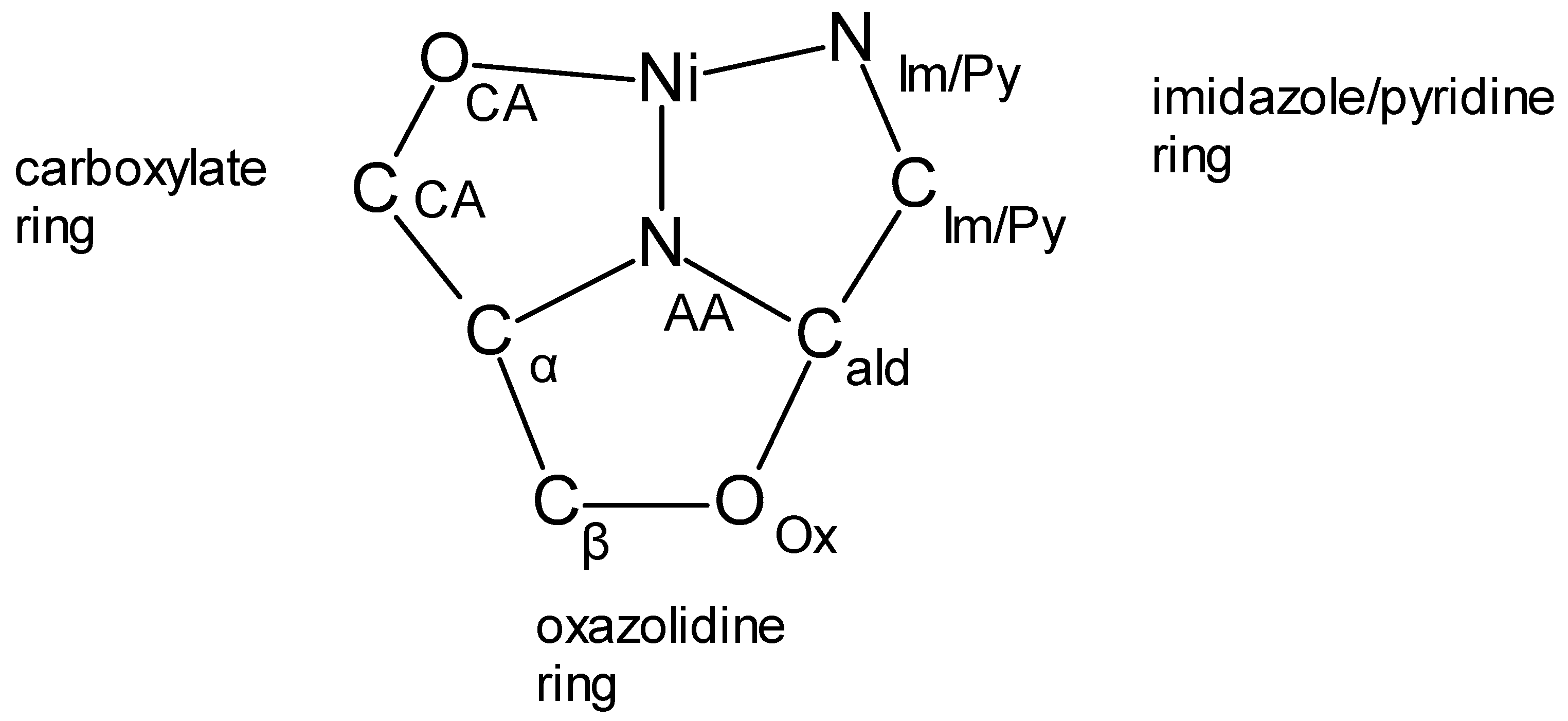
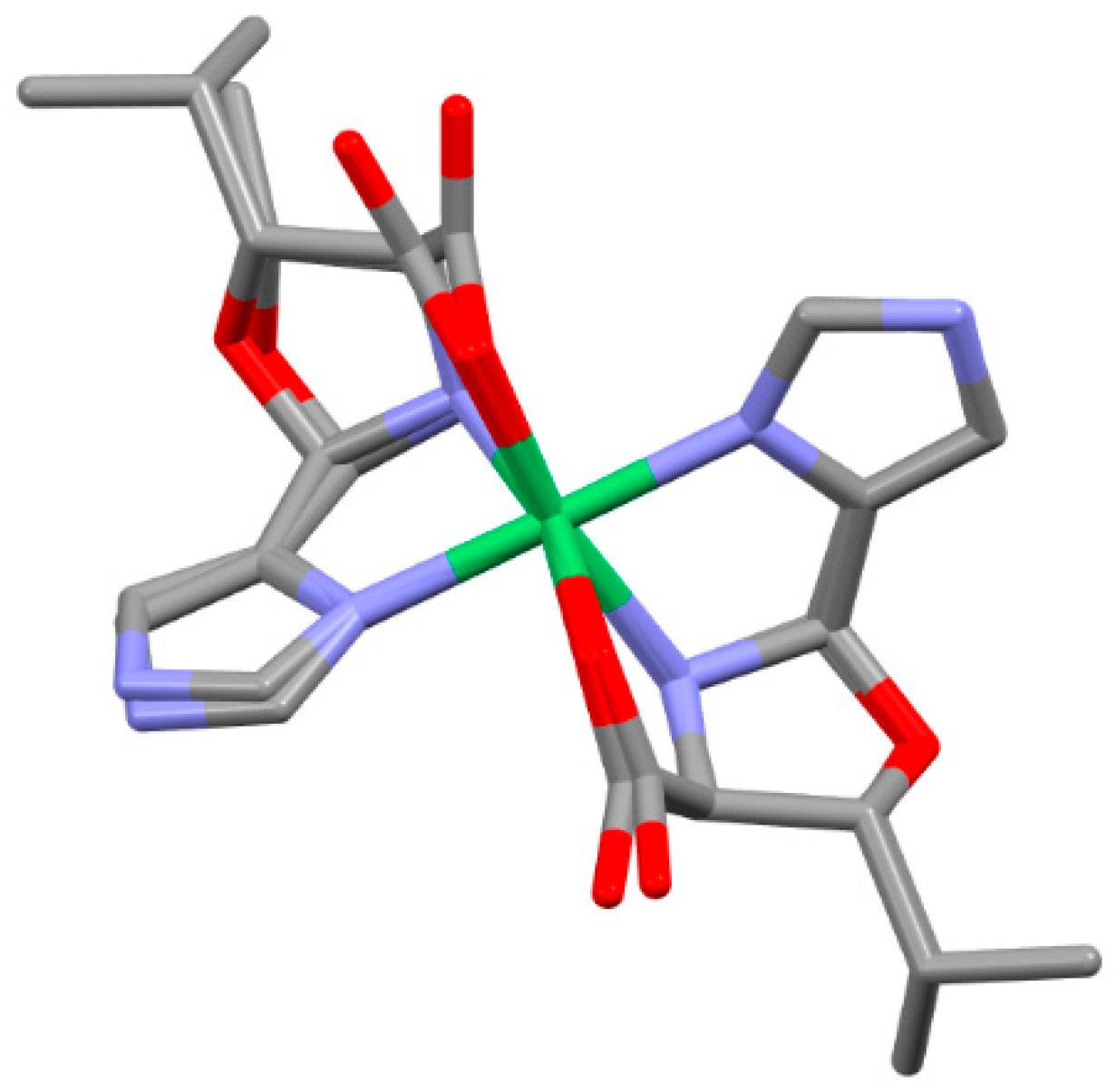
2.3. Nickel Coordination Environment
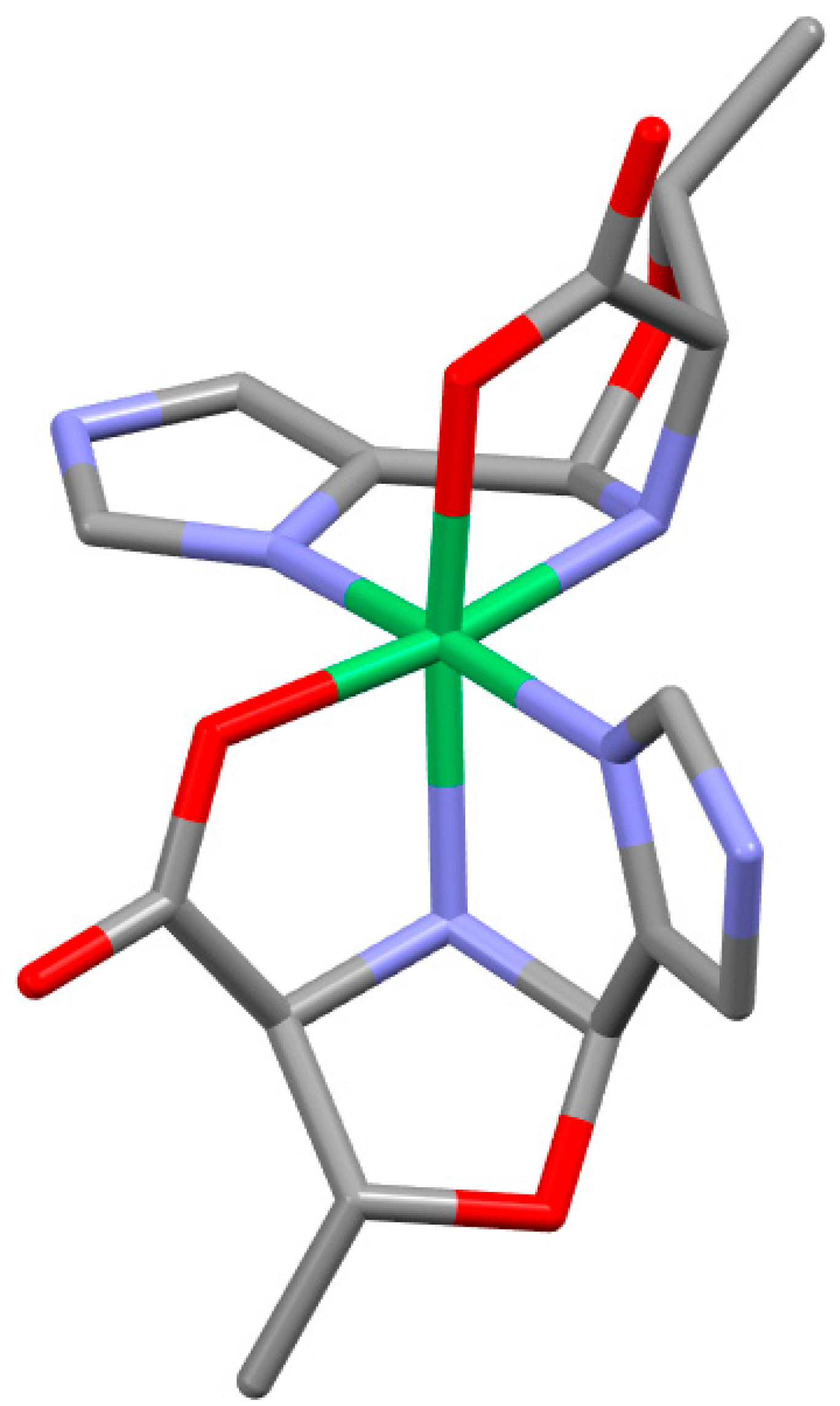
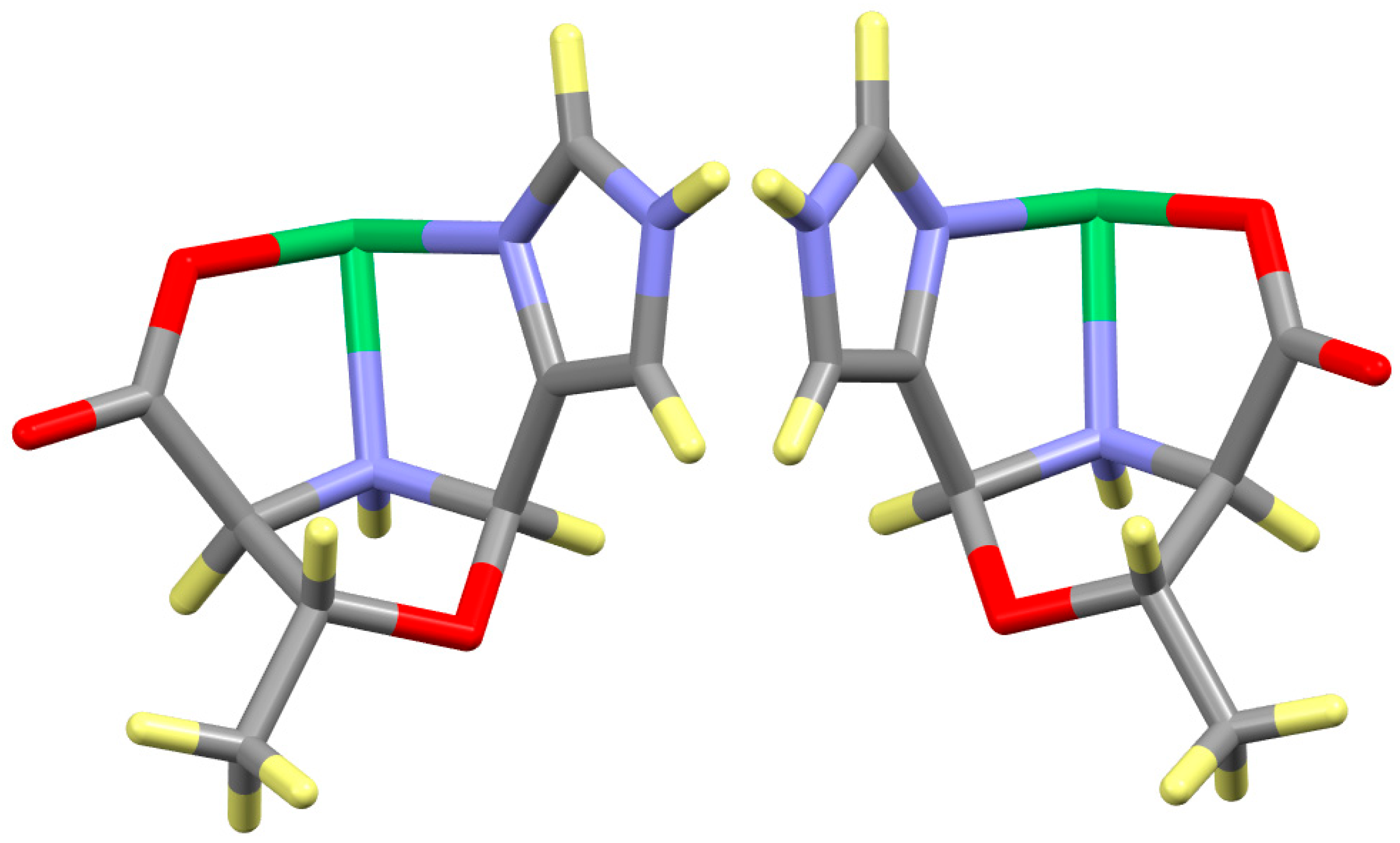
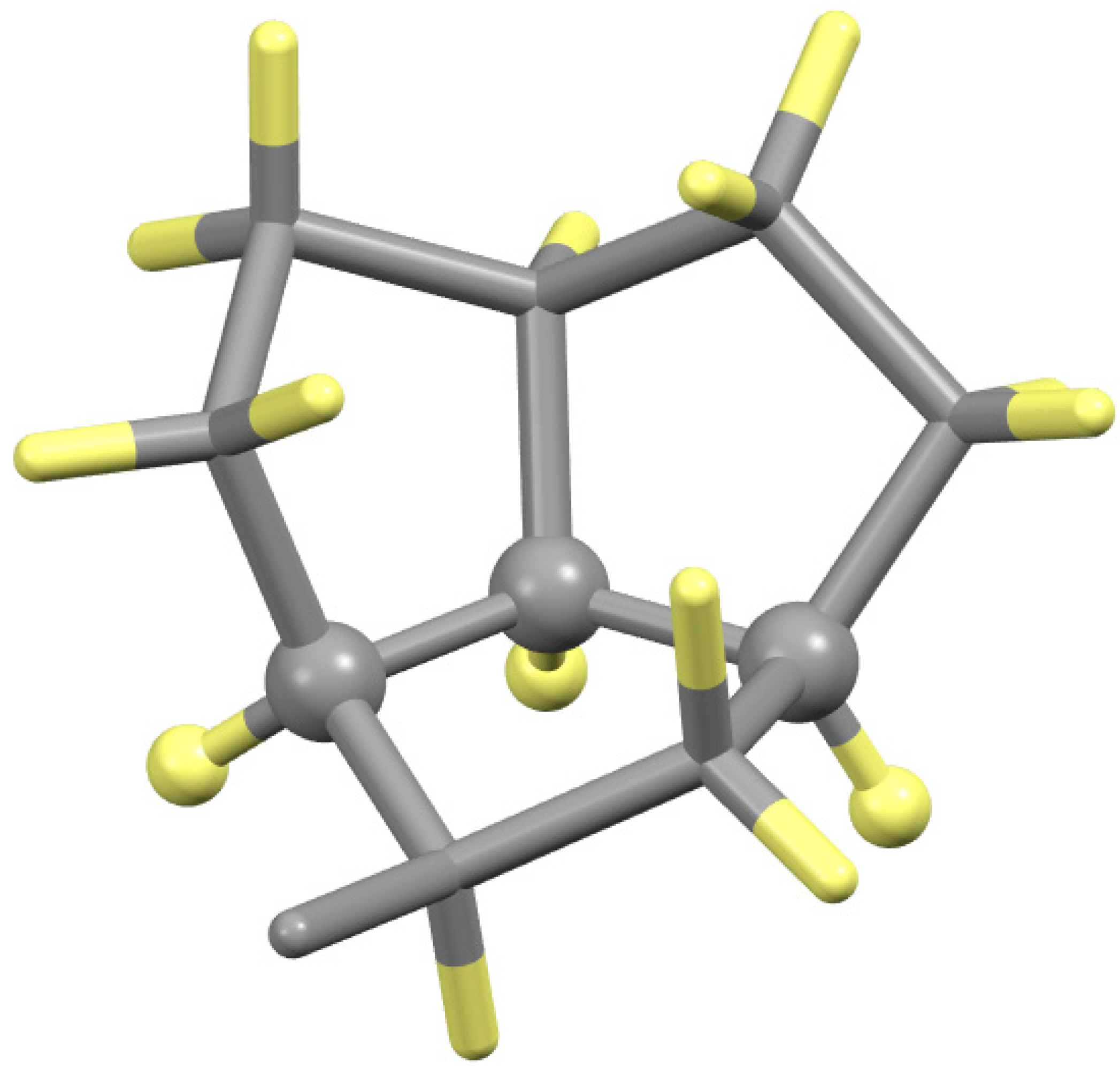
2.4. Overall Shape of the Oxazolidine Complexes
2.5. Chirality Transfer
2.6. Preliminary Reactivity Comparison
3. Experimental Procedure
3.1. General
3.2. External Laboratories
3.3. X-Ray Crystallography
3.4. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pichon-Barre, D.; Zhang, Z.; Cador, A.; Vives, T.; Roisnel, T.; Basle, O.; Jarrige, L.; Cavallo, L.; Falivene, L.; Mauduit, M. Chiral oxazolidines acting as transientlhydroxy-alkyl-functionalized N-heterocyclic carbenes: An efficient route to gold and copper complexes for asymmetric catalysis. Chem. Sci. 2022, 13, 8773–8780. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Xu, H. Asymmetric catalysis with chiral oxazolidine ligands. Chem. Commun. 2011, 47, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Kobielska, P.A.; Harms, K.; Katsikogianni, M.G.; Telford, R.; Novitchi, G.; Nayak, S. Transition metal complexes of a versatile polyalkoxy oxazolidine-based ligand derived from in situ cyclization. Dalton Trans. 2018, 47, 6156–6165. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.-H.; Wang, W.-S.; Chong, K.-V.; Win, Y.-F.; Neo, K.-E.; Lee, H.-B.; San, S.-L.; Rahman, R.; Leong, W. Ternary copper(II)-polypyridyl enantiomers: Aldol-type condensation, characterization, DNA-binding recognition, BSA-binding and anticancer property. Dalton Trans. 2013, 42, 10233–10243. [Google Scholar] [CrossRef]
- Lee, K.; Ng, Y.; Wang, W.; Ng, P.; Chan, C.; Lai, J.; Davamani, F.; Chitra, E.; Lim, W.; Ganguly, R.; et al. Enantiomeric pairs of ternary copper(ii) complexes and their aldol-type condensation products: Synthesis, characterization, and anticancer and epigenetic properties. Dalton Trans. 2019, 48, 4987–4999. [Google Scholar] [CrossRef]
- Bannerjee, A.; Ganguly, S.; Chattopadhyay, T.; Banu, K.; Patra, A.; Bhattacharya, S.; Zangrando, E.; Das, D. Metal-Assisted Oxazolidine/Oxazine Ring Formation in Dinuclear Zinc(II) Complexes: Synthesis, Structural Aspects, and Bioactivity. Inorg. Chem. 2009, 48, 8695–8702. [Google Scholar] [CrossRef]
- Tuo, S.-X.; Li, Y.; He, P.; Zhou, J.-L.; Yan, J. Synthesis and characterization of catalytic active oxazolidine/Schiff complexes using 2-quinoline formaldehyde and tris-base for carbon dioxide chemical fixation. Inorg. Chim. Acta 2024, 568, 122075. [Google Scholar] [CrossRef]
- Wylde, J.; Taylor, G.; Sorbie, K.; Samaniego, W.; Allan, B. Oxazolidine-Based H2S and Mercaptan Scavengers: Uncovering the Myths. Energy Fuels 2021, 35, 12993–13010. [Google Scholar] [CrossRef]
- Mena-Cervantes, V.; Hernandez-Altamirano, R.; Buenrostro-Gonzalez, E.; Beltran, H.; Zamudio-Rivera, L. Development of oxazolidines derived from polyisobutylene succinimides as multifunctional stabilizers of asphaltenes in oil industry. Fuel 2013, 110, 293–301. [Google Scholar] [CrossRef]
- Ding, C.; Zeng, F.; Ni, J.; Wang, B.; Zie, Y. Polynuclear Complexes of Ligands Containing in Situ Formed Oxazinane and Oxazolidine Rings with Appended Alkoxyl and Phenol Groups. Cryst. Growth Des. 2012, 12, 2089–2096. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Li, X.-L.; Ke, H.; Xu, Z. A series of tetranuclear lanthanide compounds constructed by in situ polydentate ligands: Synthesis, structure, and SMM behaviour of the Dy4 compound. CrystEngComm 2015, 17, 9167–9174. [Google Scholar] [CrossRef]
- Liu, H.; Li, X. Serine/Threonine Ligation: Origin, Mechanistic Aspects, and Applications. Acc. Chem. Res. 2018, 51, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Li, T.; Lam, H.; Zhang, Y.; Li, X. Realizing serine/threonine ligation: Scope and limitations and mechanistic implication thereof. Front. Chem. 2014, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Lam, H.; Lee, C.; Li, X. Protein chemical synthesis by serine and threonine ligation. Proc. Natl. Acad. Sci. USA 2013, 110, 6657–6662. [Google Scholar] [CrossRef]
- Lee, C.; Li, X. Serine/threonine ligation for the chemical synthesis of proteins. Curr. Opin. Chem. Biol. 2014, 22, 108–114. [Google Scholar] [CrossRef]
- Belokon, Y.; Belikov, V.; Vitt, S.; Savel’eva, T.; Burbelo, V.; Bakhmutov, V.; Aleksandrov, G.; Struchov, Y. A simple stereochemical model of pyridoxal-dependent aldolase: Asymmetric conversions of the amino acid fragment in chiral complexes of potassium Ʌ and Δ bis-/n-salicylideneaminoacidato/cobaltate(III). Tetrahedron 1977, 33, 2251–2564. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Wang, Y.; Liu, Z. A pair of enantiomeric trinuclear nickel (II) clusters based on chiral Schiff-base: Synthesis, structures, circular dichroism and magnetic properties. Inorg. Chem. Commun. 2017, 86, 281–284. [Google Scholar] [CrossRef]
- Ebel, M.; Rehder, D. Interaction of Vanadyl (VO2+) with Ligands Containing Serine, Tyrosine, and Threonine. Inorg. Chem. 2006, 45, 7083–7090. [Google Scholar] [CrossRef]
- Krivosudsky, L.; Schwendt, P.; Gyeps, R.; Zak, Z. X-ray structure analysis, electronic and vibrational circular dichroism of chiral-at-metal dioxidovanadium(V) complexes with amino acids derived Schiff base ligands. Polyhedron 2014, 81, 421–427. [Google Scholar] [CrossRef]
- Garcia-Raso, A.; Fiol, J.; Lopez-Zafra, A.; Tasada, A.; Mata, I.; Espinosa, E.; Molins, E. Different ways of interaction between binary copper(II)-Schiff bases (Cu–N-salicylideneserinato) and pyrimidine derivatives. Polyhedron 2006, 25, 2295–2302. [Google Scholar] [CrossRef]
- Pratihar, S. Electrophilicity and nucleophilicity of commonly used aldehydes. Org. Biomol. Chem. 2014, 12, 5781–5788. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, D.; Lau, S. 3-Pyridinecarboxaldehyde: A Model System for Superelectrophilic Activation and the Observation of a Diprotonated Electrophile. J. Org. Chem. 1999, 64, 7309–7311. [Google Scholar] [CrossRef]
- Kamps, J.; Hopkinson, R.; Schofield, C.; Claridge, T. How formaldehyde reacts with amino acids. Commun. Chem. 2019, 2, 126. [Google Scholar] [CrossRef]
- Teo, S.; Ng, C.-H.; Horn, E.; Tiekink, E. Synthesis and structural studies of some metal(II) complexes of 5-methyloxazolidine-4-carboxylic acid. Inorg. Chim. Acta 1991, 183, 25–30. [Google Scholar] [CrossRef]
- Ng, C.; Chong, T.; Teoh, S.; Adams, F.; Ng, S. Reactions of a series of bis(α amino acidato) copper(II) complexes with formaldehyde and benzamide: Aldol type condensation versus Mannich aminomethylation. J. Chem. Soc. Dalton Trans. 2002, 17, 3361–3366. [Google Scholar] [CrossRef]
- Ng, C.; Wang, W.; Win, Y.; Neo, K.; Ganguly, S.; Er, H.; Ooi, I. Rac-aqua(4-methyl-oxazolidine-4-carboxylato)(nitrato)(1,10-phenanthroline)copper(II), the aldol condensation product of formaldehyde with the D- and L-(alaninato)aqua(1,10-phenanthroline)copper(II) nitrate. Transit. Met. Chem. 2016, 41, 563–571. [Google Scholar] [CrossRef]
- Reid, J. Open questions on the transfer of chirality. Commun. Chem. 2021, 4, 171. [Google Scholar] [CrossRef]
- Miao, T.; Cheng, X.; Guo, Y.; Zhang, G.; Zhang, W. Preparation of chiral polymers: Precise chirality transfer from natural species to achiral artificial polymers. Giant 2023, 14, 100161–100178. [Google Scholar] [CrossRef]
- Crassous, J. Chiral transfer in coordination complexes: Towards molecular materials. Chem. Soc. Rev. 2009, 38, 830–845. [Google Scholar] [CrossRef]
- Mayans, J.; Gomez, D.; Font–Bardia, M.; Escuer, A. Chiral oxazolidine complexes derived from phenolic Schiff bases. Cryst. Growth Des. 2020, 20, 4176–4184. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Bi, C.; Zuo, J.; Wang, Q. Synthesis and Crystal Structure of a New Nickel(II) Coordination Polymer Based on Schiff Base. Russ. Coord. Chem. 2012, 38, 349–352. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.; Zavalij, P. Formation of Ketimines from Aldimines in Schiff Base Condensation of Amino Acids and Imidazole-2-Carboxaldehydes: Tautomerization of Schiff Bases of Amino Acids Resulting in the Loss of Stereogenic Center. Inorganics 2023, 11, 381–399. [Google Scholar] [CrossRef]
- Brewer, G.; Brewer, C.; Butcher, R.; Zavalij, P. Selective Generation of Aldimine and Ketimine Tautomers of the Schiff Base Condensates of Amino Acids with Imidazole Aldehydes or of Imidazole Methanamines with Pyruvates—Isomeric Control with 2- vs. 4-Substituted Imidazoles. Molecules 2024, 29, 1324. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Garcia-Rodriguez, R.; Miguel, D. Iminopyridine complexes of manganese, rhenium and molybdenum derived from amino ester (L)-methylserine, and peptides Gly-Gly, GlyVal, and Gly-Gly-Gly. Self-assembly of the peptide chains. Inorg. Chem. 2012, 51, 2984–2996. [Google Scholar] [CrossRef] [PubMed]
- Juraj, N.; Kirin, S. Inorganic stereochemistry: Geometric isomerism in bis-tridentate ligand complexes. Coord. Chem. Rev. 2021, 445, 214051–214071. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Qu, B.; Chen, Q.; He, M. Synthesis, supramolecular assemblies and luminescence of nickel (II) complexes based on a series of N-(2-pyridylmethyl) amino acid derivatives. Inorg. Chim. Acta 2014, 418, 59–65. [Google Scholar] [CrossRef]
- Lewis, W.; Steele, P. Chiral heterocyclic ligands XVI: Synthesis and crystal structures of four metal complexes of a tridentate, biheterocyclic ligand derived from l-cysteine. Polyhedron 2010, 29, 2220–2224. [Google Scholar] [CrossRef]
- Clardy, J.; Solheim, B.A.; Springer, J.P.; Itoh, I.; Paquette, L.A. Crystal and molecular structural studies of (±) and meso-bivalvane. J. Chem. Soc. Perkin Trans. 2 1979, 3, 296–299. [Google Scholar] [CrossRef]
- Grajda, M.; Staros, G.; Jędrzejewska, H.; Szumna, A. Toward Coordination Cages with Hybrid Chirality: Amino Acid-Induced Chirality on Metal Centers. Inorg. Chem. 2022, 61, 11410–11418. [Google Scholar] [CrossRef]
- Kim, H.; Im, S.-W.; Kim, R.-M.; Cho, N.-H.; Lee, H.-E.; Ahn, H.-Y.; Nam, K.-T. Chirality control of inorganic materials and metals by peptides or amino acids. Mater. Adv. 2020, 1, 512–524. [Google Scholar] [CrossRef]
- Subramaniyam, V.; Ravi, P.V.; Pichumani, M. Structure co-ordination of solitary amino acids as ligands in metal-organic frameworks (MOFs): A Comprehensive Review. J. Mol. Struct. 2022, 1251, 131931. [Google Scholar] [CrossRef]
- Kundu, T.; Sahoo, S.; Banerjee, R. Variable Water Adsorption in Amino Acid Derivative Based Homochiral Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 4633–4640. [Google Scholar] [CrossRef]
- Kumar, N.; Khullar, S.; Mandal, S. Controlling the self-assembly of homochiral coordination architectures of CuII by substitution in amino acid based ligands: Synthesis, crystal structures and physicochemical properties. Dalton Trans. 2015, 44, 5672–5687. [Google Scholar] [CrossRef] [PubMed]
- Baul, T.; Kehie, P.; Chanu, O.; Duthie, A.; Hopfl, H. Synthesis and structural studies of diorganotin(IV) compounds derived from (E)-3-hydroxy-2-((2-hydroxybenzylidene)amino)propanoate and (E)-3-hydroxy-2-((1-(2-hydroxyphenyl)ethylidene)amino)propanoate. J. Organomet. Chem. 2013, 733, 36–43. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97: Program for Crystal Structure Refinement; ScienceOpen, Inc.: Boston, MA, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: FORTRAN Program for Crystal Structure Analysis; Gottingen University, Institute Fr Anorganisheche Chemie der Universitat: Gottingen, Germany, 1998. [Google Scholar]
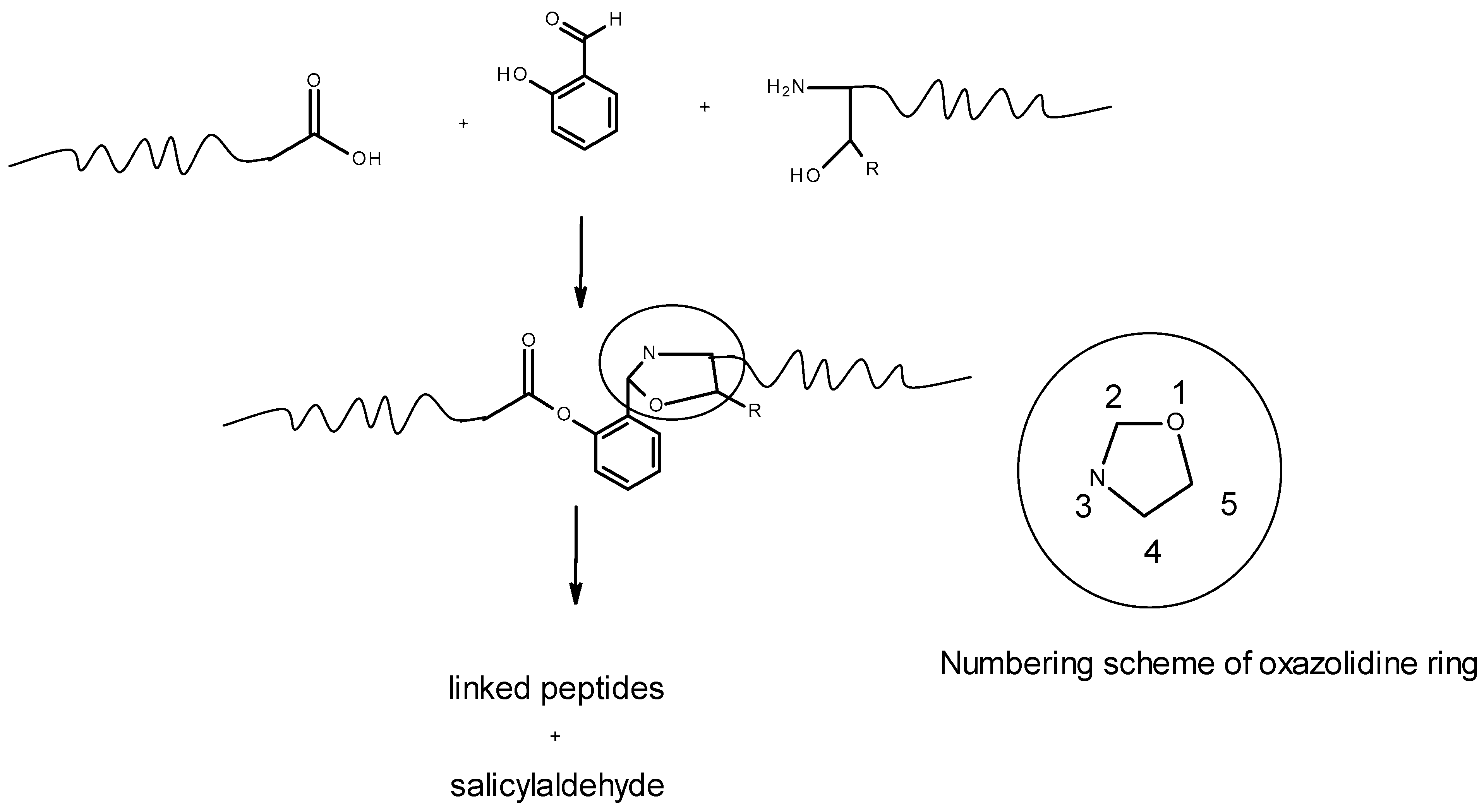
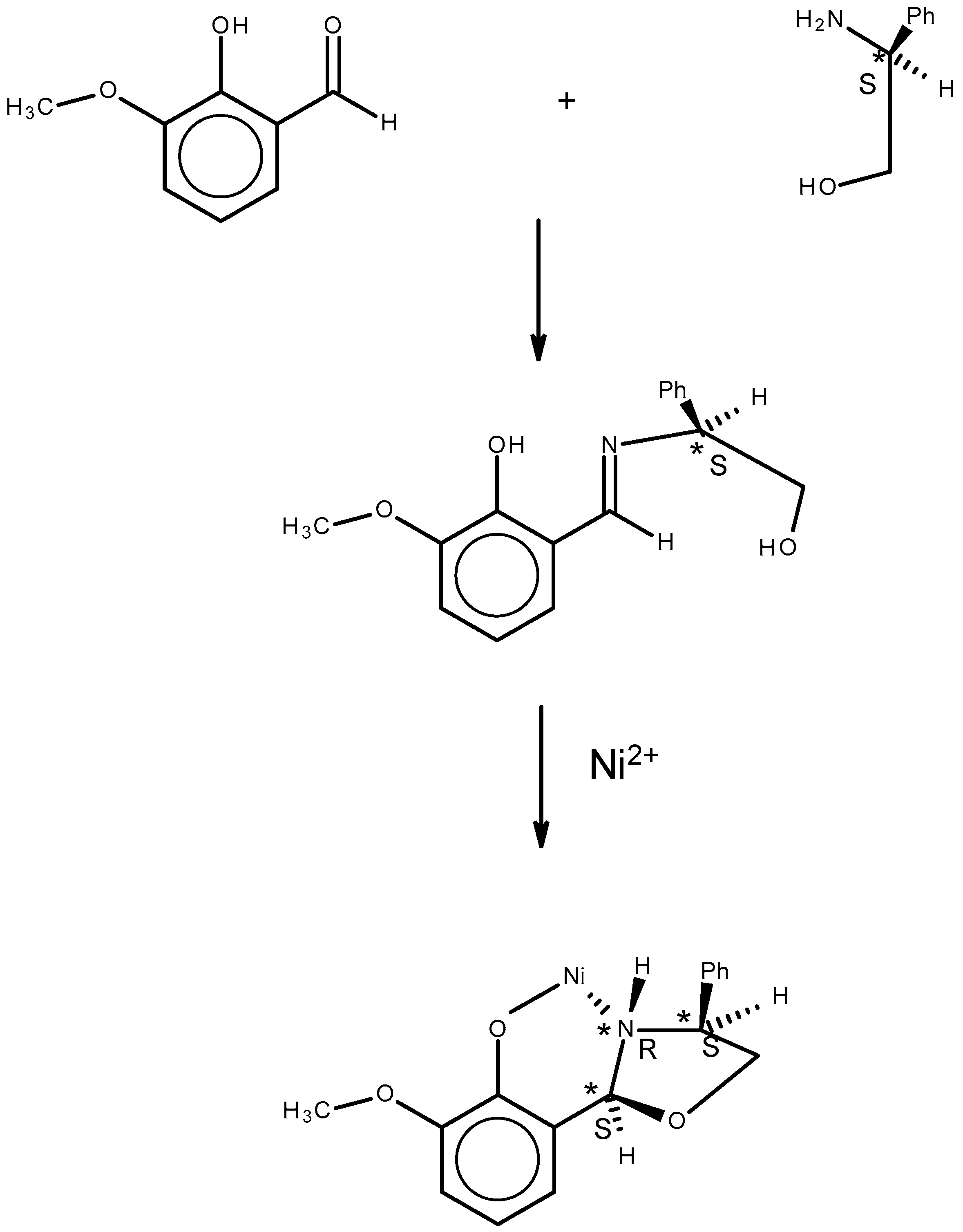
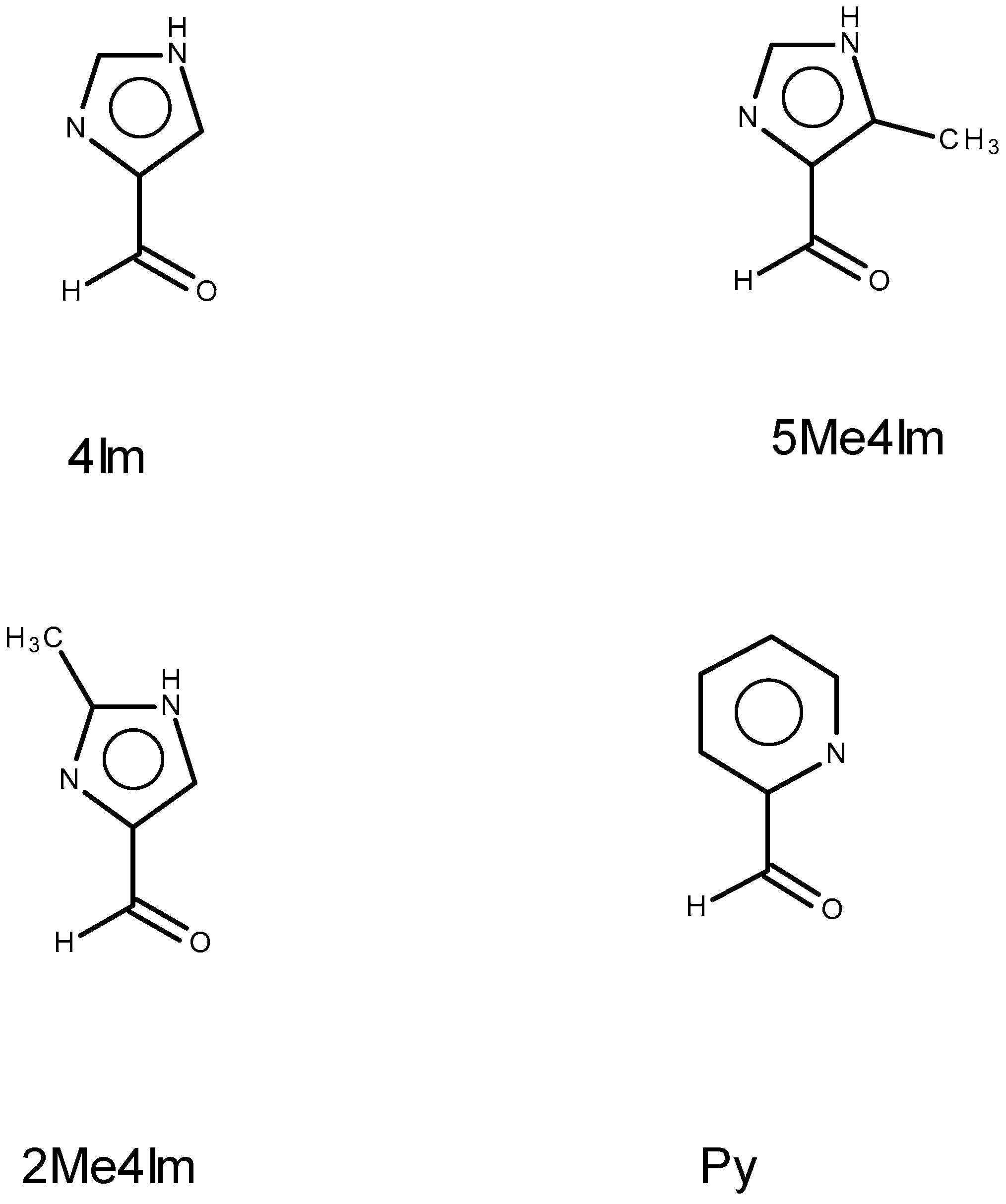
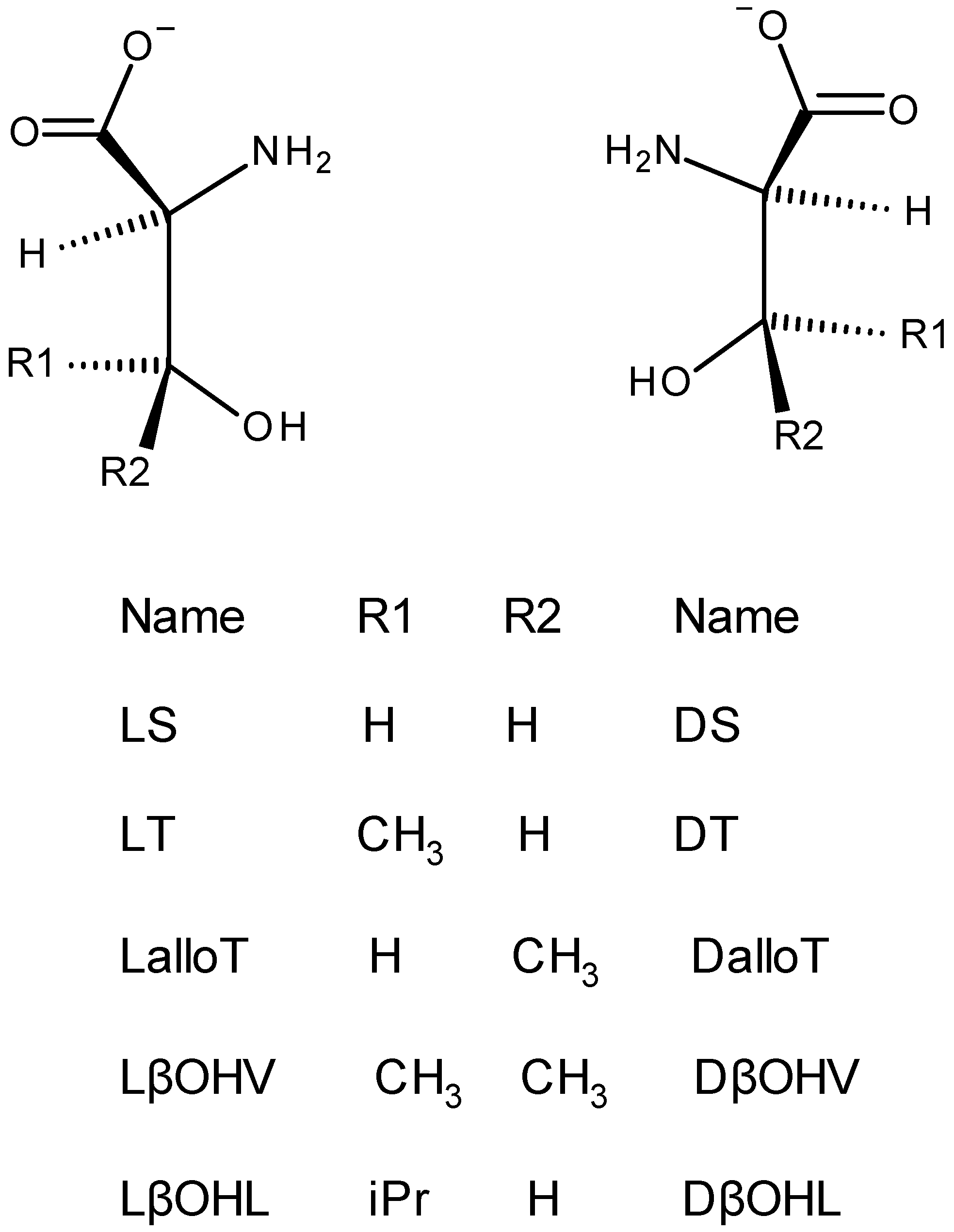
| Compound | Complex | Cα | Cβ | NAA | CAld |
|---|---|---|---|---|---|
| Ni(LTOx4Im)2 | Ʌ | S | R | R | R |
| Ni(DTOx4Im)2 | Δ | R | S | S | S |
| Ni(LTOxPy)2 | Ʌ | S | R | R | R |
| Ni(DTOxPy)2 | Δ | R | S | S | S |
| Ni(LβOHVOx4Im)2 | Ʌ | S | --- | R | R |
| Ni(DβOHVOx4Im)2 | Δ | R | --- | S | S |
| Ni(LβOHLOx4Im)2 | Λ | S | R | R | R |
| Ni(DβOHLOx4Im)2 | Δ | R | S | S | S |
| Ni(LSAld5Me4Im)2 | Δ | S | --- | --- | --- |
| Ni(DSAld 5Me4m)2 | Λ | R | --- | --- | --- |
| Ni(LalloTAld5Me4Im)2 | Δ | S | S | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, C.T.; Brewer, G.; Butcher, R.J. Chirality Transfer and Oxazolidine Formation in Reaction of L and D Enantiomers of β-Hydroxy Amino Acids with Nitrogenous Carboxaldehydes and Nickel(II). Molecules 2025, 30, 2913. https://doi.org/10.3390/molecules30142913
Brewer CT, Brewer G, Butcher RJ. Chirality Transfer and Oxazolidine Formation in Reaction of L and D Enantiomers of β-Hydroxy Amino Acids with Nitrogenous Carboxaldehydes and Nickel(II). Molecules. 2025; 30(14):2913. https://doi.org/10.3390/molecules30142913
Chicago/Turabian StyleBrewer, Cynthia T., Greg Brewer, and Raymond J. Butcher. 2025. "Chirality Transfer and Oxazolidine Formation in Reaction of L and D Enantiomers of β-Hydroxy Amino Acids with Nitrogenous Carboxaldehydes and Nickel(II)" Molecules 30, no. 14: 2913. https://doi.org/10.3390/molecules30142913
APA StyleBrewer, C. T., Brewer, G., & Butcher, R. J. (2025). Chirality Transfer and Oxazolidine Formation in Reaction of L and D Enantiomers of β-Hydroxy Amino Acids with Nitrogenous Carboxaldehydes and Nickel(II). Molecules, 30(14), 2913. https://doi.org/10.3390/molecules30142913








