Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of BTD Derivatives
2.2. Phase Transition Behaviors of BTD Derivatives
2.2.1. Me2NCn, Me2NOCn (n = 7 or 8), and Me2NOEH
2.2.2. C8NMeC7, C6NOC6, EHNOC6, and EHNOEH
2.2.3. AldC8, ActC8, TFActC8, and TFMeC8
2.2.4. C6NCN and C6NTFMe
2.2.5. A Comparison of Liquid Crystallinity with Previous Studies
2.3. Photophysical Properties of BTD Derivatives
2.3.1. Absorption Properties in THF Solution
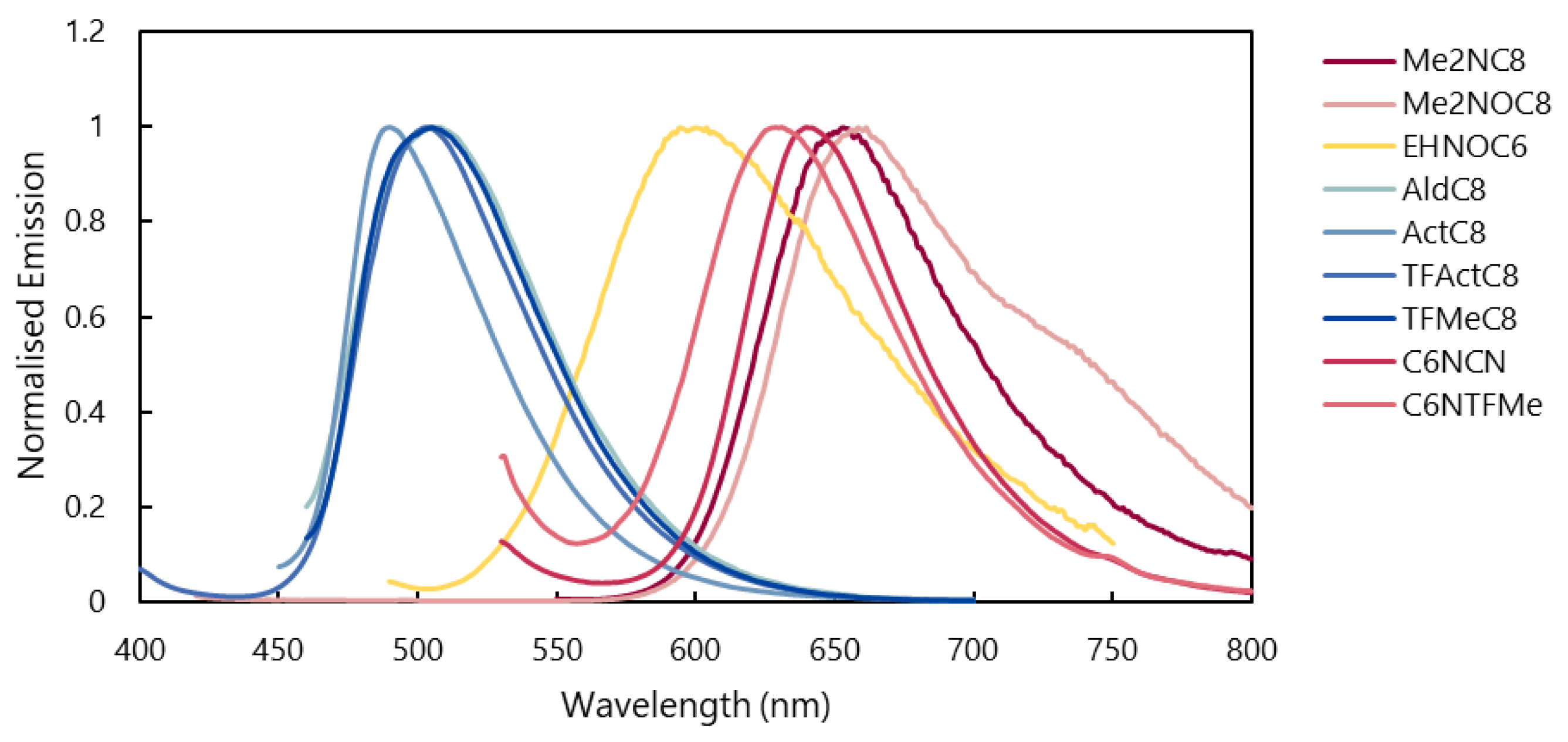
2.3.2. Fluorescence Properties in THF Solution
2.3.3. Fluorescence Properties in Polycrystalline State
2.3.4. Fluorescence Properties in Mesophase
3. Experimental Method
3.1. Materials
3.2. Instruments
3.3. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About Supramolecular Assemblies of π-Conjugated Systems. Chem. Rev. 2005, 105, 1491. [Google Scholar] [CrossRef]
- Fleischmann, E.-K.; Zentel, R. Liquid-Crystalline Ordering as a Concept in Materials Science: From Semiconductors to Stimuli-Responsive Devices. Angew. Chem. Int. Ed. 2013, 52, 8810. [Google Scholar] [CrossRef] [PubMed]
- Niori, T.; Sekine, T.; Watanabe, J.; Furukawa, T.; Takezoe, H. Distinct ferroelectric smectic liquid crystals consisting of banana shaped achiral molecules. J. Mater. Chem. 1996, 6, 1231. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and higher oligomers: Between monomers and polymers, Chem. Soc. Rev. 2007, 36, 2096. [Google Scholar] [CrossRef]
- Swager, T.M. Molecular Shape and Polar Order in Columnar Liquid Crystals. Acc. Chem. Res. 2022, 55, 3010. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Ordered Materials for Organic Electronics and Photonics. Adv. Mater. 2010, 23, 566. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Liquid crystals for charge transport, luminescence, and photonics. Adv. Mater. 2003, 15, 1135. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Liquid Crystals in Photovoltaics: A New Generation of Organic Photovoltaics. Polym. J. 2017, 49, 85. [Google Scholar] [CrossRef]
- Hwang, J.; Song, M.H.; Park, B.; Nishimura, S.; Toyooka, T.; Wu, J.W.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Electro-tunable optical diode based on photonic bandgap liquid-crystal heterojunctions. Nat. Mater. 2005, 4, 383. [Google Scholar] [CrossRef]
- Kobashi, J.; Yoshida, H.; Ozaki, M. Planar optics with patterned chiral liquid crystals. Nat. Photonics 2016, 10, 389. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Tsutsui, Y.; Sakurai, T.; Sakamaki, D.; Tohnai, N.; Kato, K.; Takata, M.; Akutagawa, T.; Sakai, K.; Seki, S. Optical and structural properties of ESIPT inspired HBT–fluorene molecular aggregates and liquid crystals. J. Phys. Chem. B 2017, 121, 10407. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Taguchi, D.; Kajitani, T.; Fukushima, T.; Kubo, S.; Shishido, A. Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties. Molecules 2023, 28, 7849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sakurai, T.; Aotani, M.; Watanabe, G.; Yoshida, H.; Padalkar, V.S.; Tsutsui, Y.; Sakamaki, D.; Ozaki, M.; Seki, S. Highly fluorescent liquid crystals from excited-state intramolecular proton transfer molecules. Adv. Opti. Mater. 2019, 7, 1801349. [Google Scholar] [CrossRef]
- Shigeyama, T.; Matsumoto, K.; Hisano, K.; Tsutsumi, O. Tunable Reflection through Size Polydispersity of Chiral-Nematic Liquid Crystal Polymer Particles. Molecules 2023, 28, 7779. [Google Scholar] [CrossRef]
- Uchimura, M.; Watanabe, Y.; Araoka, F.; Watanabe, J.; Takezoe, H.; Konishi, G. Development of Laser Dyes to Realize Low Threshold in Dye-Doped Cholesteric Liquid Crystal Lasers. Adv. Mater. 2010, 22, 4473. [Google Scholar] [CrossRef]
- Oladepo, S.A. Development and Application of Liquid Crystals as Stimuli-Responsive Sensors. Molecules 2022, 27, 1453. [Google Scholar] [CrossRef]
- Wang, L.; Urbas, L.M.; Li, Q. Nature-Inspired Emerging Chiral Liquid Crystal Nanostructures: From Molecular Self-Assembly to DNA Mesophase and Nanocolloids. Adv. Mater. 2020, 32, 1801335. [Google Scholar] [CrossRef]
- Gao, J.; Tang, Y.; Martella, D.; Guo, J.; Wiersma, D.S.; Li, Q. Stimuli-responsive photonic actuators for integrated biomimetic and intelligent systems. Responsive Mater. 2023, 1, e20230008. [Google Scholar] [CrossRef]
- Kang, W.; Tang, Y.; Meng, X.; Lin, S.; Zhang, X.; Guo, J.; Li, Q. A Photo- and Thermo-Driven Azoarene-Based Circularly Polarized Luminescence Molecular Switch in a Liquid Crystal Host. Angew. Chem. 2023, 62, e202311486. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887. [Google Scholar] [CrossRef]
- De Castro, L.D.C.; Lub, J.; Oliveira, O.N.; Schenning, A.P.H.J. Mechanochromic Displays Based on Photoswitchable Cholesteric Liquid Crystal Elastomers. Angew. Chem. Int. Ed. 2025, 64, e202413559. [Google Scholar] [CrossRef] [PubMed]
- Fellert, M.; Hein, R.; Ryabchun, A.; Gisbert, Y.; Stindt, C.N.; Feringa, B.L. A Multiresponsive Ferrocene-Based Chiral Overcrowded Alkene Twisting Liquid Crystals. Angew. Chem. Int. Ed. 2025, 64, e202413047. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Komiyama, R.; Kobayashi, M.; Kosaka, A.; Kajitani, T.; Haruki, R.; Kumai, R.; Adachi, S.I.; Tada, T.; Karasawa, N.; et al. Collective bending motion of a two-dimensionally correlated bowl-stacked columnar liquid crystalline assembly under a shear force. Sci. Adv. 2023, 9, eadg8202. [Google Scholar] [CrossRef]
- Yagai, S.; Okamura, S.; Nakano, Y.; Yamauchi, M.; Kishikawa, K.; Karatsu, T.; Kitamura, A.; Ueno, A.; Kuzuhara, D.; Yamada, H.; et al. Design amphiphilic dipolar π-systems for stimuli-responsive luminescent materials using metastable states. Nat. Commun. 2014, 5, 4013. [Google Scholar] [CrossRef]
- Strachan, G.J.; Górecka, E.; Hobbs, J.; Pociecha, D. Fluorination: Simple Change but Complex Impact on Ferroelectric Nematic and Smectic Liquid Crystal Phases. J. Am. Chem. Soc. 2025, 147, 6058. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Liao, R.; Xie, M.; Song, X.; Zhang, A.; Fang, Y.; Zhang, C.; Yu, H. Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals. Molecules 2025, 30, 799. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Wang, J.; Chen, X. Aggregation-Induced Emission-Active Cyanostilbene-Based Liquid Crystals: Self-Assembly, Photophysical Property, and Multiresponsive Behavior. Molecules 2024, 29, 5811. [Google Scholar] [CrossRef]
- Hang, Z.; Jiang, S.; Wu, Z.; Gong, J.; Zhang, L. A Novel Near-Infrared Tricyanofuran-Based Fluorophore Probe for Polarity Detection and LD Imaging. Molecules 2024, 29, 5069. [Google Scholar] [CrossRef]
- Bui, T.-T.; Péralta, S.; Dumur, F. Synthesis and Optical Properties of a Series of Push-Pull Dyes Based on Pyrene as the Electron Donor. Molecules 2023, 28, 1489. [Google Scholar] [CrossRef]
- Singh, A.P.; Gupta, S.K.; Mohiuddin, G.; Khan, R.K.; Karunakar, M. Synthesis, structure and mesogenic properties of benzoxazole derivatives. Liq. Cryst. 2024, 51, 1195. [Google Scholar] [CrossRef]
- Da Silva, F.N.; Silva, E.O.; dos Santos, G.; Postacchini, B.B.; Cazati, T.; Bechtold, I.H.; Vieira, A.A. Unlocking the potential of 2,1,3-benzoxadiazole-based luminescent liquid crystals. Liq. Cryst. 2023, 50, 1688. [Google Scholar] [CrossRef]
- Cruickshank, E. The Emergence of a Polar Nematic Phase: A Chemist’s Insight into the Ferroelectric Nematic Phase. ChemPlusChem 2024, 89, e202300726. [Google Scholar] [CrossRef] [PubMed]
- Mandle, R.J.; Sebastián, N.; Martinez-Perdiguero, J.; Mertelj, A. On the molecular origins of the ferroelectric splay nematic phase. Nat. Commun. 2021, 12, 4962. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Korblova, E.; Dong, D.; Wei, X.; Shao, R.; Radzihovsky, L.; Glaser, M.A.; Maclennan, J.E.; Bedrov, D.; Walba, D.M.; et al. First-principles experimental demonstration of ferroelectricity in a thermotropic nematic liquid crystal: Polar domains and striking electro-optics. Proc. Natl. Acad. Sci. USA 2020, 117, 14021. [Google Scholar] [CrossRef]
- Nishikawa, H.; Okada, D.; Kwaria, D.; Nihonyanagi, A.; Kuwayama, M.; Hoshino, M.; Araoka, F. Emergent Ferroelectric Nematic and Heliconical Ferroelectric Nematic States in an Achiral “Straight” Polar Rod Mesogen. Adv. Mater. 2024, 39, 2405718. [Google Scholar] [CrossRef]
- Czerwiński, M.; Dmochowska, E.; Herman, J.; Filipow, M.; Biełaga, G.; Lubiński, N.; Perkowski, P.; Kula, P. Chiral liquid crystal dimers with smectic phases for stabilization of anticlinic state in surface-stabilised geometry. J. Mol. Liq. 2024, 412, 125824. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Haraguchi, N.; Itsuno, S.; Tsuji, H. Synthesis, phase transitions and birefringence of novel liquid crystalline 1,4-phenylene bis(4-alkylthio benzoates) and insights into the cybotactic nematic behaviour. Liq. Cryst. 2017, 45, 821. [Google Scholar] [CrossRef]
- Arakawa, Y.; Kang, S.; Tsuji, H.; Watanabe, J.; Konishi, G. The design of liquid crystalline bistolane-based materials with extremely high birefringence. RSC Adv. 2016, 6, 92845. [Google Scholar] [CrossRef]
- Arakawa, Y.; Kang, S.; Tsuji, H.; Watanabe, J.; Konishi, G. Development of novel bistolane-based liquid crystalline molecules with an alkylsulfanyl group for highly birefringent materials. RSC Adv. 2016, 6, 16568. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, S.; Igawa, K.; Tokita, M.; Konishi, G.; Tsuji, H. Birefringence and photoluminescence properties of diphenylacetylene-based liquid crystal dimers. New J. Chem. 2020, 44, 17531. [Google Scholar] [CrossRef]
- Long, G.Y.; Deng, Y.P.; Zhao, W.; Zhou, G.F.; Broer, D.J.; Feringa, B.; Chen, J.W. Photoresponsive Biomimetic Functions by Light-Driven Molecular Motors in Three Dimensionally Printed Liquid Crystal Elastomers. J. Am. Chem. Soc. 2024, 146, 13894. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Greenfield, J.L.; Cowieson, N.; Fuchter, M.J.; Evans, R.C. Light-Driven Hexagonal-to-Cubic Phase Switching in Arylazopyrazole Lyotropic Liquid Crystals. J. Am. Chem. Soc. 2024, 146, 12315. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Peterca, M.; Percec, V. Designing Highly Ordered Helical and Nonhelical Porous Crystalline and Disordered Nonhelical Columnar Liquid Crystalline Self-Organizations. J. Am. Chem. Soc. 2024, 146, 22943. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, W.; Yan, H.; Zhang, X.; Han, D.; He, Y.; Li, C.; Sun, L. Programmable Complex Shape Changing of Polysiloxane Main-Chain Liquid Crystalline Elastomers. Molecules 2023, 28, 4858. [Google Scholar] [CrossRef]
- Shaya, J.; Ribierre, J.-C.; Correia, G.; Dappe, Y.J.; Mathevet, F.; Mager, L.; Heinrich, B.; Méry, S. Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization. Molecules 2023, 28, 2038. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, L.L.; Gao, M.Y.; Xu, C.J.; Ge, Y.F.; Bai, T.X.; Wen, J.; Cheng, Y.H.; Zhu, M.F. Confinement fluorescence effect of an aggregation-induced emission luminogen in crystalline polymer. Aggregate 2023, 4, e338. [Google Scholar] [CrossRef]
- Anders, C.; Tan, T.; Fischer, V.M.; Ruoyu, W.; Alaasar, M.; Waldecker, R.; Cao, Y.; Liu, F.; Tschierske, C. Engineering “Meso-Atom” bonding: Honeycomb-network transitions in reticular liquid crystals. Aggregate 2025, 6, e728. [Google Scholar] [CrossRef]
- Sakurai, T.; Kato, K.; Shimizu, M. Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems. Crystals 2023, 13, 1473. [Google Scholar] [CrossRef]
- Wu, X.; Niu, X.; Zhu, S.; Tian, M.; Liu, W. A novel multi-stimuli responsive fluorescence liquid crystal material with aggregationinduced emission effect. Liq. Cryst. 2023, 50, 1035. [Google Scholar] [CrossRef]
- Sawatari, Y.; Shimomura, Y.; Takeuchi, M.; Iwai, R.; Tanaka, T.; Tsurumaki, E.; Tokita, M.; Watanabe, J.; Konishi, G. Supramolecular liquid crystals from the dimer of L-shaped molecules with tertiary amide end groups. Aggregate 2024, 5, e507. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kawamura, A.; Tokita, M.; Watanabe, J.; Konishi, G. Fluorinated Poly(pentylene 4,4′-bibenzoate)s with Low Isotropization Temperatures and Unique Phase Transition Behavior. Macromolecules 2023, 56, 5152. [Google Scholar] [CrossRef]
- Kang, S.; Nakajima, S.; Arakawa, Y.; Tokita, M.; Watanabe, J.; Konishi, G. Highly birefringent side-chain LC polymethacrylate with a dinaphthyl-acetylene mesogenic unit. Polym. Chem. 2014, 5, 2253. [Google Scholar] [CrossRef]
- Voskuhl, J.; Giese, M. Mesogens with aggregation-induced emission properties: Materials with a bright future. Aggregate 2022, 3, e124. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, S.; Li, B.; McEllin, A.J.; Liao, J.; Fang, Z.; Xiao, C.; Bruce, D.W.; Zhu, W.; Wang, Y. Liquid-Crystalline Thermally Activated Delayed Fluorescence: Design, Synthesis, and Application in Solution-Processed Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 15437. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Guo, Z.; Ma, T.; Duan, Y.; Yang, Y.; Cheng, X. Fluorescent Columnar Liquid Crystals Based on BTD Hexacatenars with Salicylaldimine and Benzaldimine Core: Self-Assembly and Multifunctional Properties. J. Mol. Liq. 2024, 403, 124803. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, D.; Zhang, H.; Lu, X.; Lu, Q. Thermally Tunable Circular Dichroism and Circularly Polarized Luminescence of Tetraphenylethene with Two Cholesterol Pendants. J. Mater. Chem. C 2015, 3, 6997. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liang, Y.; Liu, X.; Xiao, Y. The Effect of the Number of Cyanostilbene Building Blocks on the Self-Assembly and Photophysical Property of Pyrene-Based Liquid Crystals. Tetrahedron 2023, 149, 133742. [Google Scholar] [CrossRef]
- Dhingra, S.; Gupta, S.P.; Shah, A.; Singh, D.P.; Pal, S.K. Temperature-Dependent Hole Mobility in Pyrene–Thiophene-Based Room-Temperature Discotic Liquid Crystals. Chem. Commun. 2024, 60, 2922. [Google Scholar] [CrossRef]
- Irla, S.; Pruthvi, M.; Raghunathan, V.A.; Kumar, S. Design and Synthesis of Extended Pyrene Based Discotic Liquid Crystalline Dyes. Dyes Pigment. 2021, 194, 109574. [Google Scholar] [CrossRef]
- Sagara, Y.; Yamane, S.; Mutai, T.; Araki, K.; Kato, T. A Stimuli-Responsive, Photoluminescent, Anthracene-Based Liquid Crystal: Emission Color Determined by Thermal and Mechanical Processes. Adv. Func. Mater. 2009, 19, 1869. [Google Scholar] [CrossRef]
- Venkatesh, S.; Yadav, P.K.; Hasija, D.C.; Patil, V.R.; Ramana, M.M.V. Novel Blue-Light Emitting Fluorescent Liquid Crystals Based on 4, 4′-(2-(Tert-Butyl)- Anthracene-9, 10-Diyl)Diphenol and Their Optical Behavior. J. Mol. Liq. 2020, 310, 113265. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, D.; Fu, T.; Ali, M.U.; Shi, Y.; He, Y.; Hu, Z.; Yan, C.; Mei, Z.; Meng, H. Anthracene Derivative Based Multifunctional Liquid Crystal Materials for Optoelectronic Devices. Mater. Chem. Front. 2020, 4, 3546–3555. [Google Scholar] [CrossRef]
- Giménez, R.; Piñol, M.; Serrano, J.L. Luminescent liquid crystals derived from 9,10-bis(phenylethynyl)anthracene. Chem. Mater. 2004, 16, 1377. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, X.; Liu, T.; Guo, W.; Wang, H.; Zeng, Z. Adjustable 2-Cyano-3,4-Difluoro-1H-Pyrrole-Based Luminescent Liquid Crystals: Synthesis, Properties and Substituent Effect. J. Mol. Liq. 2018, 264, 425. [Google Scholar] [CrossRef]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.W.; Vill, V. Physical Properties of Liquid Crystals; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1999. [Google Scholar]
- Heilmeier, G.H.; Zanoni, L.A.; Barton, L.A. Guest-host interactions in nematic liquid crystals. A new electro-optic effect. Appl. Phys. Lett. 1968, 13, 91. [Google Scholar] [CrossRef]
- Heilmeier, G.H. Liquid crystal displays: An experiment in interdisciplinary research that worked. IEEE Trans Electron Devices 1976, 23, 780. [Google Scholar] [CrossRef]
- Demus, D. One Century Liquid Crystal Chemistry: From Vorländer’s Rods to Disks, Stars and Dendrites. Mol. Cryst. Liq. Cryst. 2006, 364, 25. [Google Scholar] [CrossRef]
- Iwai, R.; Yoshida, H.; Arakawa, Y.; Sasaki, S.; Iida, Y.; Igawa, K.; Sakurai, T.; Suzuki, S.; Tokita, M.; Watanabe, J.; et al. Near-room-temperature π-conjugated nematic liquid crystals in molecules with a flexible seven-membered ring structure. Aggregate 2024, 5, e660. [Google Scholar] [CrossRef]
- Shimomura, Y.; Iida, Y.; Tsurumaki, E.; Konishi, G. Innovative Molecular Design of Bridged Biphenyls for Calamitic Nematic Liquid Crystals with Extensive π-Conjugated Mesogens. Mater. Chem. Front. 2025, 9, 1127. [Google Scholar] [CrossRef]
- Konishi, G.; Sawatari, Y.; Iwai, R.; Tanaka, T.; Shimomura, Y.; Tokita, M. Synthesis of Side-Chain Liquid Crystalline Polyacrylates with Bridged Stilbene Mesogens. Molecules 2024, 29, 5220. [Google Scholar] [CrossRef]
- Iida, Y.; Shimomura, Y.; Tokita, M.; Konishi, G. Push-Pull Biphenyl and Tolane Derivatives as Novel Luminescent Liquid Crystals: Synthesis and Properties. Liq. Cryst. 2024, 51, 2032. [Google Scholar] [CrossRef]
- Vieira, A.A.; Cristiano, R.; Bortoluzzi, A.J.; Gallardo, H. Luminescent 2,1,3-Benzothiadiazole-Based Liquid Crystalline Compounds. J. Mol. Struct. 2008, 875, 364. [Google Scholar] [CrossRef]
- Aguiar, L.d.O.; Regis, E.; Tuzimoto, P.; Girotto, E.; Bechtold, I.H.; Gallardo, H.; Vieira, A.A. Investigation of Thermal and Luminescent Properties in 4,7-Diphenylethynyl-2,1,3-Benzothiadiazole Systems. Liq. Cryst. 2018, 45, 49. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Leclère, P.; Surin, M.; Lazzaroni, R.; Lin, T.T.; Dodabalapur, A.; Sellinger, A. Synthesis, Characterization and Comparative Study of Thiophene–Benzothiadiazole Based Donor–Acceptor–Donor (D–A–D) Materials. J. Mater. Chem. 2009, 19, 3228. [Google Scholar] [CrossRef]
- Echeverri, M.; Martín, I.; Concellón, A.; Ruiz, C.; Anselmo, M.S.; Gutiérrez-Puebla, E.; Serrano, J.L.; Gómez-Lor, B. Fluorescent and Electroactive Monoalkyl BTD-Based Liquid Crystals with Tunable Self-Assembling and Electronic Properties. ACS Omega 2018, 3, 11857. [Google Scholar] [CrossRef]
- Hori, A.; Matsumoto, A.; Ikenouchi, J.; Konishi, G. D−π–A Fluorophores with Strong Solvatochromism for Single-Molecule Ratiometric Thermometers. J. Am. Chem. Soc. 2025, 147, 9953. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsumoto, A.; Klymchenko, A.S.; Tsurumaki, E.; Ikenouchi, J.; Konishi, G. Fluorescent Solvatochromic Probes for Long-Term Imaging of Lipid Order in Living Cells. Adv. Sci. 2024, 11, 2309721. [Google Scholar] [CrossRef]
- Rybczyński, P.; Bousquet, M.H.E.; Kaczmarek-Kędziera, A.; Jędrzejewska, B.; Jacquemin, D.; Ośmiałowski, B. Controlling the Fluorescence Quantum Yields of Benzothiazole-Difluoroborates by Optimal Substitution. Chem. Sci. 2022, 13, 13347. [Google Scholar] [CrossRef]
- Benevides, T.O.; Regis, E.; Nicoleti, C.R.; Bechtold, I.H.; Vieira, A.A. Phase-Dependent Photoluminescence of Non-Symmetric 2,1,3-Benzothiadiazole Liquid Crystals. Dye. Pigment. 2019, 163, 300. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xiao, Q.; Pei, J. Benzothiadiazole-Based D−π-A−π-D Organic Dyes with Tunable Band Gap: Synthesis and Photophysical Properties. Org. Lett. 2010, 12, 4164. [Google Scholar] [CrossRef] [PubMed]
- Ishi-i, T.; Tanaka, H.; Youfu, R.; Aizawa, N.; Yasuda, T.; Kato, S.; Matsumoto, T. Mechanochromic Fluorescence Based on a Combination of Acceptor and Bulky Donor Moieties: Tuning Emission Color and Regulating Emission Change Direction. New J. Chem. 2019, 43, 4998. [Google Scholar] [CrossRef]
- Gierschner, J.; Shi, J.; Milián-Medina, B.; Roca-Sanjuán, D.; Varghese, S.; Park, S. Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids. Adv. Opt. Mater. 2021, 9, 2002251. [Google Scholar] [CrossRef]
- Shimomura, Y.; Konishi, G. Push-Pull Bridged Distyrylbenzene with Highly Bright Solid-State Red-Orange Aggregation-Induced Emission. Chem. Eur. J. 2023, 29, e202301191. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Konishi, G. Flexible Alkylene Bridges as a Tool To Engineer Crystal Distyrylbenzene Structures Enabling Highly Fluorescent Monomeric Emission. Chem. Eur. J. 2022, 28, e202201884. [Google Scholar] [CrossRef]
- Iwasaki, T.; Murakami, S.; Takeda, Y.; Fukuhara, G.; Tohnai, N.; Yakiyama, Y.; Sakurai, H.; Kambe, N. Molecular Packing and Solid-State Photophysical Properties of 1,3,6,8-Tetraalkylpyrenes. Chem. Eur. J. 2019, 25, 14817. [Google Scholar] [CrossRef]
- Mandle, R.J.; Bevis, E.; Goodby, J.W. Phase Structures of Nematic Liquid Crystals. In Handbook of Liquid Crystals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–27. ISBN 978-3-527-67140-3. [Google Scholar]
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160. [Google Scholar] [CrossRef]
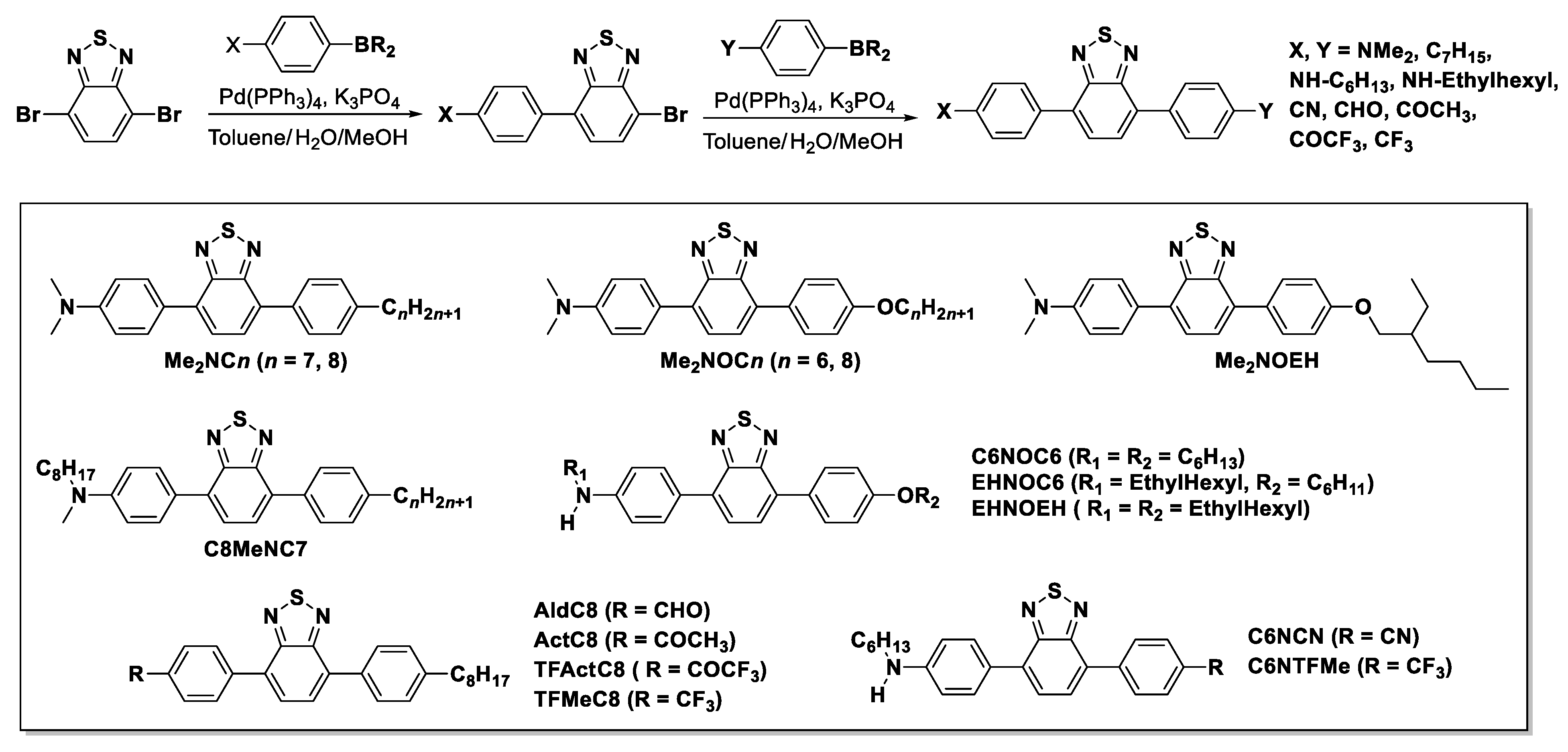
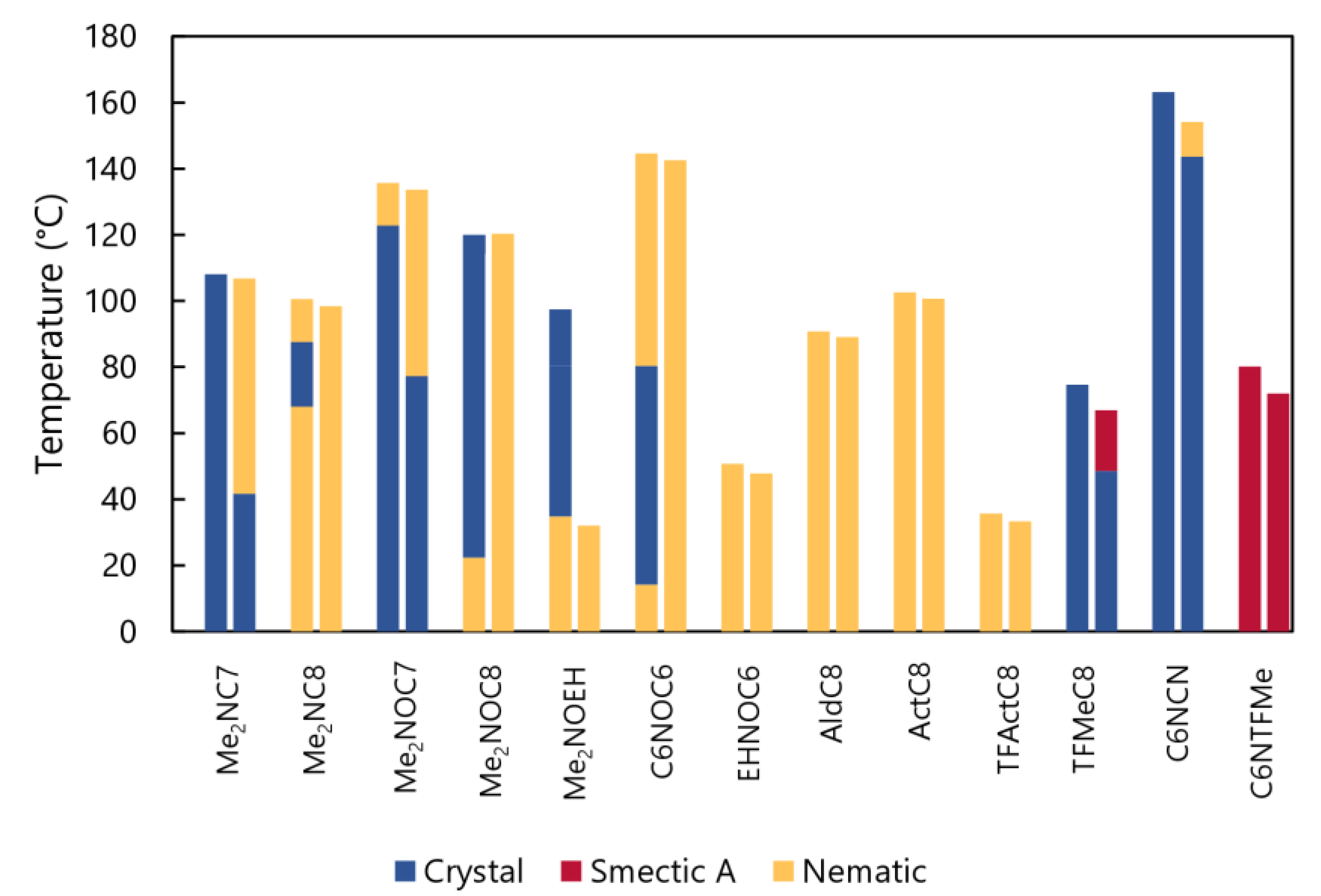

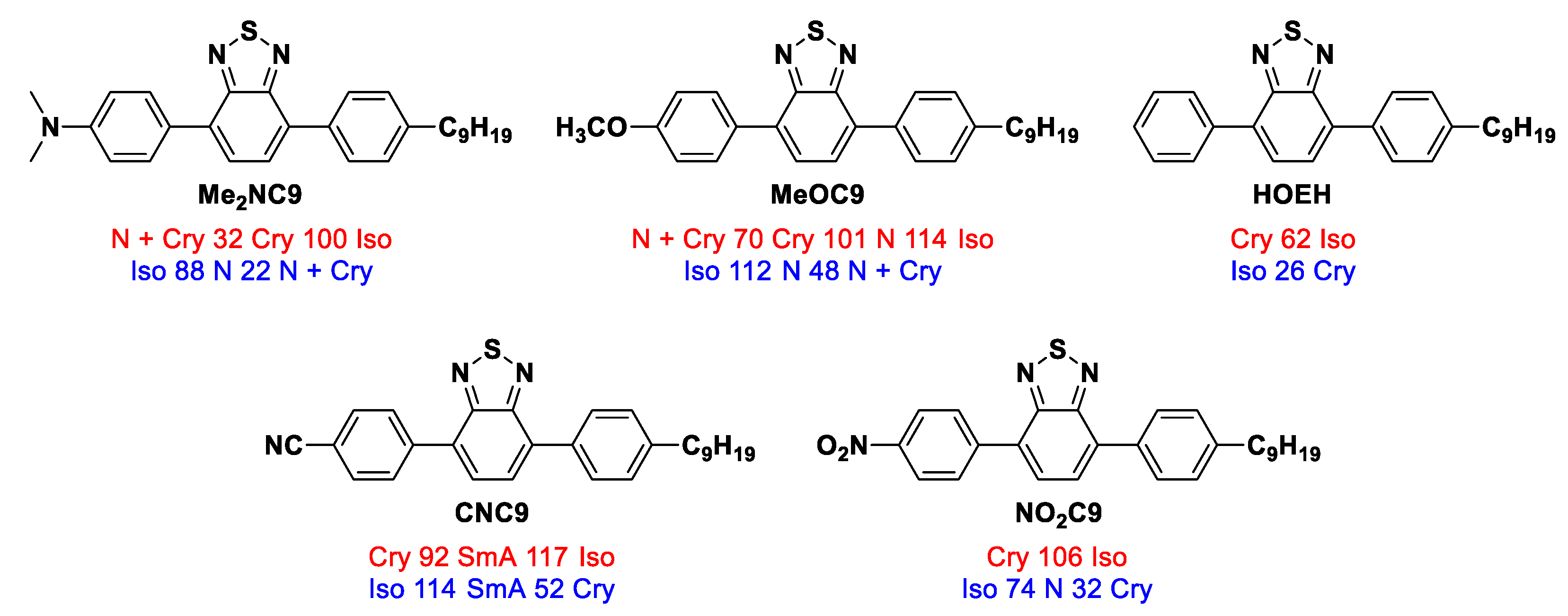


| Entry | Phase Transition Behavior [°C] (ΔH [kJ mol−1]) | |
|---|---|---|
| Heating | Cooling | |
| Me2NC7 | Cry 108.1 (29.0) Iso | Iso 106.8 (−0.5) N 41.7 (−17.7) Cry |
| Me2NC8 | N 68.0 (−2.7) Cry 87.6 (3.8) N 100.6 (0.5) Iso | Iso 98.4 (−0.6) N |
| Me2NOC7 | Cry 122.8 (42.2) N 135.7 (0.5) Iso | Iso 133.7 (−0.6) N 77.3 (−32.5) Cry |
| Me2NOC8 | N 22.4 (−7.7) Cry1 36.3 (−9.4) Cry2 114.2, 120.0 (35.2) Iso | Iso 120.3 (−0.70) N |
| Me2NOEH | N 34.8 (−26.3) Cry1 80.4 (33.0) Cry2 97.3 (3.4) Iso | Iso 32.0 (−1.0) N |
| C8MeNC7 | Iso | Iso |
| C6NOC6 | N 14.3 (−5.60) Cr1 48.4 (−5.80) Cr2 80.4 (26.0) N 144.6 (0.97) Iso | Iso 142.6 (−0.97) N |
| EHNOC6 | N 50.7 (0.02) Iso | Iso 47.8 (−0.04) N |
| EHNOEH | Iso | Iso |
| AldC8 | N 90.8 (0.48) Iso | Iso 89.1 (−0.76) N |
| ActC8 | N 58.2 (0.19) N1 66.4 (0.03) N2 75.6 (0.16) N3 102.6 (0.48) Iso | Iso 100.7 (−0.54) N |
| TFActC8 | N 35.7 (0.2) Iso | Iso 33.3 (−0.2) N |
| TFMeC8 | Cry 74.7 (19.1) Iso | Iso 66.9 (−3.2) SmA 48.6 (−13.1) Cry |
| C6NCN | Cry 163.2 (37.7) Iso | Iso 154.2 (−0.37) N 143.6 (−36.4) Cry |
| C6NTFMe [a] | SmA 80 Iso | Iso 72-55 SmA |
| Entry | THF | Mesophase | Solid | |||||
|---|---|---|---|---|---|---|---|---|
| εa (L mol−1 cm−1) | λabsb (nm) | λemc (nm) | Φflu (-) | λem (nm) | Φflu (-) | λem (nm) | Φflu (-) | |
| Me2NC8 | 35,000 12,000 | 302 451 | 661 | >0.99 0.86 | 647 | 0.36 | 592 | 0.80 |
| Me2NOC8 | 31,000 12,000 | 305 454 | 657 | 0.67 0.57 | 652 | 0.28 | 658 | 0.56 |
| EHNOC6 | 38,000 14,000 | 305 459 | 650 | 0.55 0.77 | 640 | 0.06 | 591 | 0.50 |
| AldC8 | 27,000 15,000 | 304 389 | 493 | 0.56 >0.99 | 513 | 0.12 | 502 | 0.50 |
| ActC8 | 27,000 15,000 | 296 389 | 494 | 0.70 >0.99 | 519 | 0.13 | 490 | 0.66 |
| TFActC8 | 20,000 18,000 | 310 391 | 495 | 0.53 >0.99 | 522 | 0.09 | 505 | 0.28 |
| TFMeC8 | 24,000 11,000 | 279 384 | 493 | 0.30 >0.99 | 519 | 0.56 | 502 | 0.59 |
| C6NCN | 28,000 13,000 | 313 465 | 674 | 0.44 0.52 | 631 | -- | 648 | 0.08 |
| C6NTFMe | 28,000 12,000 | 301 456 | 667 | 0.21 0.41 | 652 | 0.03 | 643 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzair, M.S.b.; Shimomura, Y.; Tanaka, T.; Kajitani, T.; Konishi, G.-i. Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals. Molecules 2025, 30, 2438. https://doi.org/10.3390/molecules30112438
Uzair MSb, Shimomura Y, Tanaka T, Kajitani T, Konishi G-i. Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals. Molecules. 2025; 30(11):2438. https://doi.org/10.3390/molecules30112438
Chicago/Turabian StyleUzair, Muhammad Suhail bin, Yoshimichi Shimomura, Takuya Tanaka, Takashi Kajitani, and Gen-ichi Konishi. 2025. "Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals" Molecules 30, no. 11: 2438. https://doi.org/10.3390/molecules30112438
APA StyleUzair, M. S. b., Shimomura, Y., Tanaka, T., Kajitani, T., & Konishi, G.-i. (2025). Development of 2,1,3-Benzothiadiazole-Based Room-Temperature Fluorescent Nematic Liquid Crystals. Molecules, 30(11), 2438. https://doi.org/10.3390/molecules30112438






