Selection of Roasting Conditions in the Valorization Process of Cornelian Cherry Stones
Abstract
1. Introduction
2. Results and Discussion
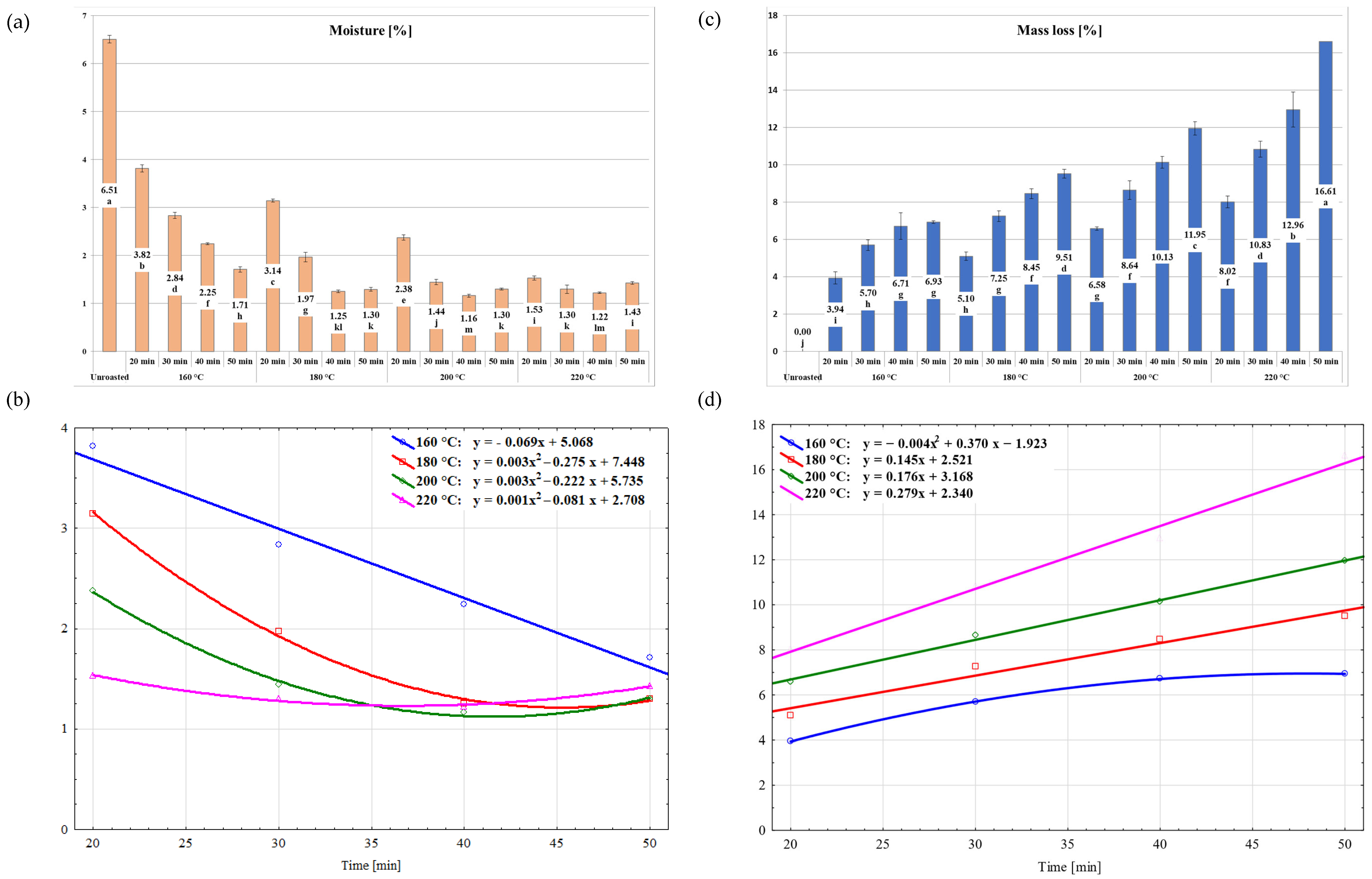
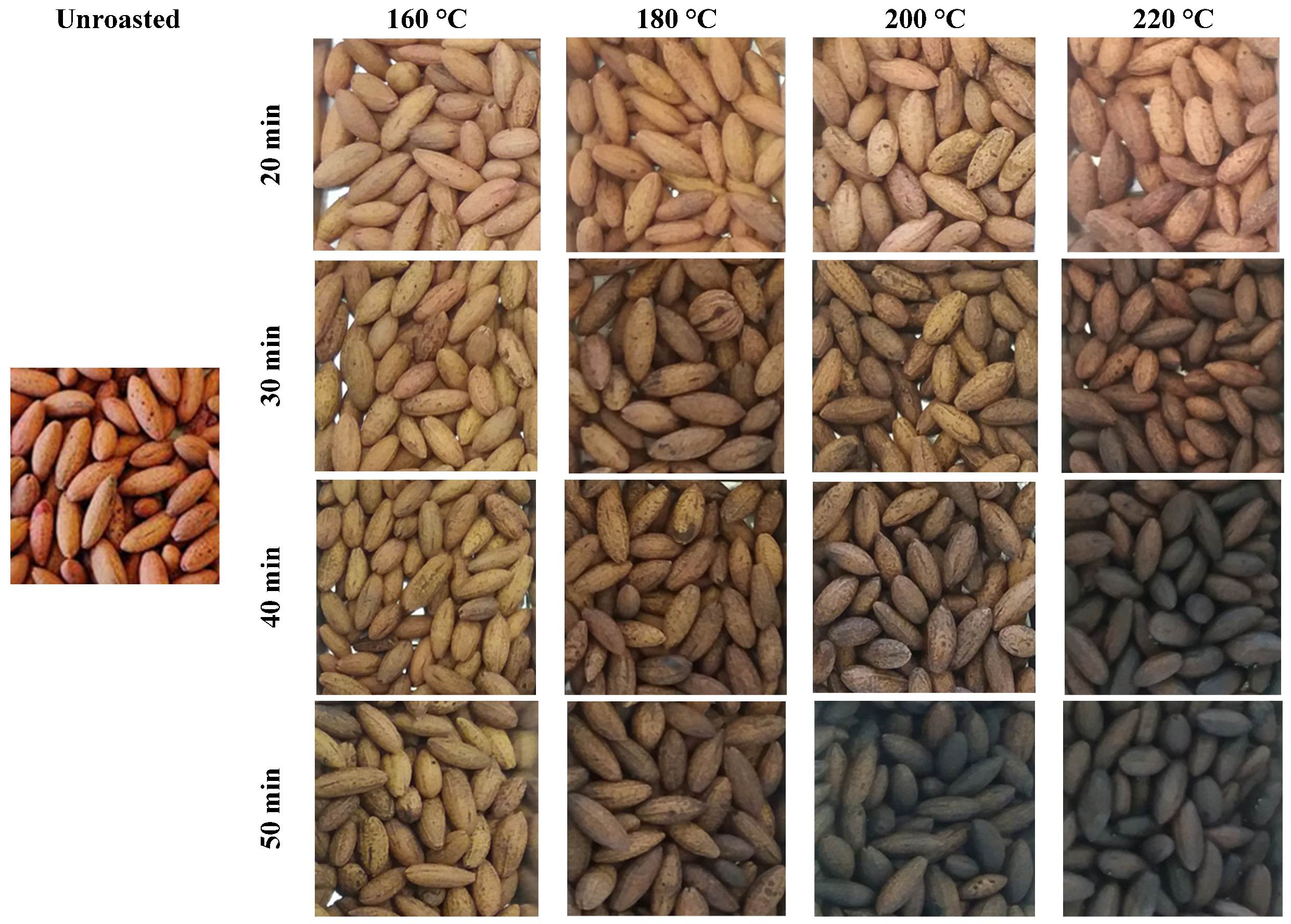
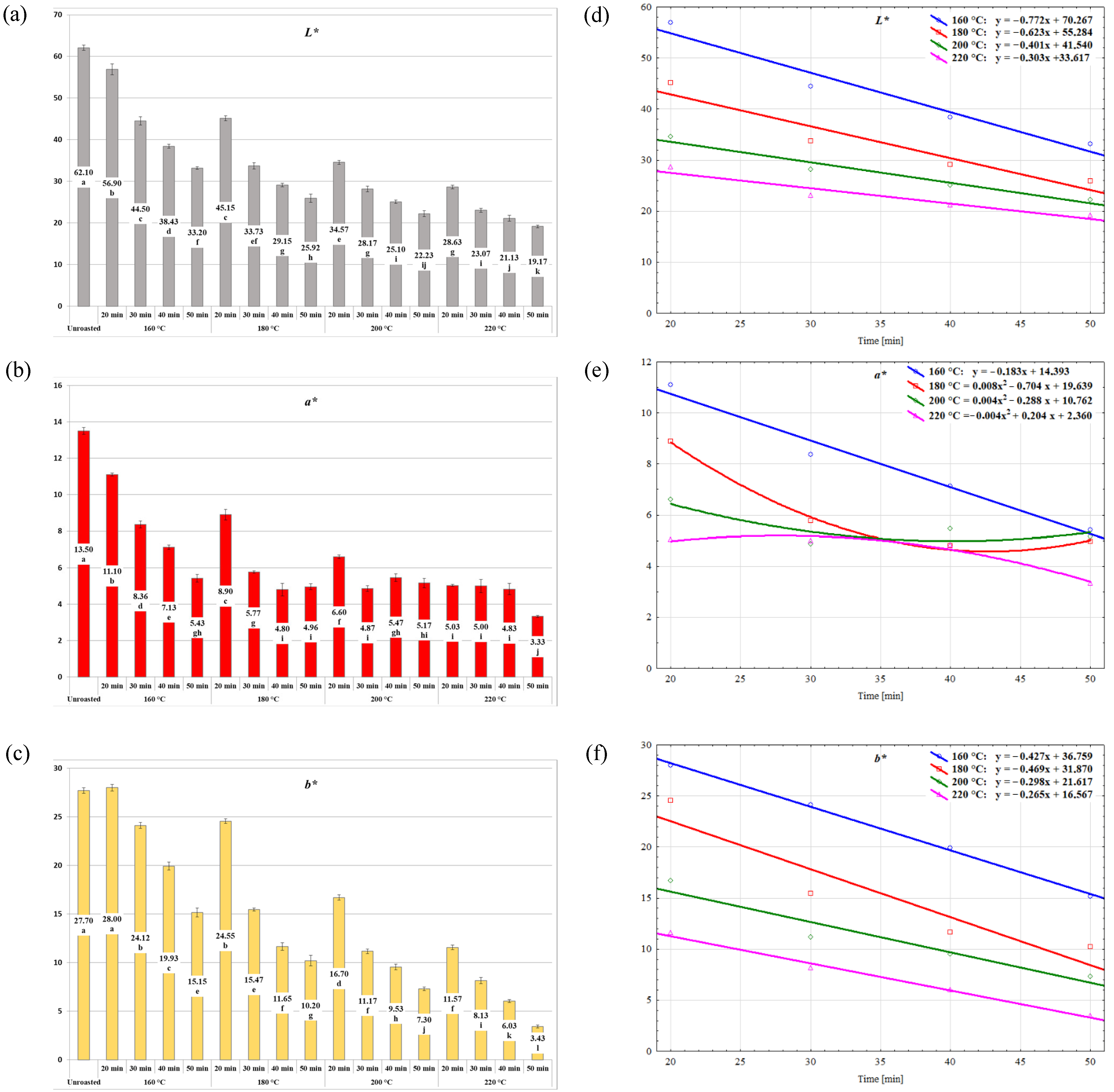
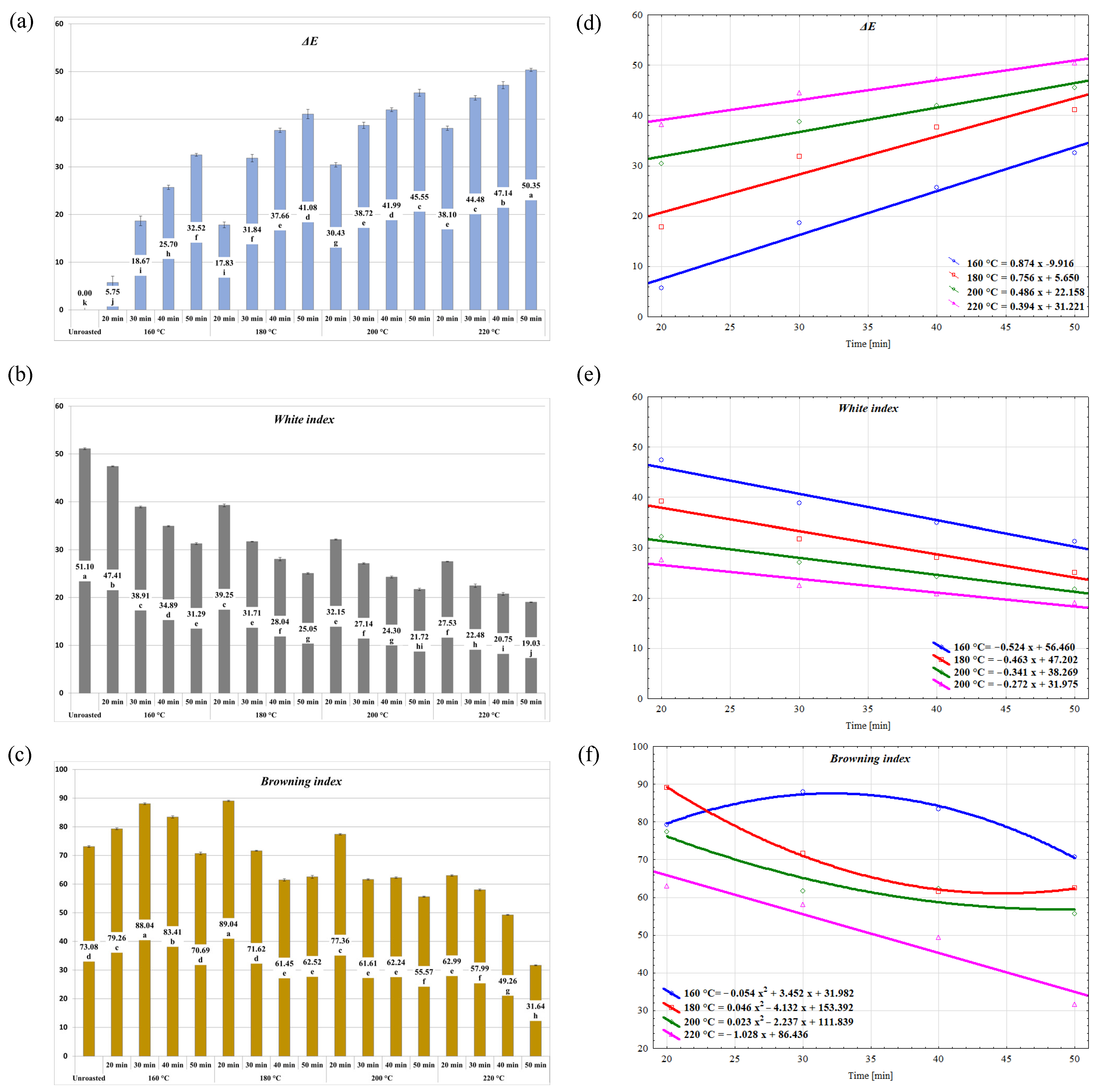
2.1. Evaluation of the Roasting Effect on the Physical Properties of the Stones
2.2. The Contents of Bioactive Compounds and HMF by the HPLC-PDA Method
2.3. Determination of Total Phenolic Content (TPC) and Antioxidant Capacity
2.4. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Chemicals and Reagents
3.2.2. Sample Preparation
3.2.3. Evaluation of the Roasting Process
3.2.4. Determination of Bioactive Compounds and HMF by the HPLC-PDA Method
3.2.5. Determination of Total Phenolic Content and Antioxidant Capacity
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kucharska, A.Z. Active Compounds of Cornelian Cherry Fruit (Cornus mas L.), 1st ed.; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu (MON. CXLVIII): Wrocław, Poland, 2012; Available online: https://www.dbc.wroc.pl/dlibra/publication/21069/edition/18811?language=en (accessed on 4 July 2025). (In Polish)
- Morozowska, M.; Wysakowska, I. Anatomical study of Cornus mas L. and Cornus officinalis Seib. et Zucc. (Cornaceae) endocarps during their development. Steciana 2016, 20, 21–32. [Google Scholar] [CrossRef]
- Atkinson, B.A.; Stockey, R.A.; Rothwell, G.W. Cretaceous origin of dogwoods: An anatomically preserved Cornus (Cornaceae) fruit from the Campanian of Vancouver Island. PeerJ 2016, 4, e2808. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—Analogies and differences of two medicinal plants traditionally used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Antoniewska-Krzeska, A.; Ivanišová, E.; Klymenko, S.; Bieniek, A.A.; Šramková, K.F.; Brindza, J. Nutrients content and composition in different morphological parts of cornelian cherry (Cornus mas L.). Agrobiodiversity Improv. Nutr. Health Life Qual. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A review on traditional uses and pharmacological properties. J. Complement. Integr. Med. 2017, 14, 1–16. [Google Scholar] [CrossRef]
- Lietava, J.; Beerova, N.; Klymenko, S.V.; Panghyova, E.; Varga, I.; Pechanova, O. Effects of cornelian cherry on atherosclerosis and its risk factors. Oxidative Med. Cell. Longev. 2019, 2019, 2515270. [Google Scholar] [CrossRef]
- Bijelić, S.; Gološin, B.; Ninić Todorović, J.; Cerović, S.; Bogdanović, B. Promising cornelian cherry (Cornus mas L.) genotypes from natural population in Serbia. Agric. Conspec. Sci. 2012, 77, 5–10. [Google Scholar]
- Kucharska, A.Z.; Sokol-Letowska, A.; Piórecki, N. Morphological, physical & chemical, and antioxidant profiles of polish varieties of cornelian cherry fruit (Cornus mas L.). Zywnosc Nauka Technol. Jakosc 2011, 18, 78–89. Available online: https://wydawnictwo.pttz.org/wp-content/uploads/2015/02/07_Kucharska.pdf (accessed on 4 July 2025). (In Polish) [CrossRef]
- Vidrih, R.; Čejić, Ž.; Hribar, J. Content of certain food components in flesh and stones of the cornelian cherry (Cornus mas L.) genotypes. Croat. J. Food Sci. Technol. 2012, 4, 64–70. [Google Scholar]
- Brindza, P.; Brindza, J.; Tóth, D.; Klimenko, S.V.; Grigorieva, O. Biological and commercial characteristics of cornelian cherry (Cornus mas L.) population in the Gemer region of Slovakia. Acta Hortic. 2009, 818, 85–94. [Google Scholar] [CrossRef]
- Spychaj, R.; Kucharska, A.Z.; Szumny, A.; Przybylska, D.; Pejcz, E.; Piórecki, N. Potential valorization of Cornelian cherry (Cornus mas L.) stones: Roasting and extraction of bioactive and volatile compounds. Food Chem. 2021, 358, 129802. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. stones: A valuable by-product as an ellagitannin source with high antioxidant potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef] [PubMed]
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling 2018, 3, 9. [Google Scholar] [CrossRef]
- Matos, M.; Barreiro, M.F.; Gandini, A. Olive stone as a renewable source of biopolyols. Ind. Crops Prod. 2010, 32, 7–12. [Google Scholar] [CrossRef]
- Haimour, N.M.; Emeish, S. Utilization of date stones for production of activated carbon using phosphoric acid. Waste Manag. 2006, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- García, M.C.; González-García, E.; Vásquez-Villanueva, R.; Marina, M.L. Apricot and other seed stones: Amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016, 7, 4693–4701. [Google Scholar] [CrossRef]
- Nehdi, I.; Omri, S.; Khalil, M.I.; Al-Resayes, S.I. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Akbari, M.; Razavizadeh, R.; Mohebbi, G.H.; Barmak, A. Oil characteristics and fatty acid profile of seeds from three varieties of date palm (Phoenix dactylifera) cultivars in Bushehr-Iran. Afr. J. Biotech. 2012, 11, 12088–12093. [Google Scholar] [CrossRef]
- Babiker, E.E.; Atasoy, G.; Özcan, M.M.; Juhaimi, F.A.; Ghafoor, K.; Ahmed, I.A.M.; Almusallam, I.A. Bioactive compounds, minerals, fatty acids, color, and sensory profile of roasted date (Phoenix dactylifera L.) seed. J. Food Process. Preserv. 2020, 44, e14495. [Google Scholar] [CrossRef]
- Padilla-Rascón, C.; Ruiz, E.; Romero, I.; Castro, E.; Oliva, J.M.; Ballesteros, I.; Manzanares, P. Valorisation of olive stone by-product for sugar production using a sequential acid/steam explosion pretreatment. Ind. Crops Prod. 2020, 148, 112279. [Google Scholar] [CrossRef]
- García Martín, J.F.; Cuevas, M.; Feng, C.H.; Álvarez Mateos, P.; Torres García, M.; Sánchez, S. Energetic valorisation of olive biomass: Olive-tree pruning, olive stones and pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Mousa, E.; Chang, L.S. Kinetics modelling of the colour, hardness, grinding energy consumption and oil yield changes during the conventional roasting of palm date seeds. Food Sci. Technol. Res. 2019, 25, 351–362. [Google Scholar] [CrossRef]
- Ghnimi, S.; Almansoori, R.; Jobe, B.; Hassan, M.H.; Afaf, K.E. Quality evaluation of coffee-like beverage from date seeds (Phoenix dactylifera, L.). J. Food Process. Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Ghazali, H.M. Antioxidative and quality properties of full-fat date seeds brew as influenced by the roasting conditions. Antioxidants 2019, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Mousa, E.; Chang, L.S. Effect of the roasting conditions on the physicochemical, quality and sensory attributes of coffee-like powder and brew from defatted palm date seeds. Foods 2019, 8, 61. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L. Prediction of the shelf-life of date seeds brew by integration of acceptability and quality indices. J. Food Meas. Charact. 2020, 14, 1158–1171. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Dupak, R.; Kalafova, A.; Schneidgenova, M.; Ivanisova, E.; Brindza, J.; Capcarova, M. Antioxidant activity and hypoglycaemic effect of cornelian cherry stone in diabetic rats. Sci. Pap. Anim. Sci. Biotechnol. 2020, 53, 164. [Google Scholar]
- Akalın, M.K.; Tekin, K.; Karagöz, S. Hydrothermal liquefaction of cornelian cherry stones for bio-oil production. Bioresour. Technol. 2012, 110, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Jakovljević, M.; Moslavac, T.; Bilić, M.; Aladić, K.; Bakula, F.; Jokić, S. Supercritical CO2 extraction of oil from rose hips (Rosa canina L.) and cornelian cherry (Cornus mas L.) seeds. Croat. J. Food Sci. Technol. 2018, 10, 197–205. [Google Scholar] [CrossRef]
- Cho, A.R.; Park, K.W.; Kim, K.M.; Kim, S.Y.; Han, J. Influence of roasting conditions on the antioxidant characteristics of colombian coffee (Coffea arabica L.). Beans. J. Food Biochem. 2014, 38, 271–280. [Google Scholar] [CrossRef]
- Cämmerer, B.; Kroh, L.W. Antioxidant activity of coffee brews. Eur. Food Res. Technol. 2006, 223, 469–474. [Google Scholar] [CrossRef]
- Wong, C.W.; Wijayanti, H.B.; Bhandari, B.R. Maillard reaction in limited moisture and low water activity environment. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Springer: Berlin/Heidelberg, Germany, 2015; pp. 41–63. [Google Scholar] [CrossRef]
- Votavova, L.; Voldřich, M.; Ševčík, R.; Čížková, H.; Mlejnecka, J.; Stolař, M.; Fleišman, T. Changes of antioxidant capacity of robusta coffee during roasting. Czech J. Food Sci. 2009, 27, S49–S52. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Piórecki, N.; Sozański, T. The health-promoting quality attributes, polyphenols, iridoids and antioxidant activity during the development and ripening of cornelian cherry (Cornus mas L.). Antioxidants 2024, 13, 229. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E.; Karpowicz, D.; Leśniewska, B. Antioxidant properties of coffee substitutes rich in polyphenols and minerals. Food Chem. 2019, 278, 101–109. [Google Scholar] [CrossRef]
- Murkovic, M.; Bornik, M.A. Formation of 5-hydroxymethyl-2-furfural (HMF) and 5-hydroxymethyl-2-furoic acid during roasting of coffee. Mol. Nutr. Food Res. 2007, 51, 390–394. [Google Scholar] [CrossRef]
- Murkovic, M.; Pichler, N. Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine. Mol. Nutr. Food Res. 2006, 50, 842–846. [Google Scholar] [CrossRef]
- Karaaslan, M.G.; Karaaslan, N.M.; Burhan, A.T.E.S. Investigation of mineral components and antioxidant properties of a healthy red fruit: Cornelian cherry (Cornus mas L.). J. Turk. Chem. Soc. A Chem. 2018, 5, 1319–1326. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Zia-Ul-Haq, M.; Bojovic, B.M.; Topuzovic, M.D. Total phenolics, flavonoid content and antioxidant power of leaf, flower and fruits from cornelian cherry (Cornus mas L.). Bulg. J. Agric. Sci. 2014, 20, 358–363. [Google Scholar]
- Gillani, F.; Raftani Amiri, Z.; Esmaeilzadeh Kenari, R. Assay of antioxidant activity and bioactive compounds of cornelian cherry (Cornus mas L.) fruit extracts obtained by green extraction methods: Ultrasound-assisted, supercritical fluid, and subcritical water extraction. Pharm. Chem. J. 2022, 56, 692–699. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]

| Roasting Parameters | Loganic Acid [mg/100 g] | Gallic Acid [mg/100 g] | Elagic Acid [mg/100 g] | HMF [mg/100 g] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature [°C] | Time [min] | ||||||||||||
| Solvent | Water | Methanol | Water | Methanol | Water | Methanol | Water | Methanol | |||||
| 50% | 80% | 50% | 80% | 50% | 80% | 50% | 80% | ||||||
| Unroasted | 185.44 ± 15.26 a*; A** | 137.27 ± 4.51 e; B | 120.84 ± 8.19 d; B | 182.15 ± 61.56 f; A | 158.46 ± 3.47 g; A | 152.72 ± 25.39 k; A | 204.32 ± 16.15 f; B | 182.00 ± 8.94 k; B | 362.73 ± 98.40 j; A | nd | nd | nd | |
| 160 | 20 | 139.93 ±3.68 c; B | 144.88 ± 5.22 e; AB | 157.85 ± 2.53 c; A | 245.89 ± 6.78 e; B | 419.79 ± 52.94 e; A | 510.33 ± 3.72 h; A | 653.68 ± 0.65 e; B | 717.83 ± 41.04 j; B | 1685.59 ± 27.76 i; A | 48.74 ± 1.36 h; C | 61.99 ± 4.09 j; B | 81.84 ± 0.47 e; A |
| 30 | 178.10 ±1.22 a; C | 188.95 ± 2.22 a; B | 210.06 ± 2.89 ab; A | 600.19 ± 4.00 b; B | 834.10 ± 14.62 b; B | 873.63 ± 35.49 e; A | 1574.53 ± 73.62 ab; C | 2584.16 ± 82.58 gh; B | 5007.34 ± 119.97 g; A | 164.49 ± 0.31 b; C | 187.84 ± 0.04 c; B | 223.10 ± 25.77 a; A | |
| 40 | 158.59 ± 1.09 b; C | 183.69 ± 0.89 a; B | 204.58 ± 5.83 a; A | 705.78 ± 0.11 a; C | 905.16 ± 11.80 a; B | 977.45 ± 17.81 d; A | 1471.13 ± 15.88 b; C | 4369.54 ± 81.82 f; B | 7111.10 ± 352.69 f; A | 149.26 ± 0.21 c; B | 196 69 ± 4.25 b; A | 199.75± 6.62 b; A | |
| 50 | 123.15 ± 2.4 d; B | 151.11 ± 0.13 c; A | 155.04 ± 2.11 c; A | 722.92 ± 4.42 a; C | 901.50 ± 18.09 a; B | 1201.55 ± 14.37 a; A | 1252.14 ± 0.82 c; C | 5855.42 ± 39.75 a; B | 8731.58 ± 92.47 de; A | 133.04 ± 3.43 d; B | 159.03 ± 5.05 e; A | 165.99 ± 0.59 c; A | |
| 180 | 20 | 173.52 ±3.19 ab; C | 189.56 ± 3.25 a; B | 212.54 ± 5.51 a; A | 579.86 ± 3.43 b; C | 824.49 ± 1.61 b; B | 891.79 ± 1.02 e; A | 1631.29 ± 51.83 a; B | 2328.62 ± 127.98 h; A | 2467.13 ± 219.04 hi; A | 147.27 ± 4.59 c; C | 172.57 ± 3.52 d; B | 188.12 ± 1.05 b; A |
| 30 | 121.82 ± 0.45 d; C | 145.36 ± 3.11 d; B | 152.61 ± 084 c; A | 723.91 ± 4.94 a; C | 903.55 ± 27.79 a; B | 1171.85 ± 7.60 ab; A | 1087.13 ± 51.84 d; C | 4822.74. ± 186.94 cd; B | 8043.72 ± 629.74 ef; A | 170.23 ± 0.16 b; B | 198.16 ± 9.65 b; A | 198.28 ± 9.39 b; A | |
| 40 | 77.48 ± 1.69 e; A | 73.42 ± 0.38 g; B | 70.08 ± 0.23 f; C | 669.67 ± 5.33 a; C | 806.96 ± 16.66 b; B | 1043.56 ± 23.90 c; A | 311.88 ± 0.01 f; C | 5065.98 ± 77.04 b; B | 10381.01 ± 188.72 bc; A | 100.27 ± 0.60 f; B | 115.14 ± 5.11 g; A | 122.10 ± 3.25 d; A | |
| 50 | 49.59 ±0.10 f; A | 47.8 ± 0.71 h; A | 50.84 ± 2.82 g; A | 512.17 ± 15.41 c; C | 690.10 ± 10.61 c; B | 770.39 ± 19.70 f; A | 70.00 ± 6.18 g; C | 4643.29 ± 111.74 de; B | 11571.11 ± 59.18 a; A | 62.63 ± 2.70 g; C | 86.91 ± 1.15 h; A | 78.76 ± 0.52 e; A | |
| 200 | 20 | 137.40 ± 12.10 c; B | 156.43 ± 0.13 b; B | 184.13 ± 1.88 b; A | 714.16 ± 35.96 a; C | 899.81 ± 17.03 a; B | 1136.86 ± 11.43 b; A | 1213.88 ± 240.17 c; C | 4442.17 ± 135.84 ef; B | 7981.32 ± 247.81 ef; A | 181.36 ± 12.14 a; B | 214.05 ± 4.47 a; A | 203.47 ± 0.00 ab; A |
| 30 | 70.32 ± 3.07 e; B | 72.16 ± 0.75 g; B | 87.53 ± 5.88 e; A | 662.56 ± 8.84 a; C | 823.79 ± 8.82 b; B | 1033.77 ± 63.74 c; A | 196.83 ± 7.45 f; C | 5130.71 ± 108.77 b; B | 11586.11 ± 770.74 a; A | 111.30 ± 0.08 e; B | 132.29 ± 5.31 f; A | 135.51 ± 9.82 d; A | |
| 40 | 42.05 ± 1.05 fg; B | 41.11 ± 1.47 i; B | 52.74 ± 1.07 g; A | 405.11 ± 2.94 d; C | 551.49 ± 23.00 d; B | 609.31 ± 5.36 g; A | 45.69 ± 3.75 g; C | 2735.57 ± 205.5 g; B | 11072.61 ± 63.73 ab; A | 62.64 ± 0.28 g; B | 82.65 ± 1.96 hi; A | 44.93 ± 31.77 f; A | |
| 50 | 18.23 ± 0.09 hi; AB | 15.38 ± 0.91 k; B | 23.01 ± 2.70 i; A | 13383 ± 0.01 f; C | 203.93 ± 4.48 f; B | 229.33 ±1.58 j; A | 21.42 ± 7.75 g; C | 2801.01 ±50.58 g; B | 9800.27 ± 545.69 cd; A | 24.29 ± 1.33 i; B | 40.0 ± 0.08 k; A | 0.00± 0.00 g; A | |
| 220 | 20 | 76.09 ±0.50 e; C | 85.30 ± 0.19 f; B | 96.31 ± 2.41 e: A | 682.30 ± 15.03 a; C | 824 ± 1.39 b; B | 883.80 ± 10.41 e; A | 220.22 ± 38.89 f; C | 4895.07 ± 2.10 bc; B | 9700.54 ± 678.54 cd; A | 132.52 ± 0.82 d; B | 153.05 ± 0.30 e; A | 155.31 ± 3.10 c; A |
| 30 | 29.74 ±2.12 gh; B | 31.30 ± 0.01 j; B | 38.22 ± 2.81 h; A | 279.65 ± 4.33 e; C | 386.30 ± 0.28 e; B | 421.02 ± 3.92 i; A | 46.36 ± 4.10 g; C | 2809.72 ± 156.44 g; B | 4755.82 ± 261.44 g; A | 54.84 ± 1.40 h; B | 75.5 ± 0.17 i; A | 78.03 ± 1.15 e; A | |
| 40 | 11.76 ± 1.27 ij; C | 13.39 ± 0.15 k; B | 17.16 ± 4.94 i; A | 60.24 ± 7.70 g; C | 96.10 ± 2.90 h; B | 123.56 ± 0.73 k; A | 28.32 ± 6.57 g; C | 1446.77 ± 125.13 i; B | 3155.82 ± 433.42 h; A | 15.57 ± 2.34 j; B | 33.35 ± 2.45 k; A | 39.04 ± 0.75 f; A | |
| 50 | 4.91 ± 0.07 j; C | 12.37 ± 0.15 k; B | 18.30 ± 0.24 i; A | 0.00 ± 0.00 h; B | 0.15 ± 0.09 i; B | 6.93 ± 2.22 l; A | 17.47 ± 2.40 g; C | 658.41 ± 4.60 j; B | 1978.86 ± 121.40 i; A | 0.00 ± 0.00 k; B | 5.52 ± 0.75 l; A | 0.00 ± 0.00 g; B | |
| Roasting Parameters | Total Phenolic Content [mg GEA/g] | ABTS [µmol TE/g] | FRAP [µmol TE/g] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature [°C] | Time [min] | |||||||||
| Solvent | Water | Methanol | Water | Methanol | Water | Methanol | ||||
| 50% | 80% | 50% | 80% | 50% | 80% | |||||
| Unroasted | 3939.10 ± 66.66 a; A | 3438.7 ± 156.63 cd; A | 4159.5 ± 135.16 ab; A | 353.20 ± 0.55 a; A | 360.3 ± 0.39 e; A | 421.1 ± 4.26 j; A | 273.7 ± 1.01 g; A | 286.0 ± 1.16 c; A | 334.1 ± 4.58 i; A | |
| 160 | 20 | 2445.38 ± 166.14 b; C | 3553.50 ± 150.97 bc; B | 3952.00 ± 169.55 bc; A | 289.43 ± 82.49 ab; C | 509.26 ± 37.02 b; B | 1214.84 ± 4.27 a; A | 933.47 ± 38.78 a; A | 339.81 ± 20.90 b; B | 792.60 ± 12.08 bc; B |
| 30 | 1148.76 ± 14.80 d; C | 3896.00 ± 84.76 ab; B | 4299.50 ± 71.18 ab; A | 238.34 ± 58.82 bc; C | 522.73 ± 5.52 b; B | 1173.31 ± 0.36 ab; A | 829.68 ± 9.99 b; A | 357.87 ± 19.51 b; B | 826.76 ± 3.72 ab; A | |
| 40 | 670.56 ± 85.99 e; C | 3787.00 ± 235.86 abc; B | 4402.00 ± 127.23 a; A | 173.84 ± 40.58 cd; C | 515.18 ± 3.03 b; B | 1205.28 ± 24.92 a; A | 768.92 ± 27.17 c; A | 355.58 ± 16.72 b; B | 817.57 ± 4.64 ab; A | |
| 50 | 159.84 ± 40.22 fgh; C | 2769.90 ± 613.83 f; B | 4196.00 ± 145.40 ab; A | 140.69 ± 5.54 de; C | 442.32 ± 21.36 c; B | 1055.78 ± 43.42 cd; A | 655.11 ± 12.08 d; B | 296.62 ± 9.99 c; C | 793.92 ± 10.22 bc; A | |
| 180 | 20 | 1691.05 ± 55.20 c; C | 3985.00 ± 285.66 a; B | 4232.00 ± 113.49 ab; A | 171.74 ± 45.91 cd; C | 582.63 ± 18.33 a; B | 1233.47 ± 35.59 a; A | 432.90 ± 4.53 e; B | 385.63 ± 8.59 a; C | 878.33 ± 4.18 a; A |
| 30 | 1115.25 ± 134.50 d; C | 3044.30 ± 181.31 ef; B | 3989.5 ± 146.47 bc; A | 111.96 ± 12.19 def; C | 415.77 ± 26.16 cd; B | 1024.07 ± 47.69 cd; A | 308.10 ± 1.74 f; B | 283.98 ± 17.19 c; B | 705.57 ± 63.63 de; A | |
| 40 | 665.75 ± 112.37 f; C | 2142.10 ± 92.42 g; B | 3730.00 ±146.07 cd; A | 55.02 ± 5.88 fg; C | 302.64 ± 13.88 f; B | 942.02 ± 15.66 de; A | 217.04 ± 2.55 h; C | 227.65 ± 1.16 d; B | 626.41 ± 2.79 fg; A | |
| 50 | 205.27 ± 43.33 fgh; C | 1615.45 ± 81.50 hi; B | 3105.5 ± 95.37 fg; A | 41.59 ± 0.40 fg; C | 245.13 ± 20.11 g; B | 756.28 ± 14.95 gh; A | 151.02 ± 5.34 ij; B | 204.16 ± 12.08 e; B | 461.21 ± 28.33 h; A | |
| 200 | 20 | 694.12 ± 31.00 e; C | 3178.00 ± 58.96 de; B | 4100.00 ± 154.69 ab; A | 66.43 ± 36.31 efg; C | 394.94 ± 8.90 d; B | 1087.99 ± 66.91 bc; A | 174.86 ± 21.95 i; C | 299.89 ± 3.37 c; B | 724.29 ± 39.02 cd; A |
| 30 | 430.05 ± 58.41 efg; C | 2296.65 ± 132.68 g; B | 3494.00 ± 221.18 de; A | 56.45 ± 7.07 fg; C | 299.24 ± 2.05 f; B | 881.62 ± 12.81 ef; A | 112.05 ± 3.25 k; C | 232.31 ± 0.23 d; B | 637.83 ± 70.52 ef; A | |
| 40 | 206.10 ± 91.29 fgh; C | 1279.85 ± 99.47 ij; B | 2927.00 ± 125.43 fg; A | 37.52 ± 2.34 fg; C | 203.16 ± 3.92 h; B | 650.83 ± 3.20 hi; A | 62.70 ± 0.70 l; C | 158.98 ± 1.97 f; B | 560.07 ± 0.93 fg; A | |
| 50 | 27.20 ± 18.11 h; C | 1109.25 ± 81.79 jk; B | 2484.7 ± 113.10 hi; A | 30.04 ± 1.45 g; C | 145.22 ± 2.76 ij; B | 469.11 ± 117.81 j; A | 39.46 ± 6.62 mL; B | 115.06 ± 8.13 g; B | 273.01 ± 73.9 i; A | |
| 220 | 20 | 494.14 ± 133.49 efg; C | 1938.40 ± 226.20 gh; B | 3237.50 ± 154.19 ef; A | 69.46 ± 16.39 efg; C | 304.65 ± 4.18 f; B | 821.72 ± 4.98 fg; A | 127.98 ± 7.55 jk; C | 243.64 ± 0.46 d; B | 556.13 ± 5.57 h; A |
| 30 | 265.89 ± 33.93 fgh; C | 1089.95 ± 68.75 jk; B | 2783.1 ± 167.17 gh; A | 40.21 ± 1.09 fg; C | 175.73 ± 0.18 hi; B | 622.89 ± 11.39 i; A | 52.81 ± 23.86 l; B | 137.80 ± 1.51 f; B | 413.26 ± 41.34 h; A | |
| 40 | 99.50 ± 33.00 gh; C | 948.75 ± 46.51 jk; B | 2380.90 ± 85.25 i; A | 32.95 ± 15.57 fg; C | 131.69 ± 0.36 j; B | 500.07 ± 135.96 j; A | 36.10 ± 1.31 mL; C | 97.48 ± 0.46 gh; B | 296.00 ± 10.22 i; A | |
| 50 | 30.58 ± 14.69 h; C | 864.90 ± 65.56 k; B | 2237.80 ± 126.82 i; A | 23.11 ± 5.56 g; C | 100.16 ± 2.40 k; B | 448.98 ± 21.0 j; A | 19.76 ± 1.39 m; C | 84.10 ± 2.67 h; B | 261.52 ± 6.97 i; A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spychaj, R.; Przybylska, D.; Szachniewicz, M.; Piórecki, N.; Kucharska, A.Z. Selection of Roasting Conditions in the Valorization Process of Cornelian Cherry Stones. Molecules 2025, 30, 2900. https://doi.org/10.3390/molecules30142900
Spychaj R, Przybylska D, Szachniewicz M, Piórecki N, Kucharska AZ. Selection of Roasting Conditions in the Valorization Process of Cornelian Cherry Stones. Molecules. 2025; 30(14):2900. https://doi.org/10.3390/molecules30142900
Chicago/Turabian StyleSpychaj, Radosław, Dominika Przybylska, Małgorzata Szachniewicz, Narcyz Piórecki, and Alicja Z. Kucharska. 2025. "Selection of Roasting Conditions in the Valorization Process of Cornelian Cherry Stones" Molecules 30, no. 14: 2900. https://doi.org/10.3390/molecules30142900
APA StyleSpychaj, R., Przybylska, D., Szachniewicz, M., Piórecki, N., & Kucharska, A. Z. (2025). Selection of Roasting Conditions in the Valorization Process of Cornelian Cherry Stones. Molecules, 30(14), 2900. https://doi.org/10.3390/molecules30142900







