From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications
Abstract
1. Introduction
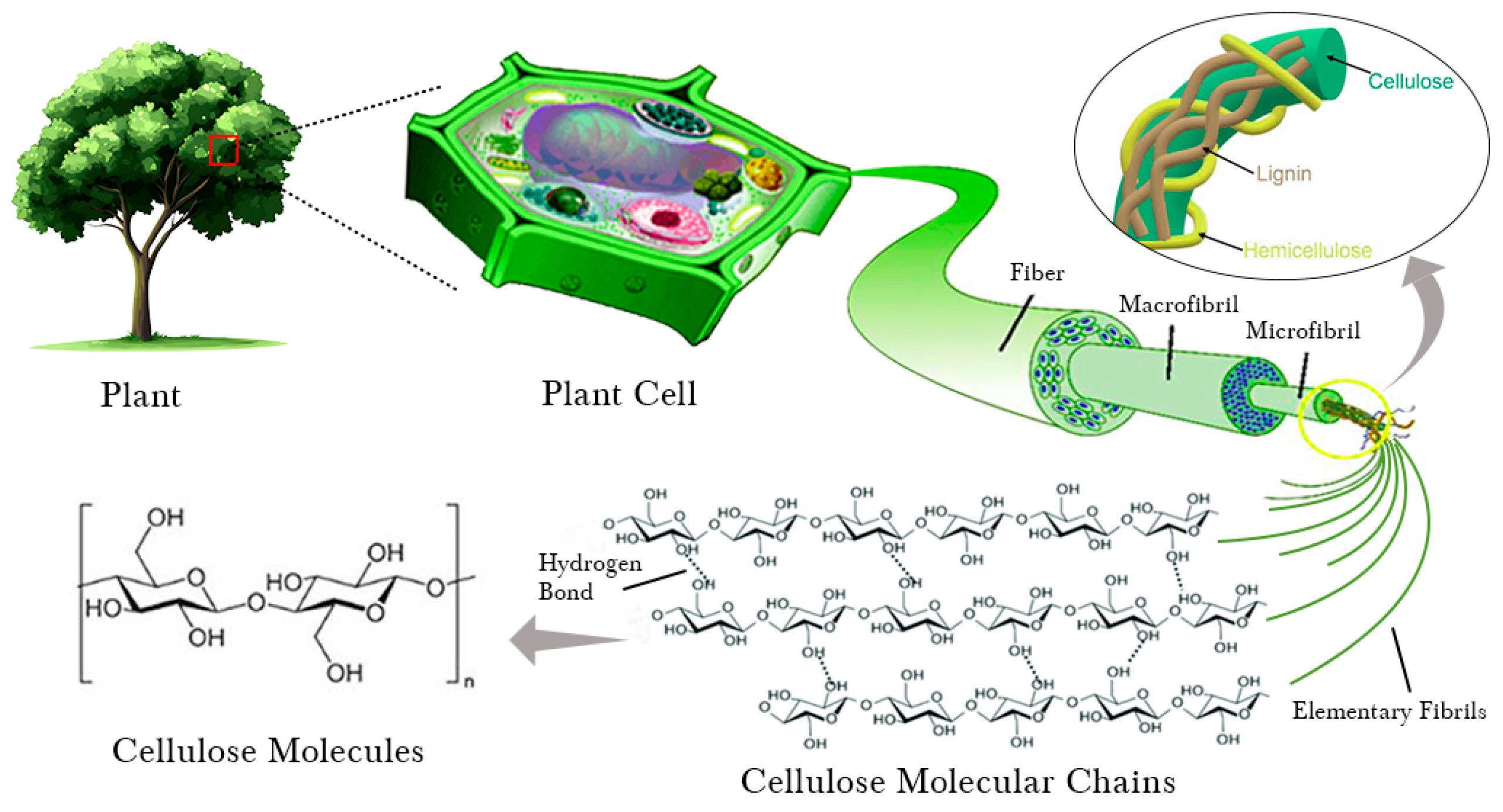
1.1. Cellulose and Nanocellulose
1.2. Types of Nanocellulose
1.2.1. Cellulose Nanofibers (CNFs)
1.2.2. Cellulose Nanocrystals (CNCs)
1.2.3. Bacterial Nanocellulose (BNC)
2. Extraction Methods
2.1. Mechanical Methods
2.1.1. High-Pressure Homogenization (HPH)
2.1.2. Grinding
2.1.3. Cryocrushing
2.1.4. Ultrasonication
2.2. Chemical Methods
2.2.1. Acid Hydrolysis
2.2.2. Alkali Treatment and Bleaching
2.3. Biological Methods
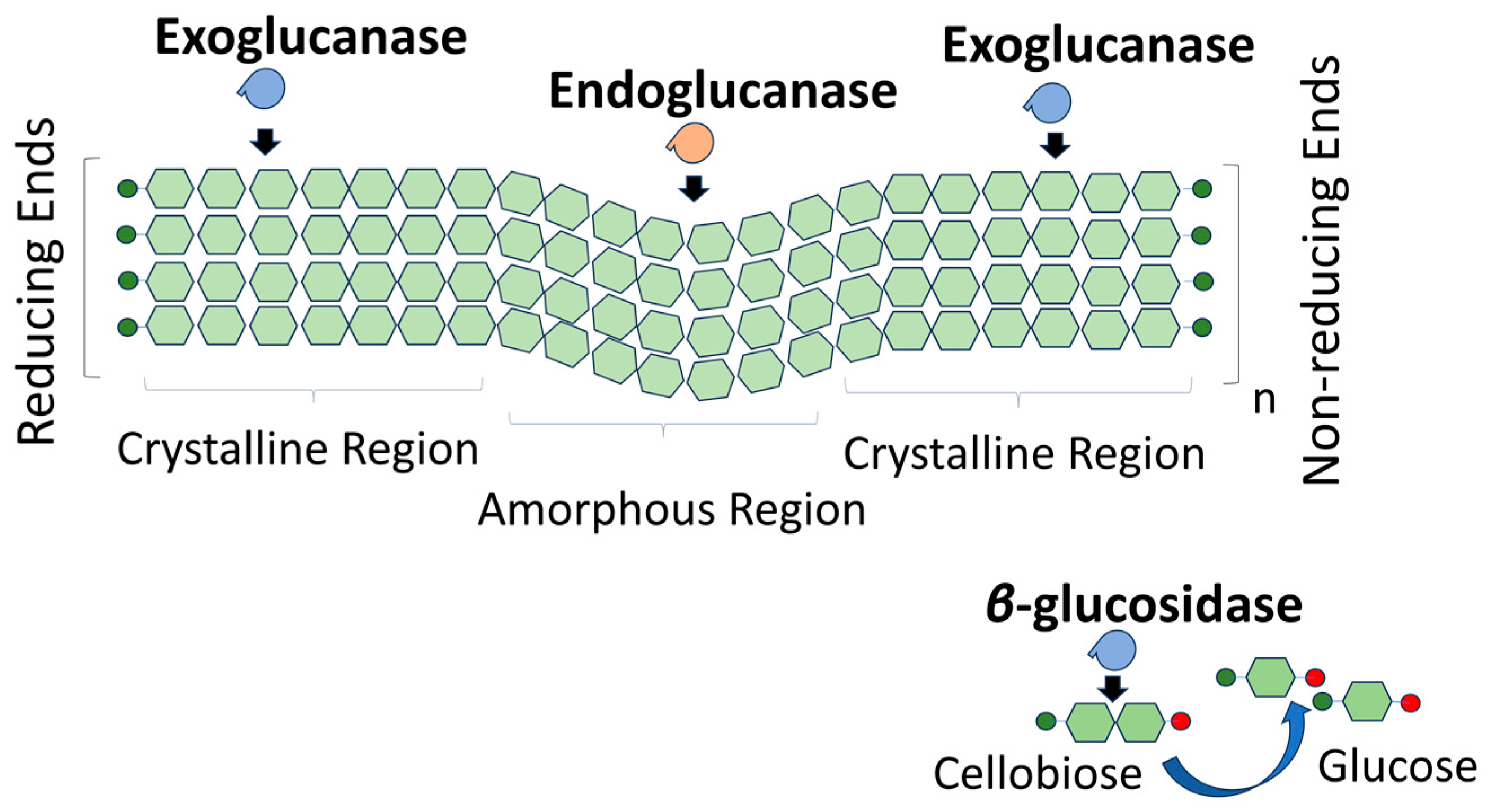
2.3.1. Enzymatic Hydrolysis
2.3.2. Microbial Fermentation
3. Applications of Nanocellulose
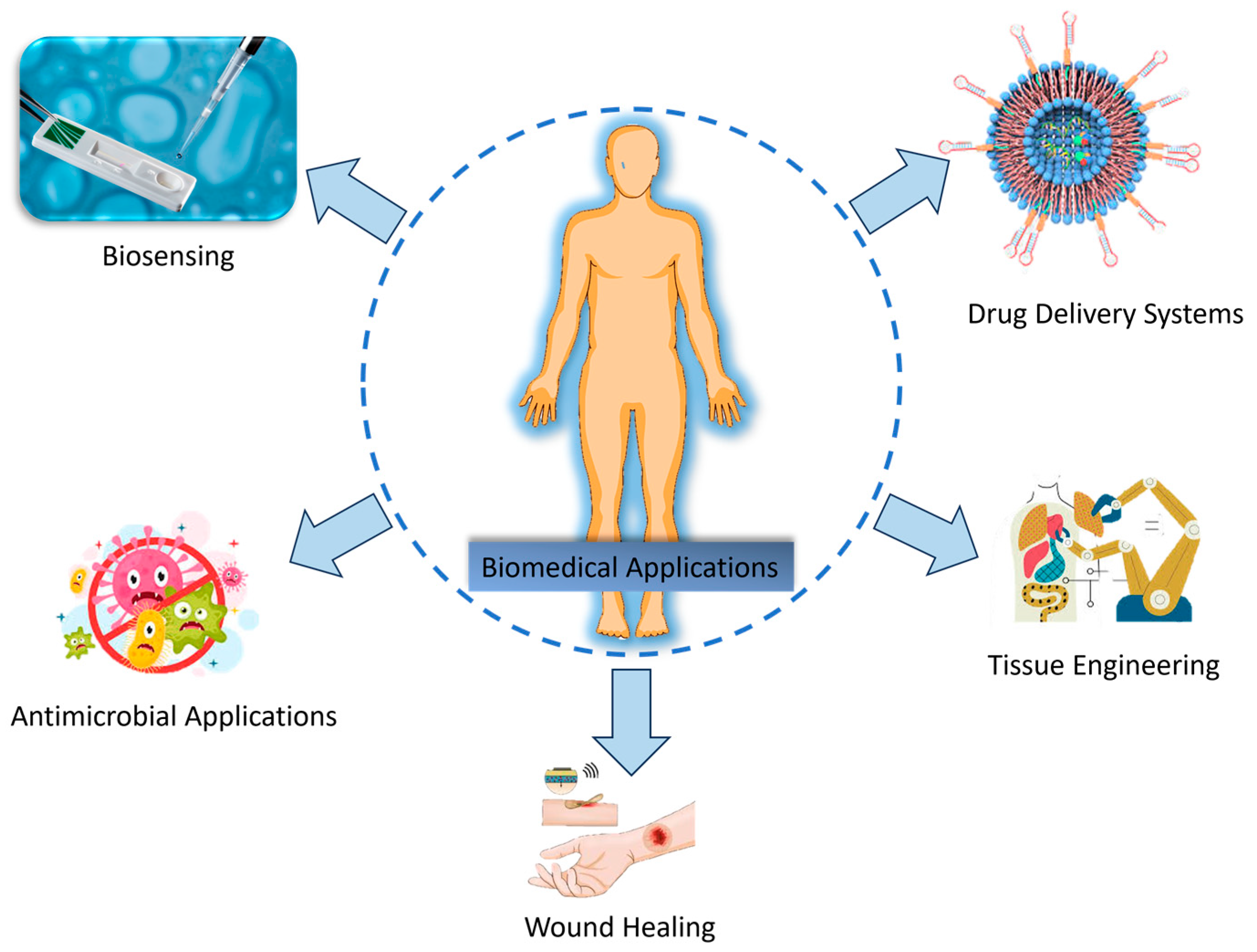
3.1. Biomedical Applications
3.1.1. Drug Delivery Systems
3.1.2. Tissue Engineering
3.1.3. Wound Healing
3.1.4. Antimicrobial Applications
3.1.5. Biosensing
3.2. Environmental Sustainability
3.2.1. Water Purification
3.2.2. Sustainable Packaging
3.2.3. Energy Storage
3.2.4. Environmental Remediation
3.3. Sensor Applications
3.3.1. Gas Sensor
3.3.2. Chemical Sensor
3.3.3. Enzyme Sensor
3.3.4. Ion Sensor
3.3.5. Glucose Sensor
3.4. Applications of Nanocellulose in Electronics
3.4.1. Flexible Electronics
3.4.2. Displays and Light-Emitting Diodes
3.4.3. Optoelectronics
3.4.4. Energy Harvesting and Storage
3.5. Applications of Nanocellulose in Thin Films
4. Challenges and Future Directions
4.1. Challenges
4.1.1. Scalability Challenges
4.1.2. Challenges Due to the Presence of Lignin
4.1.3. Functionalization Challenges
4.1.4. Regulatory Challenges
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNC | Cellulose Nanocrystal |
| CNF | Cellulose Nanofiber |
| BNC | Bacterial Nanocellulose |
| NFC | Nano-fibrillated Cellulose |
| BC | Bacterial Cellulose |
| HPH | High-pressure Homogenization |
| TEM | Transmission Electron Microscope |
| XRD | X-ray Diffraction |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl |
| xGnPs | Exfoliated Graphite nanoplatelets |
| NC | Nano Clay |
| PMDA/DCA | Pyromellitic Dianhydride and Dicyandiamide |
| GOx | Glucose Oxidase |
| CTE | Coefficient of Thermal Expansion |
| OFET | Organic Field-effect Transistors |
| CNT | Carbon Nanotubes |
| LED | Light-emitting Diodes |
| LCD | Liquid Crystal Displays |
| PAN | Polyacrylonitrile |
| PET | Polyethylene Terephthalate |
| IL | Ionic Liquid |
| DES | Deep Eutectic Solvent |
References
- Thakur, M.K.; Rana, A.K.; Thakur, V.K. Lignocellulosic polymer composites: A brief overview. In Polymer Science and Plastic Engineering; Scrivener Publishing LLC: Beverly, MA, USA, 2015; pp. 3–15. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Khowala, S.; Verma, D.; Banik, S.P. Biomolecules: (Introduction, Structure and Function), Carbohydrates, June 2008. Available online: https://www.academia.edu/1114817/Biomolecules_Introduction_Structure_and_Functions_Carbohydrates (accessed on 18 October 2024).
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Usov, I.; Nyström, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure-properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A Review of Properties of Nanocellulose, Its Synthesis, and Potential in Biomedical Applications. Appl. Sci. 2022, 12, 7090. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio- and nanocomposites: A review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Naz, S.; Ali, J.S.; Zia, M. Nanocellulose isolation characterization and applications: A journey from non-remedial to biomedical claims. Bio-Design Manuf. 2019, 2, 187–212. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, E.P.; Silvestre, A.J.D.; Freire, C.S.R.; Lobo, J.M.S.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial Synthesized Cellulose Ðarticial® Blood Vessels for Microsurgery. Available online: www.elsevier.com/locate/ppolysci (accessed on 18 October 2024).
- da Gama, F.M.P.; Dourado, F. Bacterial NanoCellulose: What future? BioImpacts 2017, 8, 1–3. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications. In Cellulose-Reinforced Nanofibre Composites: Production, Properties and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 261–276. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Giri, J.; Adhikari, R. A Brief review on extraction of nanocellulose and its application. BIBECHANA 2012, 9, 81–87. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous isolation of nanocellulose from eucalyptus pulp by high pressure homogenization. Ind. Crop. Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses and commercial potential. Appl. Polym. Sci. 1983, 37, 815–827. [Google Scholar]
- Leitner, J.; Hinterstoisser, B.; Wastyn, M.; Keckes, J.; Gindl, W. Sugar beet cellulose nanofibril-reinforced composites. Cellulose 2007, 14, 419–425. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Shakeri, A.; Misra, M.; Oksman, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4, 626–639. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Hussein, M.Z.B.; Oksman, K. Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 2009, 17, 299–307. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Ghasemi, S.; Behrooz, R.; Ghasemi, I. Extraction and characterization of nanocellulose structures from linter dissolving pulp using ultrafine grinder. J. Nanosci. Nanotechnol. 2016, 16, 5791–5797. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Gleisner, R.; Kuster, T.A.; Baxa, U.; McNeil, S.E. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Donescu, D.; Spataru, C.I.; Radovici, C.; Trusca, R.; Somoghi, R. Preparation and characterisization of PVA composites with cellulose nanofibers obtained by ultrasonication. BioResources 2011, 6, 487–512. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 2007, 67, 2521–2527. [Google Scholar] [CrossRef]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar] [CrossRef]
- Huang, W. Cellulose Nanopapers. In Nanopapers: From Nanochemistry and Nanomanufacturing to Advanced Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–173. [Google Scholar] [CrossRef]
- Wasim, M.; Shi, F.; Liu, J.; Khan, M.R.; Farooq, A.; Sanbhal, N.; Alfred, M.; Xin, L.; Yajun, C.; Zhao, X. Extraction of cellulose to progress in cellulosic nanocomposites for their potential applications in supercapacitors and energy storage devices. J. Mater. Sci. 2021, 56, 14448–14486. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J. Appl. Polym. Sci. 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Mishra, S.P.; Manent, A.-S.; Chabot, B.; Daneault, C. The use of sodium chlorite in post-oxidation of TEMPO-oxidized pulp: Effect on pulp characteristics and nanocellulose yield. J. Wood Chem. Technol. 2012, 32, 137–148. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Hai, Y.; Zhang, M.; Chen, P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose 2011, 18, 433–442. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2009, 110, 3479–3500. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Bacha, E.G. Response Surface Methodology Modeling, Experimental Validation, and Optimization of Acid Hydrolysis Process Parameters for Nanocellulose Extraction. S. Afr. J. Chem. Eng. 2022, 40, 176–185. [Google Scholar] [CrossRef]
- Filson, P.; Dawsonandoh, B. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 100, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and rapid one–step extraction of carboxylated cellulose nanocrystals by H2SO4/HNO3 mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Dorgan, J.R. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules 2008, 10, 334–341. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Ghosh, T.; Mohanty, A.K.; Misra, M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 2009, 16, 783–793. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2013, 43, 1519–1542. [Google Scholar] [CrossRef]
- Börjesson, M.; Sahlin, K.; Bernin, D.; Westman, G. Increased thermal stability of nanocellulose composites by functionalization of the sulfate groups on cellulose nanocrystals with azetidinium ions. J. Appl. Polym. Sci. 2017, 135, 45963. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Ioelovich, M. Peculiarities of Cellulose Nanoparticles; Designer Energy Ltd.: Rehovot, Israel, 2014. [Google Scholar]
- Guo, J.; Guo, X.; Wang, S.; Yin, Y. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohydr. Polym. 2016, 135, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application; KeAi Publishing Communications Ltd.: Beijing, China, 2018. [Google Scholar] [CrossRef]
- Phanthong, P.; Ma, Y.; Guan, G.; Abudula, A. Extraction of Nanocellulose from Raw Apple Stem. J. Jpn. Inst. Energy 2015, 94, 787–793. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation, and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Ghazy, M.B.; Esmail, F.A.; El-Zawawy, W.K.; Al-Maadeed, M.A.; Owda, M.E. Extraction and characterization of Nanocellulose obtained from sugarcane bagasse as agro-waste. J. Adv. Chem. 2016, 12, 4256–4264. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Morais, J.P.S.; Rosa, M.F.; Filho, M.M.S.; Nascimento, L.D.; Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Lucia, L.A.; Sain, M. Cellulosic nanocomposites, review. BioResources 2008, 3, 929–980. [Google Scholar]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and mechanical properties of wet-spun fibers made from natural cellulose nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2011, 18, 257–270. [Google Scholar] [CrossRef]
- Trifol, J.; Sillard, C.; Plackett, D.; Szabo, P.; Bras, J.; Daugaard, A.E. Chemically extracted nanocellulose from sisal fibres by a simple and industrially relevant process. Cellulose 2016, 24, 107–118. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Kallel, F.; Bettaieb, F.; Khiari, R.; García, A.; Bras, J.; Chaabouni, S.E. Isolation and structural characterization of cellulose nanocrystals extracted from garlic straw residues. Ind. Crop. Prod. 2016, 87, 287–296. [Google Scholar] [CrossRef]
- Teixeira, E.d.M.; Corrêa, A.C.; Manzoli, A.; Leite, F.d.L.; de Oliveira, C.R.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Du, C.; Li, H.; Li, B.; Liu, M.; Zhan, H. Characteristics and properties of cellulose nanofibers prepared by TEMPO oxidation of corn husk. BioResources 2016, 11, 5276–5284. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Negut, I.; Albu, C.; Bita, B. Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings 2024, 14, 256. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M.; Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl. Compos. Mater. 2007, 14, 89–103. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Sian, C.; Gea, S.; Nishino, T.; Peijs, T. All-cellulose nanocomposites by surface selective dissolution of bacterial cellulose. Cellulose 2009, 16, 435–444. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Isolation of cellulose microfibrils—An enzymatic approach. Bioresources 2006, 1, 176–188. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Himmel, M.E.; Mielenz, J.R. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of cell growth and crystalline nanocellulose production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, T.J. Mechanics of Strong and Tough Cellulose Nanopaper. Appl. Mech. Rev. 2019, 71, 040801. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Kargarzadeh, H.; Abdullah, I.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2017. [Google Scholar]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Y.; Fang, G.; Huang, C.; Rojas, O.J.; Pan, H. Highly Transparent, Strong, and Flexible Films with Modified Cellulose Nanofiber Bearing UV Shielding Property. Biomacromolecules 2018, 19, 4565–4575. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Koga, H.; Suganuma, K.; Nogi, M. Hazy Transparent Cellulose Nanopaper. Sci. Rep. 2017, 7, srep41590. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Shen, J.; Zou, L.; Wang, F.; Wang, X.; Zhang, Y.; Yao, X. Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 2016, 99, 203–211. [Google Scholar] [CrossRef]
- Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. [Google Scholar] [CrossRef]
- Fu, T.; Montes, F.; Suraneni, P.; Youngblood, J.; Weiss, J. The influence of cellulose nanocrystals on the hydration and flexural strength of Portland cement pastes. Polymers 2017, 9, 424. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef]
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 23. [Google Scholar] [CrossRef]
- Ong, X.-R.; Chen, A.X.; Li, N.; Yang, Y.Y.; Luo, H.-K. Nanocellulose: Recent Advances Toward Biomedical Applications. Small Sci. 2022, 3, 2200076. [Google Scholar] [CrossRef]
- Meziane, H.; Laita, M.; Azzaoui, K.; Boulouiz, A.; Neffa, M.; Sabbahi, R.; Nandiyanto, A.B.D.; Elidrissi, A.; Abidi, N.; Siaj, M.; et al. Nanocellulose fibers: A Review of Preparation Methods, Characterization Techniques, and Reinforcement Applications. Moroc. J. Chem. 2024, 12, 305–343. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Shishparenok, A.N.; Furman, V.V.; Dobryakova, N.V.; Zhdanov, D.D. Protein Immobilization on Bacterial Cellulose for Biomedical Application. Polymers 2024, 16, 2468. [Google Scholar] [CrossRef] [PubMed]
- Utoiu, E.; Manoiu, V.S.; Oprita, E.I.; Craciunescu, O. Bacterial Cellulose: A Sustainable Source for Hydrogels and 3D-Printed Scaffolds for Tissue Engineering. Gels 2024, 10, 387. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, P.K.; Pattnaik, R.; Kumar, S.; Ojha, S.K.; Srichandan, H.; Parhi, P.K.; Jyothi, R.K.; Sarangi, P.K. Biochemistry, Synthesis, and Applications of Bacterial Cellulose: A Review. Front. Bioeng. Biotechnol. 2022, 10, 780409. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Ogundare, S.A.; van Zyl, W.E. A review of cellulose-based substrates for SERS: Fundamentals, design principles, applications. Cellulose 2019, 26, 6489–6528. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packaging applications. Curr. Opin. Green Sustain. Chem. 2021, 31, 100512. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M.; Bousfield, D. Enhancing the Oxygen Barrier Properties of Nanocellulose at High Humidity: Numerical and Experimental Assessment. Sustain. Chem. 2020, 1, 198–208. [Google Scholar] [CrossRef]
- Rehim, A. Green Food Packaging from Nanocellulose-Based Composite Materials. In Bio-Based Packaging: Material, Environmental and Economic Aspects; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 151–164. [Google Scholar]
- Li, Y.; Zhou, Y.; Muhammad, Y.; Zhou, J.; Guo, Z.; Tan, H.; Guo, S. Nanocellulose and Its Derivatives toward Advanced Lithium Sulfur Batteries. ACS Mater. Lett. 2021, 3, 1130–1142. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current State of Applications of Nanocellulose in Flexible Energy and Electronic Devices. Front. Chem. 2020, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Agate, S.; Joyce, M.; Lucia, L.; Pal, L. Cellulose and nanocellulose-based flexible-hybrid printed electronics and conductive composites—A review. Carbohydr. Polym. 2018, 198, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Bidgoli, H.; Mortazavi, Y.; Khodadadi, A.A. A functionalized nano-structured cellulosic sorbent aerogel for oil spill cleanup: Synthesis and characterization. J. Hazard. Mater. 2019, 366, 229–239. [Google Scholar] [CrossRef]
- ben Hammouda, S.; Chen, Z.; An, C.; Lee, K. Recent advances in developing cellulosic sorbent materials for oil spill cleanup: A state-of-the-art review. J. Clean. Prod. 2021, 311, 127630. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Misenan, M.S.M.; Janudin, N.; Shah, N.A.A.; Kasim, N.; Yusoff, W.Y.W.; Noor, S.A.M.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef]
- Yin, J.; Huang, G.; An, C.; Zhang, P.; Xin, X.; Feng, R. Exploration of nanocellulose washing agent for the green remediation of phenanthrene-contaminated soil. J. Hazard. Mater. 2021, 403, 123861. [Google Scholar] [CrossRef]
- Nemoto, J.; Saito, T.; Isogai, A. Simple Freeze-Drying Procedure for Producing Nanocellulose Aerogel-Containing, High-Performance Air Filters. ACS Appl. Mater. Interfaces 2015, 7, 19809–19815. [Google Scholar] [CrossRef]
- Nemoto, J.; Soyama, T.; Saito, T.; Isogai, A. Improvement of air filters by nanocelluloses. Jpn. TAPPI J. 2016, 70, 1072–1078. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanocellulose for Sensing Applications. Adv. Mater. Interfaces 2019, 6, 1900424. [Google Scholar] [CrossRef]
- Silva, R.M.; Noremberg, B.S.; Marins, N.H.; Alano, J.H.; Santana, L.R.; Valentini, A.; Łukowiec, D.; Tański, T.; Carreño, N.L.V. Flexible cellulose-carbon nanotube paper substrate decorated with PZT: Sensor properties. MRS Adv. 2018, 3, 31–36. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, M.; Yu, J.; Sun, G. Nanofibrous polyethyleneimine membranes as sensitive coatings for quartz crystal microbalance-based formaldehyde sensors. Sens. Actuators B Chem. 2010, 144, 11–17. [Google Scholar] [CrossRef]
- Fu, J.; Li, D.; Li, G.; Huang, F.; Wei, Q. Carboxymethyl cellulose assisted immobilization of silver nanoparticles onto cellulose nanofibers for the detection of catechol. J. Electroanal. Chem. 2015, 738, 92–99. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose nanowhisker modulated 3D hierarchical conductive structure of carbon black/natural rubber nanocomposites for liquid and strain sensing application. Compos. Sci. Technol. 2016, 124, 44–51. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Benítez-Martínez, S.; Valcárcel, M. Photoluminescent sensing hydrogel platform based on the combination of nanocellulose and S,N-codoped graphene quantum dots. Sens. Actuators B Chem. 2017, 245, 946–953. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; Sethumadhavan, K.; Ullah, A.; Condon, B. Peptide conjugated cellulose nanocrystals with sensitive human neutrophil elastase sensor activity. Cellulose 2013, 20, 1223–1235. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; French, A.D.; Concha, M.; Condon, B.D. Kinetic and structural analysis of fluorescent peptides on cotton cellulose nanocrystals as elastase sensors. Carbohydr. Polym. 2015, 116, 278–285. [Google Scholar] [CrossRef]
- Schyrr, B.; Pasche, S.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors based on porous cellulose nanocrystal-poly (vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef]
- Guyomard-Lack, A.; Cerclier, C.; Beury, N.; Jean, B.; Cousin, F.; Moreau, C.; Cathala, B. Nano-structured cellulose nanocrystals-xyloglucan multilayered films for the detection of cellulase activity. Eur. Phys. J. Spec. Top. 2012, 213, 291–294. [Google Scholar] [CrossRef]
- Yin, W.; Cui, H.; Yang, Z.; Li, C.; She, M.; Yin, B.; Li, J.; Zhao, G.; Shi, Z. Facile synthesis and characterization of rhodamine-based colorimetric and “off–on” fluorescent chemosensor for Fe3+. Sens. Actuators B Chem. 2011, 157, 675–680. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Zhou, J.; Zhang, L. Synthesis and photophysical behavior of pyrene-bearing cellulose nanocrystals for Fe3+ sensing. Macromol. Chem. Phys. 2012, 213, 1612–1617. [Google Scholar] [CrossRef]
- Kacmaz, S.; Ertekin, K.; Gocmenturk, M.; Suslu, A.; Ergun, Y.; Celik, E. Selective sensing of Fe3+ at pico-molar level with ethyl cellulose based electrospun nanofibers. React. Funct. Polym. 2013, 73, 674–682. [Google Scholar] [CrossRef]
- Li, Y.; Wen, Y.; Wang, L.; He, J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Simultaneous visual detection and removal of lead(II) ions with pyromellitic dianhydride-grafted cellulose nanofibrous membranes. J. Mater. Chem. A 2015, 3, 18180–18189. [Google Scholar] [CrossRef]
- Xu, G.; Liang, S.; Fan, J.; Sheng, G.; Luo, X. Amperometric sensing of nitrite using a glassy carbon electrode modified with a multilayer consisting of carboxylated nanocrystalline cellulose and poly(diallyldimethyl ammonium) ions in a PEDOT host. Microchim. Acta 2016, 183, 2031–2037. [Google Scholar] [CrossRef]
- Tursi, A.; Gallizzi, V.; Olivito, F.; Algieri, V.; De Nino, A.; Maiuolo, L.; Beneduci, A. Selective and efficient mercury(II) removal from water by adsorption with a cellulose citrate biopolymer. J. Hazard. Mater. Lett. 2022, 3, 100060. [Google Scholar] [CrossRef]
- Hu, L.; Yan, X.-W.; Li, Q.; Zhang, X.-J.; Shan, D. Br-PADAP embedded in cellulose acetate electrospun nanofibers: Colorimetric sensor strips for visual uranyl recognition. J. Hazard. Mater. 2017, 329, 205–210. [Google Scholar] [CrossRef]
- Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy effect of nanocrystalline cellulose for the biosensing detection of glucose. Sensors 2015, 15, 24681–24697. [Google Scholar] [CrossRef]
- Yasuzawa, M.; Omura, Y.; Hiura, K.; Li, J.; Fuchiwaki, Y.; Tanaka, M. Fabrication of Amperometric Glucose Sensor Using Glucose Oxidase-Cellulose Nanofiber Aqueous Solution Rapid Communications. Anal. Sci. 2015, 31, 1111–1114. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, X.; Ren, S.; Lei, T.; Sun, X.; Qi, Y.; Wu, Q. Poly(diallyldimethylammonium chloride)-cellulose nanocrystals supported Au nanoparticles for nonenzymatic glucose sensing. RSC Adv. 2016, 6, 6436–6442. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Jia, Y.; Yang, L.; Wang, N.; Xianyu, Y.; Chen, W.; Li, X.; Cha, R.; Jiang, X. Nanocrystalline Cellulose-Assisted Generation of Silver Nanoparticles for Nonenzymatic Glucose Detection and Antibacterial Agent. Biomacromolecules 2016, 17, 2472–2478. [Google Scholar] [CrossRef]
- Seo, J.-H.; Chang, T.-H.; Lee, J.; Sabo, R.; Zhou, W.; Cai, Z.; Gong, S.; Ma, Z. Microwave flexible transistors on cellulose nanofibrillated fiber substrates. Appl. Phys. Lett. 2015, 106, 262101. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Marín-Sánchez, A.; Wicklein, B.; Salmerón, J.F.; Castillo, E.; Bobinger, M.; Salinas-Castillo, A. Cellulose nanofibers as substrate for flexible and biodegradable moisture sensors. Compos. Sci. Technol. 2021, 208, 108738. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.-H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.-W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, H.; Chen, Y.; Preston, C.; Rohrbach, K.; Cumings, J.; Hu, L. Highly transparent and flexible nanopaper transistors. ACS Nano 2013, 7, 2106–2113. [Google Scholar] [CrossRef]
- Yagyu, H.; Saito, T.; Isogai, A.; Koga, H.; Nogi, M. Chemical Modification of Cellulose Nanofibers for the Production of Highly Thermal Resistant and Optically Transparent Nanopaper for Paper Devices. ACS Appl. Mater. Interfaces 2015, 7, 22012–22017. [Google Scholar] [CrossRef]
- Nge, T.T.; Nogi, M.; Suganuma, K. Electrical functionality of inkjet-printed silver nanoparticle conductive tracks on nanostructured paper compared with those on plastic substrates. J. Mater. Chem. C 2013, 1, 5235–5243. [Google Scholar] [CrossRef]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood—Cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Yoshida, A.; Sugimoto, A.; Miyadera, T.; Miyaguchi, S. Organic Light Emitting Devices on Polymer Substrates. J. Photopolym. Sci. Technol. 2001, 14, 327–332. [Google Scholar] [CrossRef]
- Legnani, C.; Vilani, C.; Calil, V.; Barud, H.; Quirino, W.; Achete, C.; Ribeiro, S.; Cremona, M. Bacterial cellulose membrane as flexible substrate for organic light emitting devices. Thin Solid Films 2008, 517, 1016–1020. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Oulachgar, H.; Bolduc, M.; Chauve, G.; Desroches, Y.; Beaupre, P.; Bouchard, J.; Galarneau, P. Fabrication and electro-optical characterization of a nanocellulose-based spatial light modulator. MRS Adv. 2015, 1, 631–637. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, G.; Yao, J.; Liu, N.; Weil, B.; Eskilsson, M.; Karabulut, E.; Ruan, Z.; Fan, S.; Bloking, J.T.; et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 2012, 6, 513–518. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Yuan, Y.; Ha, D.; Zhu, S.; Preston, C.; Chen, Q.; Li, Y.; Han, X.; Lee, S.; et al. Novel nanostructured paper with ultrahigh transparency and ultrahigh haze for solar cells. Nano Lett. 2013, 14, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, 1536. [Google Scholar] [CrossRef]
- Jabbour, L.; Gerbaldi, C.; Chaussy, D.; Zeno, E.; Bodoardo, S.; Beneventi, D. Microfibrillated cellulose–graphite nanocomposites for highly flexible paper-like Li-ion battery electrodes. J. Mater. Chem. 2010, 20, 7344–7347. [Google Scholar] [CrossRef]
- Hu, L.; Liu, N.; Eskilsson, M.; Zheng, G.; McDonough, J.; Wågberg, L.; Cui, Y. Silicon-conductive nanopaper for Li-ion batteries. Nano Energy 2012, 2, 138–145. [Google Scholar] [CrossRef]
- Jabbour, L.; Bongiovanni, R.; Chaussy, D.; Gerbaldi, C.; Beneventi, D. Cellulose-based Li-ion batteries: A review. Cellulose 2013, 20, 1523–1545. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-paper flexible Li-ion battery cells through a paper-making process based on nano-fibrillated cellulose. J. Mater. Chem. A 2013, 1, 4671–4677. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Mathew, A.P. Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 21048–21058. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of chitosan/bacterial cellulose composite films containing nanodiamonds as a potential flexible platform for wound dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly stretchable and self-healing strain sensors based on nanocellulose-supported graphene dispersed in electro-conductive hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Tao, P.; Wu, Z.; Xing, C.; Liao, X.; Nie, S. Nanocellulose-graphene composites: A promising nanomaterial for flexible supercapacitors. Carbohydr. Polym. 2019, 207, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Melt-blending of unmodified and modified cellulose nanocrystals with reduced graphene oxide into PLA matrix for biomedical application. Polym. Adv. Technol. 2019, 30, 3049–3060. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Reduced graphene oxide and PEG-grafted TEMPO-oxidized cellulose nanocrystal reinforced poly-lactic acid nanocomposite film for biomedical application. Mater. Sci. Eng. C 2019, 104, 109956. [Google Scholar] [CrossRef]
- Song, N.; Cui, S.; Hou, X.; Ding, P.; Shi, L. Significant Enhancement of Thermal Conductivity in Nanofibrillated Cellulose Films with Low Mass Fraction of Nanodiamond. ACS Appl. Mater. Interfaces 2017, 9, 40766–40773. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef]
- Fotie, G.; Limbo, S.; Piergiovanni, L. Manufacturing of food packaging based on nanocellulose: Current advances and challenges. Nanomaterials 2020, 10, 1726. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; bin Abdullah, M.F.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2019, 137, 48544. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Adam, A.A.; Dennis, J.O.; Al-Hadeethi, Y.; Mkawi, E.M.; Abdulkadir, B.A.; Usman, F.; Hassan, Y.M.; Wadi, I.A.; Sani, M. State of the art and new directions on electrospun lignin/cellulose nanofibers for supercapacitor application: A systematic literature review. Polymers 2020, 12, 2884. [Google Scholar] [CrossRef]
- Li, W.; Shi, J. Lignin-derived carbon material for electrochemical energy storage applications: Insight into the process-structure-properties-performance correlations. Front. Bioeng. Biotechnol. 2023, 11, 1121027. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Water interactions and physical properties for packaging applications. Cellulose 2010, 17, 835–848. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, N.; Ashaduzzaman; Nazhad, M.M. Nanocellulose: Its applications, consequences and challenges in papermaking. J. Packag. Technol. Res. 2020, 4, 253–260. [Google Scholar] [CrossRef]
- Kautto, P.; Valve, H. Cosmopolitics of a Regulatory Fit: The Case of Nanocellulose. Sci. Cult. 2018, 28, 25–45. [Google Scholar] [CrossRef]
- Stoudmann, N.; Nowack, B.; Som, C. Prospective environmental risk assessment of nanocellulose for Europe. Environ. Sci. Nano 2019, 6, 2520–2531. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Monte, M.C.; Merayo, N.; Campano, C.; Negro, C.; Blanco, A. Industrial application of nanocelluloses in papermaking: A review of challenges, technical solutions, and market perspectives. Molecules 2020, 25, 526. [Google Scholar] [CrossRef] [PubMed]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2020, 33, e2000630. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2022, 25, 59–105. [Google Scholar] [CrossRef]


| Isolation Method | Source | Type of Nanocellulose | Dimensions and Yield of the Final Product | Ref. |
|---|---|---|---|---|
| Acid Hydrolysis (Acetic Acid) | Sisal fibers | CNF | 27 ± 13 nm width 658 ± 290 nm length 60–70 wt% cellulose | |
| 10–15 wt% hemicellulose | [68,69] | |||
| 8–12 wt% lignin | ||||
| Acid Hydrolysis (Sulfuric Acid) | Tea stalk | CNC | 4–8 nm width 49.87% yield | [69] |
| Acid Hydrolysis (Sulfuric Acid) | Garlic straw | CNC | 6 nm diameter 480 nm length 80 aspect ratio 41% cellulose 18% hemicellulose 3.6% lignin | [70] |
| Acid Hydrolysis (Sulfuric Acid) | Cotton fibers | CNF | 6–18 nm diameter 85–225 nm length 97.7 ± 2.2% cellulose 0.5 ± 0.4% hemicellulose 0.4 ± 0.1% lignin | [71] |
| Alkali Treatment + TEMPO Oxidation | Corn husks | CNF | 8–10 nm width Aspect ratio > 300 66.5% cellulose 29.3% hemicellulose 2.6% lignin | [72] |
| Steam Explosion + Acid Hydrolysis | Pineapple leaf fibers | CNF | 5–60 nm width 10.80 ± 0.50% moisture content 98.63 ± 0.54% cellulose 0.53 ± 0.03% hemicellulose 0.77 ± 0.44% lignin | [73] |
| Microwave Liquefaction + Chemical Treatment | Bamboo | CNF | 2–30 nm diameter 83.67% cellulose 0.13% lignin | [74] |
| High Pressure defibrillation + Chemical Treatment | Hemp fibers | CNF | 30–100 nm width Several micrometers in length 94% α-cellulose | [75] |
| Chemo-mechanical treatment | Wheat straw | CNF | 10–80 nm diameter Few thousand nanometers in length 84.6 ± 4.41% cellulose 6.0 ± 1.1% hemicellulose 9.4 ± 0.8% lignin | [76] |
| Chemo-mechanical treatment | Soy hulls | CNF | 20–520 nm diameter Few thousand nanometers in length | [76] |
| 94.0 ± 1.53% α-cellulose 3.5 ± 0.8% hemicellulose 2.5 ± 0.4% lignin |
| Properties of Nanocellulose | Examples | Numerical Ranges in Applications | Influencing Factors | Ref. |
|---|---|---|---|---|
| Mechanical Properties | Tensile Strength | 2–6 GPa (CNFCs) 10 GPa (CNCs) 3–4 GPa (Microalgal CNFCs) | Crystallinity, Aspect ratio, Orientation | [86,87] |
| Elastic Modulus | 79–88 GPa (Bacterial CNFs) 56–220 (CNCs) 29–36 GPa (Wood CNFs) | Fibril entanglement, Matrix bonding | [86,87] | |
| Thermal Properties | Thermal Degradation | 355.56 ± 2.4 °C (BNCs 2 weeks of production) 368 °C (CNCs from Ramie fibers) 350 °C (CNFs from bleached wood pulp) | Crystallinity, Surface Modification | [88] |
| Thermal Decomposition | - | Acid residues, Degree of oxidation | - | |
| Thermal Conductivity | ~0.03–0.06 W/m·K Nano wood with naturally aligned nanocellulose | Density, Porosity | [89] | |
| Optical properties | Transparency | Influence is negligible for B-CNF when at <10 wt% | Fibril diameter, Dispersion | [90] |
| Haze | 27.3–86.7% (Hazy transparent nanocellulose with 40 µm thickness) | Fibril uniformity, Surface roughness | [91] | |
| Transmittance | 93–97% (when B-CNF is loaded up to 10 wt% in the composite films) | Film thickness, Dispersion | [90,91] | |
| Structural Properties | Porosity | Mean pore diameter; 17.5 nm cellulose—FD 23.4 nm cellulose—SCD | Processing technique | [92] |
| Morphology | CNCs: ~500 nm length; ~20 nm width) CNFs enlarged fibrils (15–100 nm width) | Extraction method | [45] | |
| Hydrophilicity and Functionalization | WCA 156° BNC membrane improved with (tridecafluoro-1,1,2,2-tetrahydrooctyl)-trichlorosilane | Surface hydroxyl/carboxyl groups | [93] | |
| Charge properties/Zeta potential | −40 to −60 mV (CNCs in aqua suspensions) | Sulfation, Oxidation, pH | [94] | |
| Rheological Properties | Gelation | CNFs gel at ~1 wt% in water | Concentration, Fibril length | [95] |
| Shear Thinning | Viscosity decreases with shear | Fibril entanglement | - | |
| Viscosity and Stability | - | Concentration, Surface chemistry | - | |
| Environmental Properties | Biodegradability | Complete degradation within weeks/months | Origin, Treatment | - |
| Sustainability | Vary upon renewable biomass | Biomass source | - | |
| Carbon Neutrality | - | Production method | - | |
| Barrier properties | Gas Barrier Properties | - | Film density, Alignment | - |
| Moisture Sensitivity | 4.7 g mm/m2. day. kPa (Methyl cellulose-based 1 wt% CNC films) | Hydrophilicity, Treatment | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansini, A.M.P.; Galpaya, G.D.C.P.; Gunasena, M.D.K.M.; Abeysundara, P.M.; Kirthika, V.; Bhagya, L.; Gunawardana, H.D.C.N.; Koswattage, K.R. From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications. Molecules 2025, 30, 2670. https://doi.org/10.3390/molecules30132670
Hansini AMP, Galpaya GDCP, Gunasena MDKM, Abeysundara PM, Kirthika V, Bhagya L, Gunawardana HDCN, Koswattage KR. From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications. Molecules. 2025; 30(13):2670. https://doi.org/10.3390/molecules30132670
Chicago/Turabian StyleHansini, A. M. P., G. D. C. P. Galpaya, M. D. K. M. Gunasena, P. M. Abeysundara, V. Kirthika, L. Bhagya, H. D. C. N. Gunawardana, and K. R. Koswattage. 2025. "From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications" Molecules 30, no. 13: 2670. https://doi.org/10.3390/molecules30132670
APA StyleHansini, A. M. P., Galpaya, G. D. C. P., Gunasena, M. D. K. M., Abeysundara, P. M., Kirthika, V., Bhagya, L., Gunawardana, H. D. C. N., & Koswattage, K. R. (2025). From Nature to Innovation: Advances in Nanocellulose Extraction and Its Multifunctional Applications. Molecules, 30(13), 2670. https://doi.org/10.3390/molecules30132670







