Focus on PD-1/PD-L1-Targeting Antibodies in Colorectal Cancer: Are There Options Beyond Dostarlimab, Nivolumab, and Pembrolizumab? A Comprehensive Review
Abstract
1. Introduction
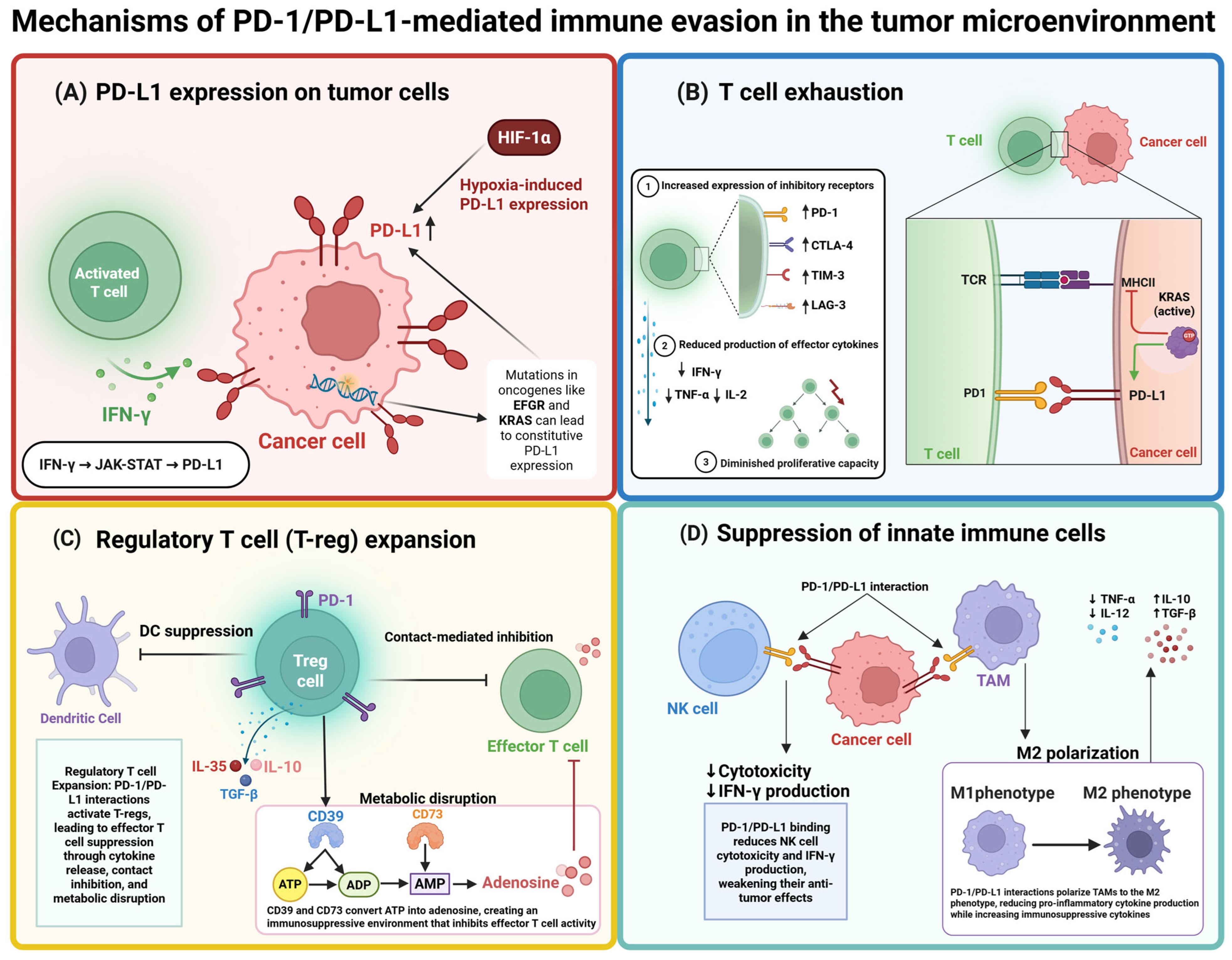
2. PD-1/PD-L1 Checkpoint
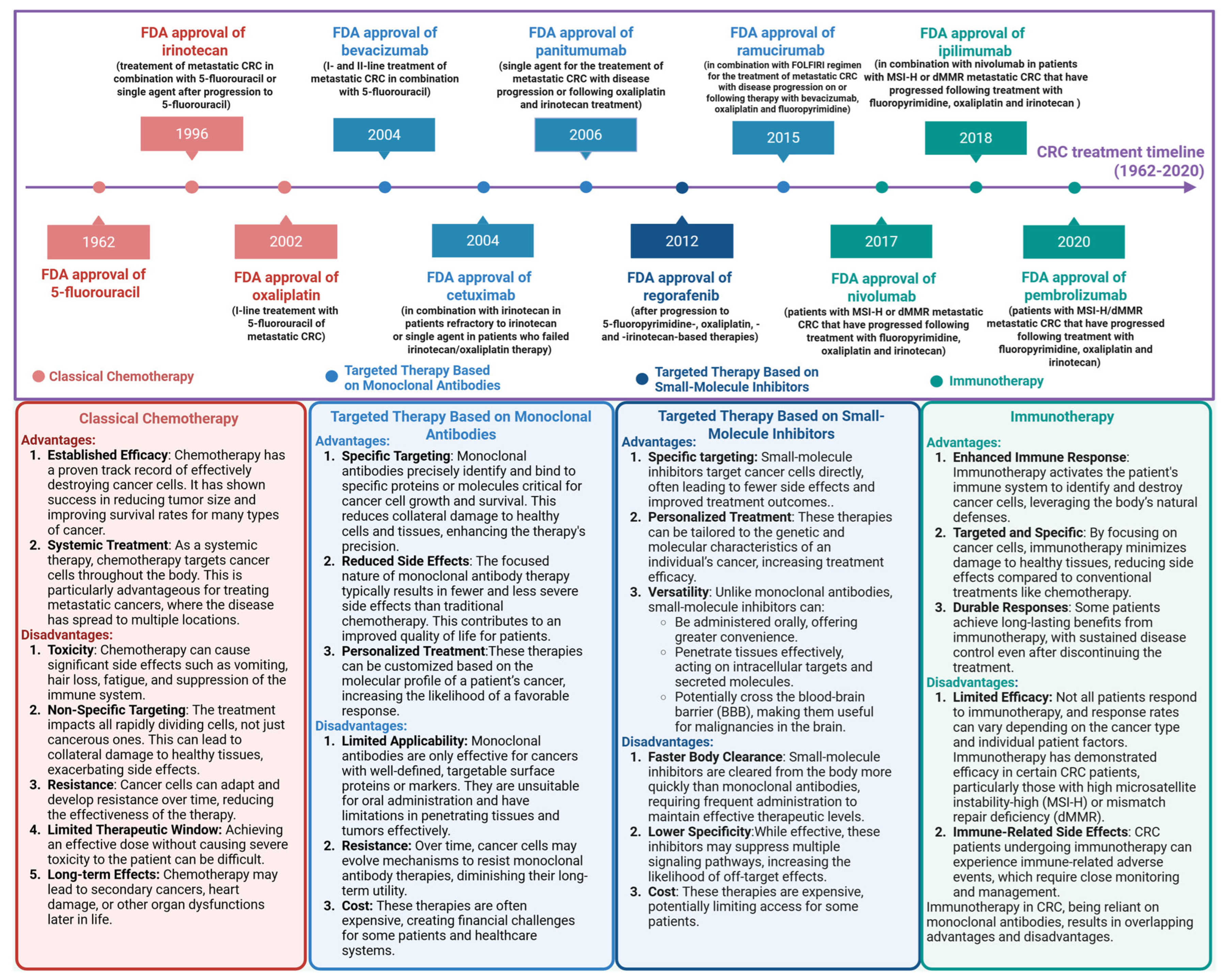
3. PD-1/PD-L1 Inhibitors Approved by the FDA
3.1. Nivolumab
3.2. Pembrolizumab
3.3. Dostarlimab (TSR-042)
4. Inhibitors Currently Not Approved for CRC Treatment
4.1. AMP-224
4.2. Atezolizumab
4.3. Avelumab
4.4. Camrelizumab
4.5. Durvalumab
4.6. Envafolimab
4.7. Sintilimab
4.8. Spartalizumab
4.9. Tislelizumab
4.10. Toripalimab
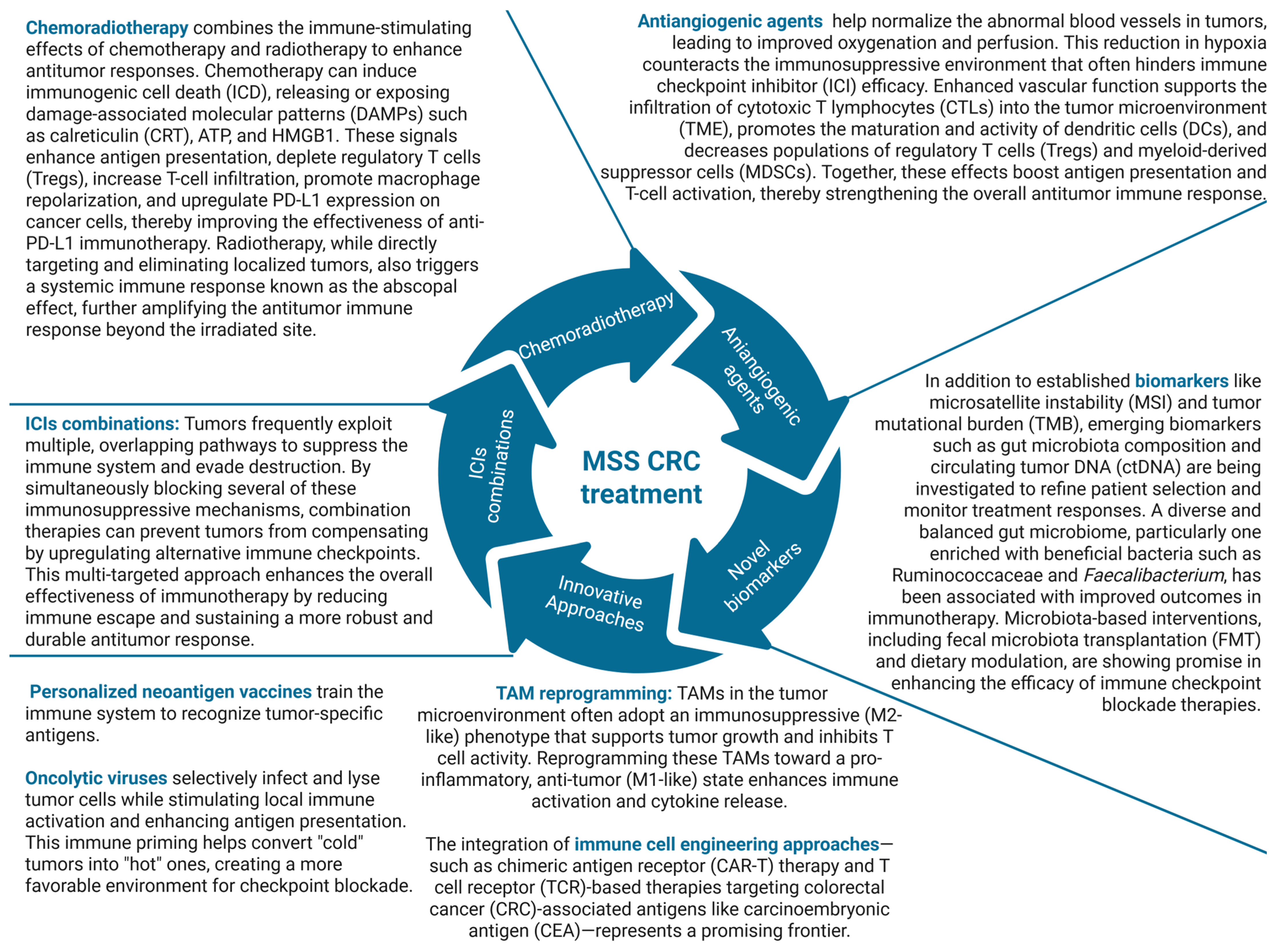
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Autologous dendritic cells |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| BRAF | Serine/threonine-protein kinase B-Raf |
| CA125 | Cancer antigen 125 |
| CA19-9 | Carbohydrate antigen 19-9 |
| CAE | Carcinoembryonic antigen |
| CAR-T | Chimeric antigen receptor therapy |
| CBR | Clinical benefit rate |
| CCR2/5 | C-C motif chemokine receptor 2/5 |
| CD39/73 | Cluster of differentiation |
| CEA | Carcinoembryonic antigen |
| CMS | Consensus molecular subtypes |
| COX-2 | Cyclooxygenase-2 |
| CPS | Combined positive score |
| CR | Complete response |
| CRC | Colorectal cancer |
| CtDNA | Circulating tumor DNA |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CTLA-4 | Cytotoxic T-cell antigen 4 |
| CTLs | Cytotoxic T cells |
| DCR | Disease control rate |
| dMMR | Mismatch repair-deficient |
| DoR | Duration of response |
| EGFR | Epidermal growth factor receptor |
| EORTC QLQ-C30 | The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 |
| Fc | Fragment crystallizable |
| FGFR | Fibroblast growth factor receptor |
| FMT | Fecal microbiota transplantation |
| GITR | Glucocorticoid-induced TNFR-related |
| HIFs | Hypoxia-inducible factors |
| HMGB1 | High mobility group box 1 |
| HPV | Human papillomavirus |
| ICIs | Immune-checkpoint inhibitors |
| IFN-γ | Interferon gamma |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| IND | Investigational new drug |
| irORR | Immune-related objective response rate |
| JNK | Janus kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LAG3 | Lymphocyte-activation gene 3 |
| mCRC | Metastatic colorectal cancer |
| MDSC | Myeloid-derived suppressor cells |
| MEK | Mitogen-activated protein kinase kinase |
| MGMT | Methylguanine methyltransferase |
| MHC | Major histocompatibility complex |
| MLH1 | MutL homolog 1 |
| MSH2 | MutS homolog 2 |
| MSH6 | MutS homolog 6 |
| MSI-H | Microsatellite instability-high |
| NK | Natural killer |
| NTRK | Neurotrophic tropomyosin receptor kinases |
| ORR | Objective response rate |
| OS | Overall survival |
| PARP | Poly (ADP-ribose) polymerase |
| PBMCs | Peripheral blood mononuclear cells |
| pCR | Pathological complete response |
| PCR | Polymerase chain reaction |
| PD-1 | Programmed death-1 |
| PDGFR | Platelet-derived growth factor receptor |
| PD-L1 | Programmed death ligand-1 |
| PFS | Progression-free survival |
| pMMR | Proficient MMR |
| PMS2 | Postmeiotic segregation increased 2 |
| POLE EDM | Polymerase ε (POLE) exonuclease domain |
| PR | Partial response |
| PVRIG | PVR-related immunoglobulin domain containing protein; CD112R |
| QoL | Quality of life |
| RCTs | Randomized controlled trials |
| RP2D | Recommended phase II dosage |
| SBRT | Stereotactic body radiation treatment |
| SCCA | Squamous cell carcinoma of the anal canal |
| SD | Stable disease |
| SHP2 | SRC homology 2 domain-containing phosphatase 2 |
| STAT | Signal transducer and activator of transcription |
| TAMs | Tumor-associated macrophages |
| TCR | T-cell receptor |
| TFD | Trifluridine |
| TGF-β | Transforming growth factor beta |
| TIGIT | Anti-T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain |
| TILs | Tumor-infiltrating lymphocytes |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TKI | Tyrosine kinase inhibitor |
| TMB | Tumor mutational burden |
| TME | Tumor microenvironment |
| TMZ | Temozolomide |
| TNF-α | Tumor necrosis factor alpha |
| TPI | Tipiraci |
| T-reg | Regulatory T cell |
| VEGF | Vascular endothelial growth factor |
| VEGFR1-3 | Vascular endothelial growth factor receptors 1-3 |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. Irinotecan Study Group. N. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer Chemotherapy: Insights into Cellular and Tumor Microenvironmental Mechanisms of Action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Selective Protection of Normal Cells from Chemotherapy, While Killing Drug-Resistant Cancer Cells. Oncotarget 2023, 14, 193–206. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Li, F.; Lin, Y.; Li, R.; Shen, X.; Xiang, M.; Xiong, G.; Zhang, K.; Xia, T.; Guo, J.; Miao, Z.; et al. Molecular Targeted Therapy for Metastatic Colorectal Cancer: Current and Evolving Approaches. Front. Pharmacol. 2023, 14, 1165666. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Peeters, M.; Siena, S.; Humblet, Y.; Hendlisz, A.; Neyns, B.; Canon, J.-L.; Van Laethem, J.-L.; Maurel, J.; Richardson, G.; et al. Open-Label Phase III Trial of Panitumumab plus Best Supportive Care Compared with Best Supportive Care Alone in Patients with Chemotherapy-Refractory Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1658–1664. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Moretto, R.; Rossini, D.; Capone, I.; Boccaccino, A.; Perrone, F.; Tamborini, E.; Masi, G.; Antoniotti, C.; Marmorino, F.; Conca, V.; et al. Rationale and Study Design of the PARERE Trial: Randomized Phase II Study of Panitumumab Re-Treatment Followed by Regorafenib Versus the Reverse Sequence in RAS and BRAF Wild-Type Chemo-Refractory Metastatic Colorectal Cancer Patients. Clin. Colorectal Cancer 2021, 20, 314–317. [Google Scholar] [CrossRef]
- Tanioka, H.; Shimada, K.; Tsuji, A.; Kochi, M.; Kim, H.M.; Takahashi, T.; Denda, T.; Takagane, A.; Watanabe, T.; Kotaka, M.; et al. Phase II Study of S-1 and Irinotecan Plus Bevacizumab as Second-Line Treatment for Patients With Metastatic Colorectal Cancer Resistant to the Fluoropyrimidine-Oxaliplatin-Cetuximab Regimen. Anticancer Res. 2022, 42, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Lipsyc-Sharf, M.; Ou, F.-S.; Yurgelun, M.B.; Rubinson, D.A.; Schrag, D.; Dakhil, S.R.; Stella, P.J.; Weckstein, D.J.; Wender, D.B.; Faggen, M.; et al. Cetuximab and Irinotecan With or Without Bevacizumab in Refractory Metastatic Colorectal Cancer: BOND-3, an ACCRU Network Randomized Clinical Trial. Oncologist 2022, 27, 292–298. [Google Scholar] [CrossRef]
- Martinelli, E.; Martini, G.; Famiglietti, V.; Troiani, T.; Napolitano, S.; Pietrantonio, F.; Ciardiello, D.; Terminiello, M.; Borrelli, C.; Vitiello, P.P.; et al. Cetuximab Rechallenge Plus Avelumab in Pretreated Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Phase 2 Single-Arm Clinical CAVE Trial. JAMA Oncol. 2021, 7, 1529–1535. [Google Scholar] [CrossRef]
- Napolitano, S.; Ciardiello, D.; De Falco, V.; Martini, G.; Martinelli, E.; Della Corte, C.M.; Esposito, L.; Famiglietti, V.; Di Liello, A.; Avallone, A.; et al. Panitumumab plus Trifluridine/Tipiracil as Anti-EGFR Rechallenge Therapy in Patients with Refractory RAS Wild-Type Metastatic Colorectal Cancer: Overall Survival and Subgroup Analysis of the Randomized Phase II VELO Trial. Int. J. Cancer 2023, 153, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, H.; Tabernero, J.; Macarulla, T. Ramucirumab in Metastatic Colorectal Cancer: Evidence to Date and Place in Therapy. Ther. Adv. Med. Oncol. 2016, 8, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Marshall, J.L. Optimizing Palliative Treatment of Metastatic Colorectal Cancer in the Era of Biologic Therapy. Oncol. Williston Park N 2007, 21, 553–564, 566; discussion 566–568, 577–578. [Google Scholar]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Mody, K.; Baldeo, C.; Bekaii-Saab, T. Antiangiogenic Therapy in Colorectal Cancer. Cancer J. Sudbury Mass 2018, 24, 165–170. [Google Scholar] [CrossRef]
- Noguerido, A.; Mulet-Margalef, N.; Matos, I.; Ros, J.; Argilés, G.; Élez, E.; Tabernero, J. The Safety of Ramucirumab for the Treatment of Colorectal Cancer. Expert Opin. Drug Saf. 2018, 17, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Manzi, J.; Hoff, C.O.; Ferreira, R.; Pimentel, A.; Datta, J.; Livingstone, A.S.; Vianna, R.; Abreu, P. Targeted Therapies in Colorectal Cancer: Recent Advances in Biomarkers, Landmark Trials, and Future Perspectives. Cancers 2023, 15, 3023. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Seufferlein, T. Regorafenib. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2018; Volume 211, pp. 45–56. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ikoma, T.; Yamamura, S.; Miura, K.; Tsuduki, T.; Watanabe, T.; Nagai, H.; Takatani, M.; Yasui, H. Regorafenib Is Suitable for Advanced Colorectal Cancer Patients Who Have Previously Received Trifluridine/Tipiracil plus Bevacizumab. Sci. Rep. 2023, 13, 2433. [Google Scholar] [CrossRef]
- Mota-George, G.; Schneider, S.M. Larotrectinib: A Targeted Therapy for Solid Tumors. Clin. J. Oncol. Nurs. 2021, 25, 181–187. [Google Scholar] [CrossRef]
- Filippi, R.; Depetris, I.; Satolli, M.A. Evaluating Larotrectinib for the Treatment of Advanced Solid Tumors Harboring an NTRK Gene Fusion. Expert Opin. Pharmacother. 2021, 22, 677–684. [Google Scholar] [CrossRef]
- Ratti, M.; Grizzi, G.; Passalacqua, R.; Lampis, A.; Cereatti, F.; Grassia, R.; Hahne, J.C. NTRK Fusions in Colorectal Cancer: Clinical Meaning and Future Perspective. Expert Opin. Ther. Targets 2021, 25, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Encorafenib: A Review in Metastatic Colorectal Cancer with a BRAF V600E Mutation. Drugs 2021, 81, 849–856. [Google Scholar] [CrossRef]
- Cornista, A.M.; Giolito, M.V.; Baker, K.; Hazime, H.; Dufait, I.; Datta, J.; Khumukcham, S.S.; De Ridder, M.; Roper, J.; Abreu, M.T.; et al. Colorectal Cancer Immunotherapy: State of the Art and Future Directions. Gastro Hep Adv. 2023, 2, 1103–1119. [Google Scholar] [CrossRef]
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical Management of Metastatic Colorectal Cancer in the Era of Precision Medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef]
- Moriarity, A.; O’Sullivan, J.; Kennedy, J.; Mehigan, B.; McCormick, P. Current Targeted Therapies in the Treatment of Advanced Colorectal Cancer: A Review. Ther. Adv. Med. Oncol. 2016, 8, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Zhu, Y.; Shen, Y.-Y.; Xu, Q.-Y.; Tang, H.-Y.; Cui, N.-X.; Jiang, L.; Dai, X.-M.; Chen, W.-Q.; Lin, Q.; et al. The Role of PD-1 Signaling in Health and Immune-Related Diseases. Front. Immunol. 2023, 14, 1163633. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Munir, S.; Lundsager, M.T.; Jørgensen, M.A.; Hansen, M.; Petersen, T.H.; Bonefeld, C.M.; Friese, C.; Met, Ö.; Straten, P.T.; Andersen, M.H. Inflammation Induced PD-L1-Specific T Cells. Cell Stress 2019, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Umansky, V. Cross-Talk between HIF and PD-1/PD-L1 Pathways in Carcinogenesis and Therapy. J. Clin. Investig. 2022, 132, e159473. [Google Scholar] [CrossRef]
- Domènech, M.; Muñoz Marmol, A.M.; Mate, J.L.; Estival, A.; Moran, T.; Cucurull, M.; Saigi, M.; Hernandez, A.; Sanz, C.; Hernandez-Gallego, A.; et al. Correlation between PD-L1 Expression and MET Gene Amplification in Patients with Advanced Non-Small Cell Lung Cancer and No Other Actionable Oncogenic Driver. Oncotarget 2021, 12, 1802–1810. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T.; Permata, T.B.M.; Hagiwara, Y.; Isono, M.; Nuryadi, E.; Sekine, R.; Oike, T.; Kakoti, S.; et al. DNA Double-Strand Break Repair Pathway Regulates PD-L1 Expression in Cancer Cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Mann, E.K.; Lee, K.J.; Chen, D.; da Silva, L.M.; Zotto, V.L.D.; Scalici, J.; Gassman, N.R. Associations between DNA Damage and PD-L1 Expression in Ovarian Cancer, a Potential Biomarker for Clinical Response. Biology 2021, 10, 385. [Google Scholar] [CrossRef]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Nanamori, H.; Sawada, Y. Epigenetic Modification of PD-1/PD-L1-Mediated Cancer Immunotherapy against Melanoma. Int. J. Mol. Sci. 2022, 23, 1119. [Google Scholar] [CrossRef]
- Dai, M.; Liu, M.; Yang, H.; Küçük, C.; You, H. New Insights into Epigenetic Regulation of Resistance to PD-1/PD-L1 Blockade Cancer Immunotherapy: Mechanisms and Therapeutic Opportunities. Exp. Hematol. Oncol. 2022, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, J.; Wang, B.; Liu, C.; Liu, L.; Sun, C. Epigenetic Modifications: Critical Participants of the PD-L1 Regulatory Mechanism in Solid Tumors (Review). Int. J. Oncol. 2022, 61, 134. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 715–723. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable Cancer Regression Off-Treatment and Effective Reinduction Therapy with an Anti-PD-1 Antibody. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 Is Upregulated by Radiochemotherapy in Rectal Adenocarcinoma Patients and Associated with a Favourable Prognosis. Eur. J. Cancer Oxf. Engl. 1990 2016, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for Previously Treated Unresectable Metastatic Anal Cancer (NCI9673): A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef]
- Cabel, L.; Bidard, F.-C.; Servois, V.; Cacheux, W.; Mariani, P.; Romano, E.; Minsat, M.; Bieche, I.; Farkhondeh, F.; Jeannot, E.; et al. HPV Circulating Tumor DNA to Monitor the Efficacy of Anti-PD-1 Therapy in Metastatic Squamous Cell Carcinoma of the Anal Canal: A Case Report. Int. J. Cancer 2017, 141, 1667–1670. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; Cohen, R.; Cochereau, D.; Duval, A.; Lascols, O.; Lopez-Trabada, D.; Afchain, P.; Trouilloud, I.; Parc, Y.; Lefevre, J.H.; et al. Immunotherapy and Patients Treated for Cancer with Microsatellite Instability. Bull. Cancer 2017, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Overman, M.J.; Ernstoff, M.S.; Morse, M.A. Where We Stand With Immunotherapy in Colorectal Cancer: Deficient Mismatch Repair, Proficient Mismatch Repair, and Toxicity Management. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 239–247. [Google Scholar] [CrossRef]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch Repair Deficiency/Microsatellite Instability-High as a Predictor for Anti-PD-1/PD-L1 Immunotherapy Efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Jácome, A.A.; Eng, C. Role of Immune Checkpoint Inhibitors in the Treatment of Colorectal Cancer: Focus on Nivolumab. Expert Opin. Biol. Ther. 2019, 19, 1247–1263. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Luis, A.; Diaz, J. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361. [Google Scholar] [CrossRef]
- He, W.-Z.; Wang, L.; Yin, C.-X.; Yi, J.-H.; Jin, Y.-N.; Jiang, C.; Guo, G.-F.; Xia, L.-P. Regorafenib with or without a Programmed Cell Death Protein 1 Antibody as Third-Line Treatment for Microsatellite Stable Metastatic Colorectal Cancer. Cancer Med. 2023, 12, 6488–6498. [Google Scholar] [CrossRef]
- Wang, S.; Cowley, L.A.; Liu, X.-S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Johnston, R.J.; Lu, L.-S.; Han, M.; Thudium, K.; Yao, D.; Quigley, M.; Valle, J.; Wang, C.; et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS ONE 2016, 11, e0161779. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Gourd, E. Nivolumab plus Ipilimumab in Metastatic Colorectal Cancer. Lancet Oncol. 2018, 19, e139. [Google Scholar] [CrossRef]
- Morse, M.A.; Overman, M.J.; Hartman, L.; Khoukaz, T.; Brutcher, E.; Lenz, H.-J.; Atasoy, A.; Shangguan, T.; Zhao, H.; El-Rayes, B. Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist 2019, 24, 1453–1461. [Google Scholar] [CrossRef]
- Cohen, R.; Bennouna, J.; Meurisse, A.; Tournigand, C.; De La Fouchardière, C.; Tougeron, D.; Borg, C.; Mazard, T.; Chibaudel, B.; Garcia-Larnicol, M.-L.; et al. RECIST and iRECIST Criteria for the Evaluation of Nivolumab plus Ipilimumab in Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The GERCOR NIPICOL Phase II Study. J. Immunother. Cancer 2020, 8, e001499. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus Low-Dose Ipilimumab in Previously Treated Patients with Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: 4-Year Follow-up from CheckMate 142. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.-C.; et al. A Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair Proficient Advanced Refractory Colorectal Cancer. Eur. J. Cancer Oxf. Engl. 1990 2022, 169, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Tam, R.; D’Adamo, D.; et al. Regorafenib plus Nivolumab in Patients with Mismatch Repair-Proficient/Microsatellite Stable Metastatic Colorectal Cancer: A Single-Arm, Open-Label, Multicentre Phase 2 Study. eClinicalMedicine 2023, 58, 101917. [Google Scholar] [CrossRef]
- Kciuk, M.; Kołat, D.; Kałuzińska-Kołat, Ż.; Gawrysiak, M.; Drozda, R.; Celik, I.; Kontek, R. PD-1/PD-L1 and DNA Damage Response in Cancer. Cells 2023, 12, 530. [Google Scholar] [CrossRef]
- Nukatsuka, M.; Fujioka, A.; Nagase, H.; Tanaka, G.; Hayashi, H. Evaluation of a Novel Combination Therapy, Based on Trifluridine/Tipiracil and Fruquintinib, against Colorectal Cancer. Chemotherapy 2023, 68, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Koumarianou, A.; Ntavatzikos, A.; Symeonidis, D.; Vallilas, C.; Giannakakou, M.; Papaxoinis, G.; Xynogalos, S.; Boukovinas, I.; Demiri, S.; Kampoli, K.; et al. RETRO-TAS, a Retrospective Observational Study of Trifluridine/Tipiracil in Chemorefractory Metastatic Colorectal Cancer. Biomedicines 2023, 11, 1267. [Google Scholar] [CrossRef]
- Patel, M.R.; Falchook, G.S.; Hamada, K.; Makris, L.; Bendell, J.C. A Phase 2 Trial of Trifluridine/Tipiracil plus Nivolumab in Patients with Heavily Pretreated Microsatellite-Stable Metastatic Colorectal Cancer. Cancer Med. 2021, 10, 1183–1190. [Google Scholar] [CrossRef]
- Bordonaro, R.; Calvo, A.; Auriemma, A.; Hollebecque, A.; Rubovszky, G.; Saunders, M.P.; Pápai, Z.; Prager, G.; Stein, A.; André, T.; et al. Trifluridine/Tipiracil in Combination with Oxaliplatin and Either Bevacizumab or Nivolumab in Metastatic Colorectal Cancer: A Dose-Expansion, Phase I Study. ESMO Open 2021, 6, 100270. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Szabolcs, A.; Allen, J.N.; Clark, J.W.; Wo, J.Y.; Raabe, M.; Thel, H.; Hoyos, D.; Mehta, A.; Arshad, S.; et al. Radiation Therapy Enhances Immunotherapy Response in Microsatellite-Stable Colorectal and Pancreatic Adenocarcinoma in a Phase II Trial. Nat. Cancer 2021, 2, 1124–1135. [Google Scholar] [CrossRef]
- Morano, F.; Raimondi, A.; Pagani, F.; Lonardi, S.; Salvatore, L.; Cremolini, C.; Murgioni, S.; Randon, G.; Palermo, F.; Antonuzzo, L.; et al. Temozolomide Followed by Combination With Low-Dose Ipilimumab and Nivolumab in Patients With Microsatellite-Stable, O6-Methylguanine-DNA Methyltransferase-Silenced Metastatic Colorectal Cancer: The MAYA Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1562–1573. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Carlsen, L.; El-Deiry, W.S. Temozolomide Combined with Ipilimumab plus Nivolumab Enhances T Cell Killing of MGMT-Expressing, MSS Colorectal Cancer Cells. Am. J. Cancer Res. 2023, 13, 216–226. [Google Scholar] [PubMed]
- Starzer, A.M.; Berghoff, A.S. New Emerging Targets in Cancer Immunotherapy: CD27 (TNFRSF7). ESMO Open 2020, 4, e000629. [Google Scholar] [CrossRef]
- Guelen, L.; Fischmann, T.O.; Wong, J.; Mauze, S.; Guadagnoli, M.; Bąbała, N.; Wagenaars, J.; Juan, V.; Rosen, D.; Prosise, W.; et al. Preclinical Characterization and Clinical Translation of Pharmacodynamic Markers for MK-5890: A Human CD27 Activating Antibody for Cancer Immunotherapy. J. Immunother. Cancer 2022, 10, e005049. [Google Scholar] [CrossRef]
- Sanborn, R.E.; Pishvaian, M.J.; Callahan, M.K.; Weise, A.; Sikic, B.I.; Rahma, O.; Cho, D.C.; Rizvi, N.A.; Sznol, M.; Lutzky, J.; et al. Safety, Tolerability and Efficacy of Agonist Anti-CD27 Antibody (Varlilumab) Administered in Combination with Anti-PD-1 (Nivolumab) in Advanced Solid Tumors. J. Immunother. Cancer 2022, 10, e005147. [Google Scholar] [CrossRef]
- Zhu, M.M.T.; Burugu, S.; Gao, D.; Yu, J.; Kos, Z.; Leung, S.; Horst, B.A.; Nielsen, T.O. Evaluation of Glucocorticoid-Induced TNF Receptor (GITR) Expression in Breast Cancer and across Multiple Tumor Types. Mod. Pathol. 2020, 33, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Wilson, D.C.; Yu, Y.; Wong, J.; Naravula, S.; Ermakov, G.; Riener, R.; Bhagwat, B.; Necheva, A.S.; Grein, J.; et al. Characterization of MK-4166, a Clinical Agonistic Antibody That Targets Human GITR and Inhibits the Generation and Suppressive Effects of T Regulatory Cells. Cancer Res. 2017, 77, 4378–4388. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Madrasi, K.; Knee, D.A.; Gruenbaum, L.; Apgar, J.F.; Burke, J.M.; Gomes, B. Quantitative Systems Pharmacology Model of GITR-Mediated T Cell Dynamics in Tumor Microenvironment. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Rakké, Y.S.; Campos Carrascosa, L.; van Beek, A.A.; de Ruiter, V.; van Gemerden, R.S.; Doukas, M.; Doornebosch, P.G.; Vermaas, M.; Ter Borg, S.; van der Harst, E.; et al. GITR Ligation Improves Anti-PD1-Mediated Restoration of Human MMR-Proficient Colorectal Carcinoma Tumor-Derived T Cells. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 77–97. [Google Scholar] [CrossRef]
- Ott, P.A.; Piha-Paul, S.A.; Munster, P.; Pishvaian, M.J.; van Brummelen, E.M.J.; Cohen, R.B.; Gomez-Roca, C.; Ejadi, S.; Stein, M.; Chan, E.; et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients with Recurrent Carcinoma of the Anal Canal. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1036–1041. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Wallmark, J.M.; Lorente, D.; Elez, E.; Raimbourg, J.; Gomez-Roca, C.; Ejadi, S.; Piha-Paul, S.A.; Stein, M.N.; Abdul Razak, A.R.; et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients with Advanced Colorectal Carcinoma. PLoS ONE 2017, 12, e0189848. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-Related Quality of Life in Patients with Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer Treated with First-Line Pembrolizumab versus Chemotherapy (KEYNOTE-177): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef]
- Casak, S.J.; Marcus, L.; Fashoyin-Aje, L.; Mushti, S.L.; Cheng, J.; Shen, Y.-L.; Pierce, W.F.; Her, L.; Goldberg, K.B.; Theoret, M.R.; et al. FDA Approval Summary: Pembrolizumab for the First-Line Treatment of Patients with MSI-H/dMMR Advanced Unresectable or Metastatic Colorectal Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4680–4684. [Google Scholar] [CrossRef]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus Chemotherapy for Microsatellite Instability-High or Mismatch Repair-Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Herting, C.J.; Farren, M.R.; Tong, Y.; Liu, Z.; O’Neil, B.; Bekaii-Saab, T.; Noonan, A.; McQuinn, C.; Mace, T.A.; Shaib, W.; et al. A Multi-Center, Single-Arm, Phase Ib Study of Pembrolizumab (MK-3475) in Combination with Chemotherapy for Patients with Advanced Colorectal Cancer: HCRN GI14-186. Cancer Immunol. Immunother. CII 2021, 70, 3337–3348. [Google Scholar] [CrossRef]
- Rahma, O.E.; Yothers, G.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Colangelo, L.H.; Lucas, P.C.; Gollub, M.J.; et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results From the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, G.; Sartore-Bianchi, A.; Lazzari, L.; Pietrantonio, F.; Amatu, A.; Macagno, M.; Barault, L.; Cassingena, A.; Bartolini, A.; Luraghi, P.; et al. Temozolomide Treatment Alters Mismatch Repair and Boosts Mutational Burden in Tumor and Blood of Colorectal Cancer Patients. Cancer Discov. 2022, 12, 1656–1675. [Google Scholar] [CrossRef]
- Fountzilas, C.; Bajor, D.L.; Mukherjee, S.; Saltzman, J.; Witkiewicz, A.K.; Maguire, O.; Minderman, H.; Nambiar, R.; Rosenheck, H.R.; Knudsen, E.S.; et al. Phase Ib/II Study of Cetuximab plus Pembrolizumab in Patients with Advanced RAS Wild-Type Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 6726–6736. [Google Scholar] [CrossRef]
- Kuang, C.; Park, Y.; Augustin, R.C.; Lin, Y.; Hartman, D.J.; Seigh, L.; Pai, R.K.; Sun, W.; Bahary, N.; Ohr, J.; et al. Pembrolizumab plus Azacitidine in Patients with Chemotherapy Refractory Metastatic Colorectal Cancer: A Single-Arm Phase 2 Trial and Correlative Biomarker Analysis. Clin. Epigenetics 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Tyan, K.; Giobbie-Hurder, A.; Brohl, A.S.; Bedard, P.L.; Renouf, D.J.; Sharon, E.; Streicher, H.; Hathaway, E.; Cunningham, R.; et al. Phase IB Study of Ziv-Aflibercept plus Pembrolizumab in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2022, 10, e003569. [Google Scholar] [CrossRef]
- Haag, G.M.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschäbitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and Maraviroc in Refractory Mismatch Repair Proficient/Microsatellite-Stable Metastatic Colorectal Cancer—The PICCASSO Phase I Trial. Eur. J. Cancer Oxf. Engl. 1990 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H.; Hansen, A.R.; Lee, J.-S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 Antagonist Navarixin in Combination with Pembrolizumab in Select Advanced Solid Tumors: A Phase 2 Randomized Trial. Invest. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef]
- Yarchoan, M.; Huang, C.-Y.; Zhu, Q.; Ferguson, A.K.; Durham, J.N.; Anders, R.A.; Thompson, E.D.; Rozich, N.S.; Thomas, D.L.; Nauroth, J.M.; et al. A Phase 2 Study of GVAX Colon Vaccine with Cyclophosphamide and Pembrolizumab in Patients with Mismatch Repair Proficient Advanced Colorectal Cancer. Cancer Med. 2020, 9, 1485–1494. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuboki, Y.; Shinozaki, E.; Hara, H.; Nishina, T.; Komatsu, Y.; Yuki, S.; Wakabayashi, M.; Nomura, S.; Sato, A.; et al. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Geva, R.; Voskoboynik, M.; Dobrenkov, K.; Mayawala, K.; Gwo, J.; Wnek, R.; Chartash, E.; Long, G.V. First-in-Human Phase 1 Study of MK-1248, an Anti-Glucocorticoid-Induced Tumor Necrosis Factor Receptor Agonist Monoclonal Antibody, as Monotherapy or with Pembrolizumab in Patients with Advanced Solid Tumors. Cancer 2020, 126, 4926–4935. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Rose, S. Dostarlimab: An Answer for Rectal Cancer? Cancer Discov. 2022, 12, 1828–1829. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.-T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef] [PubMed]
- Floudas, C.S.; Brar, G.; Mabry-Hrones, D.; Duffy, A.G.; Wood, B.; Levy, E.; Krishnasamy, V.; Fioravanti, S.; Bonilla, C.M.; Walker, M.; et al. A Pilot Study of the PD-1 Targeting Agent AMP-224 Combined with Low-Dose Cyclophosphamide and Stereotactic Body Radiation Therapy in Patients with Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2019, 18, e349–e360. [Google Scholar] [CrossRef]
- Tapia Rico, G.; Price, T.J. Atezolizumab for the Treatment of Colorectal Cancer: The Latest Evidence and Clinical Potential. Expert Opin. Biol. Ther. 2018, 18, 449–457. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Kim, T.-W.; Lee, C.B.; Goh, B.-C.; Miller, W.H.; Oh, D.-Y.; Jamal, R.; Chee, C.-E.; Chow, L.Q.M.; Gainor, J.F.; et al. Phase Ib Study of Atezolizumab Combined with Cobimetinib in Patients with Solid Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1134–1142. [Google Scholar] [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without Cobimetinib versus Regorafenib in Previously Treated Metastatic Colorectal Cancer (IMblaze370): A Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Schröder, C.; Lawrance, M.; Li, C.; Lenain, C.; Mhatre, S.K.; Fakih, M.; Reyes-Rivera, I.; Bretscher, M.T. Building External Control Arms From Patient-Level Electronic Health Record Data to Replicate the Randomized IMblaze370 Control Arm in Metastatic Colorectal Cancer. JCO Clin. Cancer Inform. 2021, 5, CCI.20.00149. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus Bevacizumab with or without Atezolizumab in the Treatment of Patients with Metastatic Colorectal Cancer (AtezoTRIBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Germani, M.M.; Moretto, R. Immune Checkpoint Inhibitors in Mismatch Repair Proficient/Microsatellite Stable Metastatic Colorectal Cancer Patients: Insights from the AtezoTRIBE and MAYA Trials. Cancers 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Mettu, N.B.; Ou, F.-S.; Zemla, T.J.; Halfdanarson, T.R.; Lenz, H.-J.; Breakstone, R.A.; Boland, P.M.; Crysler, O.V.; Wu, C.; Nixon, A.B.; et al. Assessment of Capecitabine and Bevacizumab With or Without Atezolizumab for the Treatment of Refractory Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2149040. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Grothey, A.; Arnold, D.; de Gramont, A.; Ducreux, M.; O’Dwyer, P.; Tahiri, A.; Gilberg, F.; Irahara, N.; Schmoll, H.-J.; et al. MODUL Cohort 2: An Adaptable, Randomized, Signal-Seeking Trial of Fluoropyrimidine plus Bevacizumab with or without Atezolizumab Maintenance Therapy for BRAFwt Metastatic Colorectal Cancer. ESMO Open 2022, 7, 100559. [Google Scholar] [CrossRef]
- Collins, J.M.; Gulley, J.L. Product Review: Avelumab, an Anti-PD-L1 Antibody. Hum. Vaccines Immunother. 2018, 15, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; O’Sullivan Coyne, G.; Reed, C.T.; Madan, R.A.; Strauss, J.; Steinberg, S.J.; Marté, J.; Cordes, L.; Heery, C.; Gulley, J.L. Avelumab in Patients With Metastatic Colorectal Cancer. Oncologist 2023, 28, 823-e804. [Google Scholar] [CrossRef]
- Español-Rego, M.; Fernández-Martos, C.; Elez, E.; Foguet, C.; Pedrosa, L.; Rodríguez, N.; Ruiz-Casado, A.; Pineda, E.; Cid, J.; Cabezón, R.; et al. A Phase I-II Multicenter Trial with Avelumab plus Autologous Dendritic Cell Vaccine in Pre-Treated Mismatch Repair-Proficient (MSS) Metastatic Colorectal Cancer Patients; GEMCAD 1602 Study. Cancer Immunol. Immunother. CII 2023, 72, 827–840. [Google Scholar] [CrossRef]
- Taïeb, J.; Bouche, O.; André, T.; Malicot, K.L.; Laurent-Puig, P.; Bez, J.; Toullec, C.; Borg, C.; Randrian, V.; Evesque, L.; et al. Avelumab vs Standard Second-Line Chemotherapy in Patients With Metastatic Colorectal Cancer and Microsatellite Instability: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1356. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Wang, J.; Liu, J.; et al. Phase II, Single-Arm Trial of Preoperative Short-Course Radiotherapy Followed by Chemotherapy and Camrelizumab in Locally Advanced Rectal Cancer. J. Immunother. Cancer 2021, 9, e003554. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Lin, Y.; Cai, W.; Li, X.; He, X. Preliminary Efficacy and Safety of Camrelizumab in Combination with XELOX Plus Bevacizumab or Regorafenib in Patients with Metastatic Colorectal Cancer: A Retrospective Study. Front. Oncol. 2021, 11, 774445. [Google Scholar] [CrossRef]
- Quan, M.; Chen, J.; Chen, Z.; Hai, Y.; Zhou, Y.; Chao, Q.; Chen, C.; Li, H.; Wang, M.; Gao, Y. China Special Issue on Gastrointestinal Tumors-Cetuximab Retreatment plus Camrelizumab and Liposomal Irinotecan in Patients with RAS Wild-Type Metastatic Colorectal Cancer: Cohort B of the Phase II CRACK Study. Int. J. Cancer 2023, 153, 1877–1884. [Google Scholar] [CrossRef]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Cercek, A.; Ku, G.; Wu, A.J.; Rimner, A.; Khalil, D.N.; Reidy-Lagunes, D.; Cuaron, J.; Yang, T.J.; Weiser, M.R.; et al. Phase II Single-Arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair-Proficient Metastatic Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2200–2208. [Google Scholar] [CrossRef]
- Kanikarla Marie, P.; Haymaker, C.; Parra, E.R.; Kim, Y.U.; Lazcano, R.; Gite, S.; Lorenzini, D.; Wistuba, I.I.; Tidwell, R.S.S.; Song, X.; et al. Pilot Clinical Trial of Perioperative Durvalumab and Tremelimumab in the Treatment of Resectable Colorectal Cancer Liver Metastases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.R.; Kim, J.E.; Hong, Y.S.; Kim, S.Y.; Ahn, J.B.; Baek, J.Y.; Lee, M.-A.; Kang, M.J.; Cho, S.H.; Beom, S.-H.; et al. Phase II Study of Durvalumab Monotherapy in Patients with Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient or POLE-Mutated Metastatic or Unresectable Colorectal Cancer. Int. J. Cancer 2022, 150, 2038–2045. [Google Scholar] [CrossRef]
- Monge, C.; Xie, C.; Myojin, Y.; Coffman, K.; Hrones, D.M.; Wang, S.; Hernandez, J.M.; Wood, B.J.; Levy, E.B.; Juburi, I.; et al. Phase I/II Study of PexaVec in Combination with Immune Checkpoint Inhibition in Refractory Metastatic Colorectal Cancer. J. Immunother. Cancer 2023, 11, e005640. [Google Scholar] [CrossRef] [PubMed]
- Thibaudin, M.; Fumet, J.-D.; Chibaudel, B.; Bennouna, J.; Borg, C.; Martin-Babau, J.; Cohen, R.; Fonck, M.; Taieb, J.; Limagne, E.; et al. First-Line Durvalumab and Tremelimumab with Chemotherapy in RAS-Mutated Metastatic Colorectal Cancer: A Phase 1b/2 Trial. Nat. Med. 2023, 29, 2087–2098. [Google Scholar] [CrossRef]
- Grassi, E.; Zingaretti, C.; Petracci, E.; Corbelli, J.; Papiani, G.; Banchelli, I.; Valli, I.; Frassineti, G.L.; Passardi, A.; Di Bartolomeo, M.; et al. Phase II Study of Capecitabine-Based Concomitant Chemoradiation Followed by Durvalumab as a Neoadjuvant Strategy in Locally Advanced Rectal Cancer: The PANDORA Trial. ESMO Open 2023, 8, 101824. [Google Scholar] [CrossRef]
- Patel, S.P.; Alonso-Gordoa, T.; Banerjee, S.; Wang, D.; Naidoo, J.; Standifer, N.E.; Palmer, D.C.; Cheng, L.-Y.; Kourtesis, P.; Ascierto, M.L.; et al. Phase 1/2 Study of Monalizumab plus Durvalumab in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2024, 12, e007340. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, M.; Wang, X.; Shen, L.; Li, J. Envafolimab—First PD-1/PD-L1 Antibody to Be Administered by Subcutaneous Injection for Microsatellite Instability-High or Deficient Mismatch Repair Advanced Solid Tumors. Expert Opin. Biol. Ther. 2022, 22, 1227–1232. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Zhang, W.; Zhou, A.-P.; Guo, W.; Yang, J.; Yuan, Y.; Zhu, L.; Qin, S.; Xiang, S.; et al. Subcutaneous Envafolimab Monotherapy in Patients with Advanced Defective Mismatch Repair/Microsatellite Instability High Solid Tumors. J. Hematol. Oncol. 2021, 14, 95. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Du, J.; Hu, J.; Hu, R.; Zeng, Z.; Jin-Si-Han, E.-E.-M.-B.-K.; Lian, S.; Wang, H.; Li, Y.; et al. Efficacy and Safety of Neoadjuvant Subcutaneous Envafolimab in dMMR/MSI-H Locally Advanced Colon Cancer. Target. Oncol. 2024, 19, 601–610. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, X.; Zhou, C.; Tang, Y.; Li, F.; Zhang, B.; Huang, T.; Wang, J.; Tu, S. Fruquintinib Enhances the Antitumor Immune Responses of Anti-Programmed Death Receptor-1 in Colorectal Cancer. Front. Oncol. 2022, 12, 841977. [Google Scholar] [CrossRef]
- Gou, M.; Qian, N.; Zhang, Y.; Yan, H.; Si, H.; Wang, Z.; Dai, G. Fruquintinib in Combination With PD-1 Inhibitors in Patients With Refractory Non-MSI-H/pMMR Metastatic Colorectal Cancer: A Real-World Study in China. Front. Oncol. 2022, 12, 851756. [Google Scholar] [CrossRef]
- Li, R.-R.; Yin, X.-L.; Zeng, D.-Y.; Shao, F.-J.; Yamamoto, S.; Liu, W.; Liu, Z.-Y. Efficacy and Safety of Anti-PD-1 Antibody plus Regorafenib in Refractory Microsatellite Stable Metastatic Colorectal Cancer: A Retrospective Single-Arm Cohort Study. Ann. Transl. Med. 2022, 10, 880. [Google Scholar] [CrossRef]
- Pei, F.; Wu, J.; Zhao, Y.; He, W.; Yao, Q.; Huang, M.; Huang, J. Single-Agent Neoadjuvant Immunotherapy With a PD-1 Antibody in Locally Advanced Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer. Clin. Colorectal Cancer 2023, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, W.; Ying, J.; Zhang, Y.; Pan, Y.; Qiu, W.; Fan, Q.; Xu, Q.; Ma, Y.; Wang, G.; et al. Phase 1b/2 Trial of Fruquintinib plus Sintilimab in Treating Advanced Solid Tumours: The Dose-Escalation and Metastatic Colorectal Cancer Cohort in the Dose-Expansion Phases. Eur. J. Cancer Oxf. Engl. 1990 2023, 181, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhu, N.; Zhong, C.; Wang, L.; Li, J.; Weng, S.; Hu, H.; Dong, C.; Li, D.; Song, Y.; et al. Sintilimab plus Bevacizumab, Oxaliplatin and Capecitabine as First-Line Therapy in RAS-Mutant, Microsatellite Stable, Unresectable Metastatic Colorectal Cancer: An Open-Label, Single-Arm, Phase II Trial. EClinicalMedicine 2023, 62, 102123. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Cheng, D.-X.; Chen, X.-C.; Yang, L.; Wu, H. Application of Sintilimab Combined with Anlotinib Hydrochloride in the Clinical Treatment of Microsatellite Stable Colorectal Cancer. World J. Gastrointest. Oncol. 2023, 15, 1925–1935. [Google Scholar] [CrossRef]
- Even, C.; Wang, H.-M.; Li, S.-H.; Ngan, R.K.-C.; Dechaphunkul, A.; Zhang, L.; Yen, C.-J.; Chan, P.C.; Chakrabandhu, S.; Ma, B.B.Y.; et al. Phase II, Randomized Study of Spartalizumab (PDR001), an Anti-PD-1 Antibody, versus Chemotherapy in Patients with Recurrent/Metastatic Nasopharyngeal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 6413–6423. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Geng, Z.; Hao, B.; Geng, Q. Tislelizumab: A Modified Anti-Tumor Programmed Death Receptor 1 Antibody. Cancer Control J. Moffitt Cancer Cent. 2022, 29, 10732748221111296. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Yang, Z.; Zhang, J.; Bai, Z.; Deng, W.; Chen, G.; Xu, R.; Wei, Q.; Liu, Y.; et al. Interim Result of Phase II, Prospective, Single-Arm Trial of Long-Course Chemoradiotherapy Combined with Concurrent Tislelizumab in Locally Advanced Rectal Cancer. Front. Oncol. 2023, 13, 1057947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lei, J.; Ke, S.; Chen, Y.; Xiao, J.; Tang, Z.; Wang, L.; Ren, Y.; Alnaggar, M.; Qiu, H.; et al. Fecal Microbiota Transplantation plus Tislelizumab and Fruquintinib in Refractory Microsatellite Stable Metastatic Colorectal Cancer: An Open-Label, Single-Arm, Phase II Trial (RENMIN-215). EClinicalMedicine 2023, 66, 102315. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, B.; Geng, Z.; Geng, Q. Toripalimab: The First Domestic Anti-Tumor PD-1 Antibody in China. Front. Immunol. 2021, 12, 730666. [Google Scholar] [CrossRef]
- Keam, S.J. Toripalimab: First Global Approval. Drugs 2019, 79, 573–578. [Google Scholar] [CrossRef]
- Hua, Y.; You, R.; Wang, Z.; Huang, P.; Lin, M.; Ouyang, Y.; Xie, Y.; Zou, X.; Liu, Y.; Duan, C.; et al. Toripalimab plus Intensity-Modulated Radiotherapy for Recurrent Nasopharyngeal Carcinoma: An Open-Label Single-Arm, Phase II Trial. J. Immunother. Cancer 2021, 9, e003290. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, M.-M.; Yao, Y.-C.; Zhao, X.; Wang, Z.-Q.; Jin, Y.; Luo, H.-Y.; Li, J.-B.; Wang, F.-H.; Qiu, M.-Z.; et al. Regorafenib plus Toripalimab in Patients with Metastatic Colorectal Cancer: A Phase Ib/II Clinical Trial and Gut Microbiome Analysis. Cell Rep. Med. 2021, 2, 100383. [Google Scholar] [CrossRef]
- Yu, W.; Tao, Q.; Zhang, Y.; Yi, F.; Feng, L. Efficacy and Safety of Regorafenib Combined with Toripalimab in the Third-Line and beyond Treatment of Advanced Colorectal Cancer. J. Oncol. 2021, 2021, 9959946. [Google Scholar] [CrossRef]
- Ma, S.; Chen, R.; Duan, L.; Li, C.; Yang, T.; Wang, J.; Zhao, D. Efficacy and Safety of Toripalimab with Fruquintinib in the Third-Line Treatment of Refractory Advanced Metastatic Colorectal Cancer: Results of a Single-Arm, Single-Center, Prospective, Phase II Clinical Study. J. Gastrointest. Oncol. 2023, 14, 1052–1063. [Google Scholar] [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 Blockade with Toripalimab, with or without Celecoxib, in Mismatch Repair-Deficient or Microsatellite Instability-High, Locally Advanced, Colorectal Cancer (PICC): A Single-Centre, Parallel-Group, Non-Comparative, Randomised, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Makuku, R.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Current and Future Perspectives of PD-1/PDL-1 Blockade in Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 6661406. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the Anticancer Efficacy of PD-1/PD-L1 Blockade via Combination Therapy and PD-L1 Regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Xia, Q. Role of Gut Microbiome in Cancer Immunotherapy: From Predictive Biomarker to Therapeutic Target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Shi, C.; Feng, J. The Role Of Circulating Tumor DNA In Therapeutic Resistance. OncoTargets Ther. 2019, 12, 9459–9471. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Zhang, S. Safety and Efficacy of Personalized Cancer Vaccines in Combination With Immune Checkpoint Inhibitors in Cancer Treatment. Front. Oncol. 2021, 11, 663264. [Google Scholar] [CrossRef]
- Makaremi, S.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Sgambato, A.; Ghorbaninezhad, F.; Safarpour, H.; Argentiero, A.; Brunetti, O.; Bernardini, R.; et al. Immune Checkpoint Inhibitors in Colorectal Cancer: Challenges and Future Prospects. Biomedicines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Qin, X.; Wu, F.; Chen, C.; Li, Q. Recent Advances in CAR-T Cells Therapy for Colorectal Cancer. Front. Immunol. 2022, 13, 904137. [Google Scholar] [CrossRef]
- Ghaffari Laleh, N.; Ligero, M.; Perez-Lopez, R.; Kather, J.N. Facts and Hopes on the Use of Artificial Intelligence for Predictive Immunotherapy Biomarkers in Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial Intelligence in Cancer Imaging: Clinical Challenges and Applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
| Number | Short Description and Rationale | Status | Estimated Enrollment |
|---|---|---|---|
| NCT05770102 | This clinical trial is evaluating the effectiveness of atezolizumab in treating rare or less common cancer types that exhibit high TMB, MSI-H, or constitutional dMMR. Atezolizumab is already approved in the UK for several cancers, including urothelial cancer, non-small cell lung cancer, and triple-negative breast cancer. The aim of this study is to determine whether the drug could also benefit patients with other cancers that share similar genetic features. If successful, the findings may support broader National Health Service (NHS) access to atezolizumab through the Cancer Drugs Fund. This trial is part of the larger DETERMINE programme, which is exploring targeted treatments for rare cancers based on genetic markers. | Recruiting | 30 |
| NCT05425940 | This study aims to compare the effects of a combination of XL092 and atezolizumab versus regorafenib in patients with MSS/MSI-low mCRC. Eligible participants are those whose cancer has progressed during or after, or who are intolerant to, standard-of-care treatments. The rationale for combining XL092, a next-generation tyrosine kinase inhibitor, with atezolizumab, an immune checkpoint inhibitor, is based on the potential for synergistic effects. XL092 may help reshape the TME and reduce immune suppression, potentially enhancing the anti-tumor immune response triggered by atezolizumab. This combination may offer improved outcomes compared to regorafenib, a standard treatment option for refractory mCRC. | Active, not recruiting | 874 |
| NCT05141721 | This clinical trial is evaluating a personalized cancer vaccine approach in combination with standard therapy for patients with advanced solid tumors. The study consists of two phases. In Phase 2, the goal is to assess the biological activity of patient-specific vaccines (GRT-C901 and GRT-R902) combined with ICIs and standard maintenance therapy (fluoropyrimidine and bevacizumab), compared to standard therapy alone. The primary measure of response is a reduction in ctDNA. In Phase 3, the study aims to determine the clinical effectiveness of the combination regimen by measuring PFS. The personalized vaccines are designed based on mutations unique to each patient’s tumor. These mutations can produce neoantigens that trigger an immune response when presented on the tumor cell surface. The vaccine uses a heterologous prime-boost strategy (first GRT-C901, then GRT-R902) to enhance T-cell activation against these neoantigens and support the efficacy. | Active, not recruiting | 700 |
| NCT06733038 | This trial is investigating whether adding atezolizumab to standard first-line chemotherapy (FOLFOXIRI plus bevacizumab) improves outcomes in patients with pMMR mCRC who are classified as immunoscore high. Patients will be assigned to one of two treatment groups: Arm A (control), FOLFOXIRI plus bevacizumab for up to 8 cycles, followed by maintenance therapy with 5-FU/leucovorin plus bevacizumab. Arm B (experimental), FOLFOXIRI plus bevacizumab and atezolizumab for up to 8 cycles, followed by maintenance therapy with 5-FU/leucovorin, bevacizumab, and atezolizumab. The primary goal is to determine whether the addition of atezolizumab extends PFS. | Recruiting | 238 |
| NCT05482516 | This pilot feasibility study is evaluating the use of atezolizumab and bevacizumab as adjuvant immunotherapy in patients with gastrointestinal cancers who have no evidence of disease on imaging but show minimal residual disease based on a positive Signatera™ ctDNA test. Although standard imaging may show no detectable disease after treatment, the presence of tumor DNA in the bloodstream (positive ctDNA) suggests a high risk of relapse. This study explores whether early intervention with immunotherapy (atezolizumab) and anti-angiogenic therapy (bevacizumab) can prevent or delay recurrence by targeting microscopic residual disease before it becomes clinically evident. All participants must have completed standard-of-care, curative-intent treatment (e.g., surgery, chemotherapy, radiation) and be enrolled within one year of treatment completion. Eligible patients will receive intravenous atezolizumab (1200 mg) and bevacizumab (15 mg/kg) every 21 days, continuing until disease recurrence, progression on ctDNA (molecular relapse), unacceptable toxicity, withdrawal of consent, or up to a maximum of 12 months. | Recruiting | 20 |
| NCT04157985 | PD-1/PD-L1 inhibitors have shown significant benefit in treating various cancers, but the ideal treatment duration remains unknown. Prolonged therapy may expose patients to unnecessary side effects and healthcare costs. This clinical trial aims to determine the optimal duration of PD-1/PD-L1 immunotherapy in patients with advanced solid tumors who have achieved stable disease. Patients who have been on treatment for one year and have no disease progression will be randomized to either stop therapy or continue until disease progression. The trial is being conducted within the University of Pittsburgh Medical Center (UPMC) health system, where over 2300 patients received PD-1/PD-L1 inhibitors in the past year for a range of advanced cancers. The study was initiated in response to a survey of oncologists within the system, the vast majority of whom expressed strong interest in participating in research to evaluate whether treatment can safely be stopped after one year. | Recruiting | 578 |
| NCT02912559 | dMMR tumors tend to produce more neoantigens, making them more visible to the immune system. This suggests that immunotherapy may be especially effective in this group. By combining atezolizumab with chemotherapy, the study aims to determine if this approach can reduce the risk of cancer recurrence and improve survival compared to chemotherapy alone. Participants will be randomly assigned to receive either standard chemotherapy (oxaliplatin, leucovorin calcium, and fluorouracil) alone or in combination with atezolizumab. | Active, not recruiting | 700 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT01772004 | Phase I | Avelumab | No objective responses; median PFS of 2.1 months; five grade 3 TRAEs. | 37310790 |
| NCT03152565 | Phase I/II | Avelumab plus autologous dendritic cell (ADC) vaccine | Combined therapy safe and well-tolerated; 11% of patients disease-free at 6 months; median PFS of 3.1 months; metabolic rewiring noted post-therapy. | 36083313 |
| NCT03186326 | Phase II | Avelumab vs. standard second-line chemotherapy | Avelumab superior to chemotherapy in PFS for dMMR/MSI mCRC; fewer grade 3+ TRAEs; better disease control duration with avelumab. | 37535388 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT04231552 | II | Preoperative short-course radiotherapy, CAPOX (capecitabine and oxaliplatin), and camrelizumab | pCR rate of 48.1% (13/27). Grade 1–2 AEs; no grade 4/5 AEs. Better pCR tendency without FGFR1-3 deletions. | 34725214 |
| N/A (Retrospective Study) | N/A | Camrelizumab, XELOX (capecitabine and oxaliplatin), and bevacizumab or regorafenib | ORR 72%, DCR 96%. Median PFS 11.2 months. Most AEs were grade 1 or 2; grade 3 toxicities occurred in 32% of patients. | 34900725 |
| N/A (Cohort B of CRACK Study) | II | Cetuximab, camrelizumab, and liposomal irinotecan | ORR 25%, DCR 75%. Median PFS 6.9 months, median OS 15.1 months. Grade 3 TRAEs in 15.8% of patients, no grade ≥4 TRAEs. | 37163613 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT03122509 | Phase II | Durvalumab combined with tremelimumab and radiotherapy | ORR 8.3%, median PFS 1.8 months, median OS 11.4 months, treatment-related grade 3–4 AEs in 25% | 33504552 |
| NCT02754856 | Phase II | Durvalumab combined with tremelimumab (perioperative) | 74% underwent resection, RFS 9.7 months, OS 24.5 months, 4 complete pCRs | 33811152 |
| NCT04083365 | Phase II | Durvalumab (monotherapy) | ORR 42.4%, 12-month PFS 58.2%, 12-month OS 68.3%, 36.4% with grade 3 AEs | 35179785 |
| NCT03206073 | Phase I/II | Durvalumab combined with PexaVec with/without tremelimumab | Median PFS 2.3 months, no unexpected toxicities, increased CD8+ T-cell activation | 36754451 |
| NCT03202758 | Phase 1b/2 | Durvalumab combined with tremelimumab and mFOLFOX6 | 3-month PFS 90.7%, ORR 64.5%, median PFS 8.2 months, promising clinical activity in MSS mCRC | 37563240 |
| NCT04083365 | Phase II | Durvalumab (neoadjuvant) plus capecitabine-based chemoradiotherapy | pCR 34.5%, safe toxicity profile, promising neoadjuvant strategy | 37774508 |
| NCT02671435 | Phase 1/2 | Durvalumab and monalizumab | Modest efficacy (7.7% MSS-CRC response), immune activation observed in TME | 38309722 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT03667170 | Phase 2 | Subcutaneous envafolimab monotherapy | ORR: 42.7%; DCR: 66.0%; median PFS: 11.1 months; OS at 12 months: 74.6%. | 34154614 |
| Not provided | Not provided | Neoadjuvant subcutaneous envafolimab | 66.7% pCR rate. Most common AEs: pruritus and rash (40%). No recurrences at 7.9 months follow-up. | 38691294 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT03903705 | Phase 1b/2 | Fruquintinib plus sintilimab in advanced solid tumors and metastatic colorectal cancer (mCRC). | ORR: 23.8%, median PFS: 6.9 months, median OS: 14.8 months. Grade ≥3 TRAEs: 47.7%. | 36628898 |
| N/A | N/A | Fruquintinib plus PD-1 inhibitors in refractory non-MSI-H/pMMR mCRC (real-world study). | ORR: 11.1%, DCR: 62.2%, median PFS: 3.8 months, median OS: 14.9 months. No adverse-effect-related deaths. | 35875064 |
| N/A | N/A | Anti-PD-1 antibody plus regorafenib in refractory pMMR/MSS mCRC (retrospective cohort study). | ORR: 12.7%, DCR: 41.8%, median OS: 8.4 months, median PFS: 2.5 months. Grade ≥3 TRAEs: 12.6%. | 36111036 |
| NCT04194359 | Phase II | Sintilimab plus bevacizumab, oxaliplatin, and capecitabine in RAS-mutant, MSS, unresectable mCRC. | ORR: 84%, DCR: 100%, median PFS: 18.2 months. No grade 5 TRAEs. | 37554125 |
| N/A | N/A | Sintilimab combined with anlotinib hydrochloride in MSS CRC treatment (comparative analysis). | ORR: 76.09%, improved quality of life, survival rate: 73.33%. Comparable safety profile. | 38077647 |
| N/A | N/A | Single-agent neoadjuvant PD-1 antibody (sintilimab) in locally advanced dMMR/MSI-H CRC. | pCR in 90.9%, no grade 3 or above immunotherapy-related adverse events. | 36528470 |
| Number | Short Description and Rationale | Status | Estimated Enrollment |
|---|---|---|---|
| NCT05236972 | This open-label Phase III clinical trial will compare the effectiveness of sintilimab alone versus standard chemotherapy (XELOX) in patients with locally advanced, dMMR or MSI-H CRC. Eligible patients must have no distant metastases (M0), lymph node involvement (N+), and tumors located at least 10 cm from the anal verge. Participants will be randomized into two treatment groups: Group A (Immunotherapy arm): Anti-PD-1 antibody (200 mg IV every 3 weeks) for 8 cycles. Group B (Chemotherapy arm): XELOX regimen (oxaliplatin + capecitabine) for 4 or 8 cycles, repeated every 21 days. The primary endpoint is 3-year DFS, assessed in all patients with post-randomization data. | Recruiting | 323 |
| NCT06497985 | MSS/pMMR colorectal cancers are typically resistant to immunotherapy alone. This study combines epigenetic modulation (tucidinostat), immune checkpoint inhibition (sintilimab), and anti-angiogenic therapy (bevacizumab) in an effort to sensitize these tumors to immune attack. The control, fruquintinib, represents the current standard for treatment-refractory MSS mCRC. This trial aims to determine whether the combination approach improves survival outcomes compared to existing therapies. A total of 430 patients will be enrolled and randomized in a 1:1 ratio to: Experimental arm: tucidinostat (a histone deacetylase inhibitor) + sintilimab (a PD-1 inhibitor) + bevacizumab (an anti-VEGF antibody); Control arm: fruquintinib monotherapy (a VEGFR tyrosine kinase inhibitor approved for refractory mCRC). | Recruiting | 430 |
| NCT05171660 | RAS-mutant mCRC patients typically do not benefit from anti-EGFR therapies and have limited targeted treatment options. This trial aims to determine whether the combination of immunotherapy and chemotherapy can improve clinical outcomes in this molecularly defined population, addressing an important unmet need in first-line mCRC treatment. This trial is evaluating the efficacy and safety of sintilimab in combination with XELOX (capecitabine + oxaliplatin) and bevacizumab as a first-line treatment for patients with RAS-mutant mCRC who have not received prior systemic therapy. | Recruiting | 436 |
| NCT06794086 | SBRT precisely targets liver metastases with high-dose radiation, potentially increasing tumor antigen release and enhancing immune recognition. When combined with PD-1 blockade, this local-regional approach may amplify systemic anti-tumor immune responses, offering a promising strategy for otherwise inoperable liver metastases. This trial will evaluate the efficacy and safety of combining SBRT with a PD-1 monoclonal antibody for patients with unresectable colorectal cancer liver metastases. Eligible participants are those whose liver metastases are deemed unresectable by a multidisciplinary hepatobiliary team but are [found] suitable for SBRT by a radiation oncology team. All patients will receive hypofractionated SBRT (8–12 Gy over 5 fractions) alongside systemic therapy consisting of 5–FU–based chemotherapy and PD-1 immunotherapy, administered before and after radiotherapy. | Recruiting | 24 |
| NCT06791512 | Standard immunotherapy has shown limited efficacy in pMMR/MSS CRC. However, preliminary data (from the earlier BASKET II study) suggest that combining chemotherapy and anti-angiogenic therapy with PD-1 blockade can enhance tumor immunogenicity, increase pCR rates, and improve the chance of R0 resection—a critical factor for long-term survival. This trial seeks to validate those findings on a larger scale and with longer-term endpoints. This RCT will evaluate the efficacy and safety of adding bevacizumab and a sintilimab to standard mFOLFOX6 neoadjuvant chemotherapy in patients with locally advanced pMMR/MSS CRC. | Recruiting | 122 |
| NCT05890742 | MSI-H/dMMR colon cancers are highly immunogenic and respond well to ICIs. While PD-1 blockade alone has shown promise, combining it with CTLA-4 inhibition may further enhance anti-tumor immunity by promoting a broader and more robust T-cell response. The goal of this combination is to maximize tumor shrinkage prior to surgery. This prospective clinical trial is evaluating the efficacy and safety of IBI310, a CTLA-4 monoclonal antibody, in combination with sintilimab, a PD-1 inhibitor, as neoadjuvant therapy for patients with MSI-H/dMMR resectable colon cancer. | Recruiting | 360 |
| NCT05484024 | Previous evidence from the STELLAR study demonstrated that short-course radiotherapy followed by chemotherapy (e.g., CAPOX) is non-inferior to traditional long-course chemoradiotherapy for rectal cancer. The combination of short-course radiotherapy and chemotherapy (CAPOX/mFOLFOX) may increase tumor immunogenicity, potentially making tumors more responsive to sintilimab. | Not yet recruiting | 588 |
| NCT05768503 | The trial evaluates the efficacy and safety of a novel combination—chidamide (a histone deacetylase inhibitor), sintilimab (a PD-1 inhibitor), and bevacizumab (an anti-VEGF monoclonal antibody)—compared with the standard second-line regimen of FOLFIRI plus bevacizumab in patients with MSS mCRC who have progressed after first-line oxaliplatin-based therapy. Patients with MSS colorectal cancer tend to respond poorly to immunotherapy alone due to a relatively “cold” tumor microenvironment with low immune infiltration. The study combines chidamide, which modulates gene expression and may increase tumor immunogenicity by enhancing antigen presentation and reversing immune suppression, sintilimab, a PD-1 inhibitor that restores T-cell anti-tumor activity, and bevacizumab, which normalizes tumor vasculature and can promote immune cell infiltration. Together, these agents may synergize to overcome immune resistance in MSS CRC, offering an immunomodulatory alternative to conventional chemotherapy. | Recruiting | 176 |
| NCT05374252 | Standard treatment for locally advanced anal canal squamous carcinoma involves mitomycin C + 5-FU chemotherapy combined with long-course IMRT. While this approach achieves reasonable local control, recurrence and distant metastasis remain challenges. Adding sintilimab, a PD-1 immune checkpoint inhibitor, may enhance anti-tumor immune responses during chemoradiation; improve tumor clearance, particularly in micrometastatic disease; and prolong PFS and OS compared to chemoradiotherapy alone. This trial will evaluate the efficacy and safety of adding the sintilimab to standard concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the anal canal. | Recruiting | 102 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT04911517 | II | Long-course chemoradiotherapy combined with concurrent tislelizumab | Pathological complete remission was achieved in 50% (13/26) of patients; immune-related AEs occurred in 19.2% (5/26) of patients; favorable safety and efficacy; did not increase surgical complication rate. | 36816939 |
| Not provided (ChiCTR2100046768) | II | Fecal microbiota transplantation (FMT) plus tislelizumab and fruquintinib | Median PFS: 9.6 months; Median OS: 13.7 months; ORR: 20%; DCR: 95%; CBR: 60%; 95% experienced TRAEs; 30% had grade 3–4 TRAEs; high abundance of Proteobacteria and Lachnospiraceae linked to response; manageable safety profile. | 38024475 |
| Number | Short Description and Rationale | Status | Estimated Enrollment |
|---|---|---|---|
| NCT06520683 | Stage II dMMR/MSI-H CRC typically has a favorable prognosis, but optimal adjuvant treatment is not well defined. Neoadjuvant immunotherapy trials (e.g., NICHE-2) showed exceptionally low recurrence rates with only two cycles of PD-1 blockade, suggesting short-course immunotherapy could be sufficient. A short, low-toxicity regimen may improve long-term outcomes without exposing patients to unnecessary side effects. This trial was designed to assess the efficacy and safety of two cycles of adjuvant tislelizumab compared to standard-of-care in patients with stage II dMMR)/MSI-H CRC. | Recruiting | 180 |
| NCT06332274 | MRD, detected via ctDNA, signals minimal disease presence even when imaging is clear. MRD+ patients have a significantly higher risk of relapse than those who are MRD negative. Given the efficacy of immunotherapy in advanced disease, there is strong interest in applying it earlier in the disease course, particularly in MRD+ settings. This is a biomarker-driven, single-arm clinical trial (UMBRELLA) evaluating the efficacy of tislelizumab in patients with solid tumors who are MRD+ after completing surgery and standard perioperative treatments. | Not yet recruiting | 717 |
| NCT06312982 | Neoadjuvant chemoradiotherapy is the standard approach for locally advanced rectal cancer. Adding immune checkpoint inhibitors like tirelizumab may enhance tumor response and improve long-term outcomes, including sphincter preservation and quality of life. This trial will evaluate the efficacy and safety of adding tirelizumab (tislelizumab) to standard neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. | Recruiting | 375 |
| NCT06017583 | This is a phase III RCT evaluating the efficacy and safety of combining tislelizumab with SIB-IMRT and chemotherapy (capecitabine/XELOX) in patients with locally advanced rectal cancer. | Recruiting | 48 |
| NCT06443671 | This is a prospective RCT evaluating the efficacy and safety of neoadjuvant fruquintinib and tislelizumab combined with mCapeOX versus CapeOX alone in patients with mid-high rectal cancer that is pMMR/MSS and locally advanced. | Not yet recruiting | 132 |
| NCT06507371 | Standard radiotherapy for rectal cancer often includes tumor-draining lymph nodes, which may impair local immunity and increase toxicity. Node-sparing radiotherapy targets only the tumor bed, aiming to preserve immune function and minimize side effects. Combining this approach with CAPOX (capecitabine + oxaliplatin) and tislelizumab may improve tumor response by enhancing local and systemic anti-tumor immunity in MSS patients, who typically respond poorly to immunotherapy alone. | Recruiting | 170 |
| NCT Number | Phase | Treatment | Findings | PMID |
|---|---|---|---|---|
| NCT03926338 | Phase II | Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high colorectal cancer | pCR response: 88% with toripalimab + celecoxib, 65% with toripalimab alone. No treatment-related surgical delays. High pCR rate and acceptable safety profile. | 34688374 |
| Not available | Phase Ib/II | Regorafenib plus toripalimab in metastatic colorectal cancer | ORR: 15.2%, DCR: 36.4%, median PFS: 2.1 months, median OS: 15.5 months. Patients with liver metastases had lower ORR. High-abundance Fusobacterium linked to shorter PFS. | 34622226 |
| Not available | Phase II | Regorafenib combined with toripalimab in third-line-and-beyond treatment of advanced colorectal cancer | ORR: 12.12%, DCR: 48.48%, median PFS: 113 days. TRAEs included hand–foot syndrome (33.33%) and liver dysfunction (27.27%). | 34603452 |
| Not available | Phase II | Toripalimab with fruquintinib in refractory advanced metastatic colorectal cancer | ORR: 21.05%, median PFS: 5.98 months, median OS: 11.10 months. Peritoneal metastasis was associated with longer PFS. Common AEs: fatigue (57.89%), hepatic dysfunction (42.11%), hypertension (36.84%). | 37201046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kciuk, M.; Wanke, K.; Kruczkowska, W.; Marciniak, B.; Kontek, R. Focus on PD-1/PD-L1-Targeting Antibodies in Colorectal Cancer: Are There Options Beyond Dostarlimab, Nivolumab, and Pembrolizumab? A Comprehensive Review. Molecules 2025, 30, 2686. https://doi.org/10.3390/molecules30132686
Kciuk M, Wanke K, Kruczkowska W, Marciniak B, Kontek R. Focus on PD-1/PD-L1-Targeting Antibodies in Colorectal Cancer: Are There Options Beyond Dostarlimab, Nivolumab, and Pembrolizumab? A Comprehensive Review. Molecules. 2025; 30(13):2686. https://doi.org/10.3390/molecules30132686
Chicago/Turabian StyleKciuk, Mateusz, Katarzyna Wanke, Weronika Kruczkowska, Beata Marciniak, and Renata Kontek. 2025. "Focus on PD-1/PD-L1-Targeting Antibodies in Colorectal Cancer: Are There Options Beyond Dostarlimab, Nivolumab, and Pembrolizumab? A Comprehensive Review" Molecules 30, no. 13: 2686. https://doi.org/10.3390/molecules30132686
APA StyleKciuk, M., Wanke, K., Kruczkowska, W., Marciniak, B., & Kontek, R. (2025). Focus on PD-1/PD-L1-Targeting Antibodies in Colorectal Cancer: Are There Options Beyond Dostarlimab, Nivolumab, and Pembrolizumab? A Comprehensive Review. Molecules, 30(13), 2686. https://doi.org/10.3390/molecules30132686






