Understanding Anti-Obesity Potential of Four Porphyrin Compounds by Investigating Pancreatic Lipase Inhibition
Abstract
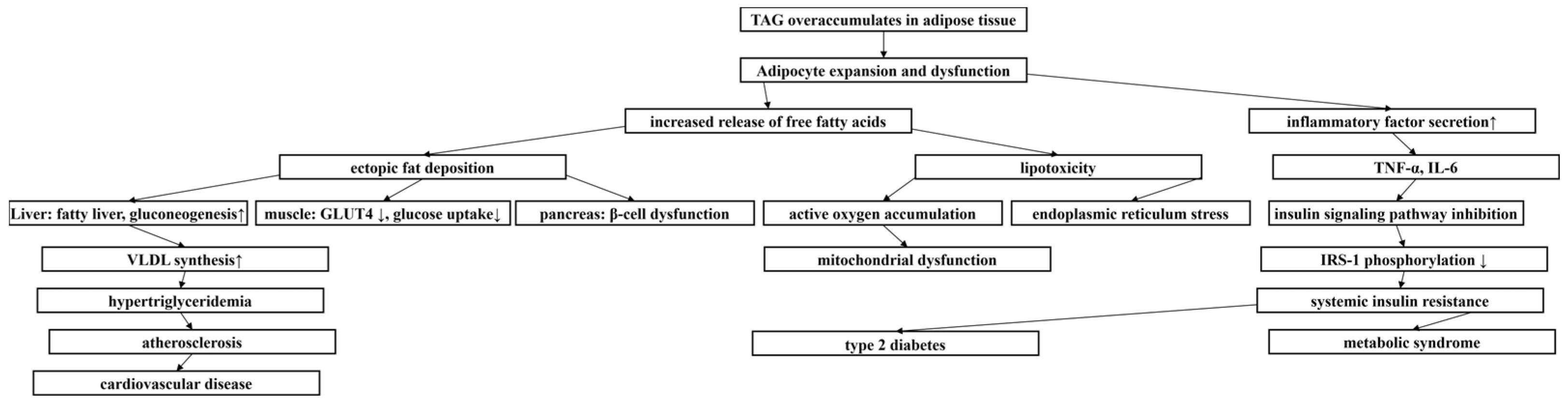
1. Introduction
2. Results
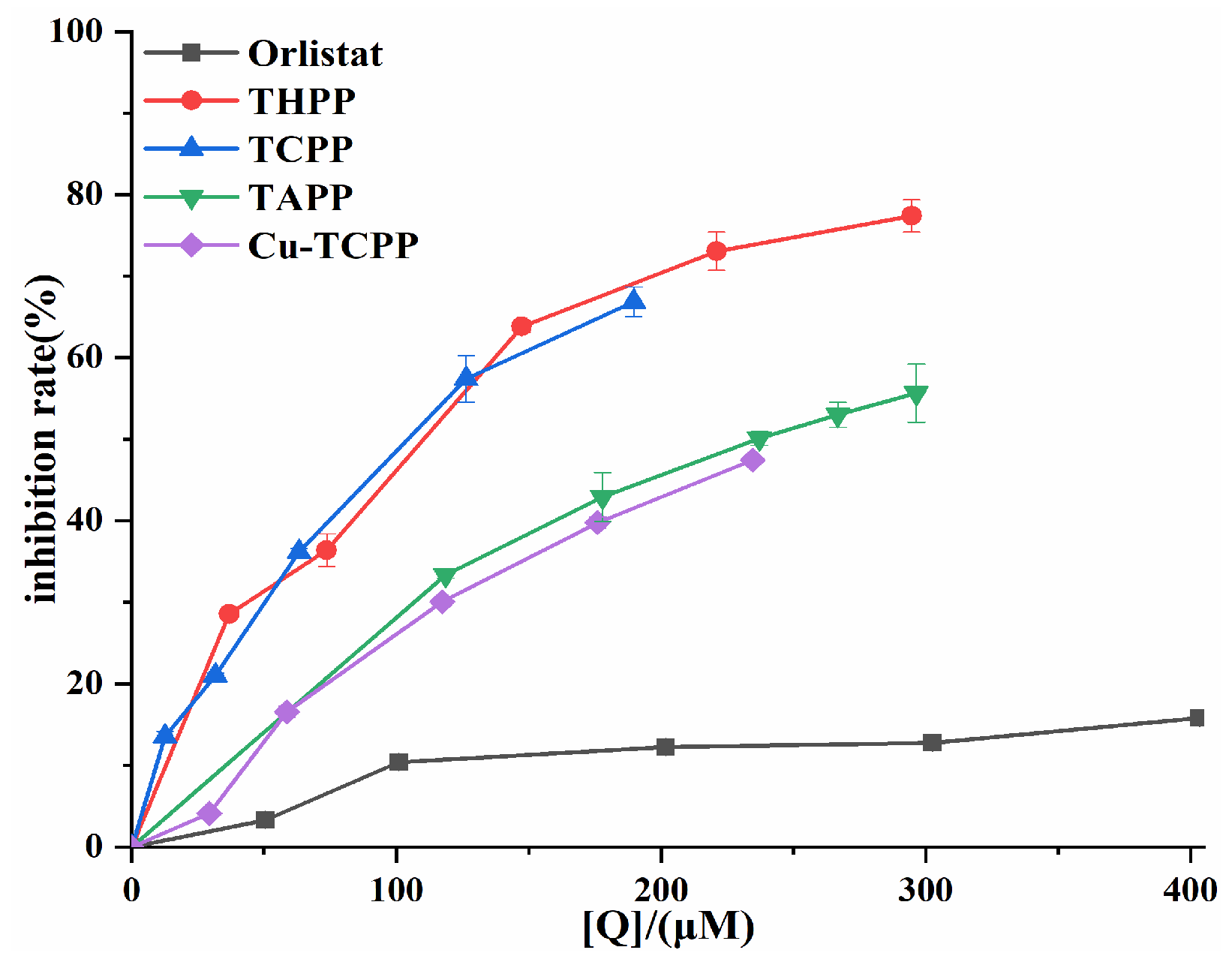
2.1. Pancreatic Lipase Inhibition by Four Porphyrin Compounds
2.2. Mode of Inhibition of Pancreatic Lipase by Four Porphyrin Compounds
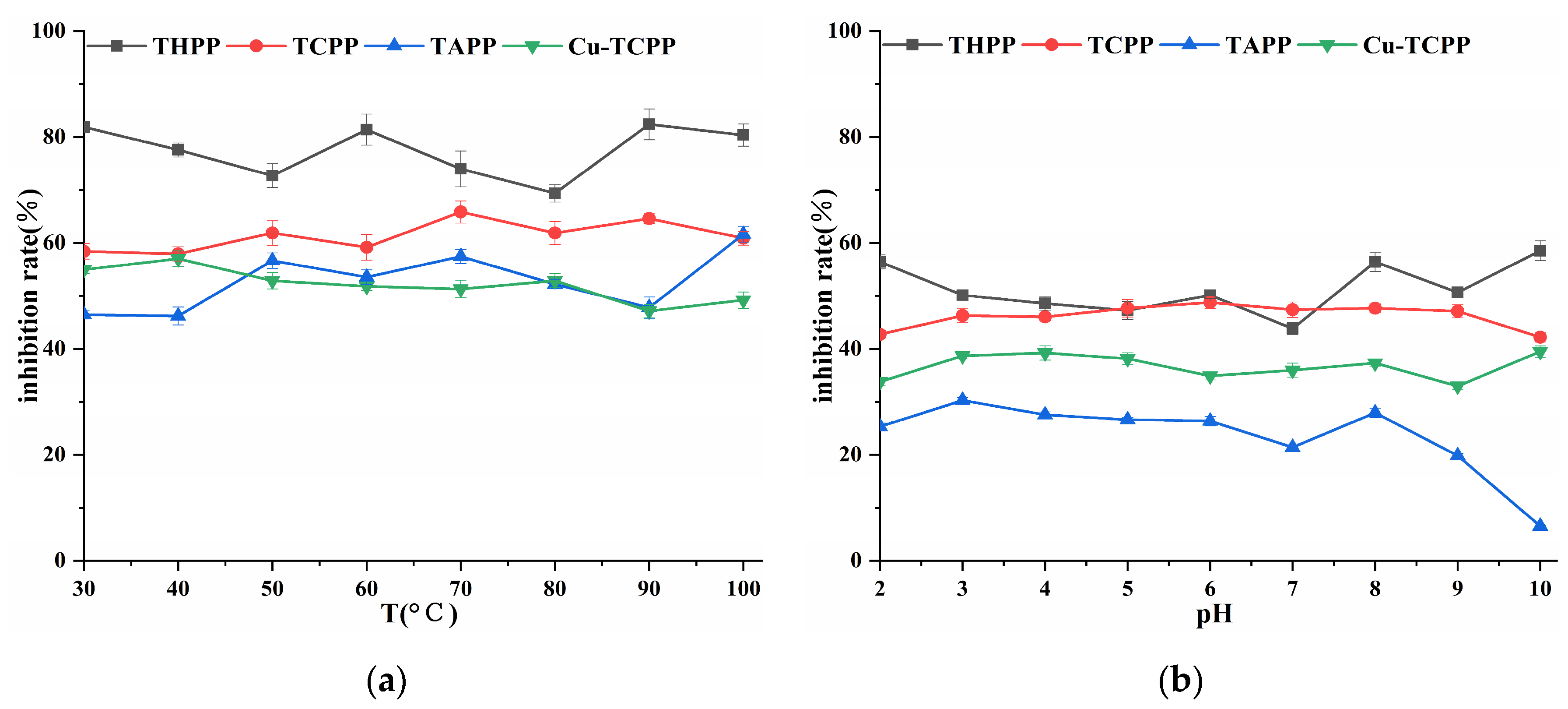
2.3. Analysis of in Vitro Stability
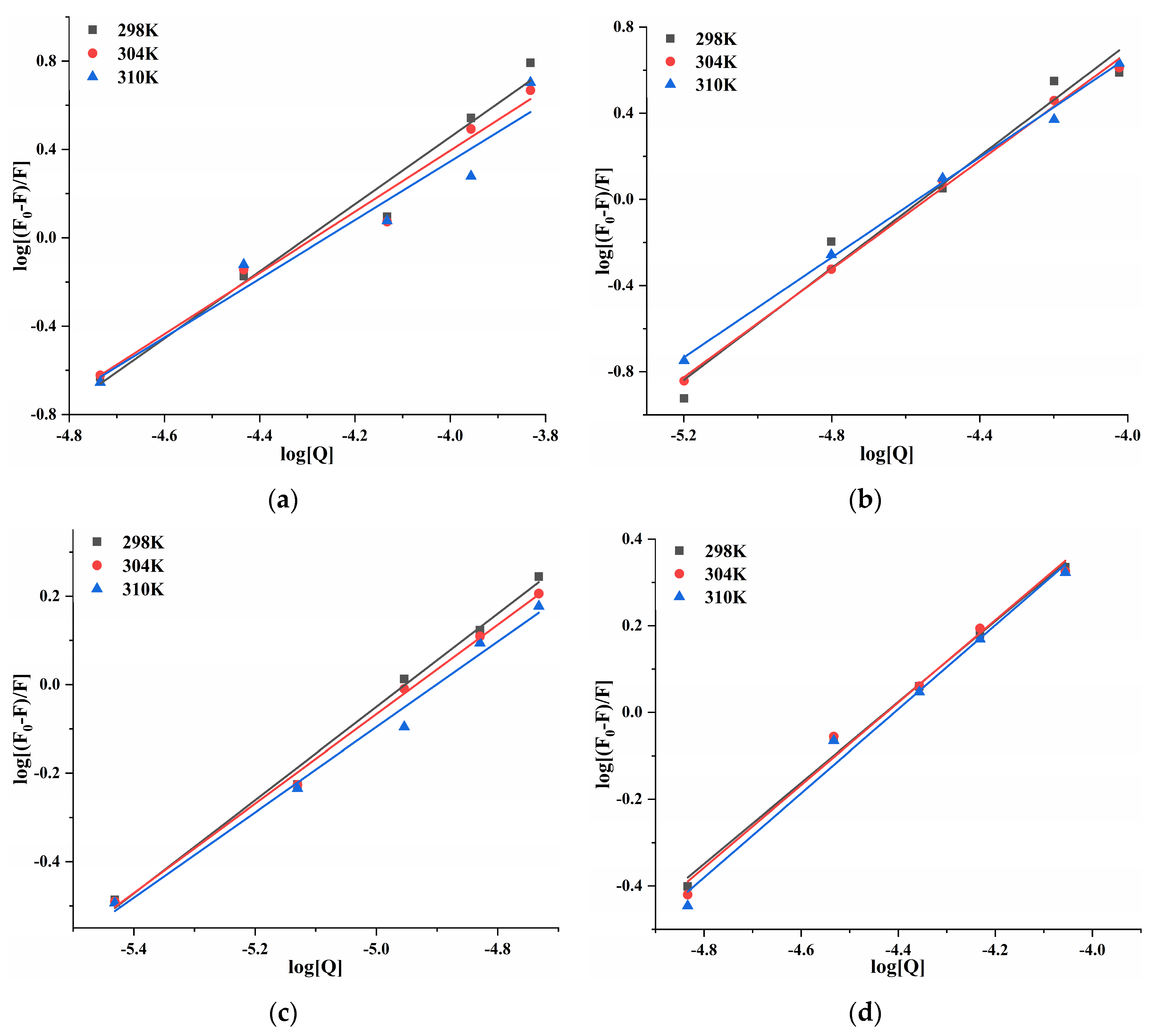
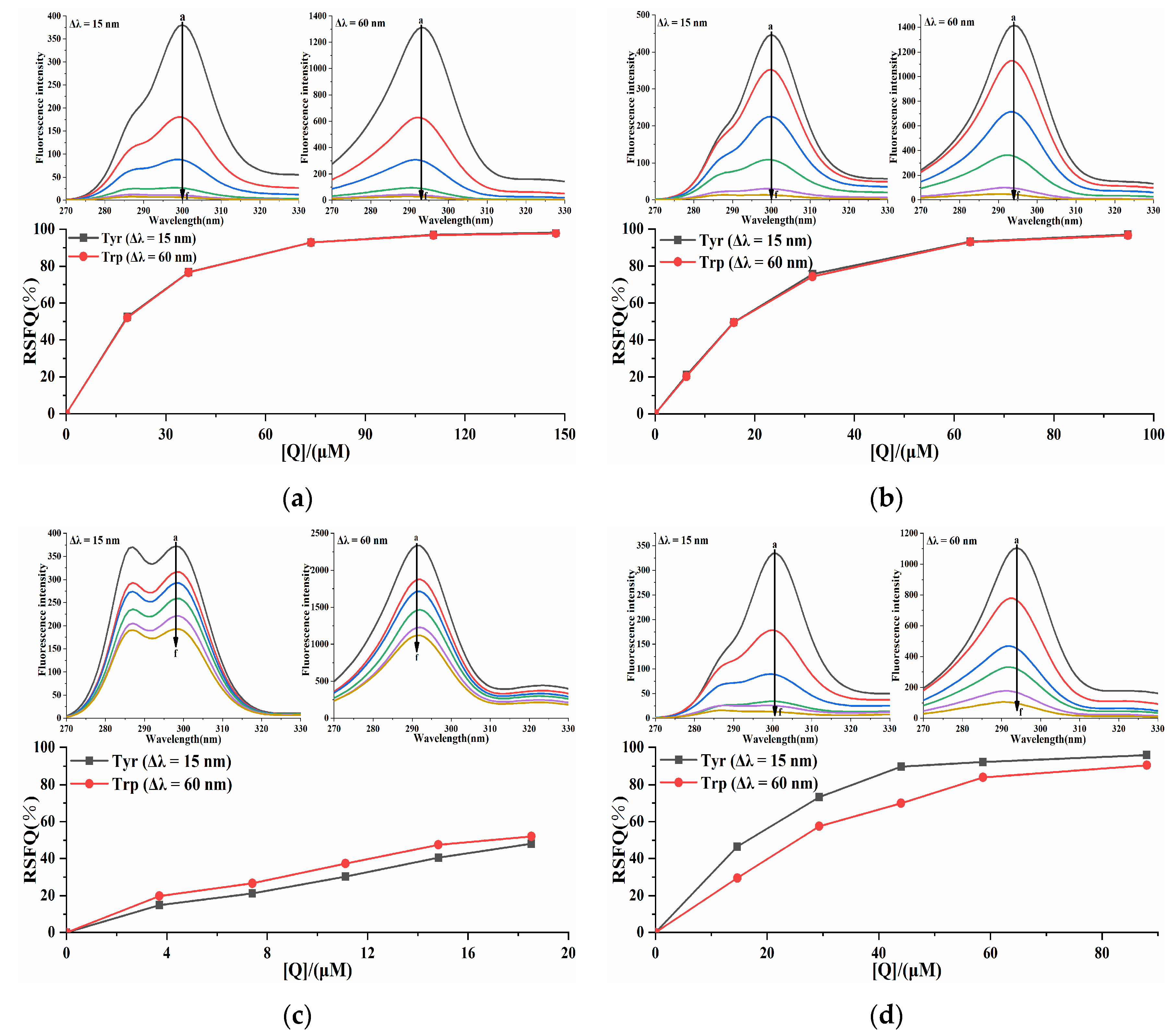
2.4. Fluorescent Quenching and Binding Interactions of Pancreatic Lipase with Four Porphyrin Compounds
2.5. Synchronous Fluorescence Analysis of the Pancreatic Lipase-Four Porphyrin Compounds Interaction
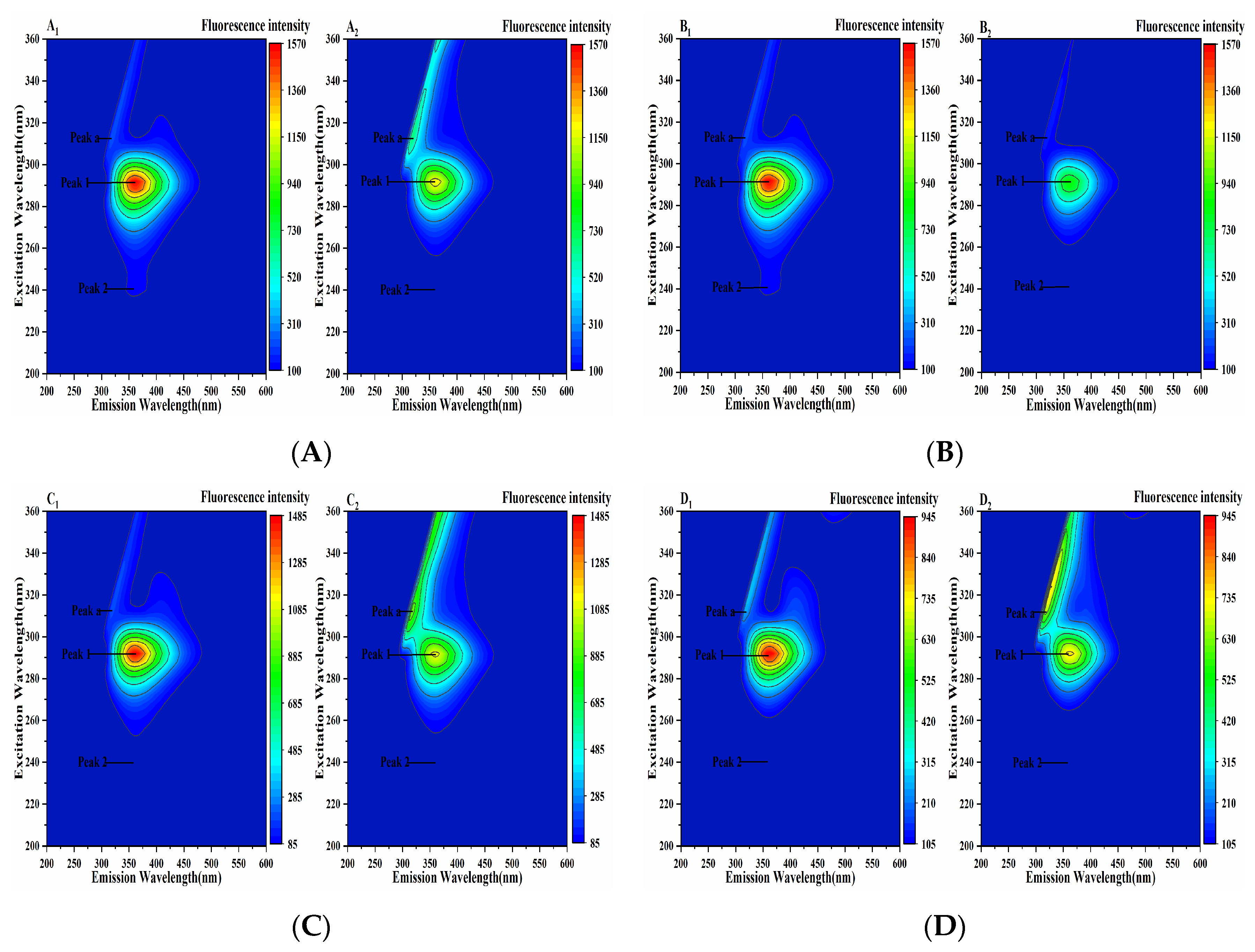
2.6. Three-Dimensional Fluorescence Analysis of the Pancreatic Lipase-Four Porphyrin Compounds Interaction
2.7. FT-IR Spectroscopy Analysis of the Pancreatic Lipase-Four Porphyrin Compounds Interaction
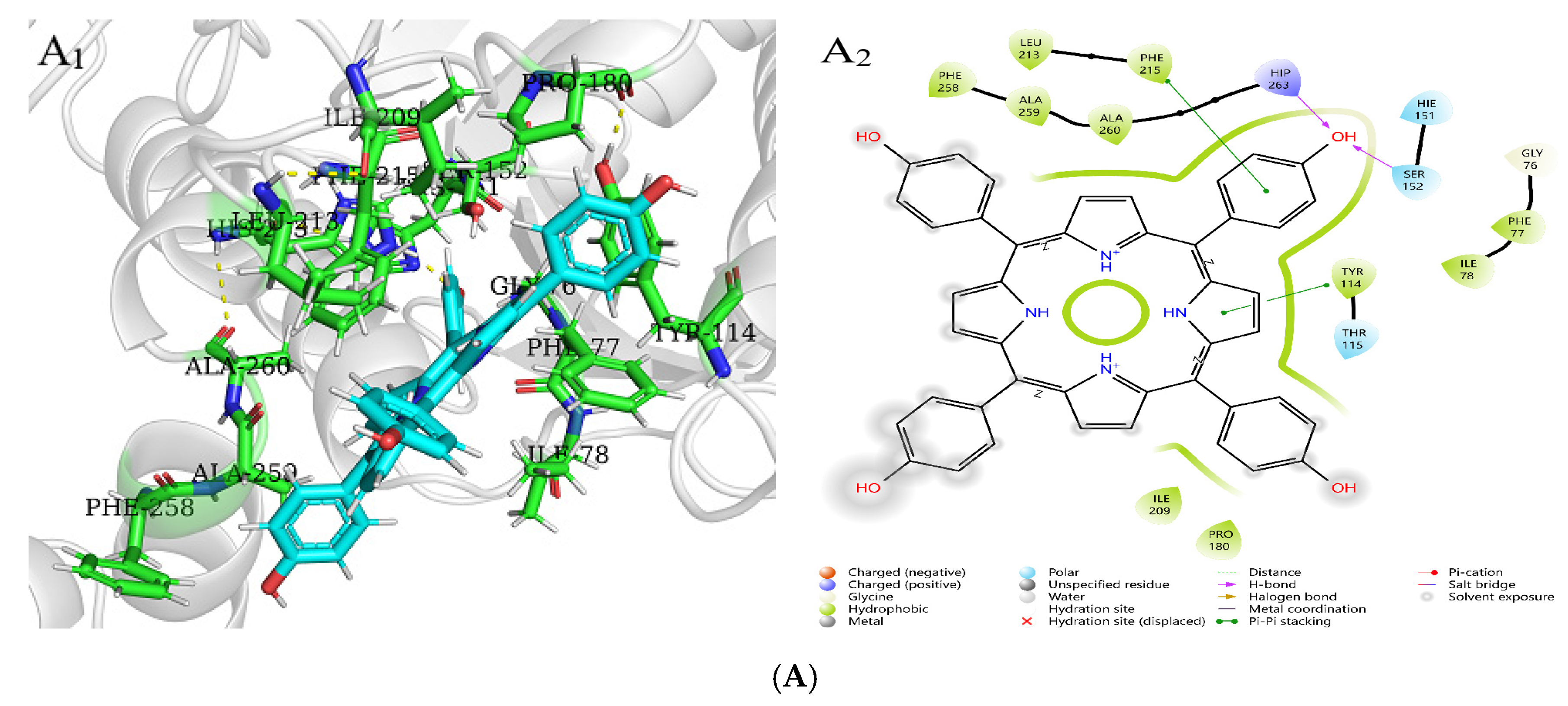
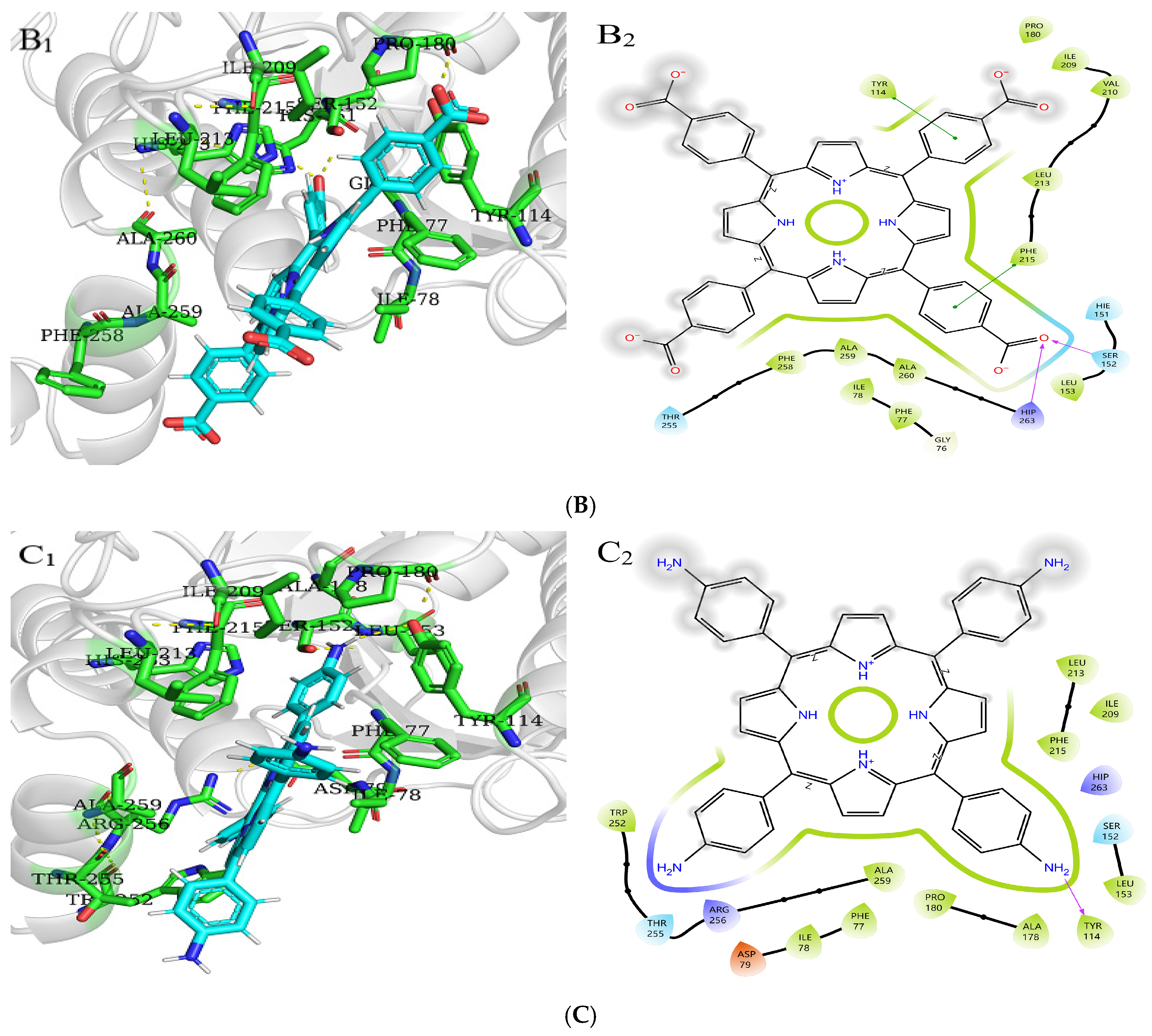
2.8. Molecular Docking Analysis of Three Porphyrin Compounds with Pancreatic Lipase
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Inhibition Studies
3.2.1. Pancreatic Lipase Inhibition Assays
3.2.2. Mode of Pancreatic Lipase Inhibition
3.2.3. Study on Stability In Vitro
3.3. Fluorescence Analysis of the Pancreatic Lipase-Inhibitor Interaction
3.4. Synchronous Fluorescence Analysis of the Pancreatic Lipase–Inhibitor Interaction
3.5. Three-Dimensional Fluorescence Analysis of the Pancreatic Lipase-Inhibitor Interaction
3.6. FT-IR Spectroscopy Analysis of the Pancreatic Lipase-Inhibitor Interaction
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daub, C.D.; Michaels, A.L.; Mabate, B.; Mkabayi, L.; Edkins, A.L.; Pletschke, B.I. Exploring the Inhibitory Potential of Sodium Alginate Against Digestive Enzymes Linked to Obesity and Type 2 Diabetes. Molecules 2025, 30, 1155. [Google Scholar] [CrossRef] [PubMed]
- Harismitha, S.; Deora, N.; Khusro, A. Molecular docking and pharmacokinetics prediction of piperine and capsaicin as human pancreatic lipase inhibitors: An in silico study. Cureus 2024, 16, e67870. [Google Scholar]
- Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. Int. J. Mol. Sci. 2023, 24, 1529. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, N.S.; Pan, X.F.; Chen, L.L.; Pan, A. Clinical management and treatment of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, N.U.A.; Ahmad, M.; Farrukh, M.; Rafique, S. Pancreatic lipase inhibitors as anti-obesity agents: A review of recent chemical scaffolds and their pancreatic lipase inhibitory potential. Med. Chem. Res. 2025, 34, 497–516. [Google Scholar] [CrossRef]
- Ahmed, N.; Asif, S.; Arfan, M.; Mahmood, Q.; Islam, A.; Gatasheh, M.K.; Zia, M. Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities. Molecules 2024, 29, 765. [Google Scholar] [CrossRef] [PubMed]
- Dechakhamphu, A.; Wongchum, N. Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs. Asian Pac. J. Trop. Biomed. 2015, 5, 1042–1045. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Louckova, A.; Jaegerova, T.; Tokarova, V.; Hajslova, J. The In Vitro Inhibitory Effect of Selected Asteraceae Plants on Pancreatic Lipase Followed by Phenolic Content Identification Through Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS). Int. J. Mol. Sci. 2022, 23, 11204. [Google Scholar] [CrossRef]
- Haddou, S.; Elrherabi, A.; Loukili, E.H.; Abdnim, R.; Hbika, A.; Bouhrim, M.; Al Kamaly, O.; Saleh, A.; Shahat, A.A.; Bnouham, M.; et al. Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts. Molecules 2024, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal plants and their inhibitory activities against pancreatic lipase: A review. eCAM 2015, 2015, 973143. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.N.; Justino, A.B.; Martins, M.M.; Peixoto, L.G.; Vilela, D.D.; Santos, P.S.; Teixeira, T.L.; da Silva, C.V.; Goulart, L.R.; Pivatto, M.; et al. Stephalagine, an alkaloid with pancreatic lipase inhibitory activity isolated from the fruit peel of Annona crassiflora Mart. Ind. Crops Prod. 2017, 97, 324–329. [Google Scholar] [CrossRef]
- Wang, P.; Song, X.; Liang, Q. Molecular Docking Studies and In Vitro Activity of Pancreatic Lipase Inhibitors from Yak Milk Cheese. Int. J. Mol. Sci. 2025, 26, 756. [Google Scholar] [CrossRef] [PubMed]
- Sukhdev, S.; Singh, K.S. Therapeutic role of phytomedicines on obesity: Importance of herbal pancreatic lipase inhibitors. Int. Res. J. Med. Sci. 2013, 1, 15–26. [Google Scholar]
- Subramaniyan, V.; Hanim, Y.U. Role of pancreatic lipase inhibition in obesity treatment: Mechanisms and challenges towards current insights and future directions. Int. J. Obes. 2025, 49, 492–506. [Google Scholar] [CrossRef]
- Khajavi, N.; Biebermann, H.; Tschöp, M.; DiMarchi, R. Treatment of diabetes and obesity by rationally designed peptide agonists functioning at multiple metabolic receptors. Endocr. Dev. 2017, 32, 165–182. [Google Scholar] [PubMed]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget Action of Xanthones from Garcinia mangostana against α-Amylase, α-Glucosidase and Pancreatic Lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.V.; Huynh, H.A.; Van Truong, D.; Tran, T.D.; Vo, C.V.T. Exploring Aurone Derivatives as Potential Human Pancreatic Lipase Inhibitors through Molecular Docking and Molecular Dynamics Simulations. Molecules 2020, 25, 4657. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Savych, A.; Marchyshyn, S. Inhibition of pancreatic lipase by water extracts of some herbal mixtures. PharmacologyOnLine 2021, 2, 1457–1463. [Google Scholar]
- Xu, Z.; Chang, J.; Qin, Z.; Liu, H.; Yan, X.; Guan, S.; Dong, X.; Wu, J.; Li, T. Preparation and characterization of a sea cucumber collagen-derived peptide and the inhibition of pancreatic lipase. Food Biosci. 2025, 65, 106013. [Google Scholar] [CrossRef]
- Almaliti, J.; Alzweiri, M.; Alhindy, M.; Al-Helo, T.; Daoud, I.; Deknash, R.; Naman, C.B.; Abu-Irmaileh, B.; Bustanji, Y.; Hamad, I. Discovery of Novel Epoxyketone Peptides as Lipase Inhibitors. Molecules 2022, 27, 2261. [Google Scholar] [CrossRef]
- Ponce Martínez, C.; Murcia García, E.; Pérez Sánchez, H.; Milagro, F.I.; Riezu-Boj, J.I.; Ramos Molina, B.; Gómez Gallego, M.; Zamora, S.; Cañavate Cutillas, R.; Hernández Morante, J.J. Effect of Silibinin on Human Pancreatic Lipase Inhibition and Gut Microbiota in Healthy Volunteers: A Randomized Controlled Trial. Int. J. Mol. Sci. 2024, 25, 12853. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Assessing the In Vitro Inhibitory Effects on Key Enzymes Linked to Type-2 Diabetes and Obesity and Protein Glycation by Phenolic Compounds of Lauraceae Plant Species Endemic to the Laurisilva Forest. Molecules 2021, 26, 2023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Sun, Z. Self-assembled porphyrin and macrocycle derivatives: From synthesis to function. MRS Bull. 2019, 44, 167–171. [Google Scholar] [CrossRef]
- Osuka, A.; Saito, S. Expanded porphyrins and aromaticity. Chem. Commun. 2011, 47, 4330–4339. [Google Scholar] [CrossRef] [PubMed]
- Oohora, K.; Hayashi, T. Hemoprotein-based supramolecular assembling systems. Curr. Opin. Chem. Biol. 2014, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Brocksom, T.J.; de Oliveira, K.T. Tetrabenzoporphyrins: Synthetic developments and applications. Chem. Soc. Rev. 2013, 42, 3302–3317. [Google Scholar] [CrossRef]
- Longevial, J.F.; Clément, S.; Wytko, J.A.; Ruppert, R.; Weiss, J.; Richeter, S. Peripherally metalated porphyrins with applications in catalysis, molecular electronics and biomedicine. Chem.-Eur. J. 2018, 24, 15442–15460. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.S.; Geetha, K.; Gayathri, M.; Shanmugam, G. Synthesis, crystal structures, spectroscopic characterization and in vitro antidiabetic studies of new Schiff base Copper(II) complexes. J. Chem. Sci. 2016, 128, 1095–1102. [Google Scholar] [CrossRef]
- Peng, X.; Liu, K.; Hu, X.; Gong, D.; Zhang, G. Hesperetin-Cu(II) complex as potential α-amylase and α-glucosidase inhibitor: Inhibition mechanism and molecular docking. Spectrochim. Acta Part A 2023, 290, 122301. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J.; Guo, Y.; Miao, M. Mechanism of binding interactions between young apple polyphenols and porcine pancreatic α-amylase. Food Chem. 2019, 283, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, J.; Wang, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Inhibitory kinetics and mechanism of flavonoids from lotus (Nelumbo nucifera Gaertn.) leaf against pancreatic α-amylase. Int. J. Biol. Macromol. 2018, 120, 2589–2596. [Google Scholar] [CrossRef]
- Ren, S.C.; Li, K.K.; Liu, Z.L. Research on the influences of five food-borne polyphenols on in vitro slow starch digestion and the mechanism of action. J. Agric. Food Chem. 2019, 67, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Poureshghi, F.; Ghandforoushan, P.; Safarnejad, A.; Soltani, S. Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): Application of spectroscopic techniques and molecular modeling methods. J. Photochem. Photobiol. B 2017, 166, 187–192. [Google Scholar] [CrossRef]
- Du, X.P.; Bai, M.L.; Huang, Y.; Jiang, Z.D.; Chen, F.; Ni, H.; Li, Q.B. Inhibitory effect of astaxanthin on pancreatic lipase with inhibition kinetics integrating molecular docking simulation. J. Funct. Foods 2018, 48, 551–557. [Google Scholar] [CrossRef]
- Almasri, I.M. Computational approaches for the discovery of natural pancreatic lipase inhibitors as antiobesity agents. Future Med. Chem. 2020, 12, 741–757. [Google Scholar] [CrossRef]
- Chen, T.; He, S.; Zhang, J.; Wang, H.; Jia, Y.; Liu, Y.; Xie, M.; Cheng, G. Effects of Ultra-High-Pressure Treatment on Chemical Composition and Biological Activities of Free, Esterified and Bound Phenolics from Phyllanthus emblica L. Fruits. Molecules 2024, 29, 3181. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Zhou, C.Y.; Pan, S.T.; Cui, Z.W.; Xie, F.; Wang, G.Q.; Ai, L.Z.; Zhang, H. Inhibitory Effects of Two Polysaccharides from Highland Barley on the Activity of Pancreatic α-amylase. Food Sci. 2023, 44, 192–199. [Google Scholar]
- Ansari, A. Decoding the binding interaction of steroidal pyridines with bovine serum albumin using spectroscopic and molecular docking techniques. Steroids 2023, 192, 109156. [Google Scholar] [PubMed]
- Alameen, A.A.; Alothman, M.R.; Al Wahibi, M.S.; Abdullah, E.M.; Ali, R.; Abdalla, M.; Fattiny, S.Z.A.; Elsayim, R. Potential Effect of Baobab’s Polyphenols as Antihyperlipidemic Agents: In Silico Study. Molecules 2023, 28, 6112. [Google Scholar] [CrossRef] [PubMed]




| System | C (μM) | Km (μM) | Vmax (ΔOD/min) | Ki (μM) |
|---|---|---|---|---|
| PL-THPP | 0 | 0.6122 ± 0.0093 | 0.0054 ± 0.0008 | 37.0317 ± 0.1864 |
| 36.84 | 0.4652 ± 0.0107 | 0.0041 ± 0.0009 | ||
| 73.67 | 0.3925 ± 0.0151 | 0.0035 ± 0.0013 | ||
| 147.34 | 0.2050 ± 0.0075 | 0.0018 ± 0.0007 | ||
| 221.01 | 0.1332 ± 0.0045 | 0.0012 ± 0.0004 | ||
| 294.68 | 0.1086 ± 0.0026 | 0.0009 ± 0.0002 | ||
| PL-TCPP | 0 | 0.6122 ± 0.0093 | 0.0054 ± 0.0008 | 53.4882 ± 0.1409 |
| 12.65 | 0.4794 ± 0.0128 | 0.0042 ± 0.0011 | ||
| 31.61 | 0.4239 ± 0.0092 | 0.0037 ± 0.0008 | ||
| 63.23 | 0.3108 ± 0.0142 | 0.0027 ± 0.0012 | ||
| 126.45 | 0.1738 ± 0.0041 | 0.0015 ± 0.0004 | ||
| 189.68 | 0.1338 ± 0.0029 | 0.0012 ± 0.0003 | ||
| PL-TAPP | 0 | 0.4584 ± 0.0145 | 0.0040 ± 0.0013 | 149.8681 ± 0.1952 |
| 118.55 | 0.2551 ± 0.0037 | 0.0023 ± 0.0003 | ||
| 177.83 | 0.2096 ± 0.0036 | 0.0018 ± 0.0003 | ||
| 237.11 | 0.1786 ± 0.0068 | 0.0016 ± 0.0006 | ||
| 266.75 | 0.1651 ± 0.0029 | 0.0015 ± 0.0003 | ||
| 296.38 | 0.1545 ± 0.0057 | 0.0014 ± 0.0005 | ||
| PL-Cu-TCPP | 0 | 0.2054 ± 0.0054 | 0.0055 ± 0.0014 | 196.1540 ± 0.1310 |
| 29.33 | 0.1920 ± 0.0049 | 0.0052 ± 0.0013 | ||
| 58.66 | 0.1643 ± 0.0037 | 0.0044 ± 0.0009 | ||
| 117.33 | 0.1333 ± 0.0022 | 0.0036 ± 0.0006 | ||
| 175.99 | 0.1115 ± 0.0046 | 0.0030 ± 0.0012 | ||
| 234.66 | 0.0988 ± 0.0017 | 0.0026 ± 0.0005 |
| System | T | Ksv (×104 L/mol) | Kq (×1012 L/mol/s) | n | Ka (×105 L/mol) |
|---|---|---|---|---|---|
| PL-THPP | 298 | 1.36 ± 0.02 | 1.36 ± 0.02 | 1.52 | 34.27 ± 0.12 |
| 304 | 1.17 ± 0.03 | 1.17 ± 0.03 | 1.38 | 10.33 ± 0.08 | |
| 310 | 1.11 ± 0.04 | 1.11 ± 0.04 | 1.33 | 4.54 ± 0.05 | |
| PL-TCPP | 298 | 4.60 ± 0.07 | 4.60 ± 0.07 | 1.30 | 8.37 ± 0.07 |
| 304 | 4.59 ± 0.06 | 4.59 ± 0.06 | 1.26 | 5.15 ± 0.04 | |
| 310 | 4.50 ± 0.06 | 4.50 ± 0.06 | 1.16 | 2.86 ± 0.03 | |
| PL-TAPP | 298 | 9.70 ± 0.11 | 9.70 ± 0.11 | 1.05 | 1.66 ± 0.01 |
| 304 | 8.80 ± 0.06 | 8.80 ± 0.06 | 1.01 | 0.97 ± 0.02 | |
| 310 | 8.17 ± 0.08 | 8.17 ± 0.08 | 0.96 | 0.54 ± 0.01 | |
| PL-Cu-TCPP | 298 | 2.36 ± 0.01 | 2.36 ± 0.01 | 0.93 | 0.13 ± 0.01 |
| 304 | 2.33 ± 0.03 | 2.33 ± 0.03 | 0.95 | 0.16 ± 0.01 | |
| 310 | 2.32 ± 0.03 | 2.32 ± 0.03 | 0.97 | 0.19 ± 0.01 |
| System | T | ΔH0 (kJ/mol) | ΔG0 (kJ/mol) | ΔS0 (J/mol/k) | R2 |
|---|---|---|---|---|---|
| PL-THPP | 298 | −129.48 ± 0.11 | −37.28 ± 0.17 | −309.86 ± 0.12 | 0.98 |
| 304 | −34.99 ± 0.11 | ||||
| 310 | −33.57 ± 0.09 | ||||
| PL-TCPP | 298 | −68.59 ± 0.07 | −33.79 ± 0.04 | −116.60 ± 0.11 | 0.99 |
| 304 | −33.24 ± 0.13 | ||||
| 310 | −32.38 ± 0.08 | ||||
| PL-TAPP | 298 | −72.32 ± 0.05 | −29.78 ± 0.05 | −142.66 ± 0.06 | 0.99 |
| 304 | −29.03 ± 0.07 | ||||
| 310 | −28.06 ± 0.06 | ||||
| PL-Cu-TCPP | 298 | 21.34 ± 0.04 | −23.56 ± 0.08 | 150.70 ± 0.07 | 0.99 |
| 304 | −24.48 ± 0.05 | ||||
| 310 | −25.37 ± 0.05 |
| System | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | β-Antiparallel (%) |
|---|---|---|---|---|---|
| PL | 25.48 ± 0.06 | 27.31 ± 0.06 | 23.12 ± 0.03 | 16.30 ± 0.04 | 7.78 ± 0.02 |
| PL-THPP | 13.96 ± 0.04 | 37.64 ± 0.05 | 19.70 ± 0.04 | 20.38 ± 0.02 | 8.32 ± 0.01 |
| PL-TCPP | 22.26 ± 0.08 | 37.79 ± 0.12 | 10.43 ± 0.03 | 24.70 ± 0.06 | 4.82 ± 0.01 |
| PL-TAPP | 12.81 ± 0.03 | 36.59 ± 0.07 | 18.09 ± 0.04 | 24.25 ± 0.07 | 8.26 ± 0.02 |
| PL-Cu-TCPP | 15.78 ± 0.05 | 46.44 ± 0.13 | 13.51 ± 0.03 | 23.40 ± 0.06 | 0.87 ± 0.01 |
| System | XP GScore (kcal/mol) | Key Residues | Hydrogen Bonds |
|---|---|---|---|
| PL-THPP | −3.49 | LEU213, PHE215, PHE258, ALA259, ALA260, HIP263, SER152, PHE77, ILE78, TYR114, PRO180, ILE209 | HIP263, SER152 |
| PL-TCPP | −2.88 | PHE215, LEU213, VAL210, ILE209, PHE258, ALA259, ALA260, HIP263, SER152, LEU153, PHE77, ILE78, TYR114, PRO180 | HIP263, SER152 |
| PL-TAPP | −2.62 | LEU213, PHE215, ALA259, TRP252, PHE77, ILE78, TYR114, PRO180, ALA178, ILE209, LEU153 | TYR114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, X.; Liu, Y.; Li, X.; Huang, M.; Bai, H.; Mo, J. Understanding Anti-Obesity Potential of Four Porphyrin Compounds by Investigating Pancreatic Lipase Inhibition. Molecules 2025, 30, 2701. https://doi.org/10.3390/molecules30132701
Zhou J, Wang X, Liu Y, Li X, Huang M, Bai H, Mo J. Understanding Anti-Obesity Potential of Four Porphyrin Compounds by Investigating Pancreatic Lipase Inhibition. Molecules. 2025; 30(13):2701. https://doi.org/10.3390/molecules30132701
Chicago/Turabian StyleZhou, Jie, Xinrui Wang, Yangyuxin Liu, Xiaochen Li, Mingze Huang, Helong Bai, and Jingang Mo. 2025. "Understanding Anti-Obesity Potential of Four Porphyrin Compounds by Investigating Pancreatic Lipase Inhibition" Molecules 30, no. 13: 2701. https://doi.org/10.3390/molecules30132701
APA StyleZhou, J., Wang, X., Liu, Y., Li, X., Huang, M., Bai, H., & Mo, J. (2025). Understanding Anti-Obesity Potential of Four Porphyrin Compounds by Investigating Pancreatic Lipase Inhibition. Molecules, 30(13), 2701. https://doi.org/10.3390/molecules30132701







