Hydroxyl Derivatives of Oils from Solid Fats as Components for Production of Polyurethane Foams
Abstract
1. Introduction
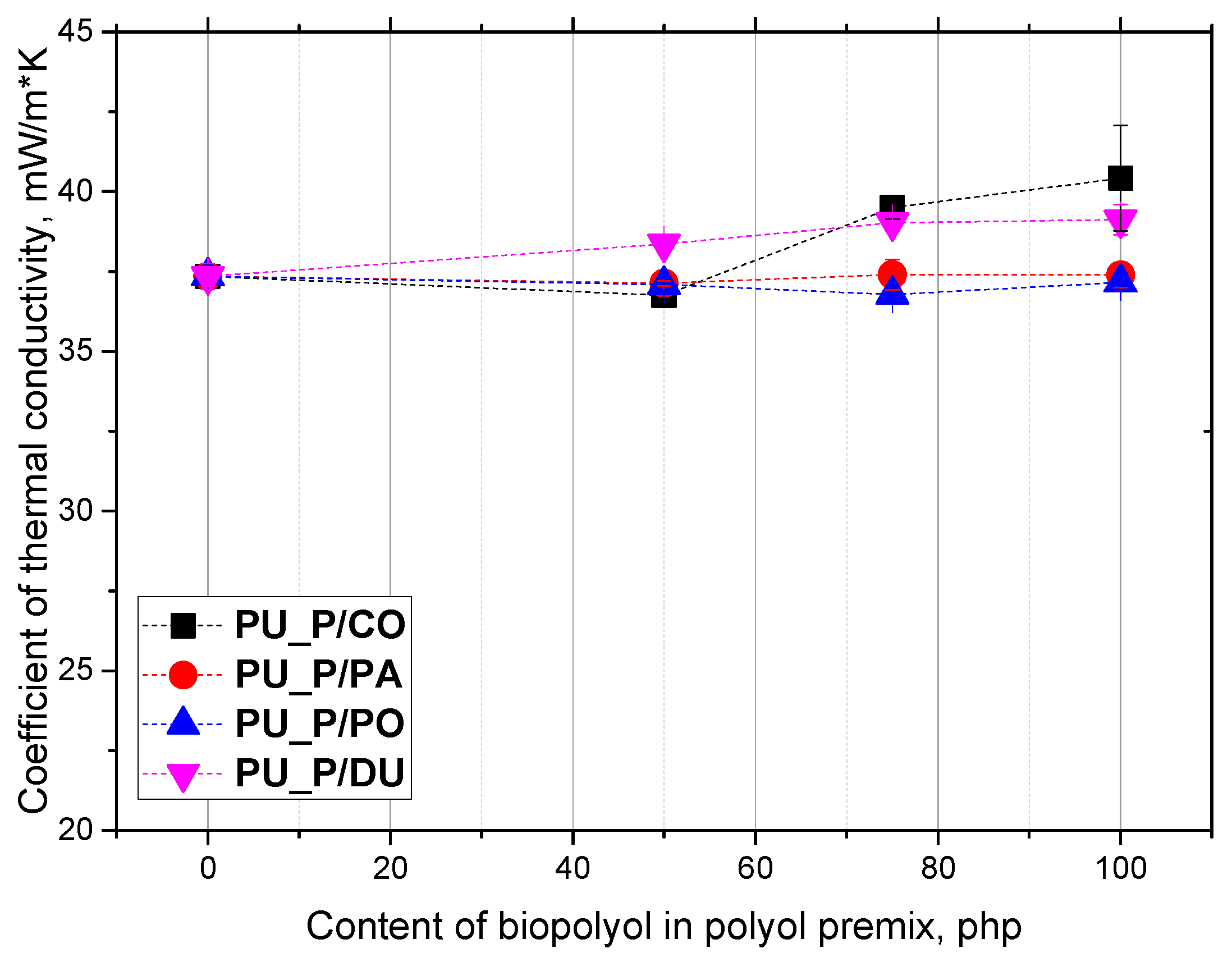
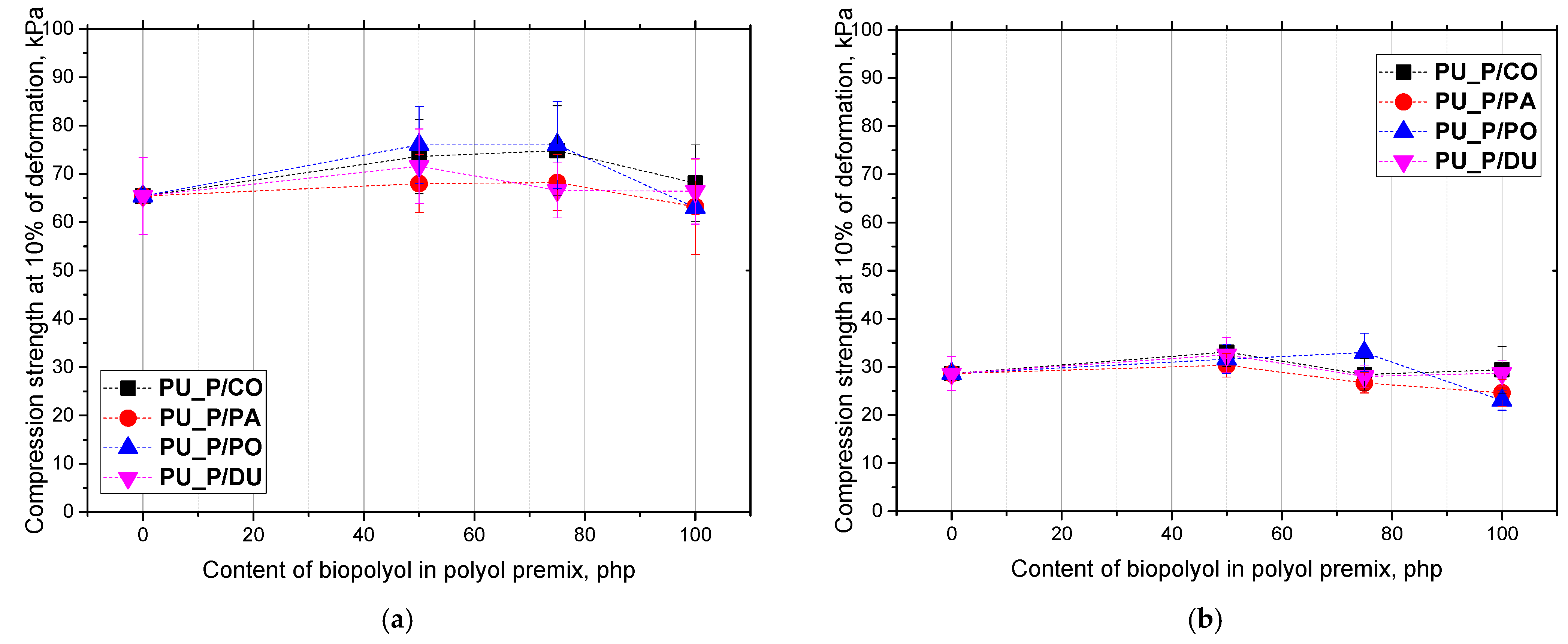
2. Results and Discussion
3. Materials and Methods
3.1. Biopolyol Synthesis and Characterization
3.2. Biofoam Manufacturing and Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M. “From seed to product”—Synthesis of high-value bio-polyol raw materials from white mustard seed processing and assessment of the influence of the degree of oxidation on the properties of the obtained products. Ind. Crops Prod. 2025, 227, 120813. [Google Scholar] [CrossRef]
- Da Silva Hyldmo, H.; Rye, S.A.; Vela-Almeida, D. A globally just and inclusive transition? Questioning policy representations of the European Green Deal. Glob. Environ. Change 2024, 89, 102946. [Google Scholar] [CrossRef]
- Wolf, S.; Teitge, J.; Mielke, J.; Schütze, F.; Jaeger, C. The European Green Deal—More Than Climate Neutrality. Intereconomics 2021, 56, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Paciorek-sadowska, J.; Borowicz, M.; Isbrandt, M. Effect of evening primrose (Oenothera biennis) oil cake on the properties of polyurethane/polyisocyanurate bio-composites. Int. J. Mol. Sci. 2021, 22, 8950. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Strakowska, A.; Kairyte, A. Application of walnut shells-derived biopolyol in the synthesis of rigid polyurethane foams. Materials 2020, 13, 2687. [Google Scholar] [CrossRef] [PubMed]
- Orjuela, A.; Clark, J. ScienceDirect Green chemicals from used cooking oils: Trends, challenges, and opportunities. Curr. Opin. Green Sustain. Chem. 2020, 26, 100369. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell polyurethane foams of very low density modified with various palm oil-based bio-polyols in accordance with cleaner production. J. Clean. Prod. 2021, 290, 125875. [Google Scholar] [CrossRef]
- Cappello, M.; Filippi, S.; Rossi, D.; Cinelli, P.; Anguillesi, I.; Camodeca, C.; Orlandini, E.; Polacco, G.; Seggiani, M. Waste-Cooking-Oil-Derived Polyols to Produce New Sustainable Rigid Polyurethane Foams. Sustainability 2024, 16, 9456. [Google Scholar] [CrossRef]
- Sarim, M.; Nikje, M.; Dargahi, M. Preparation and Characterization of Polyurethane Rigid FoamNanocomposites from Used Cooking Oil and Perlite. Int. J. Polym. Sci. 2023, 2023, 7185367. [Google Scholar] [CrossRef]
- Kurańska, M.; Polaczek, K.; Auguścik-Królikowska, M.; Prociak, A.; Ryszkowska, J. Open-cell rigid polyurethane bio-foams based on modified used cooking oil. Polymer 2020, 190, 122164. [Google Scholar] [CrossRef]
- Hasan, N.; Ratnam, M.V. Biodiesel Production from Waste Animal Fat by Transesterification Using H2SO4 and KOH Catalysts: A Study of Physiochemical Properties. Int. J. Chem. Eng. 2022, 2022, 6932320. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; Salar-García, M.J.; Veiga-del-Baño, J.M.; Chica, A.; Quesada-Medina, J. Waste animal fats as feedstock for biodiesel production using non-catalytic supercritical alcohol transesterification: A perspective by the PRISMA methodology. Energy Sustain. Dev. 2022, 69, 150–163. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Tech. 2009, 6, 677. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Lazdin, B.; Misane, M.; Vilsone, D. Biobased Polyurethanes from Rapeseed Oil Polyols: Structure, Mechanical and Thermal Properties. J. Polym. Environ. 2013, 21, 952–962. [Google Scholar] [CrossRef]
- Polaczek, K.; Kurańska, M. Hemp Seed Oil and Oilseed Radish Oil as New Sources of Raw Materials for the Synthesis of Bio-Polyols for Open-Cell Polyurethane Foams. Materials 2022, 15, 8891. [Google Scholar] [CrossRef] [PubMed]
- Malewska, E.; Kurańska, M.; Tenczyńska, M.; Prociak, A. Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials. Materials 2024, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- PN-87/C-04281; Determination of Iodine Value. Polish Committee for Standardization: Warsaw, Poland, 1981.
- PN-C-89052-03:1993; Polyethers for Polyurethanes—Test Methods—Determination of the Hydroxyl Value. Polish Committee for Standardization: Warsaw, Poland, 1993.
- ISO 845:2006; Cellular Plastics and Rubbers—Determination of Apparent Density. ISO: Geneva, Switzerland, 2006.
- ISO 8301:1991; Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties—Heat Flow Meter Apparatus. ISO: Geneva, Switzerland, 1991.
- ISO 844:2021; Rigid Cellular Plastics—Determination of Compression Properties. ISO: Geneva, Switzerland, 2021.
- ISO 2796:1986; Cellular Plastics, Rigid—Test for Dimensional Stability. ISO: Geneva, Switzerland, 1986.


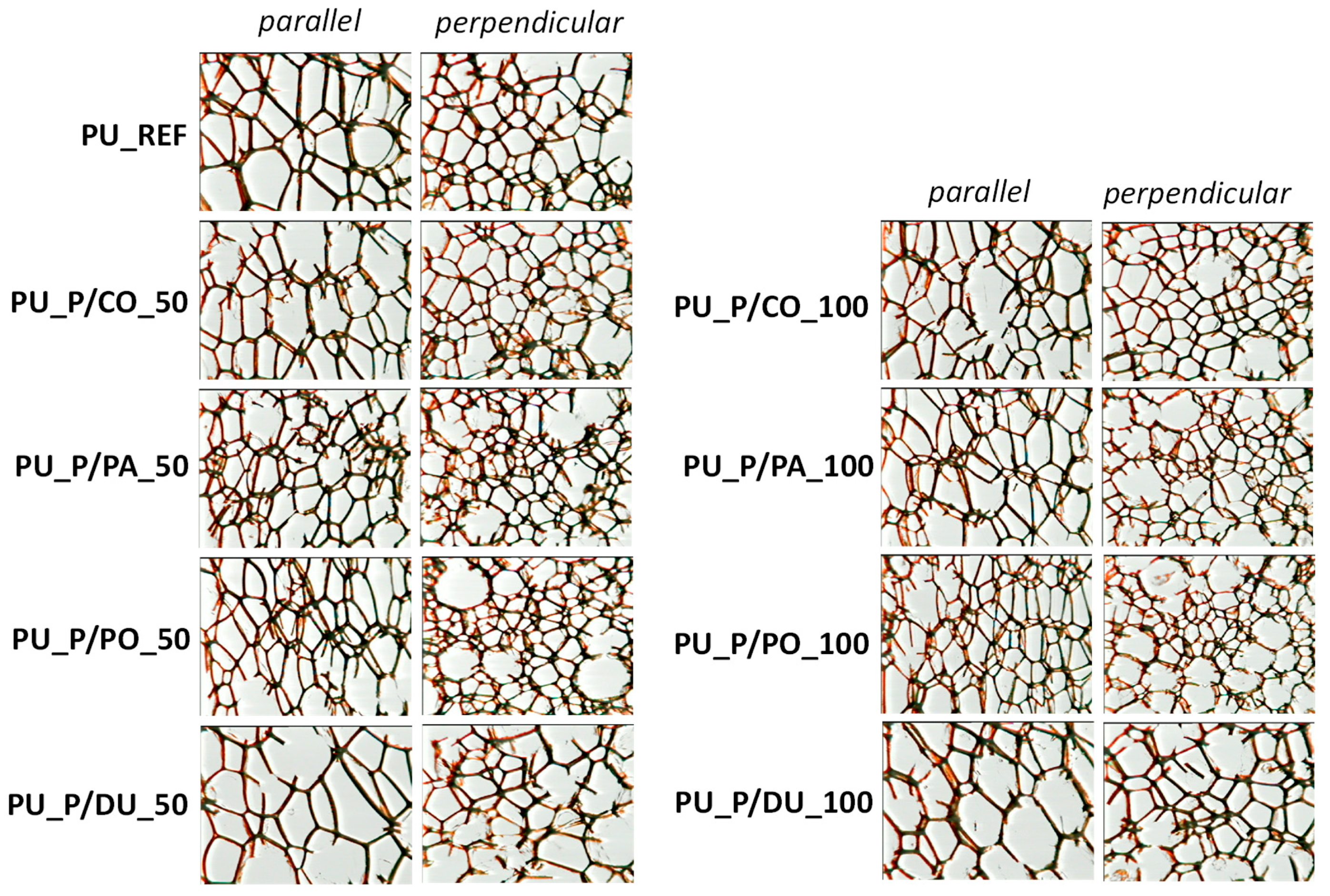


| Property | P/CO | P/PA | P/PO | P/DU |
|---|---|---|---|---|
| Hydroxyl value, mgKOH/g | 396 | 341 | 370 | 352 |
| Number molecular weight, g/mol | 330 | 510 | 510 | 510 |
| Dispersity | 1.25 | 1.27 | 1.24 | 1.25 |
| Functionality | 2.3 | 3.1 | 3.4 | 3.2 |
| Viscosity, mPa·s | 48 | 88 | 60 | 64 |
| Foam Symbol | Parallel-to-Foam-Growth Direction | Perpendicular-to-Foam-Growth Direction | ||
|---|---|---|---|---|
| Cross-Sectional Area, ·102 [mm2] | Anisotropy Index | Cross-Sectional Area, ·102 [mm2] | Anisotropy Index | |
| PU_REF | 2.52 · 10−2 ± 3.62 · 10−3 | 1.29 ± 8.32 · 10−2 | 9.64 · 10−3 ± 8.82 · 10−3 | 1.05 ± 4.73 · 10−2 |
| PU_P/CO_50 | 2.57 · 10−2 ± 4.50 · 10−3 | 1.26 ± 1.36 · 10−1 | 1.36 · 10−2 ± 1.51 · 10−3 | 1.09 ± 2.28 · 10−2 |
| PU_P/CO_100 | 2.25 · 10−2 ± 1.56 · 10−3 | 1.46 ± 3.83 · 10−2 | 1.30 · 10−2 ± 9.96 · 10−4 | 0.96 ± 3.01 · 10−2 |
| PU_P/PA_50 | 1.27 · 10−2 ± 9.37 · 10−4 | 1.19 ± 1.10 · 10−1 | 1.18 · 10−2 ± 1.17 · 10−3 | 1.03 ± 3.98 · 10−2 |
| PU_P/PA_100 | 1.59 · 10−2 ± 2.02 · 10−3 | 1.36 ± 1.07 · 10−1 | 1.48 · 10−2 ± 8.33 · 10−4 | 1.00 ± 1.69 · 10−2 |
| PU_P/PO_50 | 1.66 · 10−2 ± 2.44 · 10−3 | 1.62 ± 1.26 · 10−1 | 1.26 · 10−2 ± 6.04 · 10−4 | 0.95 ± 3.33 · 10−2 |
| PU_P/PO_100 | 1.13 · 10−2 ± 4.26 · 10−4 | 1.52 ± 3.74 · 10−2 | 1.54 · 10−2 ± 6.12 · 10−4 | 1.03 ± 3.77 · 10−2 |
| PU_P/DU_50 | 4.89 · 10−2 ± 2.62 · 10−3 | 1.38 ± 8.46 · 10−2 | 3.46 · 10−2 ± 5.60 · 10−3 | 1.01 ± 2.48 · 10−2 |
| PU_P/DU_100 | 3.33 · 10−2 ± 9.92 · 10−4 | 1.20 ± 6.16 · 10−2 | 1.94 · 10−2 ± 1.05 · 10−3 | 0.95 ± 3.65 · 10−2 |
| Symbols of Samples | −25 °C | 70 °C | ||||
|---|---|---|---|---|---|---|
| Length | Width | Thickness | Length | Width | Thickness | |
| REF | 0.32 | 0.28 | 0.71 | 0.33 | 0.18 | 0.35 |
| PU_P/CO_50 | 0.25 | 0.54 | 0.56 | 0.02 | 0.54 | −0.12 |
| PU_P/CO_75 | 0.97 | 0.65 | 0.35 | 0.44 | 0.27 | 0.13 |
| PU_P/CO_100 | 0.49 | 0.94 | 0.62 | 0.18 | 0.41 | 0.17 |
| PU_P/PA_50 | 0.23 | 0.38 | 1.04 | 0.42 | 0.42 | −0.43 |
| PU_P/PA_75 | 0.08 | 0.30 | 0.89 | 0.23 | 0.29 | −0.33 |
| PU_P/PA_100 | 0.13 | 0.30 | 0.96 | 0.13 | 0.26 | −0.21 |
| PU_P/PO_50 | 0.06 | 0.27 | 0.62 | 0.61 | 0.40 | 0.15 |
| PU_P/PO_75 | 0.10 | 0.12 | 0.70 | 0.55 | 0.39 | −0.14 |
| PU_P/PO_100 | 0.10 | 0.17 | 0.58 | 0.15 | 0.20 | 0.13 |
| PU_P/DU_50 | 0.15 | 0.14 | 0.22 | 0.19 | 0.21 | 0.07 |
| PU_P/DU_75 | 0.09 | 0.17 | 0.59 | 0.08 | 0.07 | 0.16 |
| PU_P/DU_100 | 0.06 | 0.18 | 0.40 | 0.03 | −0.10 | 0.21 |
| Properties | CO | PA | PO | DU |
|---|---|---|---|---|
| Iodine value, gI2/100 g | 10.3 | 47.5 | 54.8 | 64.8 |
| Number average molecular weight, g/mol | 748 | 927 | 853 | 929 |
| Dispersity | 1.01 | 1.04 | 1.01 | 1.01 |
| Viscosity, mPa·s | 19 | 47 | 32 | 28 |
| Foam Symbol/ Polyol | RF551 | P/CO | P/PA | P/PO | P/DU |
|---|---|---|---|---|---|
| REF | 100 | 0 | 0 | 0 | 0 |
| PU_P/CO_50 | 50 | 50 | 0 | 0 | 0 |
| PU_P/CO_75 | 25 | 75 | 0 | 0 | 0 |
| PU_P/CO_100 | 0 | 100 | 0 | 0 | 0 |
| PU_P/PA_50 | 50 | 0 | 50 | 0 | 0 |
| PU_P/PA_75 | 25 | 0 | 75 | 0 | 0 |
| PU_P/PA_100 | 0 | 0 | 100 | 0 | 0 |
| PU_P/PO_50 | 50 | 0 | 0 | 50 | 0 |
| PU_P/PO_75 | 25 | 0 | 0 | 75 | 0 |
| PU_P/PO_100 | 0 | 0 | 0 | 100 | 0 |
| PU_P/DU_50 | 50 | 0 | 0 | 0 | 50 |
| PU_P/DU_75 | 25 | 0 | 0 | 0 | 75 |
| PU_P/DU_100 | 0 | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malewska, E.; Kurańska, M.; Grelowska, K.; Put, A.; Ożóg, H.; Sędzimir, J.; Kowalik, N.; Kucała, M.; Prociak, A. Hydroxyl Derivatives of Oils from Solid Fats as Components for Production of Polyurethane Foams. Molecules 2025, 30, 2703. https://doi.org/10.3390/molecules30132703
Malewska E, Kurańska M, Grelowska K, Put A, Ożóg H, Sędzimir J, Kowalik N, Kucała M, Prociak A. Hydroxyl Derivatives of Oils from Solid Fats as Components for Production of Polyurethane Foams. Molecules. 2025; 30(13):2703. https://doi.org/10.3390/molecules30132703
Chicago/Turabian StyleMalewska, Elżbieta, Maria Kurańska, Klara Grelowska, Aleksandra Put, Hubert Ożóg, Julia Sędzimir, Natalia Kowalik, Michał Kucała, and Aleksander Prociak. 2025. "Hydroxyl Derivatives of Oils from Solid Fats as Components for Production of Polyurethane Foams" Molecules 30, no. 13: 2703. https://doi.org/10.3390/molecules30132703
APA StyleMalewska, E., Kurańska, M., Grelowska, K., Put, A., Ożóg, H., Sędzimir, J., Kowalik, N., Kucała, M., & Prociak, A. (2025). Hydroxyl Derivatives of Oils from Solid Fats as Components for Production of Polyurethane Foams. Molecules, 30(13), 2703. https://doi.org/10.3390/molecules30132703








