Dispersive Liquid–Liquid Microextraction Method Utilizing a Novel Peripherally Tetra-Substituted Ni(II) Phthalocyanine as a Sensor Prior to UV-Visible Spectrophotometry for the Determination of Co2+
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of MAMA-Ni(II)Pc 2
2.2. Optimization of DLLME
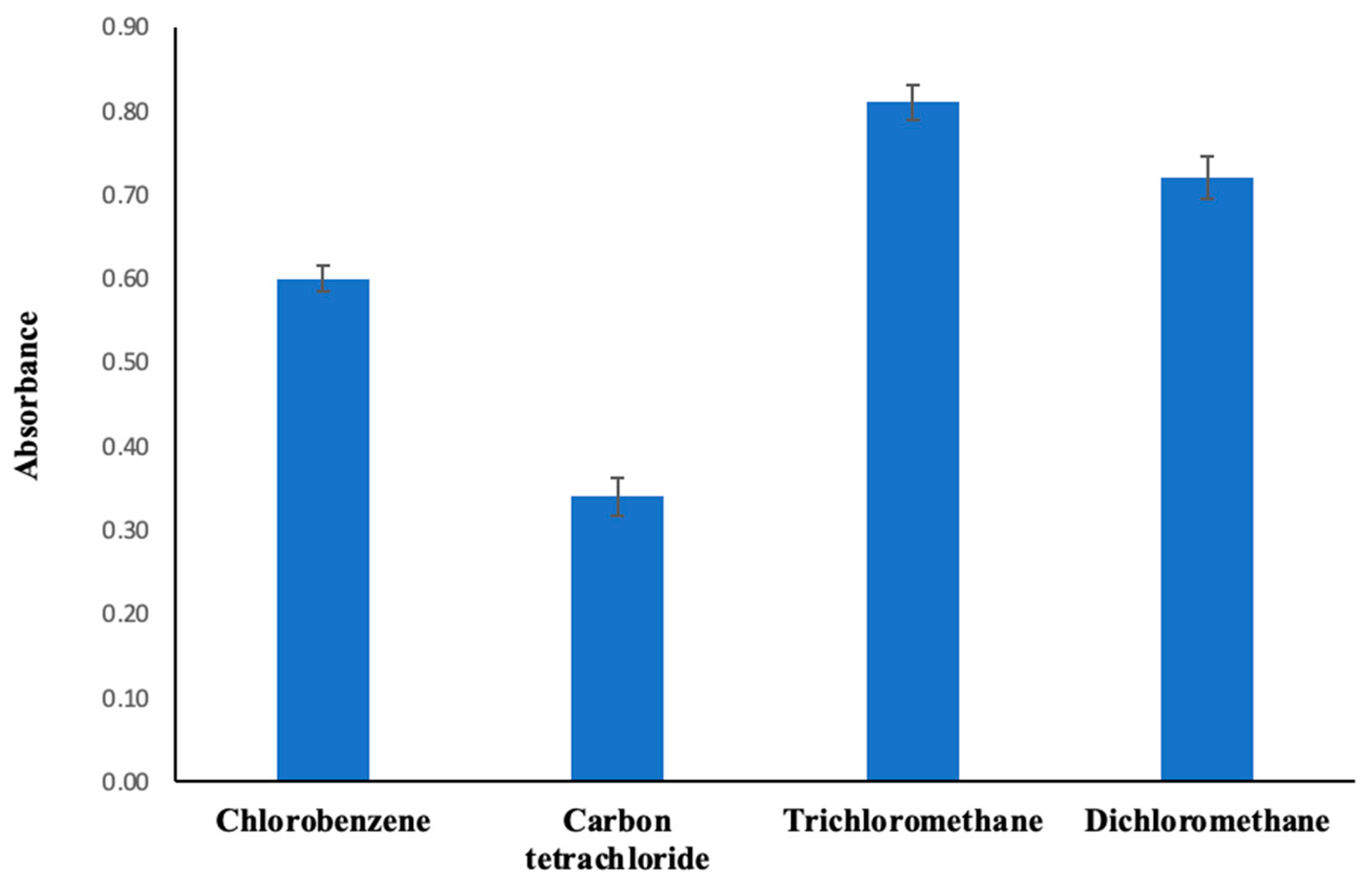
2.2.1. Specification of Extraction Solvent Kind and Quantity
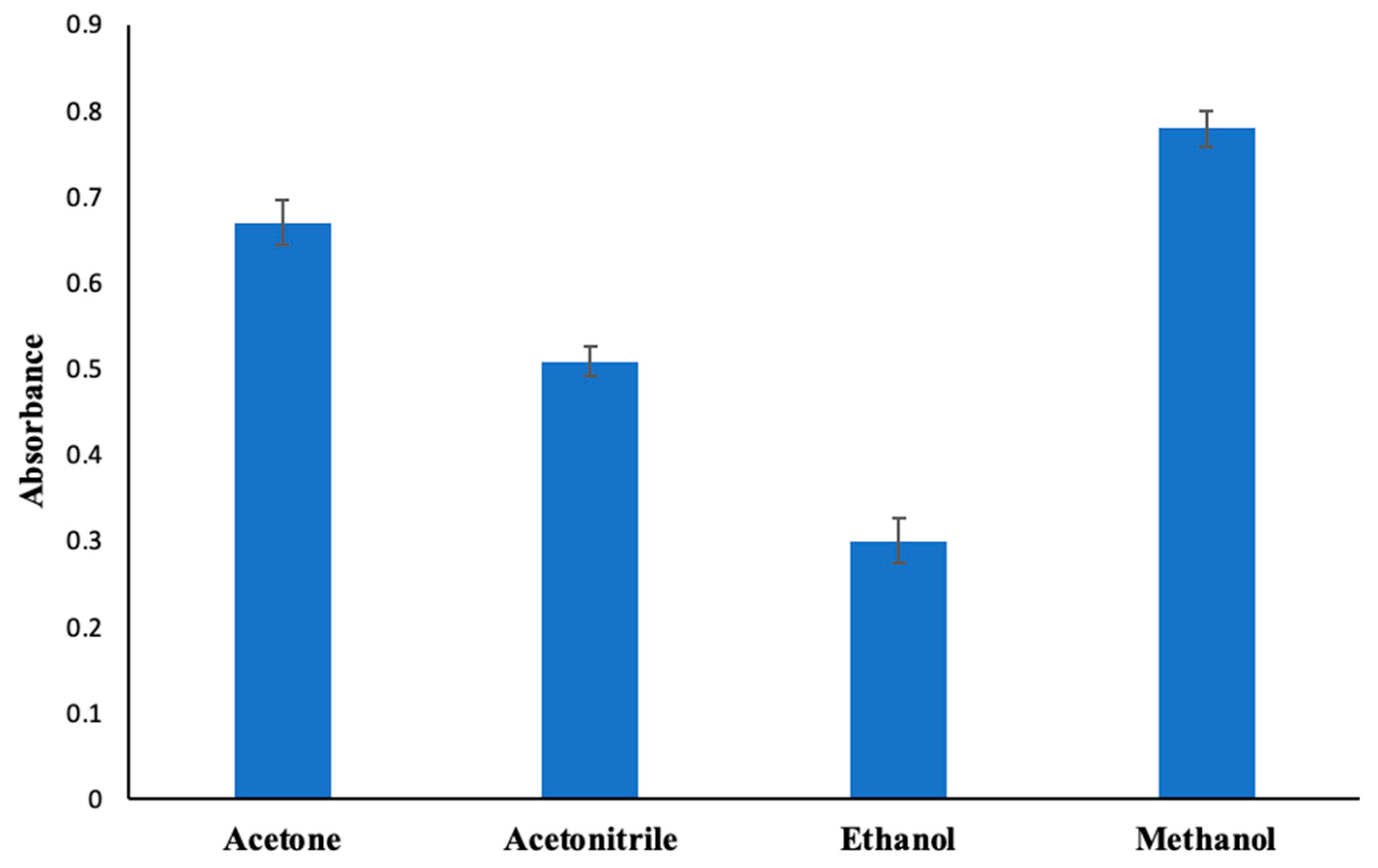
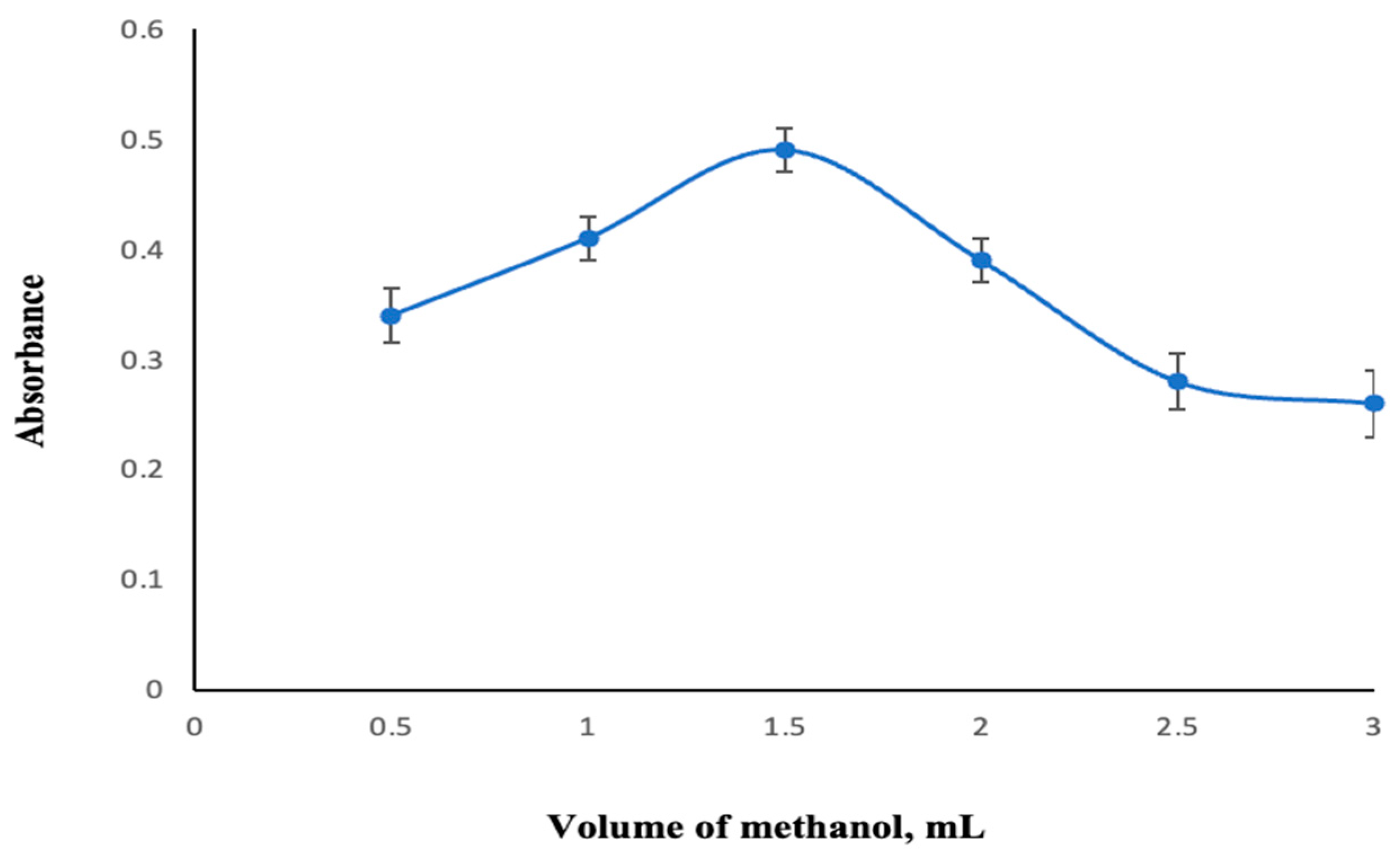
2.2.2. Specification of Dispersive Solvent Kind and Quantity
2.2.3. Effect of pH
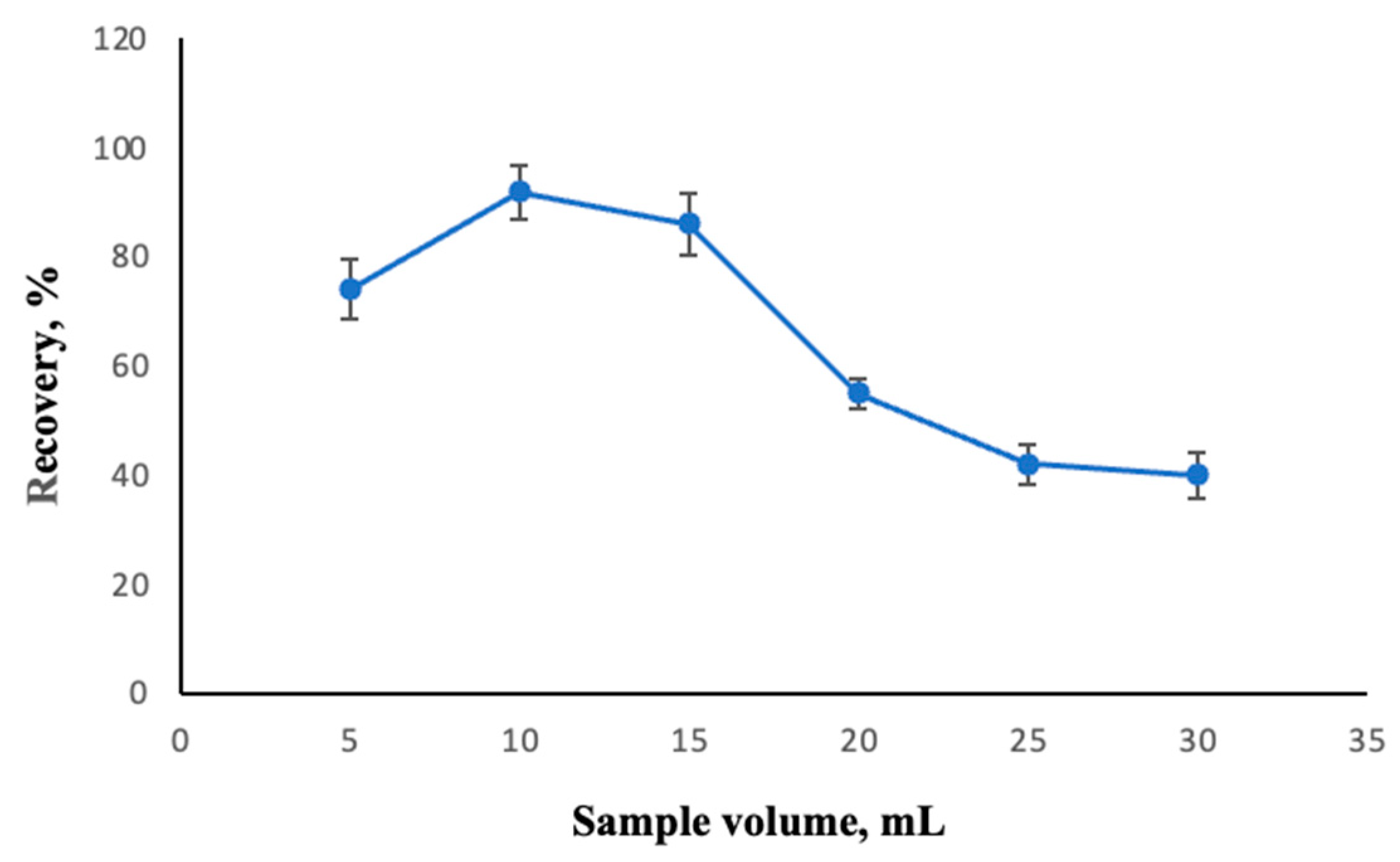
2.2.4. Effect of Extraction Time
2.2.5. Concentration of Sensor
2.2.6. Centrifuge Parameters
2.3. Interferences
2.4. Analytical Figures of Merit
2.5. Comparison with Other Studies
3. Materials and Methods
3.1. Reagents and Instrumentation
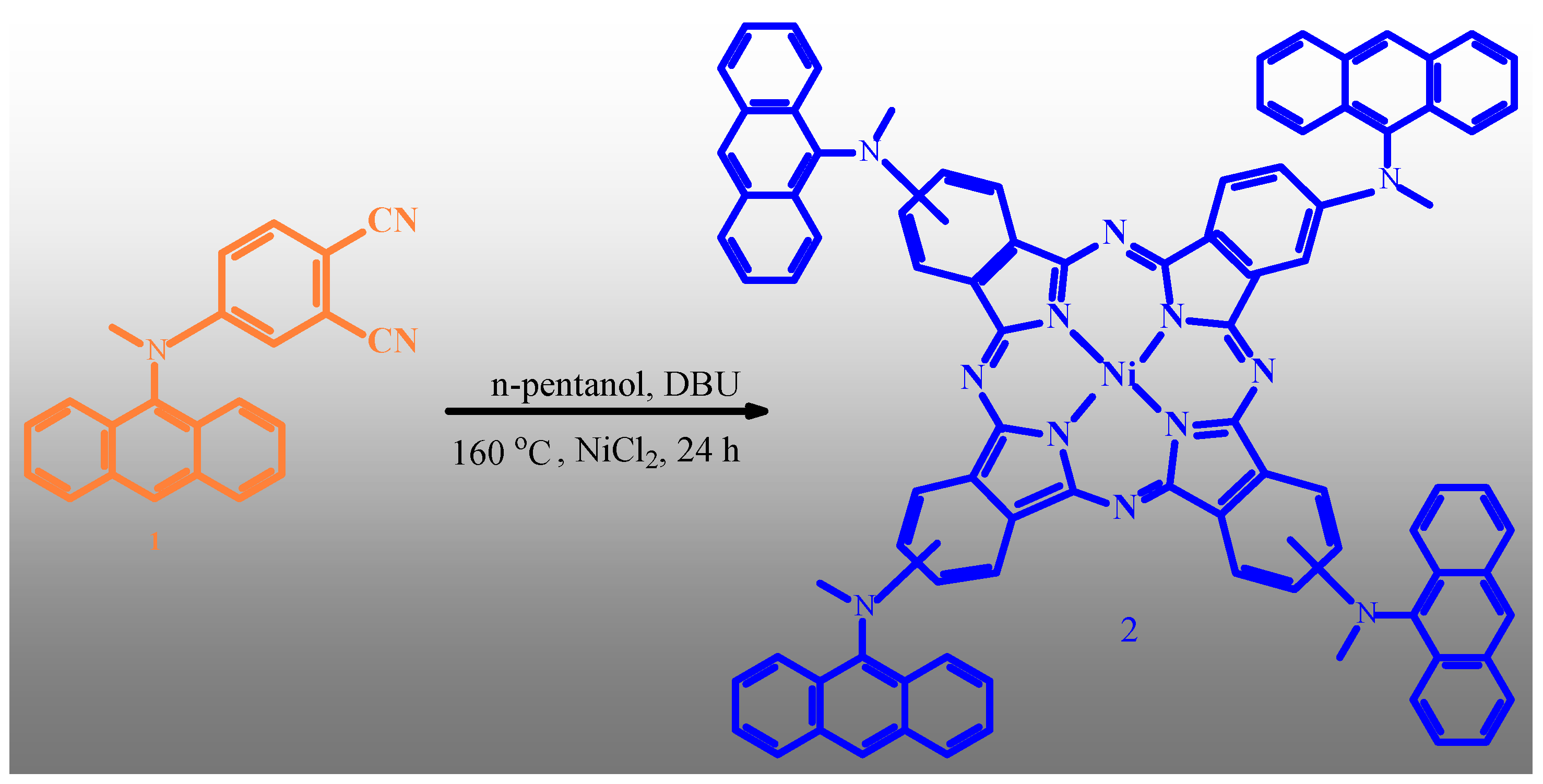
3.2. Synthesis of MAMA-Ni(II)Pc 2
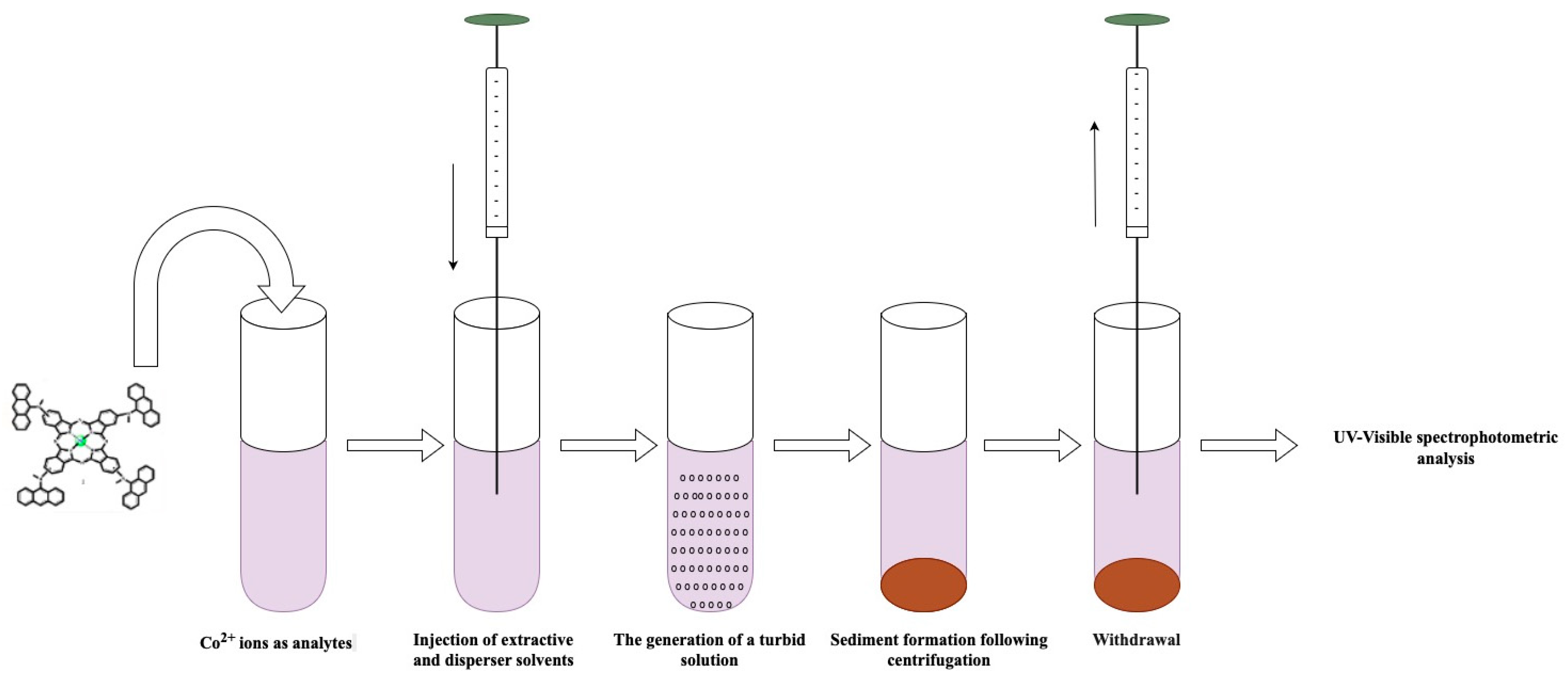
3.3. DLLME Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, A.; Kumar, A. Recent Advances on Rapid Detection and Remediation of Environmental Pollutants Utilizing Nanomaterials-Based (Bio)Sensors. Sci. Total Environ. 2022, 834, 155219. [Google Scholar] [CrossRef]
- Cerrato, A.; Cannazza, G.; Capriotti, A.L.; Citti, C.; La Barbera, G.; Laganà, A.; Montone, C.M.; Piovesana, S.; Cavaliere, C. A New Software-Assisted Analytical Workflow Based on High-Resolution Mass Spectrometry for the Systematic Study of Phenolic Compounds in Complex Matrices. Talanta 2020, 209, 120573. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wei, X.; Huang, L.; Wang, H. Worldwide Research on Extraction and Recovery of Cobalt through Bibliometric Analysis: A Review. Environ. Sci. Pollut. Res. 2023, 30, 16930–16946. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Eppinger, R.G. Distribution of Cu, Co, As, and Fe in Mine Waste, Sediment, Soil, and Water in and around Mineral Deposits and Mines of the Idaho Cobalt Belt, USA. Appl. Geochem. 2012, 27, 1053–1062. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpilowska, S.; Siwicki, A.K. Selected Aspects of the Action of Cobalt Ions in the Human Body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt Metabolism and Toxicology—A Brief Update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Santos, A.J.M.; Khemiri, S.; Simões, S.; Prista, C.; Sousa, I.; Raymundo, A. The Importance, Prevalence and Determination of Vitamins B6 and B12 in Food Matrices: A Review. Food Chem. 2023, 426, 136606. [Google Scholar] [CrossRef]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef]
- Behbahani, M.; Zarezade, V.; Veisi, A.; Omidi, F.; Bagheri, S. Modification of Magnetized MCM-41 by Pyridine Groups for Ultrasonic-Assisted Dispersive Micro-Solid-Phase Extraction of Nickel Ions. Int. J. Environ. Sci. Technol. 2019, 16, 6431–6440. [Google Scholar] [CrossRef]
- Rohani Moghadam, M.; Poorakbarian Jahromi, S.M.; Darehkordi, A. Simultaneous Spectrophotometric Determination of Copper, Cobalt, Nickel and Iron in Foodstuffs and Vegetables with a New Bis Thiosemicarbazone Ligand Using Chemometric Approaches. Food Chem. 2016, 192, 424–431. [Google Scholar] [CrossRef]
- Tekin, Z.; Erarpat, S.; Şahin, A.; Selali Chormey, D.; Bakırdere, S. Determination of Vitamin B12 and Cobalt in Egg Yolk Using Vortex Assisted Switchable Solvent Based Liquid Phase Microextraction Prior to Slotted Quartz Tube Flame Atomic Absorption Spectrometry. Food Chem. 2019, 286, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Habibollahi, S.; Akbari, A. Centrifuge-Less Deep Eutectic Solvent Based Magnetic Nanofluid-Linked Air-Agitated Liquid–Liquid Microextraction Coupled with Electrothermal Atomic Absorption Spectrometry for Simultaneous Determination of Cadmium, Lead, Copper, and Arsenic in Food Samples and Non-Alcoholic Beverages. Food Chem. 2019, 281, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.A.; El Sheikh, R.; El Sayed, H.M.; Khedr, A.M.; Al Ezz, S.A.; Gamil, W.; Hamdy, M. Ultrasound-Assisted Dispersive Microsolid-Phase Extraction for Preconcentration of Trace Cobalt and Nickel in Environmental Samples Prior to Their Determination by Flame Atomic Absorption Spectrometry. J. Appl. Spectrosc. 2022, 89, 567–578. [Google Scholar] [CrossRef]
- Jamila, N.; Khan, N.; Hwang, I.M.; Saba, M.; Khan, F.; Amin, F.; Khan, S.N.; Atlas, A.; Javed, F.; Minhaz, A.; et al. Characterization of Natural Gums via Elemental and Chemometric Analyses, Synthesis of Silver Nanoparticles, and Biological and Catalytic Applications. Int. J. Biol. Macromol. 2020, 147, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Min Hwang, I.; Yang, J.-S.; Hyun Kim, S.; Jamila, N.; Khan, N.; Su Kim, K.; Seo, H.-Y.; Hwang, M. Elemental Analysis of Sea, Rock, and Bamboo Salts by Inductively Coupled Plasma-Optical Emission and Mass Spectrometry. Anal. Lett. 2016, 49, 2807–2821. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Ilin, D.V.; Pletnev, I.V. Extraction and ICP-OES Determination of Heavy Metals Using Tetrabutylammonium Bromide Aqueous Biphasic System and Oleophilic Collector. Talanta 2021, 221, 121485. [Google Scholar] [CrossRef]
- Sadia, M.; Rasul Jan, M.; Shah, J.; Greenway, G.M. Simultaneous Preconcentration and Determination of Nickel and Cobalt Using Functionalised Mesoporous Silica Spheres by ICP-OES. Int. J. Environ. Anal. Chem. 2013, 93, 1537–1556. [Google Scholar] [CrossRef]
- Feist, B.; Mikula, B. Preconcentration of Heavy Metals on Activated Carbon and Their Determination in Fruits by Inductively Coupled Plasma Optical Emission Spectrometry. Food Chem. 2014, 147, 302–306. [Google Scholar] [CrossRef]
- Shirani, M.; Salari, F.; Habibollahi, S.; Akbari, A. Needle Hub In-Syringe Solid Phase Extraction Based a Novel Functionalized Biopolyamide for Simultaneous Green Separation/Preconcentration and Determination of Cobalt, Nickel, and Chromium (III) in Food and Environmental Samples with Micro Sampling Flame Atomic Absorption Spectrometry. Microchem. J. 2020, 152, 104340. [Google Scholar] [CrossRef]
- Arain, M.B.; Yilmaz, E.; Soylak, M. Deep Eutectic Solvent Based Ultrasonic Assisted Liquid Phase Microextraction for the FAAS Determination of Cobalt. J. Mol. Liq. 2016, 224, 538–543. [Google Scholar] [CrossRef]
- Loṕez-García, I.; Vicente-Martińez, Y.; Hernańdez-Córdoba, M. Determination of Lead and Cadmium Using an Ionic Liquid and Dispersive Liquid-Liquid Microextraction Followed by Electrothermal Atomic Absorption Spectrometry. Talanta 2013, 110, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Chan, K.H.; Azimi, G. Quantification of Nickel, Cobalt, and Manganese Concentration Using Ultraviolet-Visible Spectroscopy. RSC Adv. 2021, 11, 28014–28028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, C.; Zhu, H.; Li, Y. Determination of Trace Ions of Cobalt and Copper by UV–Vis Spectrometry in Purification Process of Zinc Hydrometallurgy. Optik 2019, 184, 227–233. [Google Scholar] [CrossRef]
- Ünaldı, M.; Yıldız, B.; Durukan, İ. Green Surfactant Assisted-Solidified Floating Organic Drop Micro-Extraction for the Preconcentration of Trace Cobalt and Determination by Flame Atomic Absorption Spectrometry. Anal. Lett. 2024, 1–18. [Google Scholar] [CrossRef]
- Ghoochani Moghadam, A.; Rajabi, M.; Hemmati, M.; Asghari, A. Development of Effervescence-Assisted Liquid Phase Microextraction Based on Fatty Acid for Determination of Silver and Cobalt Ions Using Micro-Sampling Flame Atomic Absorption Spectrometry. J. Mol. Liq. 2017, 242, 1176–1183. [Google Scholar] [CrossRef]
- Yazıcı, E.; Fırat, M.; Selali Chormey, D.; Gülhan Bakırdere, E.; Bakırdere, S. An Accurate Determination Method for Cobalt in Sage Tea and Cobalamin: Slotted Quartz Tube-Flame Atomic Absorption Spectrometry after Preconcentration with Switchable Liquid-Liquid Microextraction Using a Schiff Base. Food Chem. 2020, 302, 125336. [Google Scholar] [CrossRef]
- Mandal, S.; Lahiri, S. A Review on Extraction, Preconcentration and Speciation of Metal Ions by Sustainable Cloud Point Extraction. Microchem. J. 2022, 175, 107150. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of Organic Compounds in Water Using Dispersive Liquid-Liquid Microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Emara, A.A.A. The Recent Developments in Dispersive Liquid-Liquid Microextraction for Preconcentration and Determination of Inorganic Analytes. J. Saudi Chem. Soc. 2014, 18, 745–761. [Google Scholar] [CrossRef]
- Faraji, H. Advancements in Overcoming Challenges in Dispersive Liquid-Liquid Microextraction: An Overview of Advanced Strategies. TrAC—Trends Anal. Chem. 2024, 170, 117429. [Google Scholar] [CrossRef]
- Kamal El-Deen, A.; Elmansi, H.; Belal, F.; Magdy, G. Recent Advances in Dispersion Strategies for Dispersive Liquid–Liquid Microextraction from Green Chemistry Perspectives. Microchem. J. 2023, 191, 108807. [Google Scholar] [CrossRef]
- Oflu, S.; Zaman, B.T.; Kılınç, Y.; Bakırdere, S.; Turak, F. Determination of Trace Amounts of Metobromuron Herbicide Residues in Fruits by QuEChERS and DLLME Methods. J. Food Compos. Anal. 2024, 133, 106449. [Google Scholar] [CrossRef]
- Senge, M.O.; Sergeeva, N.N.; Hale, K.J. Classic Highlights in Porphyrin and Porphyrinoid Total Synthesis and Biosynthesis. Chem. Soc. Rev. 2021, 50, 4730–4789. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; van Zyl, W.E. Recent Advances in Phthalocyanines for Chemical Sensor, Non-Linear Optics (NLO) and Energy Storage Applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Lu, H.; Kobayashi, N. Optically Active Porphyrin and Phthalocyanine Systems. Chem. Rev. 2016, 116, 6184–6261. [Google Scholar] [CrossRef] [PubMed]
- Souza, F. Recent Advances in the Electrochemistry of Porphyrins and Phthalocyanines. J. Porphyr. Phthalocyanines 2002, 6, 285–288. [Google Scholar] [CrossRef]
- Beduoğlu, A.; Budak, Ö.; Sevim, A.M.; Koca, A.; Bayır, Z.A. Double-Decker Lutetium Phthalocyanine Functionalized with 4-Phenylthiazol-2-Thiol Moieties: Synthesis, Characterization, Electrochemistry, Spectroelectrochemistry and Electrochromism. Polyhedron 2021, 209, 115479. [Google Scholar] [CrossRef]
- Rawling, T.; McDonagh, A.M.; Colbran, S.B. Synthesis, Electrochemistry and Spectroscopic Properties of Ruthenium Phthalocyanine and Naphthalocyanine Complexes with Triphenylarsine Ligands. Inorganica Chim. Acta 2008, 361, 49–55. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Qi, C.; Bing, T.; Cheng, X.; Shangguan, D. Highly Selective Phthalocyanine-Thymine Conjugate Sensor for Hg 2+ Based on Target Induced Aggregation. Anal. Chem. 2009, 81, 3699–3704. [Google Scholar] [CrossRef]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From Outstanding Electronic Properties to Emerging Applications. Chem. Rec. 2008, 8, 75–97. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The Unique Features and Promises of Phthalocyanines as Advanced Photosensitisers for Photodynamic Therapy of Cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; He, Q.X.; Li, X.; Yoon, J.; Huang, J.D. Phthalocyanines as Contrast Agents for Photothermal Therapy. Coord. Chem. Rev. 2021, 426, 213548. [Google Scholar] [CrossRef]
- Galstyan, A. Turning Photons into Drugs: Phthalocyanine-Based Photosensitizers as Efficient Photoantimicrobials. Chem.-Eur. J. 2021, 27, 1903–1920. [Google Scholar] [CrossRef] [PubMed]
- Reheman, A.; Hu, S.; Cao, L.; Xie, D.; Yan, G.; Wang, J. Liquid-Crystalline Behaviour and Electrorheological Effect of Phthalocyanine-Based Ionic Liquid Crystals. Liq. Cryst. 2021, 48, 1321–1330. [Google Scholar] [CrossRef]
- Yenilmez, H.Y.; Farajzadeh, N.; Tollu, G.; Kuşçulu, N.G.; Bahar, D.; Özdemir, S.; Bayır, Z.A. Silicon Phthalocyanines as Anti-Infectious, Antioxidant, and Anticancer Agents. Chem. Sel. 2023, 8, e202300856. [Google Scholar] [CrossRef]
- Li, B.; Wei, P.; de Leon, A.; Frey, T.; Pentzer, E. Polymer Composites with Photo-Responsive Phthalocyanine for Patterning in Color and Fluorescence. Eur. Polym. J. 2017, 89, 399–405. [Google Scholar] [CrossRef]
- Seto, J.; Tamura, S.I.; Asai, N.; Kishii, N.; Kijima, Y.; Matsuzawa, N. Macrocyclic Functional Dyes: Applications to Optical Disk Media, Photochemical Hole Burning and Nonlinear Optics. Pure Appl. Chem. 1996, 68, 1429–1434. [Google Scholar] [CrossRef]
- Ravikanth, M.; Achim, C.; Tyhonas, J.S.; Münck, E.; Lindsey, J.S. Investigation of Phthalocyanine Catalysts for the Aerobic Synthesis of Meso-Substituted Porphyrins. J. Porphyr. Phthalocyanines 1997, 1, 385–394. [Google Scholar] [CrossRef]
- Katsurayama, Y.; Ikabata, Y.; Maeda, H.; Segi, M.; Nakai, H.; Furuyama, T. Direct Near Infrared Light–Activatable Phthalocyanine Catalysts. Chem.-Eur. J. 2022, 28, e202103223. [Google Scholar] [CrossRef]
- Spinelli, F.; D’Agostino, S.; Taddei, P.; Jones, C.D.; Steed, J.W.; Grepioni, F. Activating [4 + 4] Photoreactivity in the Solid-State: Via Complexation: From 9-(Methylaminomethyl)Anthracene to Its Silver(i) Complexes. Dalton Trans. 2018, 47, 5725–5733. [Google Scholar] [CrossRef]
- Saka, E.T. Preparation, Characterization of New Co(II) and Cu(II) Phthalocyanines and Their Catalytic Performances in Aerobic Oxidation of Substituted Phenols. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 61–69. [Google Scholar] [CrossRef]
- Saglam Ertunga, N.; Saka, E.T.; Taskin-Tok, T.; Inan Bektas, K.; Yildirim Akatin, M. Synthesis, Characterization, DNA Interaction, Molecular Docking, and α-Amylase and α-Glucosidase Inhibition Studies of a Water Soluble Zn(Ii) Phthalocyanine. Dalton Trans. 2024, 53, 11354–11367. [Google Scholar] [CrossRef] [PubMed]
- Saka, E.T.; Senocak, A.; Akkol, C. Synthesis of Phthalocyanine/C3N4 Structures and Investigation of Photocatalytic Activities in the Oxidation Reaction of 4-Nitrophenol. J. Coord. Chem. 2024, 78, 31–43. [Google Scholar] [CrossRef]
- Avlar, A.; Muzaffarzade, Y.; Tutal, B.; Bekircan, O.; Saka, E.T. Synthesis of 1,2,4-Triazole Substituted Co(II) and Cu(II) Phthalocyanine Compounds and Investigation of Their Photocatalytic Activities in the Photochemical Degradation Reaction of 4-Nitrophenol. J. Mol. Struct. 2025, 1334, 141757. [Google Scholar] [CrossRef]
- Quigley, A.; Cummins, W.; Connolly, D. Dispersive Liquid-Liquid Microextraction in the Analysis of Milk and Dairy Products: A Review. J. Chem. 2016, 2016, 4040165. [Google Scholar] [CrossRef]
- Abedi, A.-S.; Mohammadi, A.; Azadniya, E.; Mohammad Mortazavian, A.; Khaksar, R. Simultaneous Determination of Sorbic and Benzoic Acids in Milk Products Using an Optimised Microextraction Technique Followed by Gas Chromatography. Food Addit. Contam. Part A 2014, 31, 21–28. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Habila, M.; Yilmaz, E.; Soylak, M. A Dispersive Liquid-Liquid Microextraction Methodology for Copper(II) in Environmental Samples Prior to Determination Using Microsample Injection Flame Atomic Absorption Spectrometry. J. AOAC Int. 2013, 96, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Berijani, S.; Assadi, Y.; Anbia, M.; Milani Hosseini, M.R.; Aghaee, E. Dispersive Liquid-Liquid Microextraction Combined with Gas Chromatography-Flame Photometric Detection. Very Simple, Rapid and Sensitive Method for the Determination of Organophosphorus Pesticides in Water. J. Chromatogr. A 2006, 1123, 1–9. [Google Scholar] [CrossRef]
- Liang, P.; Sang, H. Determination of Trace Lead in Biological and Water Samples with Dispersive Liquid-Liquid Microextraction Preconcentration. Anal. Biochem. 2008, 380, 21–25. [Google Scholar] [CrossRef]
- Liang, P.; Peng, L.; Yan, P. Speciation of As(III) and As(V) in Water Samples by Dispersive Liquid-Liquid Microextraction Separation and Determination by Graphite Furnace Atomic Absorption Spectrometry. Microchim. Acta 2009, 166, 47–52. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Alharthi, S.S. Efficiency Enhancement of the Spectrophotometric Estimation of Cobalt in Waters and Pharmaceutical Preparations Using Dispersive Liquid–Liquid Microextraction and Microcells with Long Optical Paths. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119552. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Asif, M.; Ihsanullah, I. Dispersive Liquid–Liquid Microextraction of Multi-Elements in Seawater Followed by Inductively Coupled Plasma-Mass Spectrometric Analysis and Evaluation of Its Greenness. Microchem. J. 2021, 169, 106565. [Google Scholar] [CrossRef]
- Divrikli, U.; Altun, F.; Akdoğan, A.; Soylak, M.; Elçi, L. An Efficient Green Microextraction Method of Co and Cu in Environmental Samples Prior to Their Flame Atomic Absorption Spectrometric Detection. Int. J. Environ. Anal. Chem. 2021, 101, 2728–2741. [Google Scholar] [CrossRef]
- Mehdi, Z.S.; Alshamkhawy, S.A.R.A. A Univariate Optimization Strategy for Pre-Concentration of Cobalt(II) in Various Matrixes by a DLLME before Analysis Using FAAS. Indones. J. Chem. 2024, 24, 403–414. [Google Scholar] [CrossRef]
- Çağlar, Y.; Biyiklioglu, Z. Spectrophotometric Determination of Hg(II) in Water Samples by Dispersive Liquid Liquid Microextraction with Use Ionic Liquid after Derivatization with a Water Soluble Fe(II) Phthalocyanine. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 331–339. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: Oxford, UK, 1989. [Google Scholar]
- Young, Y.G.; Onyebuagu, W. Synthesis and Characterization of Di-Disubstituted Phthalocyanines. J. Org. Chem. 1990, 55, 2155–2159. [Google Scholar] [CrossRef]



| Foreign Ion Added | Tolerance Limit (µg/L) |
|---|---|
| Na+ | 650 |
| K+ | 800 |
| Mg2+ | 850 |
| Ca2+ | 400 |
| Ba2+ | 300 |
| Mn2+ | 752 |
| Fe3+ | 100 |
| Ni2+ | 50 |
| Cu2+ | 150 |
| Zn2+ | 200 |
| Cd2+ | 300 |
| Pb2+ | 300 |
| Cr2+ | 150 |
| Parameters | |
|---|---|
| Linear range, µg/L | 0.40–260 |
| Correlation coefficient (R2) | 0.9978 |
| LOD, µg/L | 0.19 |
| LOQ, µg/L | 0.46 |
| Enhancement factor, | 40 |
| RSD, % (n = 7) | 1.7 a and 2.4 b |
| Sample | Co2+ Amount µg/L | ||
|---|---|---|---|
| Added | Finded | Recovery % ± s.d. a | |
| Tap water 1 | 25 50.00 | 24.18 52.31 | 96.72 ± 4.1 104.62 ± 3.9 |
| Tap water 2 | 25 50.00 | 23.89 52.45 | 95.56 ± 2.7 104.9 ± 5.2 |
| Tap water 3 | 25 50.00 | 24.22 48.24 | 96.88 ± 2.5 96.48 ± 3.2 |
| Extraction Method | Linear Range (µg/L) | LOD (µg/L) | LOQ (µg/L) | RSD (%) | Reference |
|---|---|---|---|---|---|
| DLLME | 0.45–10.0 | 0.08 | 0.264 | 1.6 | [61] |
| DLLME a | 0.1–100 | 0.02 b | 0.07 | 2.7 | [62] |
| DLLME a | 0–6 b | 2.48 b | 9.01 | 1 | [63] |
| DLLME | 4.00–160 | 1.04 | 3.47 | 2.4–11.8 | [64] |
| DLLME | 0.40–260 | 0.19 | 0.46 | 1.7 c and 2.4 d | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çağlar, Y.; Saka, E.T. Dispersive Liquid–Liquid Microextraction Method Utilizing a Novel Peripherally Tetra-Substituted Ni(II) Phthalocyanine as a Sensor Prior to UV-Visible Spectrophotometry for the Determination of Co2+. Molecules 2025, 30, 2548. https://doi.org/10.3390/molecules30122548
Çağlar Y, Saka ET. Dispersive Liquid–Liquid Microextraction Method Utilizing a Novel Peripherally Tetra-Substituted Ni(II) Phthalocyanine as a Sensor Prior to UV-Visible Spectrophotometry for the Determination of Co2+. Molecules. 2025; 30(12):2548. https://doi.org/10.3390/molecules30122548
Chicago/Turabian StyleÇağlar, Yasemin, and Ece Tuğba Saka. 2025. "Dispersive Liquid–Liquid Microextraction Method Utilizing a Novel Peripherally Tetra-Substituted Ni(II) Phthalocyanine as a Sensor Prior to UV-Visible Spectrophotometry for the Determination of Co2+" Molecules 30, no. 12: 2548. https://doi.org/10.3390/molecules30122548
APA StyleÇağlar, Y., & Saka, E. T. (2025). Dispersive Liquid–Liquid Microextraction Method Utilizing a Novel Peripherally Tetra-Substituted Ni(II) Phthalocyanine as a Sensor Prior to UV-Visible Spectrophotometry for the Determination of Co2+. Molecules, 30(12), 2548. https://doi.org/10.3390/molecules30122548






