New 3D Spiral Microfluidic Platform Tested for Fe3O4@SA Nanoparticle Synthesis
Abstract
1. Introduction
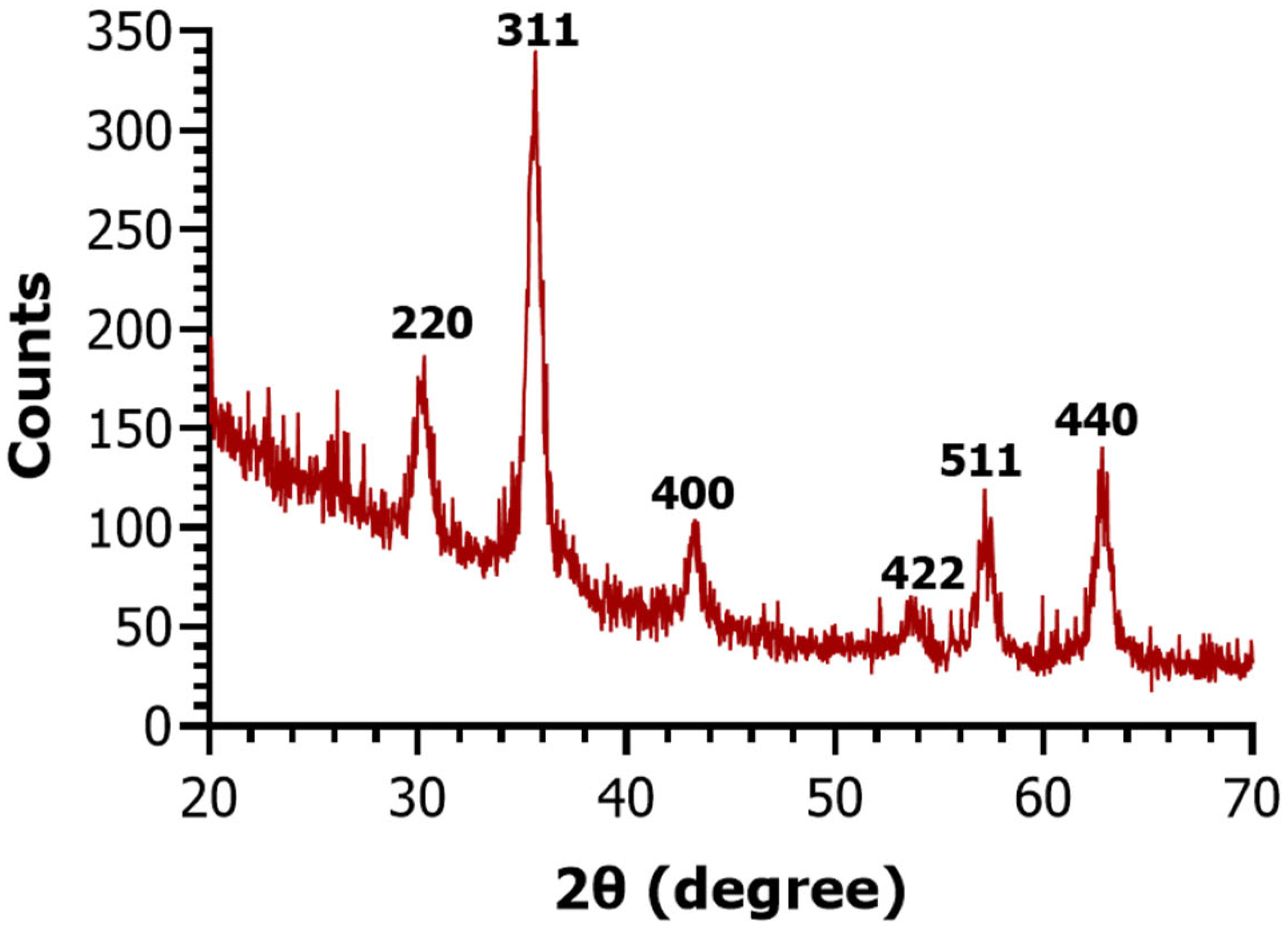
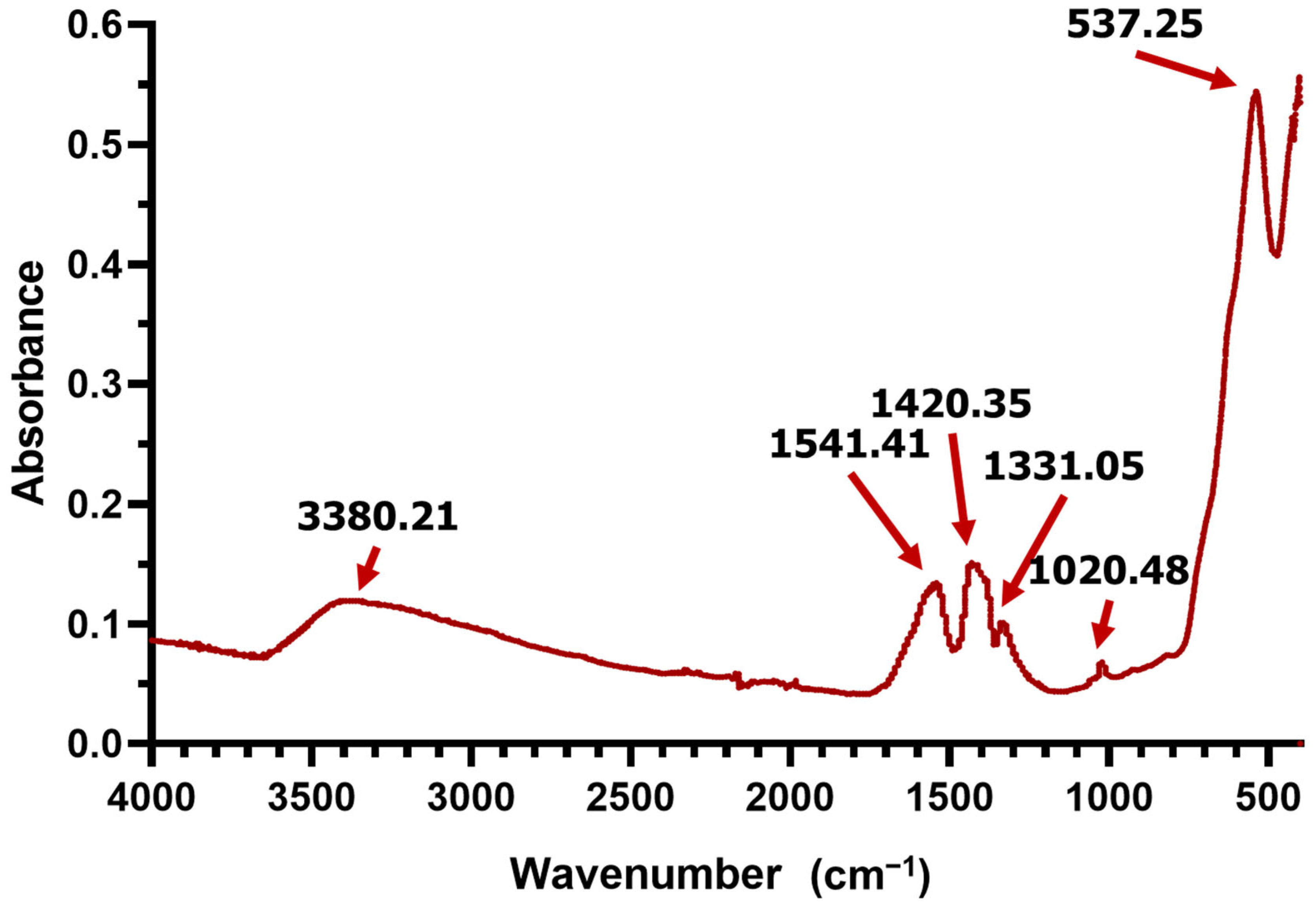
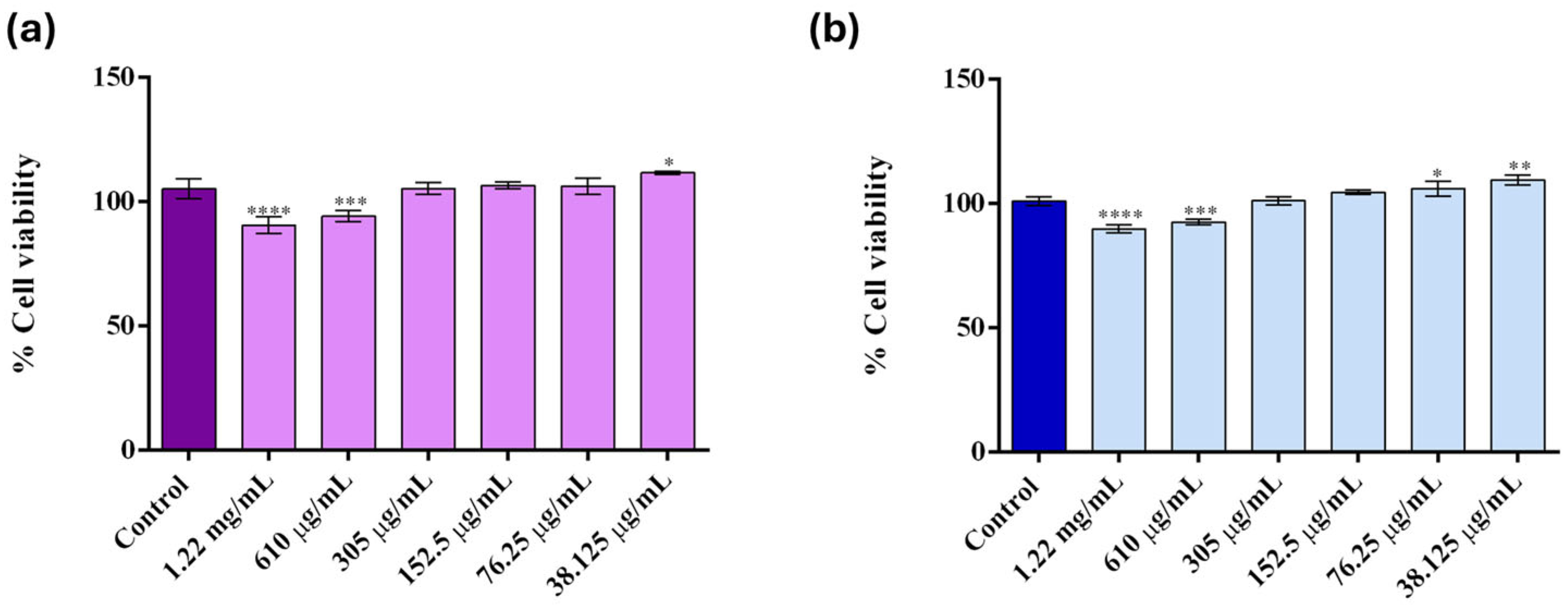
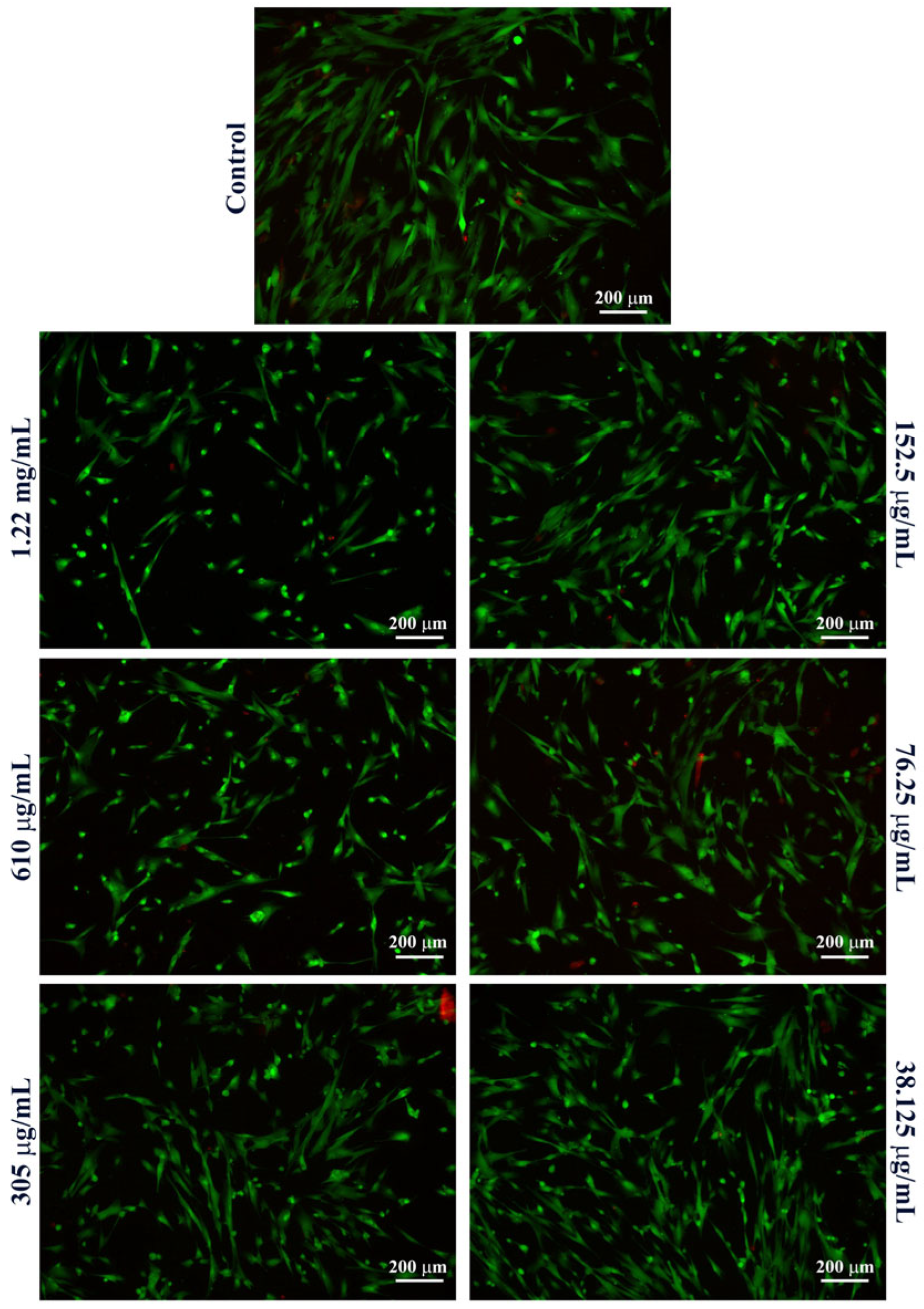
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
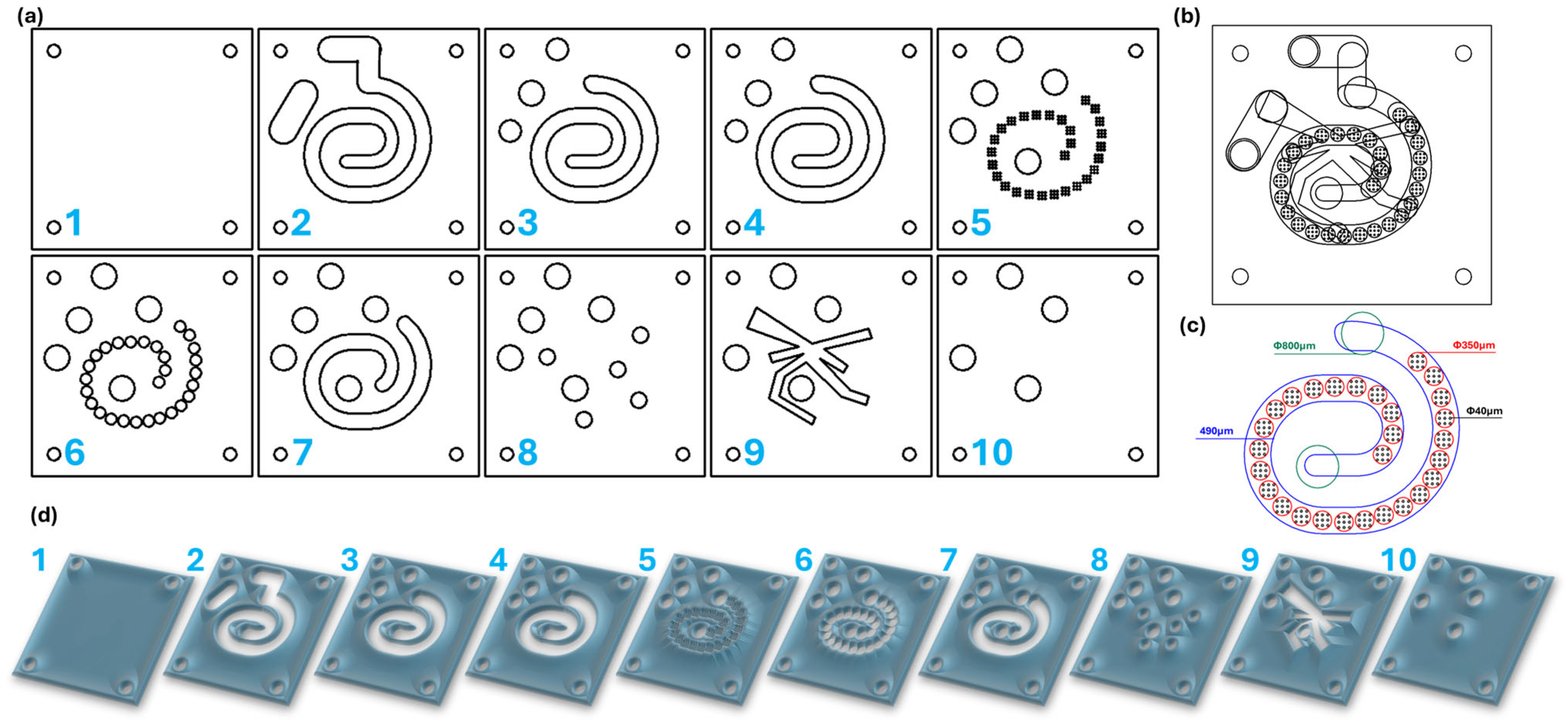
4.2. Microfluidic Platform Design
4.3. Microfluidic Platform Fabrication
4.4. Fe3O4@SA Nanoparticle Synthesis
4.5. Nanoparticle Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2023, 13, 41. [Google Scholar] [CrossRef]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.O.d. Overview of Nanomaterials and Cellular Interactions. Biointerface Res. Appl. Chem. 2023, 13, 367. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lai, Y.-P.; Wu, K.-Y. A high-productivity process for mass-producing Fe3O4 nanoparticles by co-precipitation in a rotating packed bed. Powder Technol. 2022, 395, 369–376. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, B.; Chen, D.; Li, Y.; Feng, C.; Du, W. Mechanochemistry Strategy in Metal/Fe3O4 with High Stability for Superior Chemoselective Catalysis. ACS Appl. Mater. Interfaces 2024, 16, 66219–66229. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Wu, Y.; Zong, B.; Ding, J. Size-dependent microwave absorption properties of Fe3O4 nanodiscs. RSC Adv. 2016, 6, 25444–25448. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, É.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Adv. Colloid Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A. Chapter 11—Multimodal applications of phytonanoparticles. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–219. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kwatra, N.; Abraham, J. Chapter 8—Nanoparticles fabrication by plant extracts. In Phytonanotechnology; Thajuddin, N., Mathew, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–157. [Google Scholar]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Fahmy, A.; Zaid, H.; Ibrahim, M. Optimizing the electrospun parameters which affect the preparation of nanofibers. Biointerface Res. Appl. Chem. 2019, 9, 4463–4473. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; Arenas-Alatorre, J.Á. Green synthesis of nanoparticles: A biological approach. In Green Chemistry for Environmental Sustainability—Prevention-Assurance-Sustainability (P-A-S) Approach; IntechOpen: London, UK, 2023. [Google Scholar]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Singh, H.; Desimone, M.F.; Pandya, S.; Jasani, S.; George, N.; Adnan, M.; Aldarhami, A.; Bazaid, A.S.; Alderhami, S.A. Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications. Int. J. Nanomed. 2023, 18, 4727–4750. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; El-Monaem, E.M.A.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Mou, J.; Wang, C.; Zhao, H.; Xiong, C.; Ren, Y.; Wang, J.; Jiang, D.; Zheng, Z. Centrifugal Microfluidic Synthesis of Nickel Sesquioxide Nanoparticles. Micromachines 2023, 14, 1741. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Mihaiescu, B.; Bîrcă, A.C.; Moroșan, A.; Munteanu, O.M.M.; Vasile, B.Ș.; Hadibarata, T.; Istrati, D.; Mihaiescu, D.E.; Grumezescu, A.M. Fabrication and Advanced Imaging Characterization of Magnetic Aerogel-Based Thin Films for Water Decontamination. Gels 2024, 10, 394. [Google Scholar] [CrossRef]
- Reznik, I.; Kolesova, E.; Pestereva, A.; Baranov, K.; Osin, Y.; Bogdanov, K.; Swart, J.; Moshkalev, S.; Orlova, A. Synthesis of Submicron CaCO3 Particles in 3D-Printed Microfluidic Chips Supporting Advection and Diffusion Mixing. Micromachines 2024, 15, 652. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Mihaiescu, D.E.; Grumezescu, A.M. A Review of Microfluidic Experimental Designs for Nanoparticle Synthesis. Int. J. Mol. Sci. 2022, 23, 8293. [Google Scholar] [CrossRef]
- Chircov, C.; Dumitru, I.A.; Vasile, B.S.; Oprea, O.-C.; Holban, A.M.; Popescu, R.C. Microfluidic Synthesis of Magnetite Nanoparticles for the Controlled Release of Antibiotics. Pharmaceutics 2023, 15, 2215. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Caimi, A.; Bellotti, M.; Giglio, A.; Conti, B.; Dorati, R.; Auricchio, F.; Genta, I. Effect of Micromixer Design on Lipid Nanocarriers Manufacturing for the Delivery of Proteins and Nucleic Acids. Pharmaceutics 2024, 16, 507. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Abaee, M.; Sohrabi, S.; Moraveji, M.K. CFD assisted engineering of spiral microfluidic reactors for room temperature synthesis of ZnO nanoparticles. Ceram. Int. 2024, 50, 32613–32623. [Google Scholar] [CrossRef]
- Saravanakumar, S.M.; Cicek, P.-V. Microfluidic Mixing: A Physics-Oriented Review. Micromachines 2023, 14, 1827. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Pankin, I.; Polyakov, V.; Khodakova, D.; Medvedev, P.; Zelenikhin, P.; Shamsutdinov, N.; Chapek, S.; Goncharova, A.; Soldatov, A. Single-Stage Microfluidic Synthesis Route for BaGdF5:Tb3+-Based Nanocomposite Materials: Synthesis, Characterization and Biodistribution. Int. J. Mol. Sci. 2023, 24, 17159. [Google Scholar] [CrossRef]
- Zardi, P.; Carofiglio, T.; Maggini, M. Mild Microfluidic Approaches to Oxide Nanoparticles Synthesis. Chem.—A Eur. J. 2021, 28, e202103132. [Google Scholar] [CrossRef]
- Yang, L.; Xu, F.; Chen, G. Effective mixing in a passive oscillating micromixer with impinging jets. Chem. Eng. J. 2024, 489, e151329. [Google Scholar] [CrossRef]
- Vasilescu, S.A.; Bazaz, S.R.; Jin, D.; Shimoni, O.; Warkiani, M.E. 3D printing enables the rapid prototyping of modular microfluidic devices for particle conjugation. Appl. Mater. Today 2020, 20, e100726. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, e094001. [Google Scholar] [CrossRef]
- Salieb-Beugelaar, G.B.; Gonçalves, D.; Wolf, M.P.; Hunziker, P. Microfluidic 3D Helix Mixers. Micromachines 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Munteanu, O.M.M.; Bîrcă, A.C.; Moroșan, A.; Purcăreanu, B.; Vasile, B.Ș.; Istrati, D.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. New 3D Vortex Microfluidic System Tested for Magnetic Core-Shell Fe3O4-SA Nanoparticle Synthesis. Nanomaterials 2024, 14, 902. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Niculescu, A.-G.; Bîrcă, A.C.; Cristea, D.-E.; Moroșan, A.; Tudorache, D.-I.; Purcăreanu, B.; Vasile, B.Ș.; Radu, D.; Grigoroscuta, M.A.; et al. Vortex-Mixing Microfluidic Fabrication of Micafungin-Loaded Magnetite–Salicylic Acid–Silica Nanocomposite with Sustained-Release Capacity. Materials 2024, 17, 5816. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Moroșan, A.; Bîrcă, A.C.; Gherasim, O.; Oprea, O.C.; Vasile, B.Ș.; Purcăreanu, B.; Mihaiescu, D.E.; Rădulescu, M.; Grumezescu, A.M. Microwave-Assisted Silanization of Magnetite Nanoparticles Pre-Synthesized by a 3D Microfluidic Platform. Nanomaterials 2023, 13, 2795. [Google Scholar] [CrossRef]
- Mihaiescu, D.E.; Buteică, A.S.; Neamţu, J.; Istrati, D.; Mîndrilă, I. Fe3O4/Salicylic acid nanoparticles behavior on chick CAM vasculature. J. Nanoparticle Res. 2013, 15, 1857. [Google Scholar] [CrossRef]
- Mishra, A.K.; Baek, K.-H. Salicylic acid biosynthesis and metabolism: A divergent pathway for plants and bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Mikhnavets, L.; Abashkin, V.; Khamitsevich, H.; Shcharbin, D.; Burko, A.; Krekoten, N.; Radziuk, D. Ultrasonic Formation of Fe3O4-Reduced Graphene Oxide–Salicylic Acid Nanoparticles with Switchable Antioxidant Function. ACS Biomater. Sci. Eng. 2022, 8, 1181–1192. [Google Scholar] [CrossRef]
- Shishehbore, M.R.; Afkhami, A.; Bagheri, H. Salicylic acid functionalized silica-coated magnetite nanoparticles for solid phase extraction and preconcentration of some heavy metal ions from various real samples. Chem. Cent. J. 2011, 5, 41. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, D.-I.T.; Niculescu, A.-G.; Bîrcă, A.-C.; Florea, D.A.; Rădulescu, M.; Vasile, B.-Ș.; Trușcă, R.; Mihaiescu, D.-E.; Hadibarata, T.; Grumezescu, A.-M. Microfluidic Synthesis of Magnetic Silica Aerogels for Efficient Pesticide Removal from Water. Gels 2025, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Kresnik, L.; Majerič, P.; Feizpour, D.; Črešnar, K.P.; Rudolf, R. Hybrid Nanostructures of Fe3O4 and Au Prepared via Coprecipitation and Ultrasonic Spray Pyrolysis. Metals 2024, 14, 1324. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Surya, R.; Filser, S.; Wimmer, A.; Weigl, F.; Fraga-García, P.; Berensmeier, S. Formation of iron oxide nanoparticles for the photooxidation of water: Alteration of finite size effects from ferrihydrite to hematite. Sci. Rep. 2017, 7, 12609. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Spoiala, A.; Chircov, C.; Dolete, G.; Oprea, O.-C.; Vasile, B.-S.; Crainiceanu, S.A.; Nicoara, A.-I.; Marinas, I.C.; Stan, M.S.; et al. Antioxidant, Antitumoral, Antimicrobial, and Prebiotic Activity of Magnetite Nanoparticles Loaded with Bee Pollen/Bee Bread Extracts and 5-Fluorouracil. Antioxidants 2024, 13, 895. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Microfluidic Flow Synthesis of Functional Mesoporous Silica Nanofibers with Tunable Aspect Ratios. ACS Sustain. Chem. Eng. 2018, 6, 1522–1526. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Gong, J.; Ma, J. Microfluidic Fabrication of Monodisperse Microcapsules for Thermo-Triggered Release of Liposoluble Drugs. Polymers 2020, 12, 2200. [Google Scholar] [CrossRef]
- Campaña, A.L.; Sotelo, D.C.; Oliva, H.A.; Aranguren, A.; Ornelas-Soto, N.; Cruz, J.C.; Osma, J.F. Fabrication and characterization of a low-cost microfluidic system for the manufacture of alginate–lacasse microcapsules. Polymers 2020, 12, 1158. [Google Scholar] [CrossRef]
- Duran, M.; Serrano, A.; Nikulin, A.; Dauvergne, J.-L.; Derzsi, L.; del Barrio, E.P. Microcapsule production by droplet microfluidics: A review from the material science approach. Mater. Des. 2022, 223, 111230. [Google Scholar] [CrossRef]
- Huang, L.; Wu, K.; He, X.; Yang, Z.; Ji, H. One-Step microfluidic synthesis of spherical and bullet-like alginate microcapsules with a core–shell structure. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 608, 125612. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, H.; Li, X.; Li, F.; Joo, S.W. Generation and Dynamics of Janus Droplets in Shear-Thinning Fluid Flow in a Double Y-Type Microchannel. Micromachines 2021, 12, 149. [Google Scholar] [CrossRef]
- Zeng, C.; Li, J.; Gong, J.; Zhang, L.; Yu, L. Synthesis of ZIF-8/PVA microspheres with the assistance of a microfluidic device and their controlled drug release properties. Microporous Mesoporous Mater. 2024, 376, 113187. [Google Scholar] [CrossRef]
- Lashkaripour, A.; McIntyre, D.P.; Calhoun, S.G.K.; Krauth, K.; Densmore, D.M.; Fordyce, P.M. Design automation of microfluidic single and double emulsion droplets with machine learning. Nat. Commun. 2024, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y. Microencapsulation of solid cores to prepare double emulsion droplets by microfluidics. Int. J. Heat Mass Transf. 2019, 135, 158–163. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Limiti, E.; Mozetic, P.; Pinelli, F.; Han, X.; Abbruzzese, F.; Basoli, F.; Del Rio, D.; Scialla, S.; Rossi, F.; et al. Droplet-based microfluidic synthesis of nanogels for controlled drug delivery: Tailoring nanomaterial properties via pneumatically actuated flow-focusing junction. Nanoscale 2022, 14, 11415–11428. [Google Scholar] [CrossRef]
- Mutlu, B.; Farhan, M.; Kucuk, I. T-Shaped Microfluidic Junction Processing of Porous Alginate-Based Films and Their Characteristics. Polymers 2019, 11, 1386. [Google Scholar] [CrossRef]
- Xia, H.; Li, A.; Man, J.; Li, J.; Li, J. Fabrication of Multi-Layered Microspheres Based on Phase Separation for Drug Delivery. Micromachines 2021, 12, 723. [Google Scholar] [CrossRef]
- Chung, C.H.Y.; Cui, B.; Song, R.; Liu, X.; Xu, X.; Yao, S. Scalable Production of Monodisperse Functional Microspheres by Multilayer Parallelization of High Aspect Ratio Microfluidic Channels. Micromachines 2019, 10, 592. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Yang, C.-H.; Wu, C.-T.; Grumezescu, A.M.; Wang, C.-Y.; Hsieh, W.-C.; Chen, S.-Y.; Huang, K.-S. A Microfluidic Chip Using Phenol Formaldehyde Resin for Uniform-Sized Polycaprolactone and Chitosan Microparticle Generation. Molecules 2013, 18, 6521–6531. [Google Scholar] [CrossRef]
- Zou, L.; Huang, B.; Zheng, X.; Pan, H.; Zhang, Q.; Xie, W.; Zhao, Z.; Li, X. Microfluidic synthesis of magnetic nanoparticles in droplet-based microreactors. Mater. Chem. Phys. 2022, 276, 125384. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S.; et al. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. Int. J. Mol. Sci. 2023, 24, 11466. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Vasile, B.S.; Oprea, O.-C.; Huang, K.-S.; Grumezescu, A.M. Microfluidic Synthesis of -NH2- and -COOH-Functionalized Magnetite Nanoparticles. Nanomaterials 2022, 12, 3160. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Nozaki, Y.; Tanaka, D.; Sekiguchi, T.; Shoji, S. Integration of Horizontal and Vertical Microfluidic Modules for Core-Shell Droplet Generation and Chemical Application. Micromachines 2019, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Fan, M.; Wu, M.; Zhang, X.; Cheng, Y.; Xia, J.; Zhuang, Y.; Zhu, W.; Qian, X.; Bai, Y. Continuous-flow enzymatic synthesis of chiral lactones in a three-dimensional microfluidic reactor. Chin. Chem. Lett. 2023, 35, 108684. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Weisensee, K. Conducting Polymeric Nanocomposites with a Three-Dimensional Co-flow Microfluidics Platform. Micromachines 2019, 10, 383. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Yin, D.-F.; Chen, W.; Qi, J.; Hu, M.; Xu, J.; Cheng, Y. Real-time spectroscopic monitoring of continuous-flow synthesis of zinc oxide nano-structures in femtosecond laser fabricated 3D microfluidic microchannels with integrated on-chip fiber probe array. Lab Chip 2023, 23, 3785–3793. [Google Scholar] [CrossRef]
- Maier, M.C.; Valotta, A.; Hiebler, K.; Soritz, S.; Gavric, K.; Grabner, B.; Gruber-Woelfler, H. 3D Printed Reactors for Synthesis of Active Pharmaceutical Ingredients in Continuous Flow. Org. Process. Res. Dev. 2020, 24, 2197–2207. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Liu, H.; Li, Y.; Lang, T.; Wang, R.; Cui, B.; Zhu, W. Efficient Mixing of Microfluidic Chip with a Three-Dimensional Spiral Structure. ACS Omega 2021, 7, 1527–1536. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discov. Nano. 2023, 18, 18. [Google Scholar] [CrossRef]
- Lai, X.; Yang, M.; Wu, H.; Li, D. Modular Microfluidics: Current Status and Future Prospects. Micromachines 2022, 13, 1363. [Google Scholar] [CrossRef]
- Mishra, S.; Mondal, G.; Kumarasamy, M. Development of Low-Cost CNC-Milled PMMA Microfluidic Chips as a Prototype for Organ-on-a-Chip and Neurospheroid Applications. Organoids 2025, 4, 13. [Google Scholar] [CrossRef]
- Panatli, O.; Gurcan, C.; Ari, F.; Unal, M.A.; Yuksekkaya, M.; Yilmazer, A. Cost-Effective and Simple Prototyping PMMA Microfluidic Chip and Open-Source Peristaltic Pump for Small Volume Applications. Micro 2025, 5, 25. [Google Scholar] [CrossRef]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Foglia, S.; Ledda, M.; Fioretti, D.; Iucci, G.; Papi, M.; Capellini, G.; Lolli, M.G.; Grimaldi, S.; Rinaldi, M.; Lisi, A. In vitro biocompatibility study of sub-5 nm silica-coated magnetic iron oxide fluorescent nanoparticles for potential biomedical application. Sci. Rep. 2017, 7, 46513. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, Á.d.J.; Reyes-López, S.Y.; Méndez-Lozano, N.; Juárez-Moreno, K. Evaluation of Superparamagnetic Fe3O4-Ag Decorated Nanoparticles: Cytotoxicity Studies in Human Fibroblasts (HFF-1) and Breast Cancer Cells (MCF-7). Appl. Sci. 2024, 14, 6750. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ianăși, C.; Dudás, Z.; Coricovac, D.; Watz, C.; Len, A.; Almásy, L.; Sacarescu, L.; Dehelean, C. SiO2-PVA-Fe(acac)3 Hybrid Based Superparamagnetic Nanocomposites for Nanomedicine: Morpho-textural Evaluation and In Vitro Cytotoxicity Assay. Molecules 2020, 25, 653. [Google Scholar] [CrossRef]
- Attia, N.F.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Elashery, S.E.A.; Eltaweil, A.S.; El Kady, M.; Khalifa, S.A.M.; Hawash, H.B.; El-Seedi, H.R. Iron oxide nanoparticles and their pharmaceutical applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Tudorache, D.-I.T.; Niculescu, A.-G.; Mogoantă, L.; Mogoşanu, G.D.; Bîrcă, A.C.; Vasile, B.Ș.; Hudiță, A.; Voinea, I.C.; Stan, M.S.; et al. Antimicrobial Coatings Based on Hybrid Iron Oxide Nanoparticles. Nanomaterials 2025, 15, 637. [Google Scholar] [CrossRef]


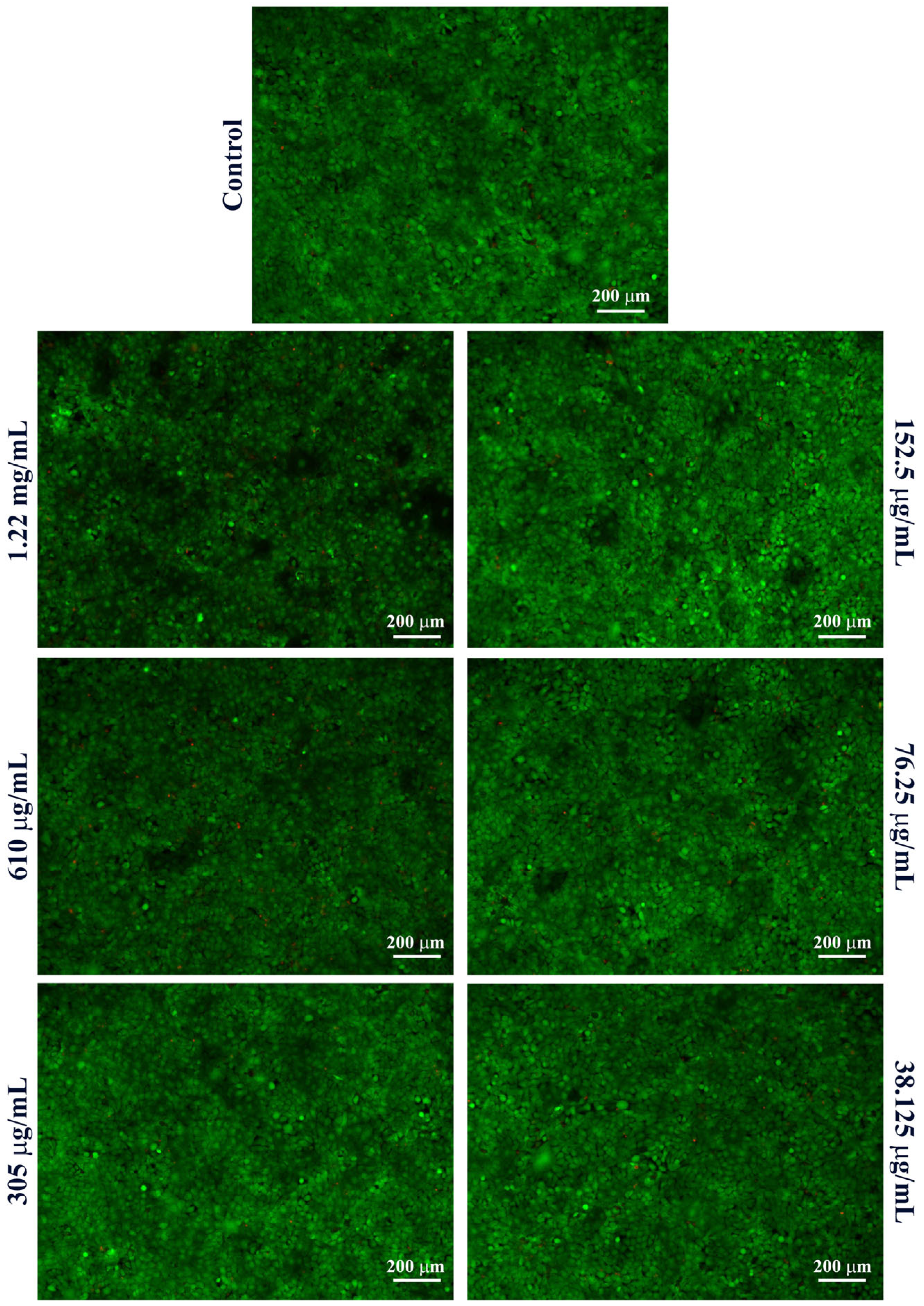

| Average Hydrodynamic Diameter (nm) | Standard Deviation | Zeta Potential (mV) | Standard Deviation | Polydispersity Index |
|---|---|---|---|---|
| 146.28 | 0.38 | 78.544 | 1.26 | 0.2 |
| Feature | Traditional Batch Synthesis | 2D Microfluidics | 3D Microfluidics (This Study) |
|---|---|---|---|
| Mixing efficiency | Low | Moderate | High (enhanced by 3D spiral flow) |
| Size control/monodispersity | Poor to moderate | Good | Excellent |
| Material consumption | High | Low | Low |
| Scalability | Moderate | Limited | Modular and stackable |
| Complexity/fabrication | Low | Low to moderate | Moderate to high |
| Functionalization integration | Post-synthesis | Limited | On-chip, simultaneous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldoveanu, E.-T.; Niculescu, A.-G.; Tudorache, D.-I.; Moroșan, A.; Bîrcă, A.-C.; Vasile, B.-Ș.; Hudita, A.; Mihaiescu, D.-E.; Hadibarata, T.; Grumezescu, A.-M. New 3D Spiral Microfluidic Platform Tested for Fe3O4@SA Nanoparticle Synthesis. Molecules 2025, 30, 2896. https://doi.org/10.3390/molecules30142896
Moldoveanu E-T, Niculescu A-G, Tudorache D-I, Moroșan A, Bîrcă A-C, Vasile B-Ș, Hudita A, Mihaiescu D-E, Hadibarata T, Grumezescu A-M. New 3D Spiral Microfluidic Platform Tested for Fe3O4@SA Nanoparticle Synthesis. Molecules. 2025; 30(14):2896. https://doi.org/10.3390/molecules30142896
Chicago/Turabian StyleMoldoveanu, Elena-Theodora, Adelina-Gabriela Niculescu, Dana-Ionela Tudorache (Trifa), Alina Moroșan, Alexandra-Cătălina Bîrcă, Bogdan-Ștefan Vasile, Ariana Hudita, Dan-Eduard Mihaiescu, Tony Hadibarata, and Alexandru-Mihai Grumezescu. 2025. "New 3D Spiral Microfluidic Platform Tested for Fe3O4@SA Nanoparticle Synthesis" Molecules 30, no. 14: 2896. https://doi.org/10.3390/molecules30142896
APA StyleMoldoveanu, E.-T., Niculescu, A.-G., Tudorache, D.-I., Moroșan, A., Bîrcă, A.-C., Vasile, B.-Ș., Hudita, A., Mihaiescu, D.-E., Hadibarata, T., & Grumezescu, A.-M. (2025). New 3D Spiral Microfluidic Platform Tested for Fe3O4@SA Nanoparticle Synthesis. Molecules, 30(14), 2896. https://doi.org/10.3390/molecules30142896














