In Vitro Evaluation of the Protective Efficacy of Crocus sativus L. Waste for the Sustainable Development of Bioactive Phytocomplexes
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Saffron Extracts
2.2. In Vitro Results
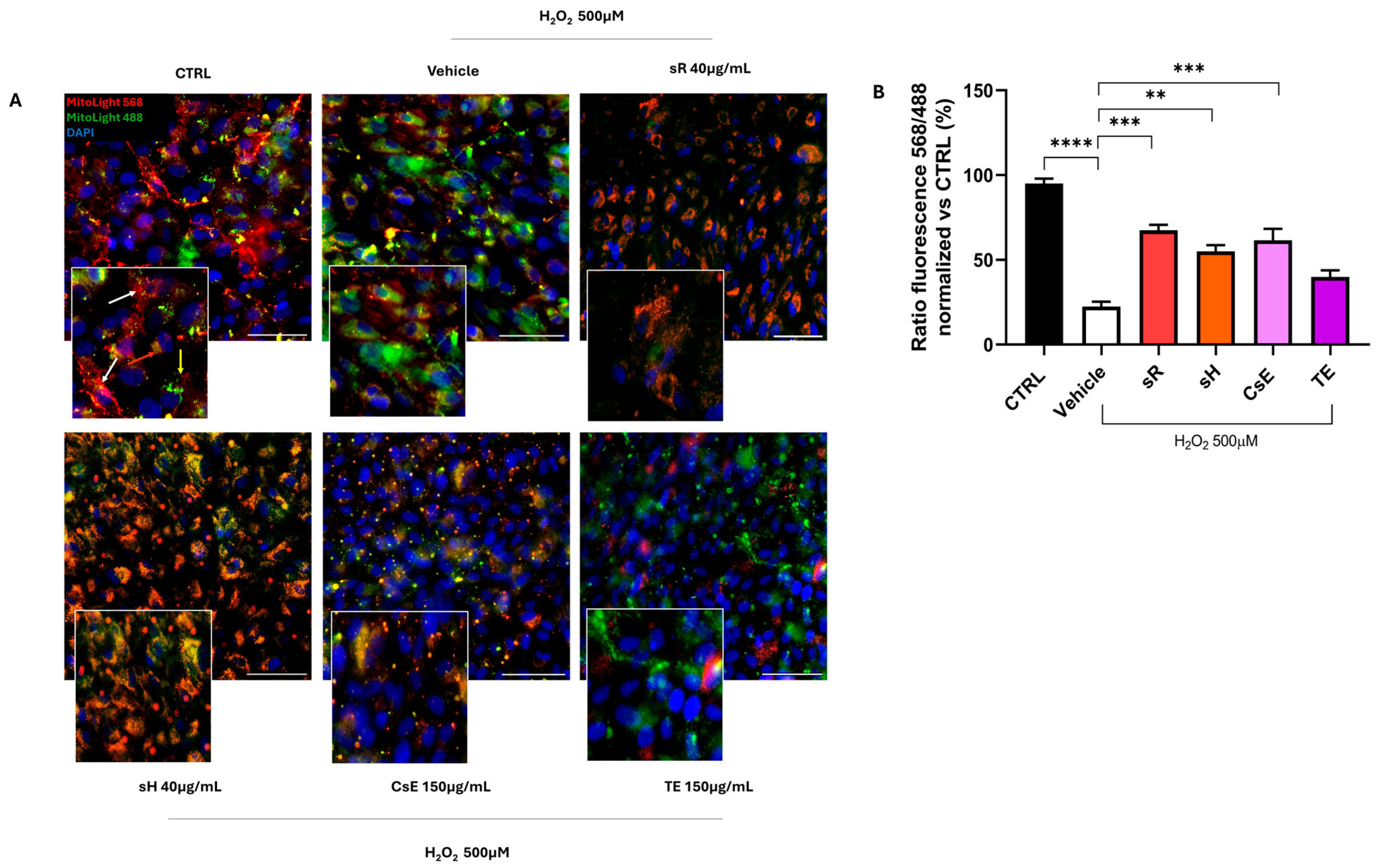
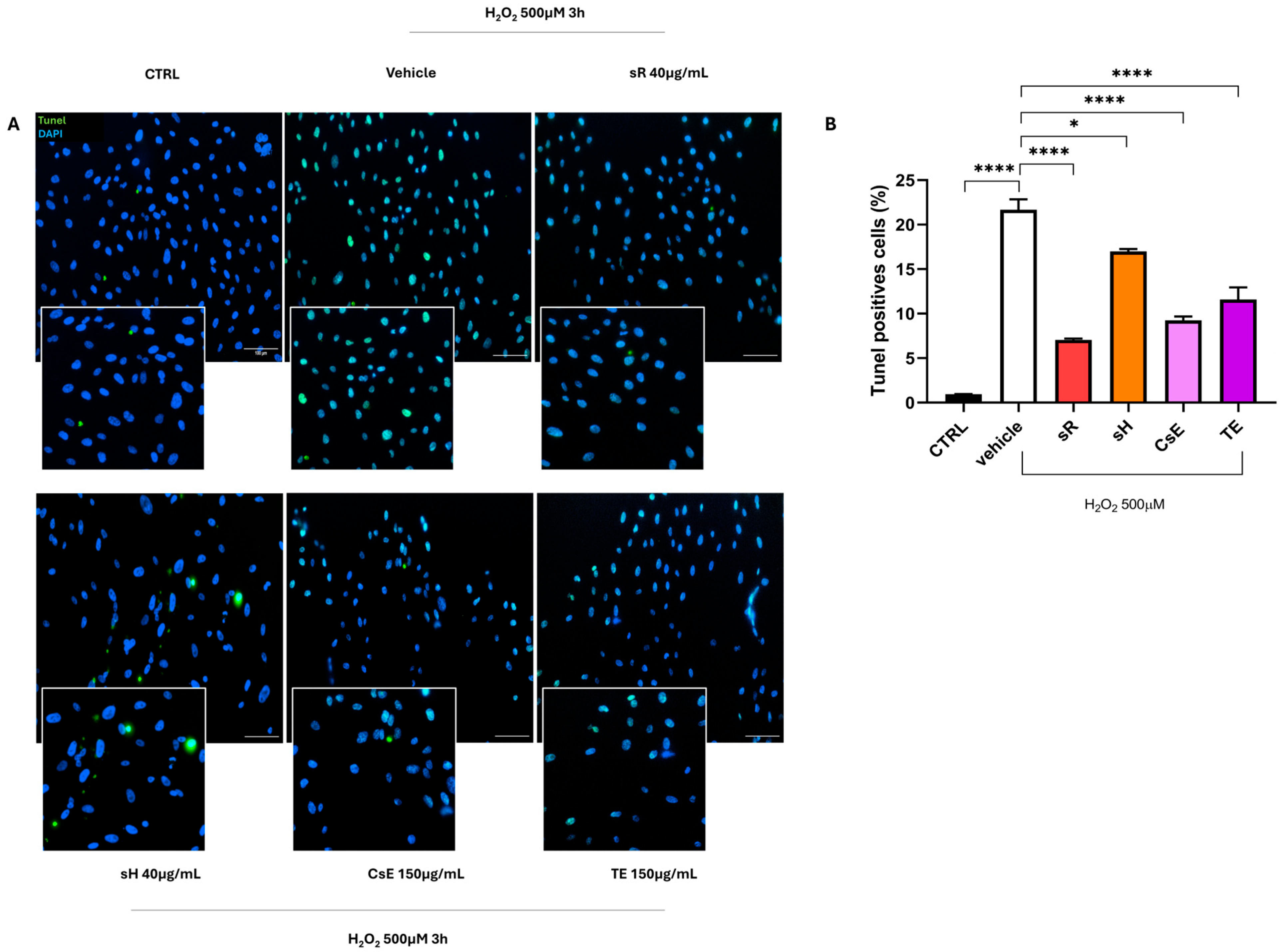
2.2.1. Different Saffron and Waste Extracts Protect Against Oxidative Stress
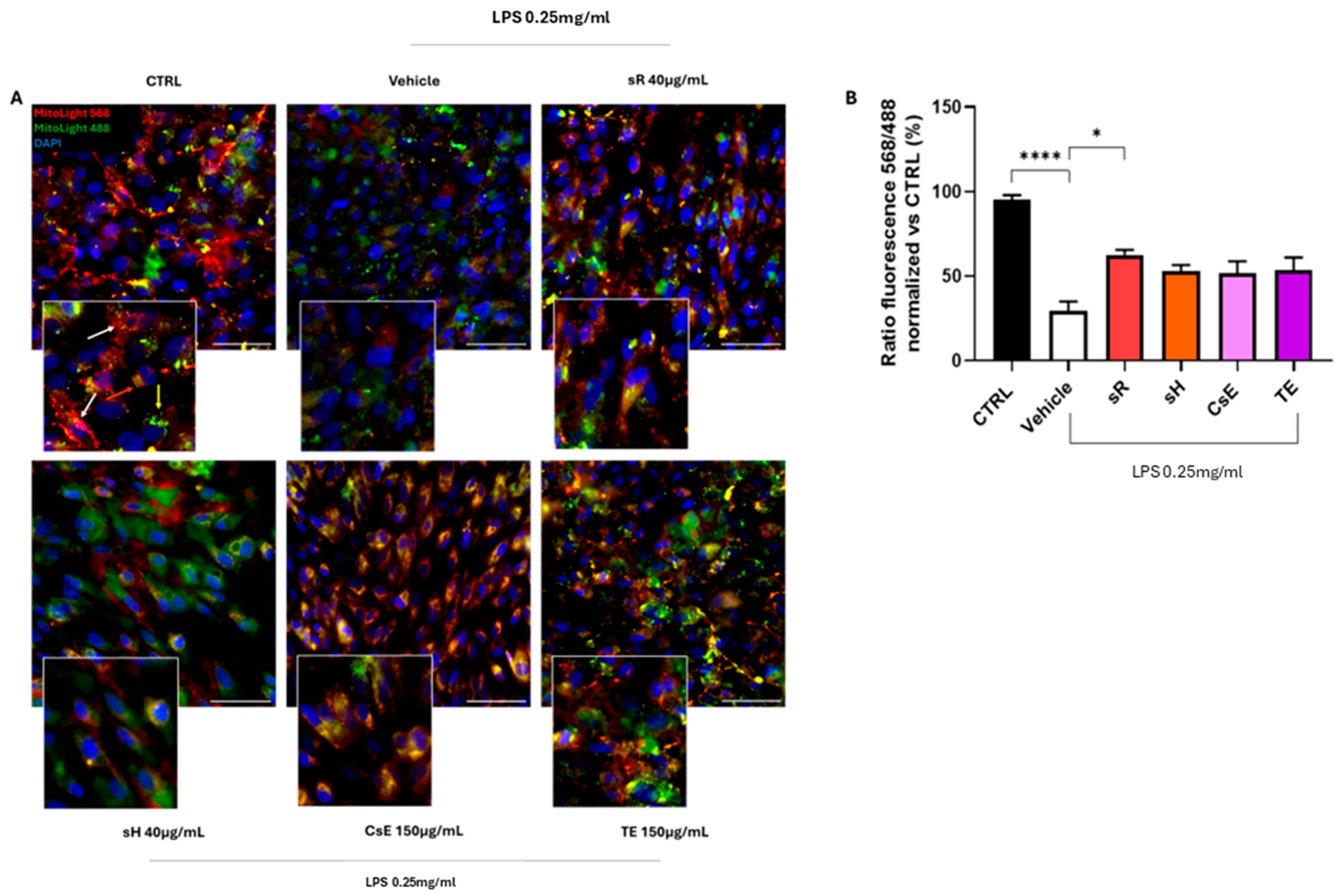
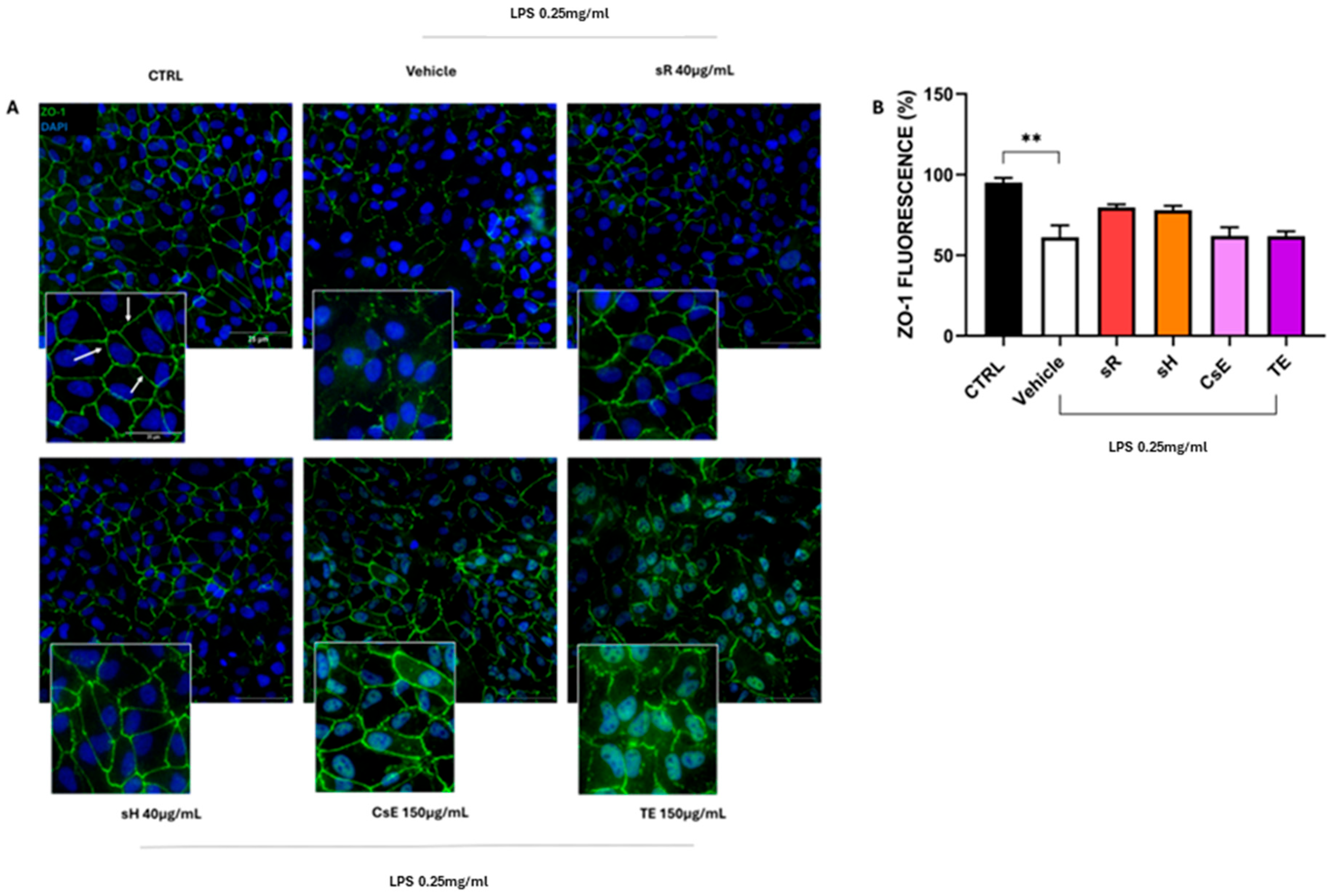
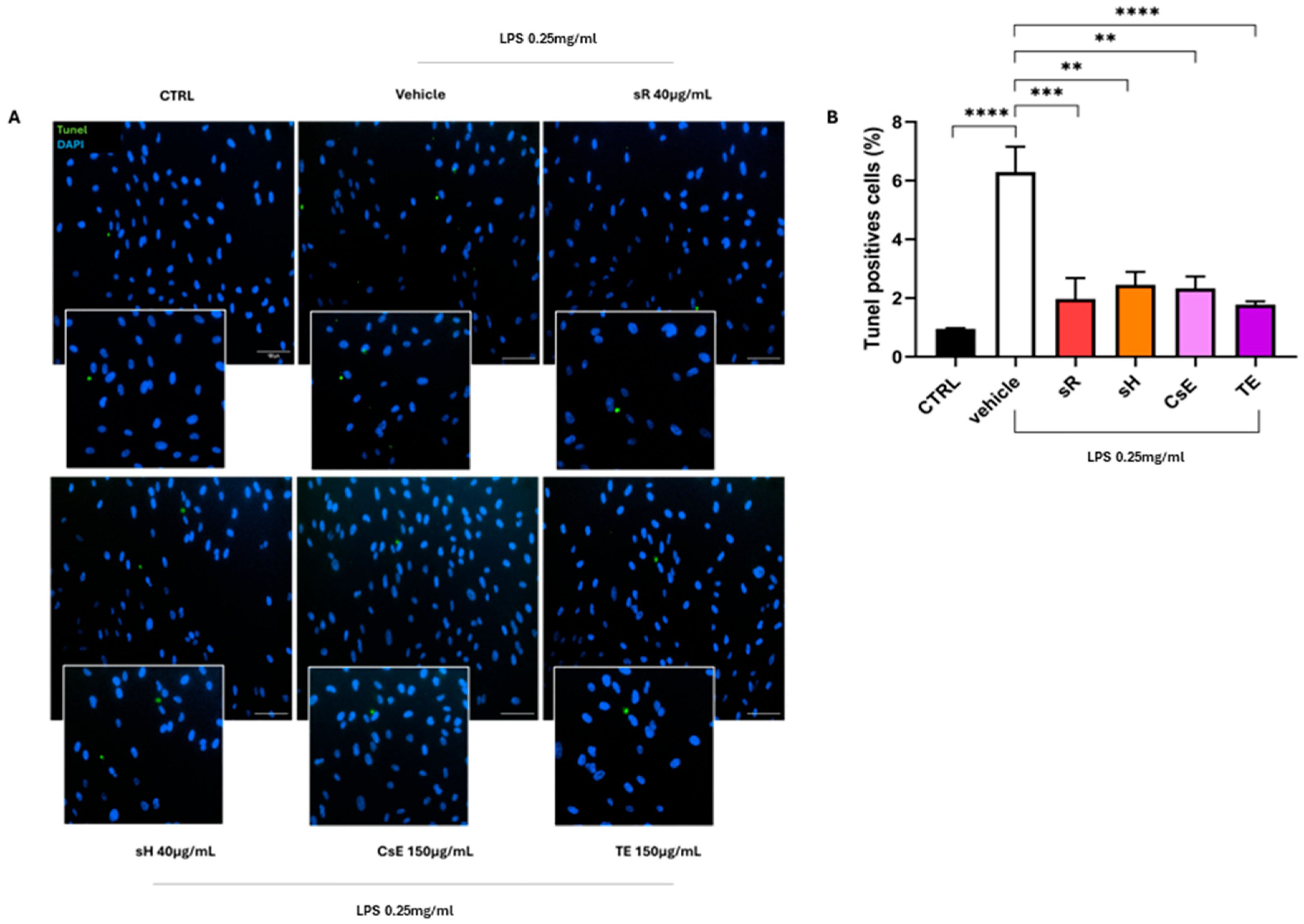
2.2.2. Different Saffron and Waste Extracts Protect from Inflammation
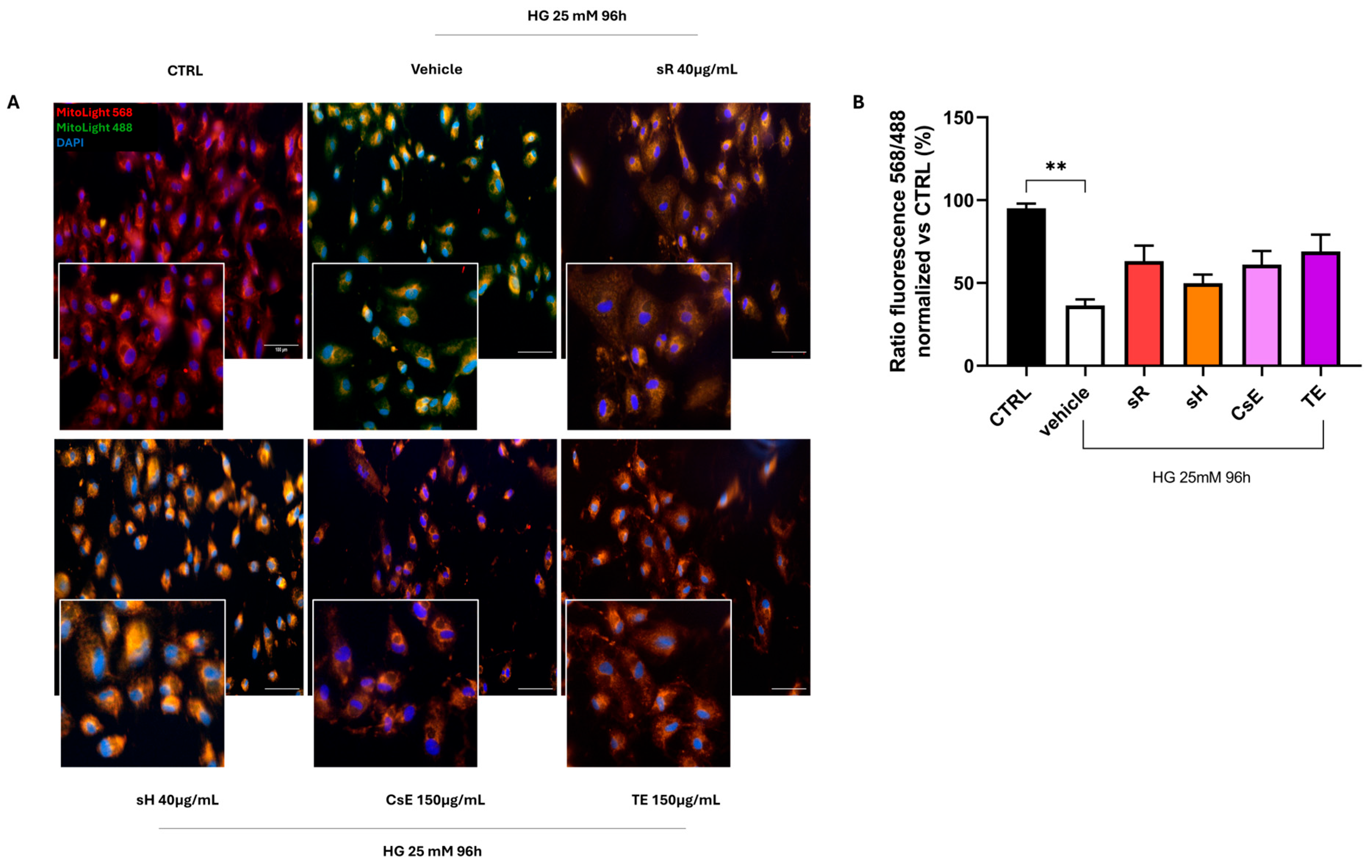
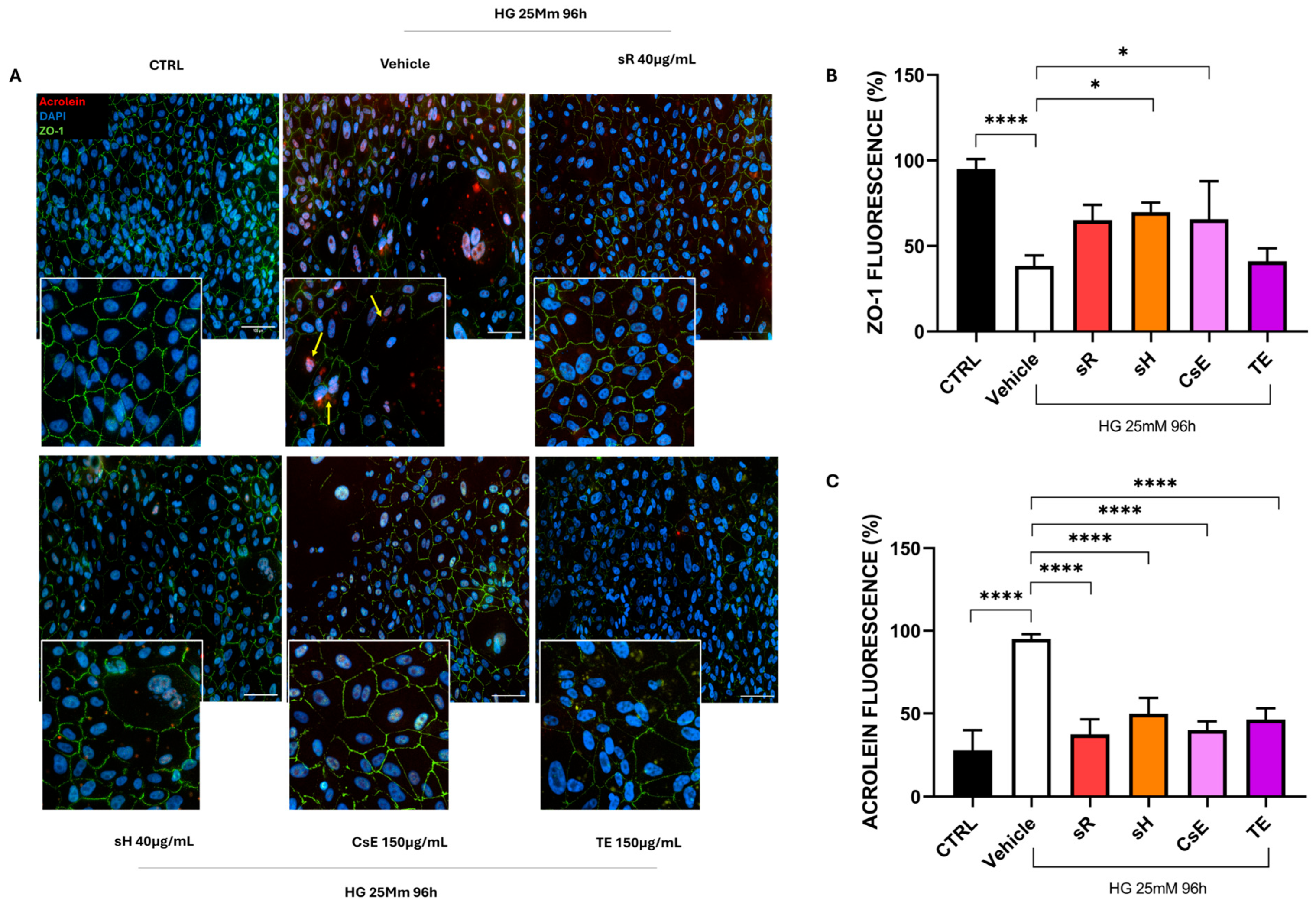
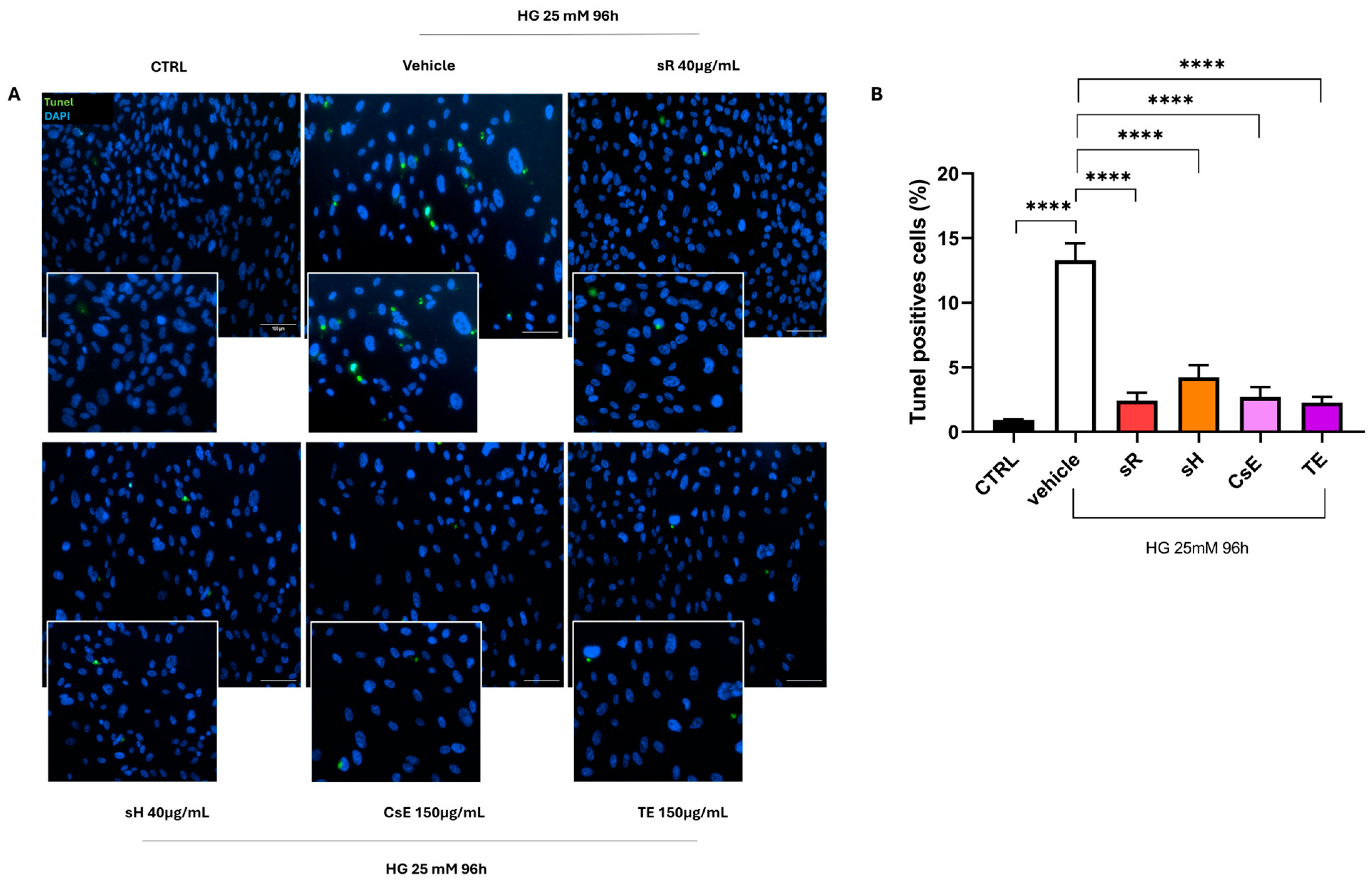
2.2.3. Different Saffron and Waste Extracts Protect from Hyperglycemia
3. Discussion
3.1. Flavonoids and Mitochondrial Protection
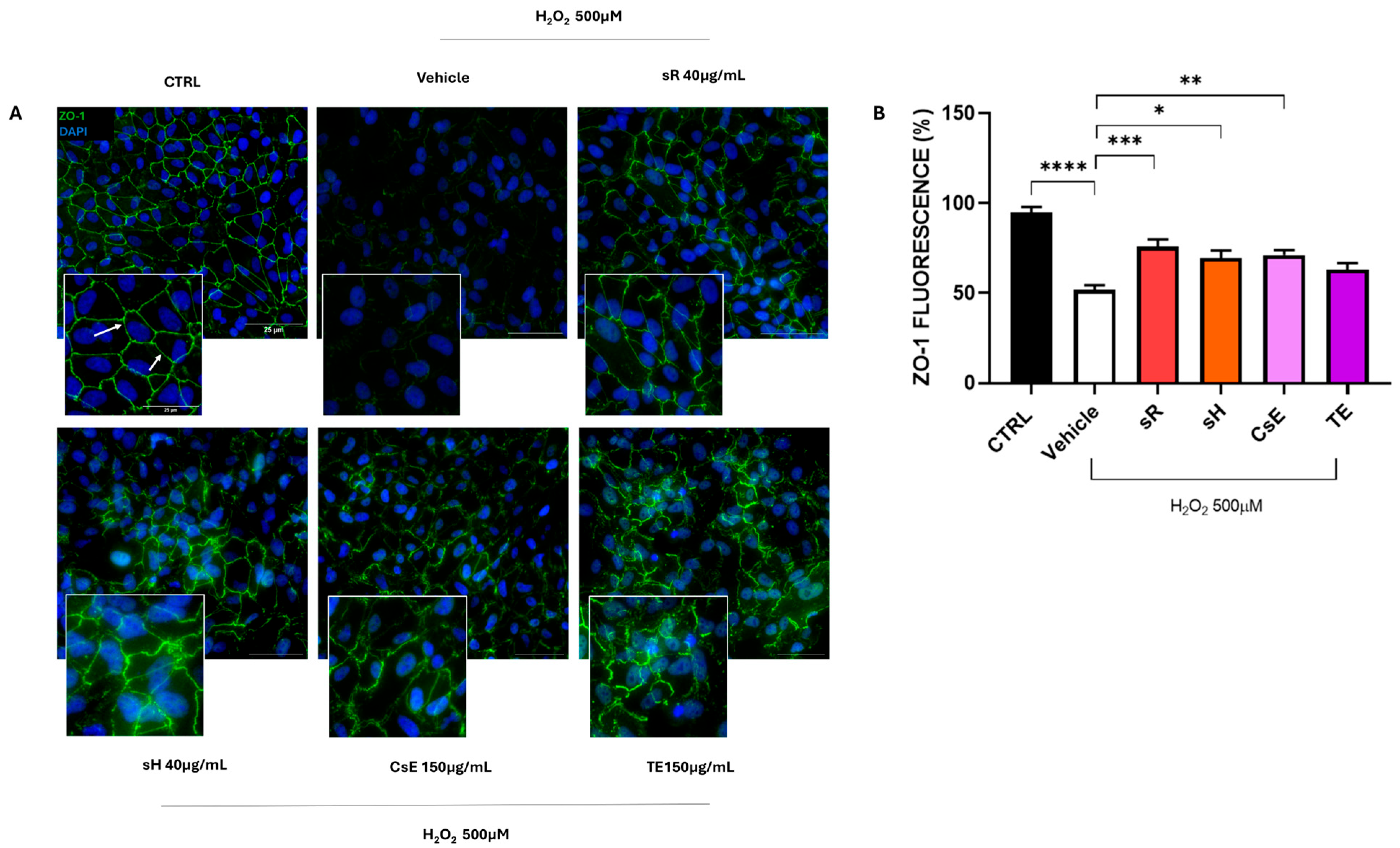
3.2. Flavonoids, Anthocyanins, and Integrity of Cellular Junctions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extract Preparation
4.3. Chemical Characterization of Extracts
4.3.1. UHPLC-DAD-HR-Orbitrap/ESI-MS2 Analyses
4.3.2. HPLC-PDA Analysis of Anthocyanins
4.4. Cell Culture
4.4.1. Pretreatment Protocol
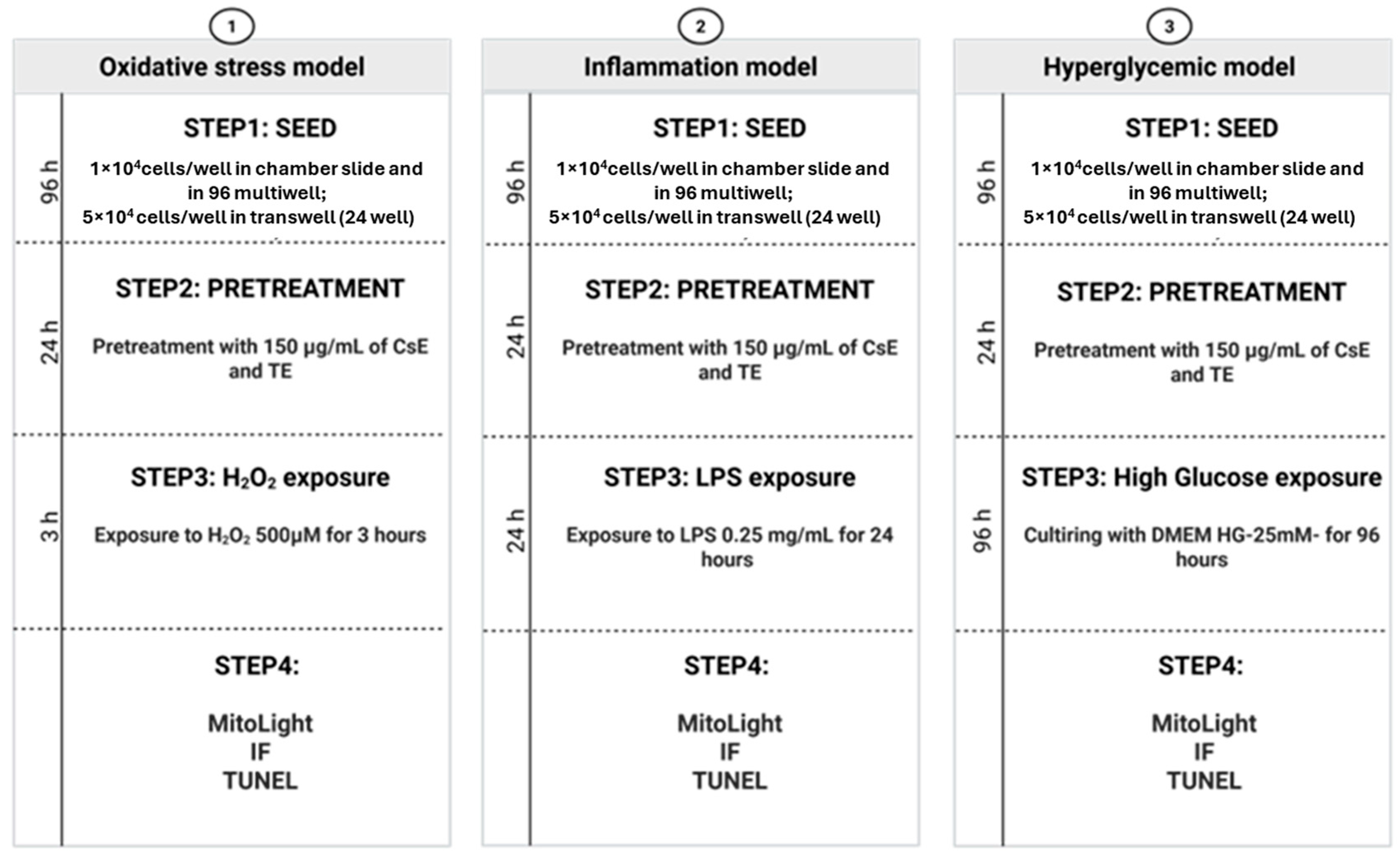
4.4.2. Retinal Degeneration Models
4.5. TUNEL Assay
4.6. Immunofluorescence
4.7. Detection of Mitochondrial Membrane Potential
4.8. Image Acquisition and Quantitative Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A Natural Product with Potential Pharmaceutical Applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef]
- Cusano, E.; Consonni, R.; Petrakis, E.A.; Astraka, K.; Cagliani, L.R.; Polissiou, M.G. Integrated Analytical Methodology to Investigate Bioactive Compounds in Crocus sativus L. Flowers. Phytochem. Anal. 2018, 29, 476–486. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Zalacain, A.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Changes in Saffron Volatile Profile According to Its Storage Time. Food Res. Int. 2010, 43, 1329–1334. [Google Scholar] [CrossRef]
- Rezaee, R.; Hosseinzadeh, H. Safranal: From an Aromatic Natural Product to a Rewarding Pharmacological Agent. Iran. J. Basic. Med. Sci. 2013, 16, 12–26. [Google Scholar]
- Anastasaki, E.G.; Kanakis, C.D.; Pappas, C.; Maggi, L.; Zalacain, A.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Quantification of Crocetin Esters in Saffron (Crocus sativus L.) Using Raman Spectroscopy and Chemometrics. J. Agric. Food Chem. 2010, 58, 6011–6017. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- Maggi, M.A.; Consonni, R.; Cagliani, L.R.; Prestipino, G.; Bisti, S.; Picco, C. Saffron and Retinal Neurodegenerative Diseases: Relevance of Chemical Composition. J. Anat. 2022, 243, joa.13722. [Google Scholar] [CrossRef]
- Di Paolo, M.; Corsi, F.; Cerri, C.; Bisti, S.; Piano, I.; Gargini, C. A Window to the Brain: The Retina to Monitor the Progression and Efficacy of Saffron Repron® Pre-Treatment in an LPS Model of Neuroinflammation and Memory Impairment. Pharmaceuticals 2023, 16, 1307. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active Constituents of Saffron (Crocus sativus L.) and Their Prospects in Treating Neurodegenerative Diseases (Review). Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Mohamadi-Zarch, S.-M.; Roghani, M. Safranal, an Active Ingredient of Saffron, Attenuates Cognitive Deficits in Amyloid β-Induced Rat Model of Alzheimer’s Disease: Underlying Mechanisms. Metab. Brain Dis. 2019, 34, 1747–1759. [Google Scholar] [CrossRef]
- Li, Y.; Kakkar, R.; Wang, J. In Vivo and in Vitro Approach to Anti-Arthritic and Anti-Inflammatory Effect of Crocetin by Alteration of Nuclear Factor-E2-Related Factor 2/Hem Oxygenase (HO)-1 and NF-κB Expression. Front. Pharmacol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and Retina: Neuroprotection and Pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Esquiva, G.; Martín-Nieto, J.; Pinilla, I.; Cuenca, N. Safranal, a Saffron Constituent, Attenuates Retinal Degeneration in P23H Rats. PLoS ONE 2012, 7, e43074. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Estevan, C.; Sogorb, M.Á.; Carmona, M.; Alonso, G.L.; Vilanova, E. Cytotoxic Effect against 3T3 Fibroblasts Cells of Saffron Floral Bio-Residues Extracts. Food Chem. 2014, 147, 55–59. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Saffron (Crocus sativus) Petal as a New Pharmacological Target: A Review. Iran. J. Basic. Med. Sci. 2018, 21, 1091–1099. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Darabi-Mahboub, E.; Mahboubi, A.; Mehrab, R.; Hakemivala, M. In-Vitro Evaluation of Crocus Sativus L. Petals and Stamens as Natural Antibacterial Agents Against Food Borne Bacterial Strains. Iran. J. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Fatehi, M.; Rashidabady, T.; Fatehi-Hassanabad, Z. Effects of Crocus Sativus Petals’ Extract on Rat Blood Pressure and on Responses Induced by Electrical Field Stimulation in the Rat Isolated Vas Deferens and Guinea-Pig Ileum. J. Ethnopharmacol. 2003, 84, 199–203. [Google Scholar] [CrossRef]
- Babaei, A.; Arshami, J.; Haghparast, A.; Danesh Mesgaran, M. Effects of Saffron (Crocus sativus) Petal Ethanolic Extract on Hematology, Antibody Response, and Spleen Histology in Rats. Avicenna J. Phytomed 2014, 4, 103–109. [Google Scholar]
- Hosseinzadeh, H.; Ghenaati, J. Evaluation of the Antitussive Effect of Stigma and Petals of Saffron (Crocus sativus) and Its Components, Safranal and Crocin in Guinea Pigs. Fitoterapia 2006, 77, 446–448. [Google Scholar] [CrossRef]
- Kianmehr, M.; Khazdair, M.R. Possible Therapeutic Effects of Crocus sativus Stigma and Its Petal Flavonoid, Kaempferol, on Respiratory Disorders. Pharm. Biol. 2020, 58, 1149–1158. [Google Scholar] [CrossRef]
- Vahdati Hassani, F.; Naseri, V.; Razavi, B.M.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Antidepressant Effects of Crocin and Its Effects on Transcript and Protein Levels of CREB, BDNF, and VGF in Rat Hippocampus. DARU J. Pharm. Sci. 2014, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and Anti-Inflammatory Effects of Crocus sativus L. Stigma and Petal Extracts in Mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.K.; Qi, X.; Goldwaser, E.L.; Godsey, G.A.; Wu, H.; Kosciuk, M.C.; Freeman, T.A.; Macphee, C.H.; Wilensky, R.L.; Venkataraman, V.; et al. Retinal Pathology Is Associated with Increased Blood–Retina Barrier Permeability in a Diabetic and Hypercholesterolaemic Pig Model: Beneficial Effects of the LpPLA 2 Inhibitor Darapladib. Diabetes Vasc. Dis. Res. 2017, 14, 200–213. [Google Scholar] [CrossRef]
- George, S.M.; Lu, F.; Rao, M.; Leach, L.L.; Gross, J.M. The Retinal Pigment Epithelium: Development, Injury Responses, and Regenerative Potential in Mammalian and Non-Mammalian Systems. Prog. Retin. Eye Res. 2021, 85, 100969. [Google Scholar] [CrossRef]
- Shao, A.; Lopez, A.J.; Chen, J.; Tham, A.; Javier, S.; Quiroz, A.; Frick, S.; Levine, E.M.; Lloyd, K.C.K.; Leonard, B.C.; et al. Arap1 Loss Causes Retinal Pigment Epithelium Phagocytic Dysfunction and Subsequent Photoreceptor Death. Dis. Models Mech. 2022, 15, dmm049343. [Google Scholar] [CrossRef]
- Montoro, P.; Tuberoso, C.I.G.; Maldini, M.; Cabras, P.; Pizza, C. Qualitative Profile and Quantitative Determination of Flavonoids from Crocus sativus L. Petals by LC-MS/MS. Nat. Prod. Commun. 2008, 3, 1934578X0800301215. [Google Scholar] [CrossRef]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Tuberoso, C.I.G. Exploring Phenolic Compounds Extraction from Saffron (C. Sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules 2024, 29, 2600. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the Flavonoid Fraction in Saffron Spice by LC/DAD/MS/MS: Comparative Study of Samples from Different Geographical Origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Lahmass, I.; Lamkami, T.; Delporte, C.; Sikdar, S.; Van Antwerpen, P.; Saalaoui, E.; Megalizzi, V. The Waste of Saffron Crop, a Cheap Source of Bioactive Compounds. J. Funct. Foods 2017, 35, 341–351. [Google Scholar] [CrossRef]
- Scupinari, T.; Mannochio Russo, H.; Sabino Ferrari, A.B.; Da Silva Bolzani, V.; Dias, W.P.; De Oliveira Nunes, E.; Hoffmann-Campo, C.B.; Zeraik, M.L. Crotalaria spectabilis as a Source of Pyrrolizidine Alkaloids and Phenolic Compounds: HPLC-MS/MS Dereplication and Monocrotaline Quantification of Seed and Leaf Extracts. Phytochem. Anal. 2020, 31, 747–755. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, G.; Guo, M. Correlations between Phytochemical Fingerprints of Moringa oleifera Leaf Extracts and Their Antioxidant Activities Revealed by Chemometric Analysis. Phytochem. Anal. 2021, 32, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Mounir, R.; Alshareef, W.A.; El Gebaly, E.A.; El-Haddad, A.E.; Ahmed, A.M.S.; Mohamed, O.G.; Enan, E.T.; Mosallam, S.; Tripathi, A.; Selim, H.M.R.M.; et al. Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals 2023, 16, 1379. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxidative Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Huang, H.; Li, Q.; Wang, X.; Sun, Q.; Wang, Q.; Li, N. In Vitro Antioxidant Analysis of Flavonoids Extracted from Artemisia Argyi Stem and Their Anti-Inflammatory Activity in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Chem. 2023, 407, 135198. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Chang, H.; Shu, Z.; Gou, H.; Zheng, Y.; Yang, Y.; Yang, Y.; Wang, Q.; Li, N. Antioxidant Analysis of Flavonoids Extracted from Artemisia Argyi Leaf and Their Antibacterial Activities against Food-Borne Pathogens Escherichia Coli and Staphylococcus aureus. Biologia 2024, 79, 975–983. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin Attenuates Oxidative Stress-induced Apoptosis via SIRT1/AMPK-mediated Inhibition of ER Stress in Rat Chondrocytes and Prevents the Progression of Osteoarthritis in a Rat Model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in Chronic Diseases: The Power of Purple. Nutrients 2022, 14, 2161. [Google Scholar] [CrossRef]
- Gallardo-Fernandez, M.; Garcia, A.R.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Brito, M.A. In Vitro Study of the Blood–Brain Barrier Transport of Bioactives from Mediterranean Foods. Food Funct. 2024, 15, 3420–3432. [Google Scholar] [CrossRef]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK Interacts with Occludin and Mediates EGF-Induced Prevention of Tight Junction Disruption by Hydrogen Peroxide. Biochem. J. 2006, 393, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin Enhances Intestinal Barrier Function through the Assembly of Zonnula Occludens-2, Occludin, and Claudin-1 and the Expression of Claudin-4 in Caco-2 Cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Tanabe, S.; Hara, H. Kaempferol Enhances Intestinal Barrier Function through the Cytoskeletal Association and Expression of Tight Junction Proteins in Caco-2 Cells. J. Nutr. 2011, 141, 87–94. [Google Scholar] [CrossRef] [PubMed]
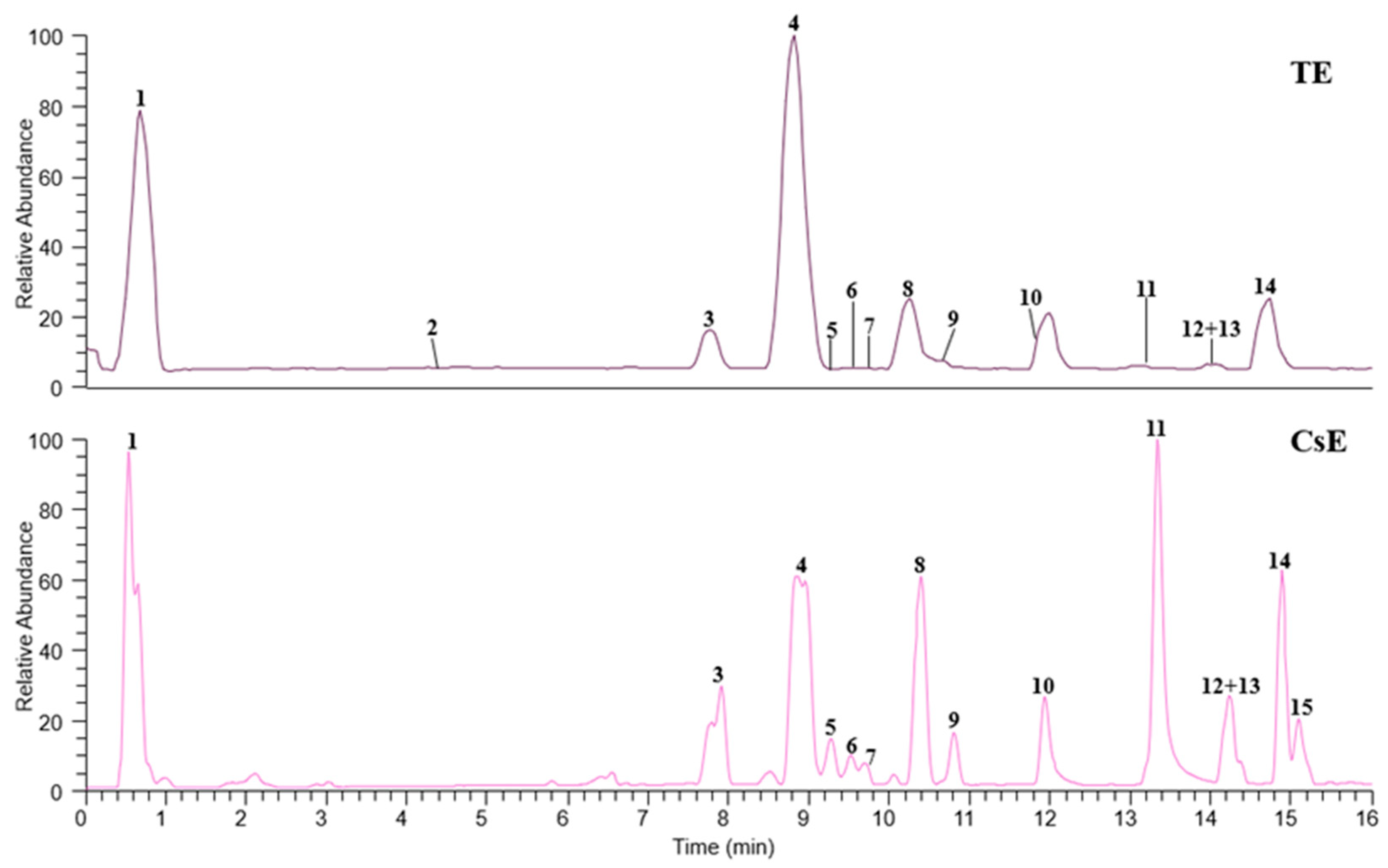
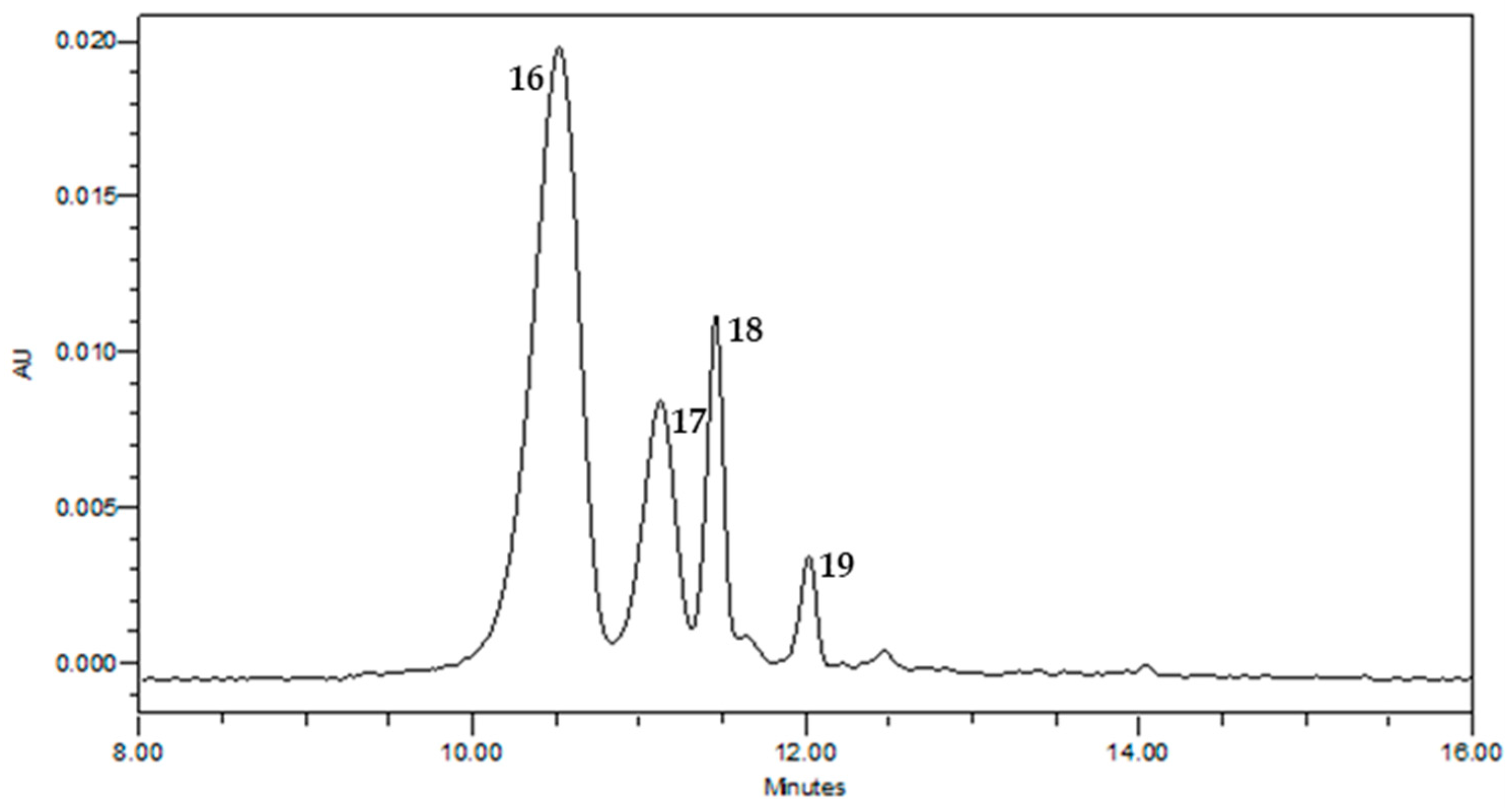
| Peak | Compound | tR (min) | [M − H]− (m/z) | ESI-MS/MS (m/z) | Molecular Formula | Error (ppm) | Extract |
|---|---|---|---|---|---|---|---|
| Organic acid | |||||||
| 1 | Citric acid | 0.53 | 191.0192 | 173.01, 129.02, 111.01 | C6H8O7 | −2.62 | CsE, TE |
| Flavonoid glycosides | |||||||
| 2 | Kaempferol tri-O-glucoside | 4.66 | 771.1974 | 609.14, 446.09, 285.04, 284.03 | C33H40O21 | −1.95 | TE |
| 3 | Quercetin O-sophoroside | 7.91 | 625.1414 | 545.05, 463.09, 301.04, 300.03 | C27H30O17 | 0.640 | CsE, TE |
| 4 | Kaempferol O-sophoroside | 8.86 | 609.1466 | 429.08, 309.04, 285.04, 284.03 | C27H30O16 | 0.821 | CsE, TE |
| 5 | Quercetin O-glucoside | 9.27 | 463.0883 | 301.04, 300.03, 271.02, 255.03 | C21H20O12 | 0.220 | CsE, TE |
| 6 | Kaempferol O-rutinoside | 9.46 | 593.1516 | 429.08, 371.78, 285.04, 284.03 | C27H30O15 | 0.674 | CsE, TE |
| 7 | Isorhamnetin O-rutinoside | 9.69 | 623.1621 | 594.16, 315.05, 314.04, 299.02 | C28H32O16 | 0.481 | CsE, TE |
| 8 | Kaempferol O-glucoside | 10.38 | 447.0935 | 285.04, 284.03, 255.03, 227.03 | C21H20O11 | 0.470 | CsE, TE |
| 9 | Isorhamnetin O-glucoside | 10.80 | 477.1038 | 357.06, 315.05, 314.04, 299.02 | C22H22O12 | −0.210 | CsE, TE |
| 10 | Quercetin | 11.93 | 301.0355 | 273.04, 193.01, 151.00, 93.03 | C15H10O7 | 0.332 | CsE, TE |
| 11 | Kaempferol | 13.33 | 285.0405 | 151.00, 117.03 | C15H10O6 | 0 | CsE, TE |
| 12 | Isorhamnetin | 14.16 | 315.0512 | 301.03, 279.86, 261.89, 219.85 | C16H12O7 | 0.635 | CsE, TE |
| Fatty acids | |||||||
| 13 | Trihydroxyoctadecadienoic acid | 14.25 | 327.2176 | 309.21, 291.20, 229.14, 211.13 | C18H32O5 | −0.31 | CsE, TE |
| 14 | Trihydroxyoctadecenoic acid I | 14.88 | 329.2332 | 311.22, 293.21, 249.22, 229.14 | C18H34O5 | 0 | CsE, TE |
| 15 | Trihydroxyoctadecenoic acid II | 15.08 | 329.2332 | 311.22, 293.21, 249.22, 229.14 | C18H34O5 | 0 | CsE |
| Peak | Compound | tR (min) | [M]+ (m/z) | ESI-MS/MS (m/z) | Molecular Formula | Error (ppm) | Extract |
|---|---|---|---|---|---|---|---|
| 16 | Delphinidin 3,5-di-O-glucoside | 10.52 | 627.1558 | 465.10, 303.05 | C27H31O17+ | −0.637 | TE |
| 17 | Petunidin 3,5-di-O-glucoside | 11.13 | 641.1704 | 479.12, 317.06 | C28H33O17+ | −1.25 | TE |
| 18 | Delphinidin 3-O-glucoside | 11.46 | 465.1030 | 303.05, 287.06 | C21H21O12+ | 0.430 | TE |
| 19 | Petunidin 3-O-glucoside | 12.06 | 479.1184 | 317.06 | C22H23O12+ | 0 | TE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galante, A.; Corsi, F.; Cioni, E.; Di Stasi, M.; Maggi, M.A.; Bisti, S.; Piano, I.; Gargini, C. In Vitro Evaluation of the Protective Efficacy of Crocus sativus L. Waste for the Sustainable Development of Bioactive Phytocomplexes. Molecules 2025, 30, 2894. https://doi.org/10.3390/molecules30142894
Galante A, Corsi F, Cioni E, Di Stasi M, Maggi MA, Bisti S, Piano I, Gargini C. In Vitro Evaluation of the Protective Efficacy of Crocus sativus L. Waste for the Sustainable Development of Bioactive Phytocomplexes. Molecules. 2025; 30(14):2894. https://doi.org/10.3390/molecules30142894
Chicago/Turabian StyleGalante, Alessia, Francesca Corsi, Emily Cioni, Mauro Di Stasi, Maria Anna Maggi, Silvia Bisti, Ilaria Piano, and Claudia Gargini. 2025. "In Vitro Evaluation of the Protective Efficacy of Crocus sativus L. Waste for the Sustainable Development of Bioactive Phytocomplexes" Molecules 30, no. 14: 2894. https://doi.org/10.3390/molecules30142894
APA StyleGalante, A., Corsi, F., Cioni, E., Di Stasi, M., Maggi, M. A., Bisti, S., Piano, I., & Gargini, C. (2025). In Vitro Evaluation of the Protective Efficacy of Crocus sativus L. Waste for the Sustainable Development of Bioactive Phytocomplexes. Molecules, 30(14), 2894. https://doi.org/10.3390/molecules30142894








