Structurally Ordered NIPUs via Catalyst-Free Synthesis with Hard Segments Based on Erythritol and a Long-Chain Diamine
Abstract
1. Introduction
2. Results and Discussion
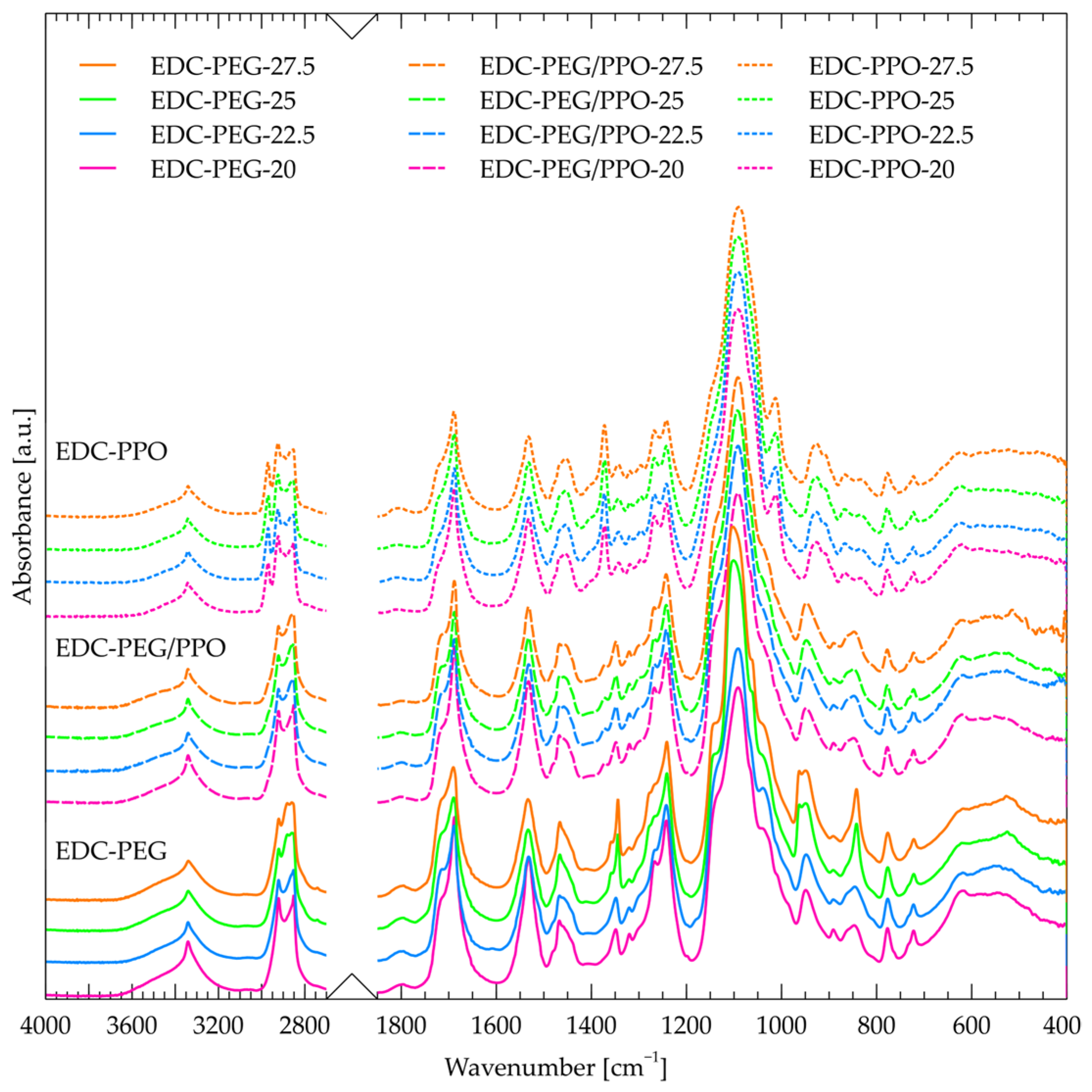
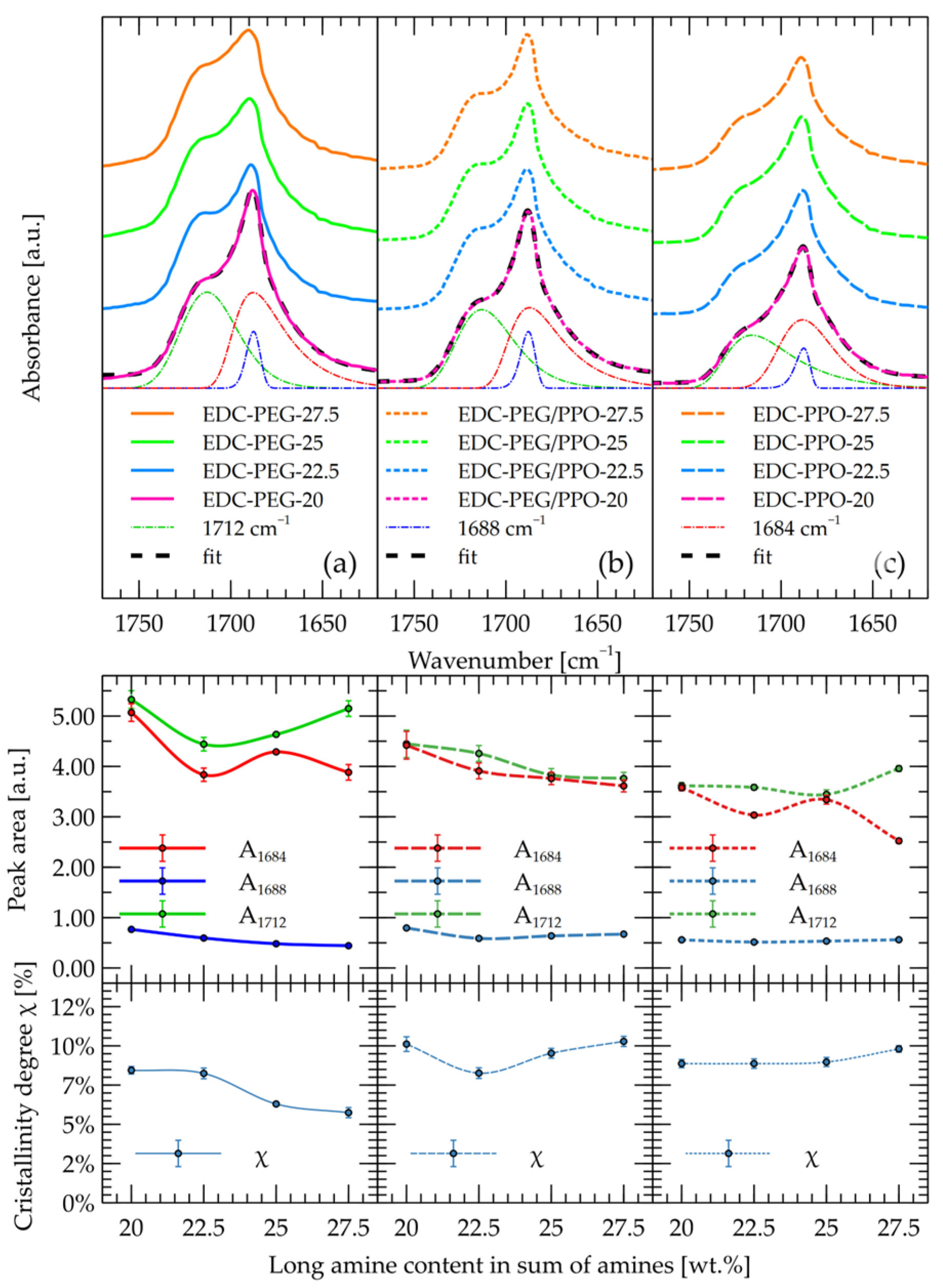
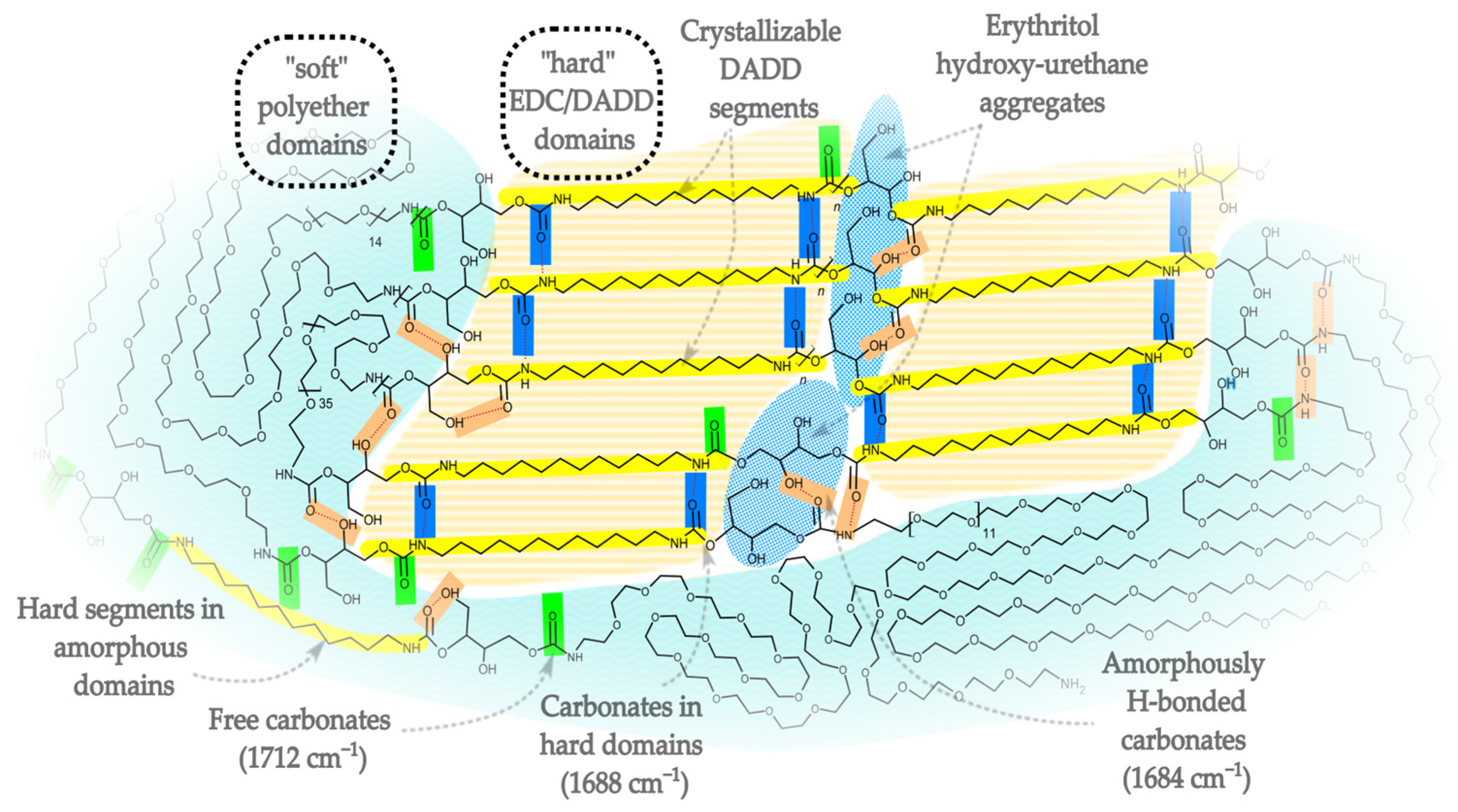
2.1. FT-IR
- -
- Free NH groups;
- -
- NH groups that are connected to carbonyl in hard segments—type I;
- -
- NH groups that are connected to ether oxygen in soft segments—type II.
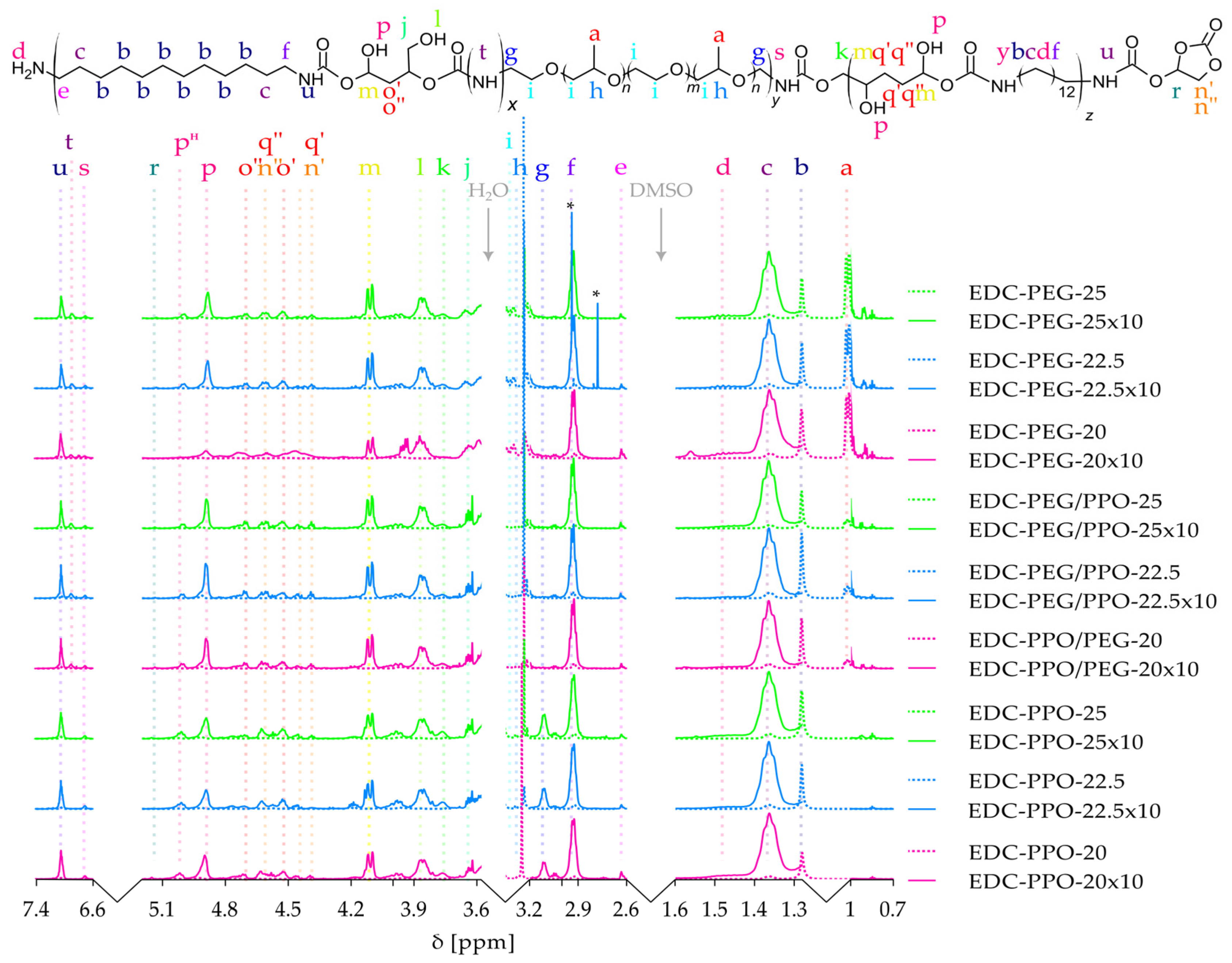
2.2. 1H NMR
- Methyl groups from PPO (a) appear at δ 0.85–0.95 ppm as a doublet, broadened in mixed PEG-PPO systems.
- Internal aliphatic CH2 groups (b) from DADD are seen as broad multiplets at δ 1.2–1.5 ppm, while β-methylene groups (c) are slightly downfield (δ ~1.6–2.0 ppm) due to proximity to urethane and ether linkages.
- Terminal NH2 and adjacent CH2 signals (d, e) are visible in some spectra as low-intensity features, indicating slight chain-end functionality.
- Methylene groups adjacent to NH (f, g) in DADD and PEO segments appear at δ ~2.9–3.1 ppm.
- PPO methine and ether CH2 (h, i) resonate at δ ~3.3–3.4 ppm and δ ~3.5–3.7 ppm, respectively, forming part of the PEO/PPO backbone.
- CHOH-associated CH2 and CH protons (j, m, o) appear from δ 4.0 to 4.7 ppm, with signal complexity reflecting regioisomeric ring opening.
- Secondary OH groups (p) show broad signals at δ 4.8–5.0 ppm, and a distinct multiplet at δ ~5.02 ppm (pH) is attributed to hydrogen-bonded CH–OH. The peak pH may be due to OH … O=C—the hydroxyl groups, bonded with the urethane group, and is responsible for dividing the FTIR band for urethanes into the 1684 cm−1 band.
- Notably, the CHOH–OH signal splitting varies with diamine structure: a triplet is observed when PPO is used (due to asymmetric, rigid environments near the urethane), while a doublet appears in PEG-containing systems where greater flexibility and symmetry may reduce additional coupling.
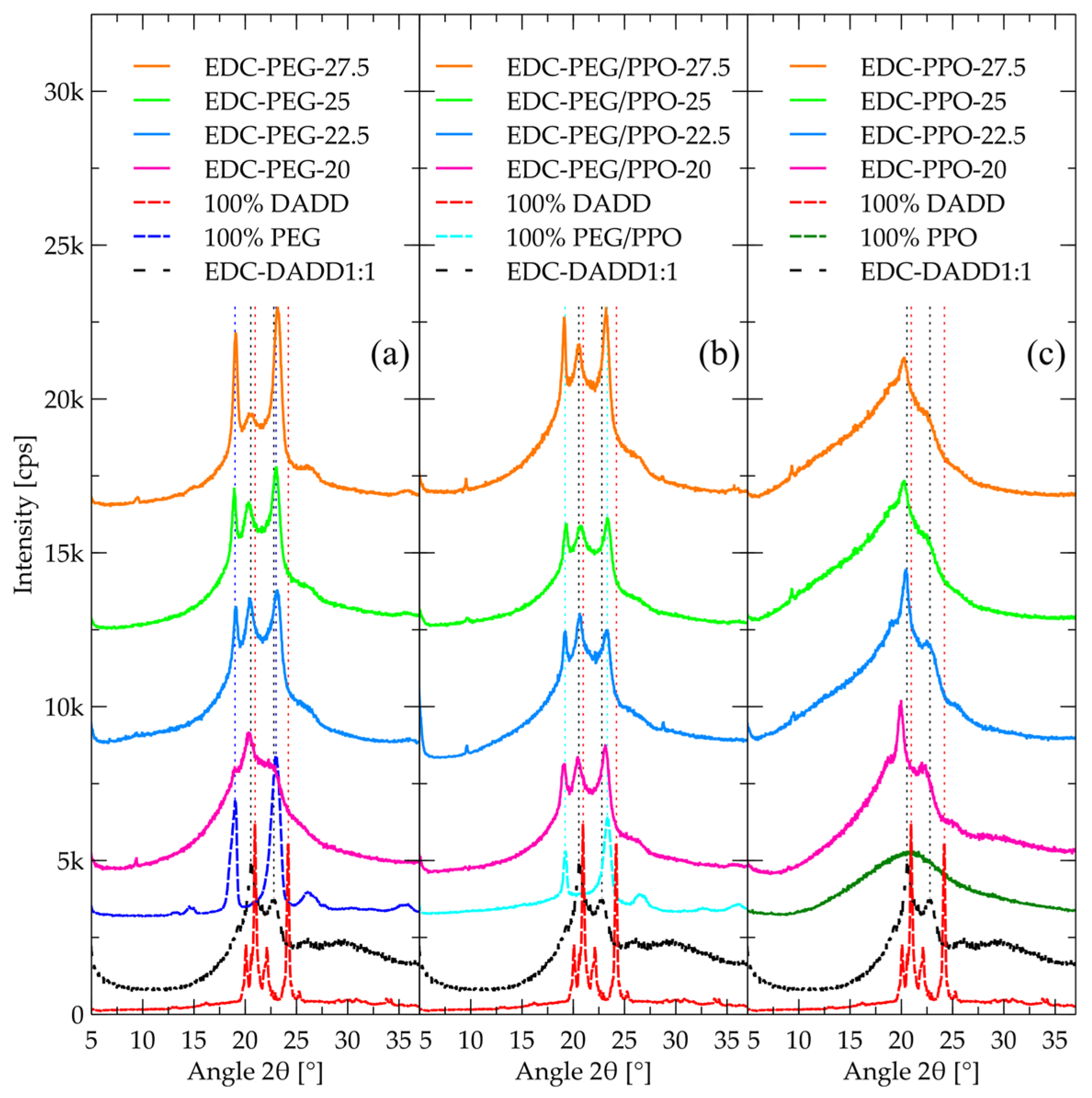
2.3. WAXD
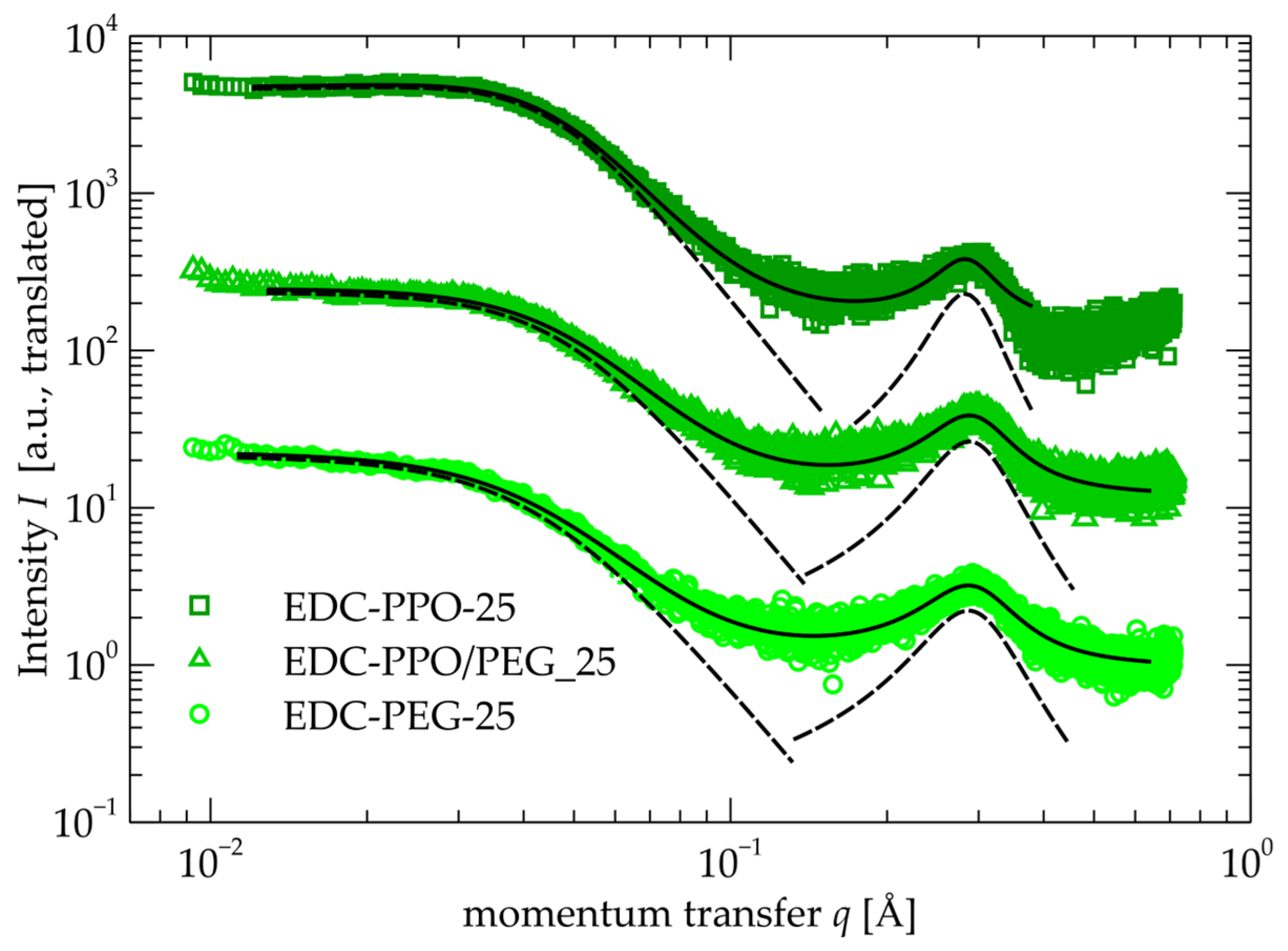
2.4. SAXS
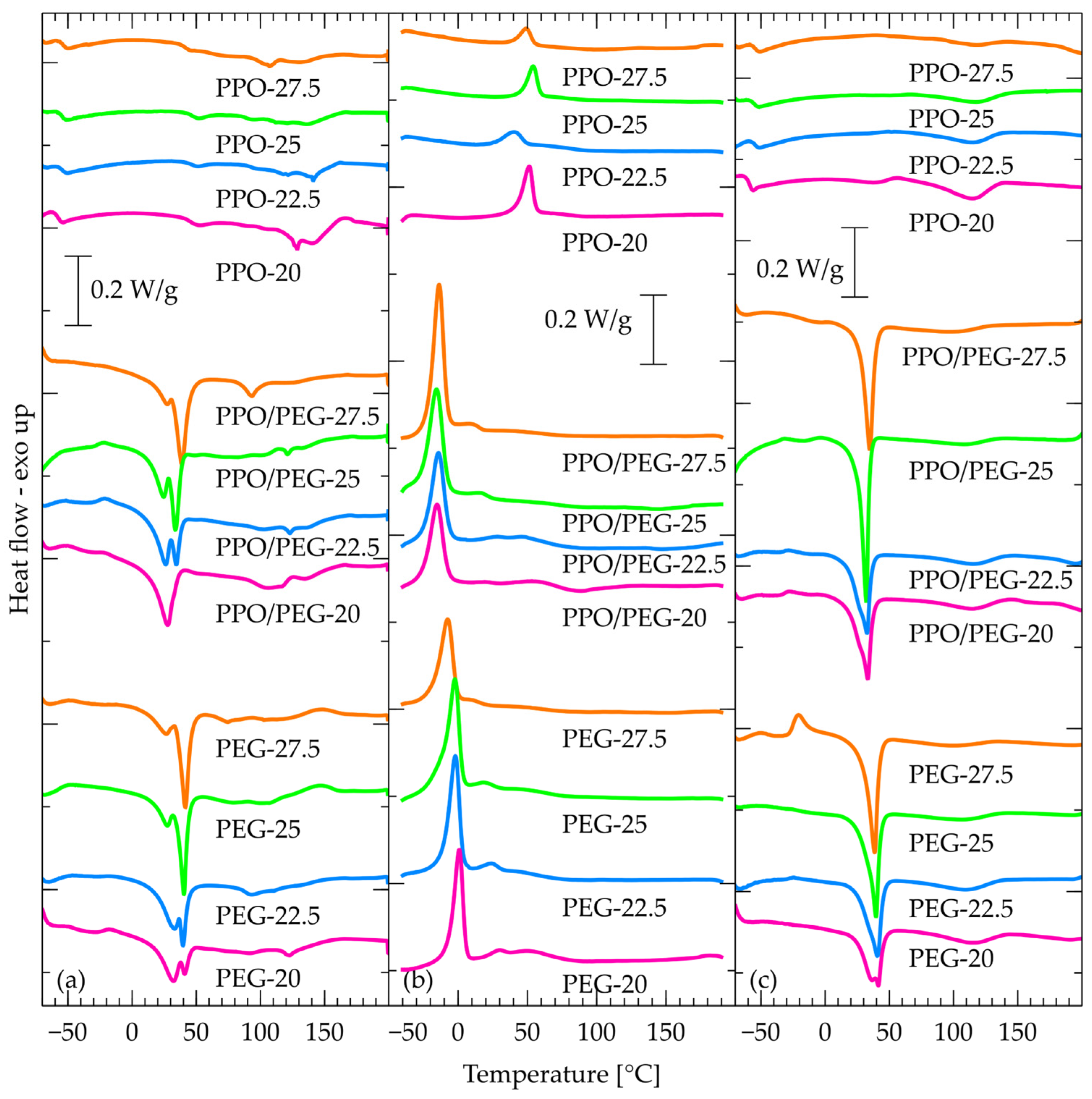
2.5. DSC
2.6. DMA
3. Materials and Methods
3.1. Materials
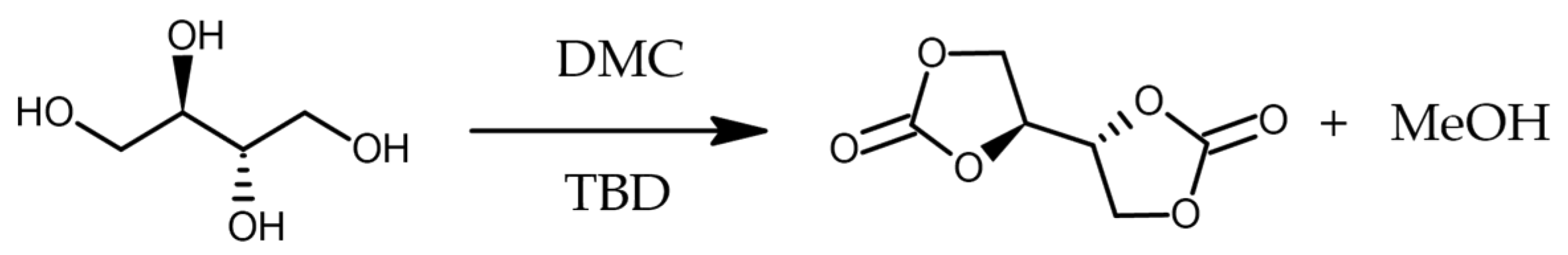
3.2. Synthesis of Erythritol Di(Carbonate)
3.3. General Procedure for Synthesis of Non-Isocyanate Polyurethanes (NIPUs)
3.4. Methods
3.4.1. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4.2. Nuclear Magnetic Resonance (NMR)
3.4.3. Differential Scanning Calorimetry (DSC)
3.4.4. Wide Angle X-Ray Diffraction (WAXD)
3.4.5. Small Angle X-Ray Scattering (SAXS)
3.4.6. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalá, J.; Guerra, I.; García-Vargas, J.M.; Ramos, M.J.; García, M.T.; Rodríguez, J.F. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers 2023, 15, 1589. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Castro, N.; Bas, O.; Saifzadeh, S.; Butler, P.; Hutmacher, D.W. The Current Versatility of Polyurethane Three-Dimensional Printing for Biomedical Applications. Tissue Eng. Part B Rev. 2020, 26, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Król, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jerome, C.; Detrembleur, C.; Sardon, H. Poly(hydroxyurethane) Adhesives and Coatings: State-of-the-Art and Future Directions. ACS Sustain. Chem. Eng. 2021, 9, 9541–9562. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Munn, A. Hazards of Isocyanates. Ann. Occup. Hyg. 1965, 8, 163–169. [Google Scholar] [CrossRef]
- Rayung, M.; Ghani, N.A.; Hasanudin, N. A review on vegetable oil-based non isocyanate polyurethane: Towards a greener and sustainable production route. RSC Adv. 2024, 14, 9273–9299. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Blain, M.; Jean-Gérard, L.; Auvergne, R.; Benazet, D.; Caillol, S.; Andrioletti, B. Rational investigations in the ring opening of cyclic carbonates by amines. Green Chem. 2014, 16, 4286–4291. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Yu, W.; Maynard, E.; Chiaradia, V.; Arno, M.C.; Dove, A.P. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials. Chem. Rev. 2021, 121, 10865–10907. [Google Scholar] [CrossRef]
- Cornille, A.; Blain, M.; Auvergne, R.; Andrioletti, B.; Boutevin, B.; Caillol, S. A study of cyclic carbonate aminolysis at room temperature: Effect of cyclic carbonate structures and solvents on polyhydroxyurethane synthesis. Polym. Chem. 2017, 8, 592–604. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Structural analysis of polyhydroxyurethane obtained by polyaddition of bifunctional five-membered cyclic carbonate and diamine based on the model reaction. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 851–859. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Model reaction for the synthesis of polyhydroxyurethanes from cyclic carbonates with amines: Substituent effect on the reactivity and selectivity of ring-opening direction in the reaction of five-membered cyclic carbonates with amine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3678–3685. [Google Scholar] [CrossRef]
- Leitsch, E.K.; Beniah, G.; Liu, K.; Lan, T.; Heath, W.H.; Scheidt, K.A.; Torkelson, J.M. Nonisocyanate Thermoplastic Polyhydroxyurethane Elastomers via Cyclic Carbonate Aminolysis: Critical Role of Hydroxyl Groups in Controlling Nanophase Separation. ACS Macro Lett. 2016, 5, 424–429. [Google Scholar] [CrossRef]
- Besse, V.; Camara, F.; Méchin, F.; Fleury, E.; Caillol, S.; Pascault, J.-P.; Boutevin, B. How to explain low molar masses in PolyHydroxyUrethanes (PHUs). Eur. Polym. J. 2015, 71, 1–11. [Google Scholar] [CrossRef]
- Sintas, J.I.; Bean, R.H.; Zhang, R.; Long, T.E. Nonisocyanate Polyurethane Segmented Copolymers from Bis-Carbonylimidazolides. Macromol. Rapid Commun. 2024, 45, 2400057. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Argyropoulos, J.N. Ambient Temperature Curable Isocyanate-Free Compositions for Preparing Crosslinked Polyurethanes. U.S. Patent No. 8,653,174 B2, 17 February 2014. [Google Scholar]
- Schimpf, V.; Heck, B.; Reiter, G.; Mülhaupt, R. Triple-Shape Memory Materials via Thermoresponsive Behavior of Nanocrystalline Non-Isocyanate Polyhydroxyurethanes. Macromolecules 2017, 50, 3598–3606. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Progress in non-isocyanate polyurethanes synthesized from cyclic carbonate intermediates and di- or polyamines in the context of structure–properties relationship and from an environmental point of view. Polym. Bull. 2016, 73, 1459–1496. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Raoul, Y.; Mouloungui, Z. Aminolysis Reaction of Glycerol Carbonate in Organic and Hydroorganic Medium. J. Am. Oil Chem. Soc. 2012, 89, 1125–1133. [Google Scholar] [CrossRef]
- Lam, K.Y.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Tan, R.Y.H. Non-isocyanate polyurethane-acrylate as UV- and thermo-responsive plasticizer for thermoplastic elastomer. J. Appl. Polym. Sci. 2024, 141, e55703. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Lin, X.; Shang, H.; Liu, Q.; Wang, H.; Liu, S.; Xu, X.; Dong, F. High-Strength, Self-Healing, Recyclable, and Catalyst-Free Bio-Based Non-Isocyanate Polyurethane. ACS Sustain. Chem. Eng. 2023, 11, 6100–6113. [Google Scholar] [CrossRef]
- Beniah, G.; Liu, K.; Heath, W.H.; Miller, M.D.; Scheidt, K.A.; Torkelson, J.M. Novel Thermoplastic Polyhydroxyurethane Elastomers as Effective Damping Materials over Broad Temperature Ranges. Eur. Polym. J. 2016, 84, 770–783. [Google Scholar] [CrossRef]
- Beniah, G.; Uno, B.; Lan, T.; Jeon, J.; Heath, W.H.; Scheidt, K.A.; Torkelson, J.M. Tuning nanophase separation behavior in segmented polyhydroxyurethane via judicious choice of soft segment. Polymer 2017, 110, 218–277. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. One-pot non-isocyanate synthesis of polyurethanes from bisepoxide, carbon dioxide, and diamine. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6613–6618. [Google Scholar] [CrossRef]
- Diakoumakos, C.D.; Kotzev, D.L. Non-Isocyanate-Based Polyurethanes Derived upon the Reaction of Amines with Cyclocarbonate Resins. Macromol. Symp. 2004, 216, 37–46. [Google Scholar] [CrossRef]
- Lambeth, R.; Henderson, T.J. Organocatalytic synthesis of (poly)hydroxyurethanes from cyclic carbonates and amines. Polymer 2013, 54, 5568–5573. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, K.; Xie, H.; Zhao, Z.K.; Bao, M.; Peng, C. Preparation of lignin/glycerol-based bis(cyclic carbonate) for the synthesis of polyurethanes. Green. Chem. 2015, 17, 4546–4551. [Google Scholar] [CrossRef]
- Prompers, G.; Keul, H.; Höcker, H. Polyurethanes with pendant hydroxy groups: Polycondensation of D-mannitol-1,2:5,6-dicarbonate with diamines. Des. Monomers Polym. 2005, 8, 547–569. [Google Scholar] [CrossRef]
- Fidalgo, D.M.; Kolender, A.A.; Varela, O. Stereoregular poly-O-methyl [m,n]-polyurethanes derived from D-mannitol. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 463–470. [Google Scholar] [CrossRef]
- Ubaghs, L.; Fricke, N.; Keul, H.; Höcker, H. Polyurethanes with Pendant Hydroxyl Groups: Synthesis and Characterization. Macromol. Rapid Commun. 2004, 25, 517–521. [Google Scholar] [CrossRef]
- Prompers, G.; Keul, H.; Höcker, H. Polyurethanes with pendant hydroxy groups: Polycondensation of 1,6-bis-O-phenoxycarbonyl-2,3:4,5 di-O-isopropylidenegalactitol and 1,6-di-O-phenoxycarbonylgalactitol with diamines. Green. Chem. 2006, 8, 467–478. [Google Scholar] [CrossRef]
- Boyer, A.; Cloutet, E.; Tassaing, T.; Gadenne, B.; Alfos, C.; Cramail, H. Solubility in CO2 and carbonation studies of epoxidized fatty acid diesters: Towards novel precursors for polyurethane synthesis. Green Chem. 2010, 12, 2205–2213. [Google Scholar] [CrossRef]
- Mazurek-Budzyńska, M.M.; Rokicki, G.; Drzewicz, M.; Guńka, P.A.; Zachara, J. Bis(cyclic carbonate) based on d-mannitol, d-sorbitol and di (trimethylolpropane) in the synthesis of non-isocyanate poly (carbonate-urethane)s. Eur. Polym. J. 2016, 84, 799–811. [Google Scholar] [CrossRef]
- Lamarzelle, O.; Durand, P.-L.; Wirotius, A.-L.; Chollet, G.; Grau, E.; Cramail, H. Activated lipidic cyclic carbonates for non-isocyanate polyurethane synthesis. Polym. Chem. 2016, 7, 1439–1451. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Schmidt, S.; Gatti, F.J.; Luitz, M.; Ritter, B.S.; Bruchmann, B.; Mulhaupt, R. Erythritol Dicarbonate as Intermediate for Solvent- and Isocyanate Free Tailoring of Bio-Based Polyhydroxyurethane Thermoplastics and Thermoplastic Elastomers. Macromolecules 2017, 50, 2296–2303. [Google Scholar] [CrossRef]
- Kihara, N.; Endo, T. Synthesis and properties of poly (hydroxyurethane)s. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2765–2773. [Google Scholar] [CrossRef]
- Salvado, V.; Dolatkhani, M.; Grau, E.; Vidil, T.; Cramail, H. Sequence-Controlled Polyhydroxyurethanes with Tunable Regioregularity Obtained from Sugar-Based Vicinal Bis-cyclic Carbonates. Macromolecules 2022, 55, 7249–7264. [Google Scholar] [CrossRef]
- Le Goupil, F.; Salvado, V.; Rothan, V.; Vidil, T.; Fleury, G.; Cramail, H.; Grau, E. Bio-Based Poly(hydroxy urethane)s for Efficient Organic High-Power Energy Storage. J. Am. Chem. Soc. 2023, 145, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, K.N.; Łukaszewska, I.; Lalik, S.; Zając, P.; Bukowczan, A.; Hebda, E.; Marzec, M.; Pielichowski, K. Structure–Glass Transition Relationships in Non Isocyanate Polyhydroxyurethanes. Molecules 2024, 29, 4057. [Google Scholar] [CrossRef]
- Mishra, A.K.; Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. FT-IR and XPS studies of polyurethane-urea-imide coatings. Prog. Org. Coat. 2006, 55, 231–243. [Google Scholar] [CrossRef]
- Koutsoumpis, S.; Ozimek, J.; Raftopoulos, K.N.; Hebda, E.; Klonos, P.; Papadakis, C.M.; Pielichowski, K.; Pissis, P. Polyurethanes with POSS pendent on flexible hard segments: Morphology and glass transition. Polymer 2018, 147, 225–236. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Gao, Z.; Li, X.; Eling, B.; Poselt, E.; Schander, E.; Wang, Z. Structure transition of aliphatic m,6-Polyurethane during heating investigated using in-situ WAXS, SAXS, and FTIR. Polymer 2022, 254, 125072. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Painter, P.C.; Coleman, M.M. Hydrogen Bonding in Polymers. 2. Infrared Temperature Studies of Nylon 11. Macromolecules 1986, 19, 699–705. [Google Scholar] [CrossRef]
- Stancik, A.L.; Brauns, E.B. A simple asymmetric lineshape for fitting infrared absorption spectra. Vib. Spectrosc. 2008, 47, 66–69. [Google Scholar] [CrossRef]
- Nobre, S.S.; Lima, P.P.; Mafra, L.; Ferreira, R.A.S.; Freire, R.O.; Fu, L.; Pischel, U.; de Zea Bermudez, V.; Malta, O.L.; Carlos, L.D. Energy Transfer and Emission Quantum Yields of Organic–Inorganic Hybrids Lacking Metal Activator Centers. J. Phys. Chem. C 2007, 111, 3275–3284. [Google Scholar] [CrossRef]
- Raut, P.W.; Shitole, A.A.; Khandwekar, A.; Sharma, N. Engineering biomimetic polyurethane using polyethylene glycol and gelatin for blood-contacting applications. J. Mater. Sci. 2019, 54, 10457–10472. [Google Scholar] [CrossRef]
- Beniah, G.; Fortman, D.J.; Heath, W.H.; Dichtel, W.R.; Torkelson, J.M. Non-Isocyanate Polyurethane Thermoplastic Elastomer: AmideBased Chain Extender Yields Enhanced Nanophase Separation and Properties in Polyhydroxyurethane. Macromolecules 2017, 50, 4425–4434. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Russell, T.P. Simultaneous SAXS-DSC study of multiple endothermic behavior in polyether-based polyurethane block copolymers. Macromolecules 1986, 19, 714–720. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Stein, R.S. Small-angle X-ray scattering studies of microdomain structure in segmented polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 1439–1472. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. Small-angle scattering analysis of hard-microdomain structure and microphase mixing in polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1883–1913. [Google Scholar] [CrossRef]
- Koutsoumpis, S.; Raftopoulos, K.N.; Oguz, O.; Papadakis, C.M.; Menceloglu, Y.Z.; Pissis, P. Dynamic glass transition of the rigid amorphous fraction in polyurethane-urea/SiO2 nanocomposites. Soft Matter 2017, 13, 4580–4590. [Google Scholar] [CrossRef]
- Koutsoumpis, S.; Raftopoulos, K.N.; Jancia, M.; Pagacz, J.; Hebda, E.; Papadakis, C.M.; Pielichowski, K.; Pissis, P. POSS moieties with PEG vertex groups as diluent in Polyurethane elastomers: Morphology and phase separation. Macromolecules 2016, 49, 6507–6517. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Koutsoumpis, S.; Jancia, M.; Lewicki, J.P.; Kyriakos, K.; Mason, H.E.; Harley, S.J.; Hebda, E.; Papadakis, C.M.; Pielichowski, K.; et al. Reduced Phase Separation and Slowing of Dynamics in Polyurethanes with Three-Dimensional POSS-Based Cross-Linking Moieties. Macromolecules 2015, 48, 1429–1441. [Google Scholar] [CrossRef]
- Teubner, M.; Strey, R. Origin of the scattering peak in microemulsions. J. Chem. Phys. 1987, 87, 3195–3200. [Google Scholar] [CrossRef]
- Endo, H.; Mihailescu, M.; Monkenbusch, M.; Allgaier, J.; Gompper, G.; Richter, D.; Jakobs, B.; Sottmann, T.; Strey, R.; Grillo, I. Effect of amphiphilic block copolymers on the structure and phase behavior of oil–water-surfactant mixtures. J. Chem. Phys. 2001, 115, 580–600. [Google Scholar] [CrossRef]
- SasView 6.0.1 Documentation » Teubner_Strey, (n.d.). Available online: https://www.sasview.org/docs/user/models/teubner_strey.html (accessed on 22 May 2025).
- SasView 6.0.1 Documentation » Peak_Lorentz, (n.d.). Available online: https://www.sasview.org/docs/user/models/peak_lorentz.html (accessed on 22 May 2025).
- Vallance, M.; Yeung, A.S.; Cooper, S.L. A dielectric study of the glass transition region in segmented polyether-urethane copolymers. Colloid Polym. Sci. 1983, 261, 541–554. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. Polyethers. In Polymeric Materials in Corrosion Inhibition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–417. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galambos, A.F. Multiple melting in segmented polyurethane block copolymers. Macromolecules 1992, 25, 5618–5624. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. DSC Annealing Study of Microphase Separation and Multiple Endothermic Behavior in Polyether-Based Polyurethane Block Copolymers. Macromolecules 1986, 19, 706–713. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Leung, L.M. Compression-molded polyurethane block copolymers. 2. Evaluation of microphase compositions. Macromolecules 1992, 25, 6205–6213. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Gancarz, I.; Clarke, T.C. The effects of morphological transitions on hydrogen bonding in polyurethanes: Preliminary results of simultaneous DSC–FTIR experiments. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 2487–2498. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galambos, A.F.; Leung, L.M. Compression-Molded Polyurethane Block Copolymers. 1. Microdomain Morphology and Thermomechanical Properties. Macromolecules 1992, 25, 6195–6204. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pielichowski, K.; Pissis, P. Molecular mobility and crystallinity in polytetramethylene ether glycol in the bulk and as soft component in polyurethanes. Eur. Polym. J. 2011, 47, 2120–2133. [Google Scholar] [CrossRef]
- Faucher, J.A.; Koleske, J.V.; Santee, E.R.; Stratta, J.J.; Wilson, C.W. Glass Transitions of Ethylene Oxide Polymers. J. Appl. Phys. 1966, 37, 3962–3964. [Google Scholar] [CrossRef]
- Bakkali-Hassani, C.; Berne, D.; Ladmiral, V.; Caillol, S. Transcarbamoylation in Polyurethanes: Underestimated Exchange Reactions? Macromolecules 2022, 55, 7974–7991. [Google Scholar] [CrossRef]
- Stachak, P.; Łukaszewska, I.; Ozimek, J.; Raftopoulos, K.N.; Bukowczan, A.; Hebda, E.; Calduch, C.B.; Pielichowski, K. Isocyanate free fabrication of sustainable polyurethane/POSS hybrid materials with tunable thermo-mechanical response. Express Polym. Lett. 2024, 18, 2–26. [Google Scholar] [CrossRef]
- Szefer, E.; Stafin, K.; Leszczyńska, A.; Zając, P.; Hebda, E.; Raftopoulos, K.N.; Pielichowski, K. Morphology, dynamics, and order development in a thermoplastic polyurethane with melt blended POSS. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1133–1142. [Google Scholar] [CrossRef]
- Dannecker, P.-K.; Meier, M.A.R. Facile and Sustainable Synthesis of Erythritol bis(carbonate), a Valuable Monomer for Non-Isocyanate Polyurethanes (NIPUs). Sci. Rep. 2019, 9, 9858. [Google Scholar] [CrossRef]
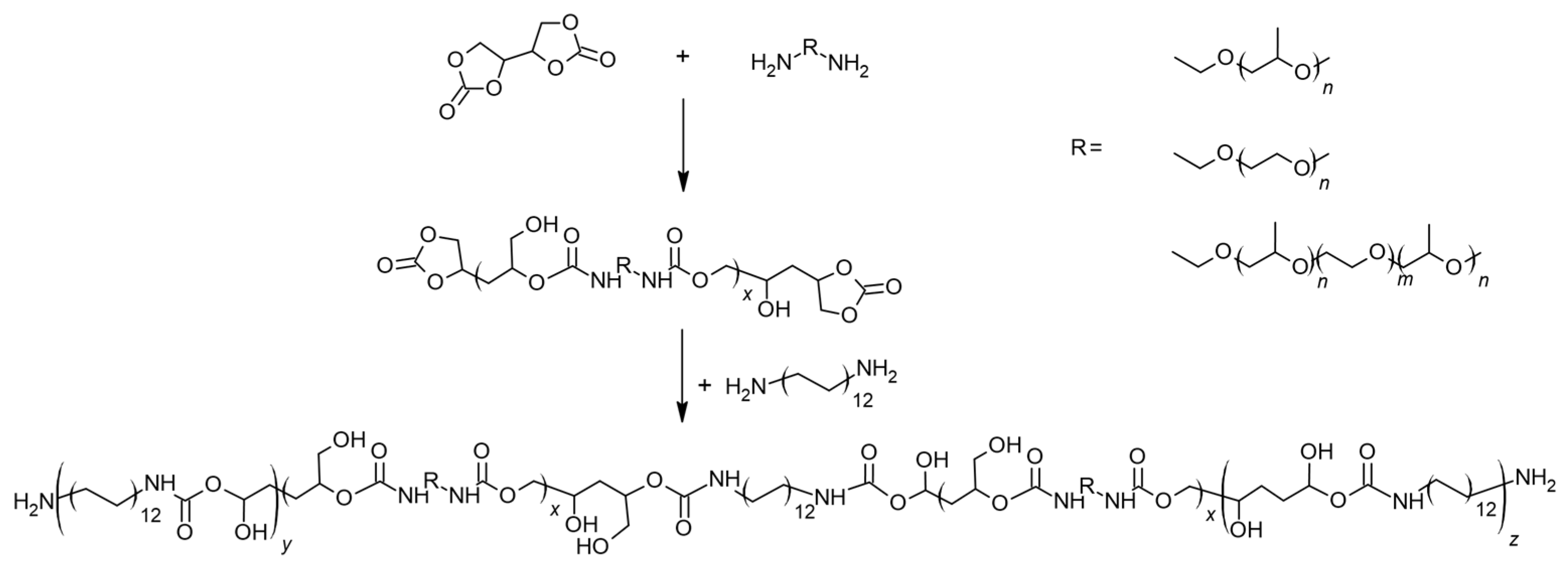

| Sample Name | Polyether | Polyether:DADD [wt%] |
|---|---|---|
| EDC-PEG_20 | H2N-PEG-NH2 | 20:80 |
| EDC-PEG_22.5 | H2N-PEG-NH2 | 22.5:77.5 |
| EDC-PEG_25 | H2N-PEG-NH2 | 25:75 |
| EDC-PEG_27.5 | H2N-PEG-NH2 | 27.5:72.5 |
| EDC-PPO_20 | H2N-PPO-NH2 | 20:80 |
| EDC-PPO_22.5 | H2N-PPO-NH2 | 22.5:77.5 |
| EDC-PPO_25 | H2N-PPO-NH2 | 25:75 |
| EDC-PPO_27.5 | H2N-PPO-NH2 | 27.5:72.5 |
| EDC-PPO/PEG_20 | H2N-PPO/PEG-NH2 | 20:80 |
| EDC-PPO/PEG_22.5 | H2N-PPO/PEG-NH2 | 22.5:77.5 |
| EDC-PPO/PEG_25 | H2N-PPO/PEG-NH2 | 25:75 |
| EDC-PPO/PEG_27.5 | H2N-PPO/PEG-NH2 | 27.5:72.5 |
| Sample | (nm) | (nm) | (nm) | (nm) |
|---|---|---|---|---|
| EDC-PEG-25 | 22.41 ± 0.10 | 3.07 ± 0.03 | 2.19 ± 0.05 | 1.46 ± 0.03 |
| EDC-PPO-25 | 17.50 ± 0.03 | 3.42 ± 0.01 | 2.26 ± 0.06 | 1.68 ± 0.06 |
| EDC-PEG/PPO-25 | 19.00 ± 0.06 | 2.86 ± 0.02 | 2.18 ± 0.04 | 1.62 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebda, E.; Wróbel, K.; Cieślik, A.; Szołdrowska, K.; Ozimek, J.; Zając, P.; Raftopoulos, K.N.; Pielichowski, K. Structurally Ordered NIPUs via Catalyst-Free Synthesis with Hard Segments Based on Erythritol and a Long-Chain Diamine. Molecules 2025, 30, 2912. https://doi.org/10.3390/molecules30142912
Hebda E, Wróbel K, Cieślik A, Szołdrowska K, Ozimek J, Zając P, Raftopoulos KN, Pielichowski K. Structurally Ordered NIPUs via Catalyst-Free Synthesis with Hard Segments Based on Erythritol and a Long-Chain Diamine. Molecules. 2025; 30(14):2912. https://doi.org/10.3390/molecules30142912
Chicago/Turabian StyleHebda, Edyta, Karolina Wróbel, Aleksandra Cieślik, Kinga Szołdrowska, Jan Ozimek, Paulina Zając, Konstantinos N. Raftopoulos, and Krzysztof Pielichowski. 2025. "Structurally Ordered NIPUs via Catalyst-Free Synthesis with Hard Segments Based on Erythritol and a Long-Chain Diamine" Molecules 30, no. 14: 2912. https://doi.org/10.3390/molecules30142912
APA StyleHebda, E., Wróbel, K., Cieślik, A., Szołdrowska, K., Ozimek, J., Zając, P., Raftopoulos, K. N., & Pielichowski, K. (2025). Structurally Ordered NIPUs via Catalyst-Free Synthesis with Hard Segments Based on Erythritol and a Long-Chain Diamine. Molecules, 30(14), 2912. https://doi.org/10.3390/molecules30142912








