Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase
Abstract
1. Introduction
2. Results
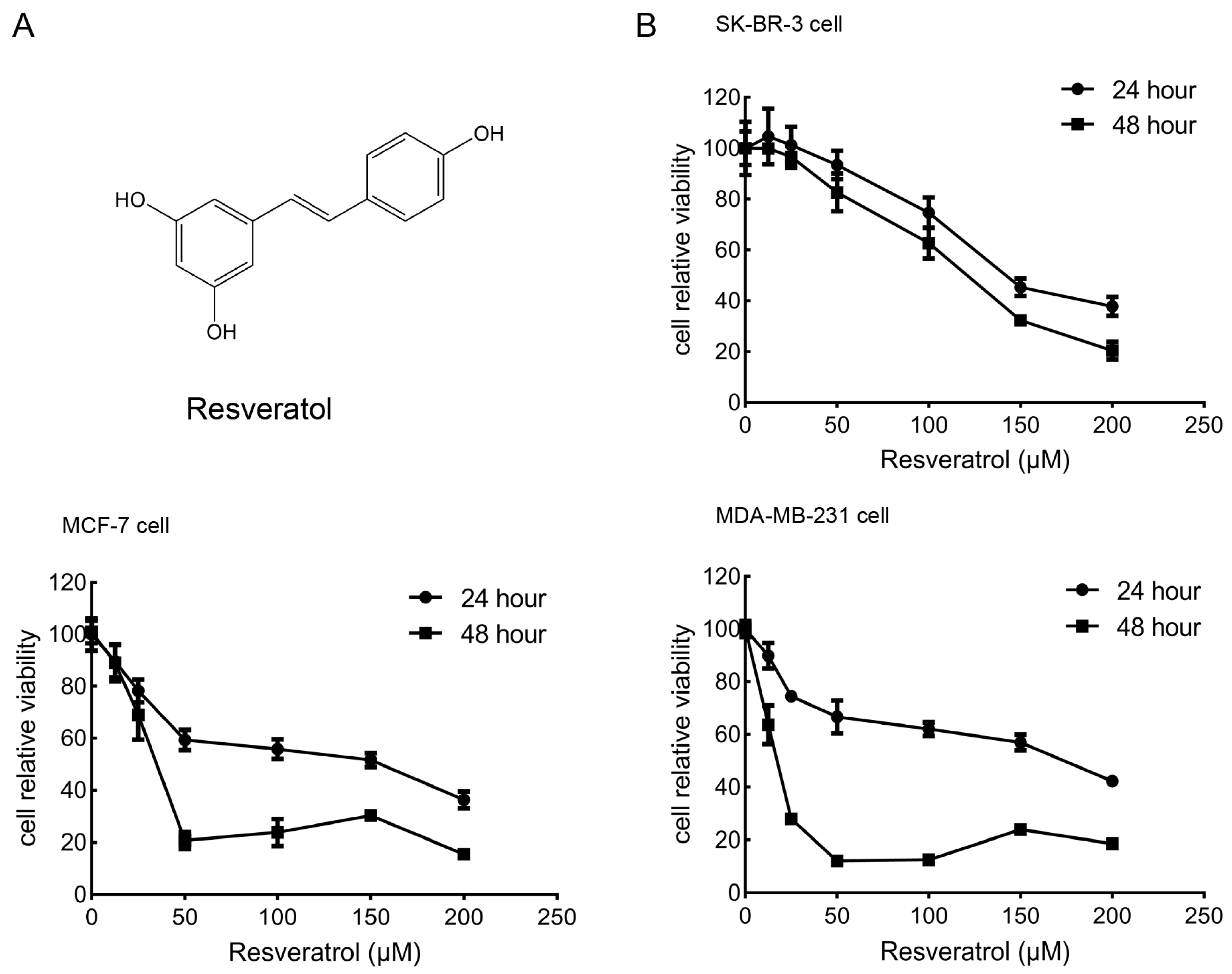
2.1. Effects of Resveratrol on the Viability of Breast Cancer Cells
2.2. Resveratrol Induces Breast Cancer Cells Apoptosis
2.3. Resveratrol Inhibits FASN Activity and Down-Regulated FASN Expression in Breast Cancer Cells
2.4. Silencing FASN via siRNA Potentiates the Cytotoxic Effect of Resveratrol in Breast Cancer Cells
2.5. Resveratrol or siRNA-Targeted FASN Reduces the Amount of Intracellular Fatty Acid
2.6. Resveratrol and FASN Knockdown Modulate Multiple Signaling Cascades Linked to Breast Cancer Cell Growth and Survival
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Apoptosis Assay
4.5. Intracellular FASN Activity Assay
4.6. Western Blotting Analysis
4.7. Small Interference RNA Transfection
4.8. Intracellular Free Fatty Acid Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Bakhshaei Shahrebabaki, P.; Fouladi, H.; Mansoori Derakhshan, S. The impact of microRNAs on the resistance of breast cancer subtypes to chemotherapy. Pathol. Res. Pract. 2023, 249, 154702. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Şener, B.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Chen, M.; He, C.; Zhu, K.; Chen, Z.; Meng, Z.; Jiang, X.; Cai, J.; Yang, C.; Zuo, Z. Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication. Theranostics 2022, 12, 782–795. [Google Scholar] [CrossRef]
- Kowsari, H.; Davoodvandi, A.; Dashti, F.; Mirazimi, S.M.A.; Bahabadi, Z.R.; Aschner, M.; Sahebkar, A.; Gilasi, H.R.; Hamblin, M.R.; Mirzaei, H. Resveratrol in cancer treatment with a focus on breast cancer. Curr. Mol. Pharmacol. 2023, 16, 346–361. [Google Scholar]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Chen, Q.L.; Zhao, M.; Su, S.B. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, L.; Chen, J.; Zhao, M. Resveratrol inhibits fatty acid synthesis and promotes apoptosis via AMPK activation in breast cancer cells. Nutrients 2023, 15, 615. [Google Scholar]
- Senga, S.S.; Grose, R.P. Hallmarks of cancer-the new testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Kuhajda, F.P. Fatty-acid synthase and human cancer: New perspectives on its role in tumor biology. Nutrition 2000, 16, 202–208. [Google Scholar] [CrossRef]
- Maier, T.; Leibundgut, M.; Boehringer, D.; Ban, N. Structure and function of eukaryotic fatty acid synthases. Q. Rev. Biophys. 2010, 43, 373–422. [Google Scholar] [CrossRef]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef]
- Bueno, A.A.; Silva, F.M.O.; Moura, F.A.; Oliveira, M. Fatty acid synthase overexpression as a metabolic driver in breast cancer: Emerging evidence and therapeutic opportunities. Front. Oncol. 2022, 12, 982159. [Google Scholar]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K.; Liu, W.; Kobayashi, A.; Xing, F.; Fukuda, K.; Hirota, S.; Sugai, T.; et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Menendez, J.A.; Decker, J.P.; Lupu, R. In support of fatty acid synthase (FAS) as a metabolic oncogene: Extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J. Cell. Biochem. 2005, 94, 1–4. [Google Scholar] [CrossRef]
- Little, J.L.; Kridel, S.J. Fatty acid synthase activity in tumor cells. In Lipids in Health and Disease; Springer: Dordrecht, The Netherlands, 2008; pp. 169–194. [Google Scholar]
- Swinnen, J.V.; Van Veldhoven, P.P.; Timmermans, L.; De Schrijver, E.; Brusselmans, K.; Vanderhoydonc, F.; Van de Sande, T.; Heemers, H.; Heyns, W.; Verhoeven, G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 2003, 302, 898–903. [Google Scholar] [CrossRef]
- Christoforou, M.; Charalambous, A.; Sfakianakis, D.; Skourides, P.A. Targeting the hydrophobic pockets of FAK/PYK2 FAT domain: A highly effective inhibitory strategy suppressing tumor growth and eliminating metastasis. Cell Commun. Signal. 2025, 23, 231. [Google Scholar] [CrossRef]
- Mondaca, J.M.; Muñoz, J.M.F.; Barraza, G.A.; Vanderhoeven, F.; Redondo, A.L.; Flamini, M.I.; Sanchez, A.M. Therapeutic potential of GNRHR analogs and SRC/FAK inhibitors to counteract tumor growth and metastasis in breast cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167826. [Google Scholar] [CrossRef]
- He, W.; Liu, W.; Liu, X.; Tan, W. The mechanism of L1 cell adhesion molecule interacting with protein tyrosine kinase 2 to regulate the focal adhesion kinase–growth factor receptor-bound protein 2–son of sevenless–rat sarcoma pathway in the identification and treatment of type I high-risk endometrial cancer. CytoJournal 2024, 21, 34. [Google Scholar]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, M.Y.; Park, S.W.; Moon, J.S.; Koh, Y.K.; Ahn, Y.H.; Park, B.W.; Kim, K.S. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J. Biol. Chem. 2007, 282, 26122–26131. [Google Scholar] [CrossRef]
- Azam, A.; Sounni, N.E. Lipid metabolism heterogeneity and crosstalk with mitochondria functions drive breast cancer progression and drug resistance. Cancers 2022, 14, 6267. [Google Scholar] [CrossRef]
- Vanauberg, D.; Schulz, C.; Lefebvre, T. Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: A promising target in anti-cancer therapies. Oncogenesis 2023, 12, 16. [Google Scholar] [CrossRef]
- Bueno, M.J.; Colomer, R. A fatty acid synthase inhibitor shows new anticancer mechanisms. EBioMedicine 2015, 2, 778–779. [Google Scholar] [CrossRef]
- Kuhajda, F.P.; Pizer, E.S.; Li, J.N.; Mani, N.S.; Frehywot, G.L.; Townsend, C.A. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 2000, 97, 3450–3454. [Google Scholar] [CrossRef]
- Puig, T.; Turrado, C.; Benhamú, B.; Aguilar, H.; Relat, J.; Ortega-Gutiérrez, S.; Casals, G.; Marrero, P.F.; Urruticoechea, A.; Haro, D.; et al. Novel inhibitors of fatty acid synthase with anticancer activity. Clin. Cancer Res. 2009, 15, 7608–7615. [Google Scholar] [CrossRef]
- Richardson, R.D.; Smith, J.W. Novel antagonists of the thioesterase domain of human fatty acid synthase. Mol. Cancer Ther. 2007, 6, 2120–2126. [Google Scholar] [CrossRef]
- Li, P.; Tian, W.; Wang, X.; Ma, X. Inhibitory effect of desoxyrhaponticin and rhaponticin, two natural stilbene glycosides from the Tibetan nutritional food Rheum. tanguticum Maxim. ex Balf., on fatty acid synthase and human breast cancer cells. Food Funct. 2014, 5, 251–256. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.-X.; Ma, X.-F. Inhibitory effects of onion (Allium cepa L.) extract on proliferation of cancer cells and adipocytes via inhibiting fatty acid synthase. Asian Pac. J. Cancer Prev. 2012, 13, 5573–5579. [Google Scholar] [CrossRef]
- Garvin, S.; Öllinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Jackowski, S.; Wang, J.; Baburina, I. Activity of the phosphatidylcholine biosynthetic pathway modulates the distribution of fatty acids into glycerolipids in proliferating cells. Biochim. Biophys. Acta Lipids Lipid Metab. 2000, 1483, 301–315. [Google Scholar] [CrossRef]
- Gnoni, G.; Paglialonga, G. Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur. J. Clin. Investig. 2009, 39, 211–218. [Google Scholar] [CrossRef]
- Menendez, J.A.; Mehmi, I.; Atlas, E.; Colomer, R.; Lupu, R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53–p21^WAF1/CIP1, ERK1/2 MAPK, p27^KIP1, BRCA1, and NF-κB. Int. J. Oncol. 2004, 24, 591–608. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK–Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Brownson, D.M.; Azios, N.G.; Fuqua, B.K.; Dharmawardhane, S.F.; Mabry, T.J. Flavonoid effects relevant to cancer. J. Nutr. 2002, 132, 3482S–3489S. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Rychahou, P.G.; Gulhati, P.; Elliott, V.A.; Mustain, W.C.; O’Connor, K.; Morris, A.J.; Sunkara, M.; Weiss, H.L.; Lee, E.Y.; et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012, 72, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Rigiracciolo, D.C.; Santolla, M.F.; Lappano, R.; Vivacqua, A.; Cirillo, F.; Galli, G.R.; Talia, M.; Muglia, L.; Pellegrino, M.; Nohata, N.; et al. Focal adhesion kinase (FAK) activation by estrogens involves GPER in triple-negative breast cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 58. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Beviglia, L.; Xu, L.-H.; Earp, H.S.; Craven, R.; Cance, W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J. Biol. Chem. 2002, 277, 38978–38987. [Google Scholar] [CrossRef]
- Dhillon, A.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Menendez, J.A.; Mehmi, I.; Papadimitropoulou, A.; Vander Steen, T.; Cuyàs, E.; Verdura, S.; Espinoza, I.; Vellon, L.; Atlas, E.; Lupu, R. Fatty acid synthase is a key enabler for endocrine resistance in heregulin-overexpressing luminal B-like breast cancer. Int. J. Mol. Sci. 2020, 21, 7661. [Google Scholar] [CrossRef]
- Nagy, P.; Vereb, G.; Sebestyén, Z.; Horváth, G.; Lockett, S.J.; Damjanovich, S.; Park, J.W.; Jovin, T.M.; SzölLősI, J. Lipid rafts and the local density of ErbB proteins influence the biological role of homo-and heteroassociations of ErbB. J. Cell Sci. 2002, 115, 4251–4262. [Google Scholar] [CrossRef]
- Danielli, M.; Marinelli, R.A. Lipid-based transfection reagents can interfere with cholesterol biosynthesis. Anal. Biochem. 2016, 495, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fiszer-Kierzkowska, A.; Vydra, N.; Wysocka-Wycisk, A.; Kronekova, Z.; Jarząb, M.; Lisowska, K.M.; Krawczyk, Z. Liposome-based DNA carriers may induce cellular stress response and change gene expression pattern in transfected cells. BMC Mol. Biol. 2011, 12, 27. [Google Scholar] [CrossRef]
- Jin, Q.; Yuan, L.X.; Boulbes, D.; Baek, J.M.; Wang, Y.N.; Gomez-Cabello, D.; Hawke, D.H.; Yeung, S.C.; Lee, M.H.; Hortobagyi, G.N.; et al. Fatty acid synthase phosphorylation: A novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010, 12, R96. [Google Scholar] [CrossRef]
- Blancafort, A.; Giró-Perafita, A.; Palafox, M.; González-Suárez, E.; Brugada, R.; Puig, T. Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs. PLoS ONE 2015, 10, e0131241. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Heng, H.H. Transient and stable vector transfection: Pitfalls, off-target effects, artifacts. Mutat. Res. Rev. Mutat. Res. 2017, 773, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. Oral resveratrol in humans: Evaluation of plasma and urine levels. Cancer Res. 2005, 65 (Suppl. 9), 1042. [Google Scholar]
- Briskey, D.; Rao, A. Trans-resveratrol oral bioavailability in humans using LipiSperse dispersion technology. Pharmaceutics 2020, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Kemper, C.; Benham, D.; Brothers, S.; Wahlestedt, C.; Volmar, C.-H.; Bennett, D.; Hayward, M. Safety and pharmacokinetics of a highly bioavailable resveratrol (JOTROL™): First-in-human study. AAPS Open 2022, 8, 11. [Google Scholar] [CrossRef]
- Marier, J.-F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.P.; Ducharme, M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Chen, T.-S.; Qu, J.-L.; Pan, W.-L.; Sun, L.; Wei, X.-B. Dihydroartemisinin (DHA) induces caspase-3-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J. Biomed. Sci. 2009, 16, 16. [Google Scholar] [CrossRef]
- Migita, T.; Ruiz, S.; Fornari, A.; Fiorentino, M.; Priolo, C.; Zadra, G.; Inazuka, F.; Grisanzio, C.; Palescandolo, E.; Shin, E.; et al. Fatty acid synthase: A metabolic enzyme and candidate oncogene in prostate cancer. J. Natl. Cancer Inst. 2009, 101, 519–532. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Liang, Y.; Ma, X. Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase. Molecules 2025, 30, 2891. https://doi.org/10.3390/molecules30142891
Li P, Liang Y, Ma X. Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase. Molecules. 2025; 30(14):2891. https://doi.org/10.3390/molecules30142891
Chicago/Turabian StyleLi, Ping, Yan Liang, and Xiaofeng Ma. 2025. "Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase" Molecules 30, no. 14: 2891. https://doi.org/10.3390/molecules30142891
APA StyleLi, P., Liang, Y., & Ma, X. (2025). Functional Role of Resveratrol in Inducing Apoptosis in Breast Cancer Subtypes via Inhibition of Intracellular Fatty Acid Synthase. Molecules, 30(14), 2891. https://doi.org/10.3390/molecules30142891




