The Pheromone Landscape of Apis mellifera: Caste-Determined Chemical Signals and Their Influence on Social Dynamics
Abstract
1. Introduction
2. Neurophysiology of the Pheromone Pathway
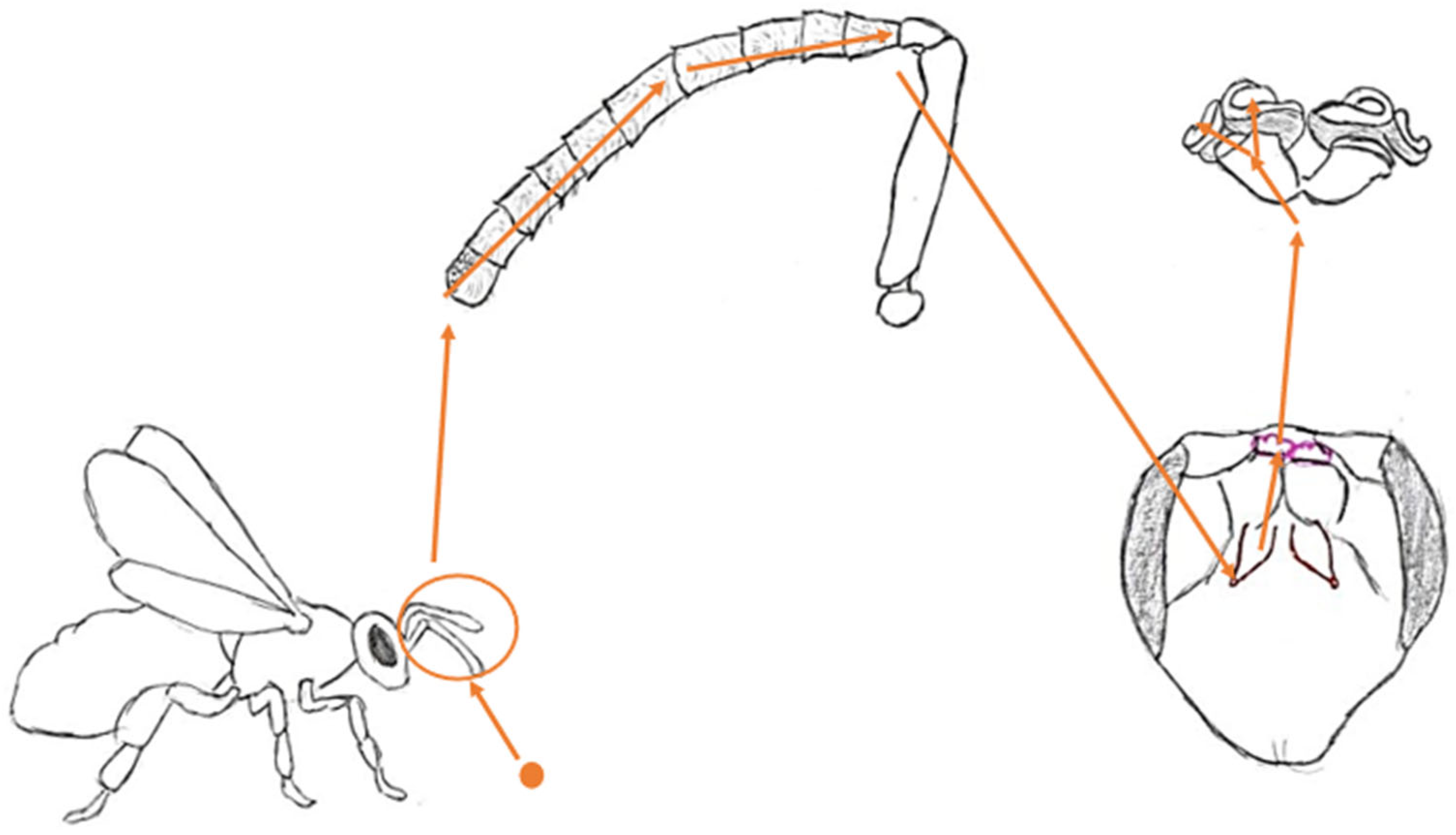
2.1. Morphology of Antennae
2.2. Sensilla
2.3. Odor Detecting Sensilla
2.4. Detection of Pheromone by the Sensilla
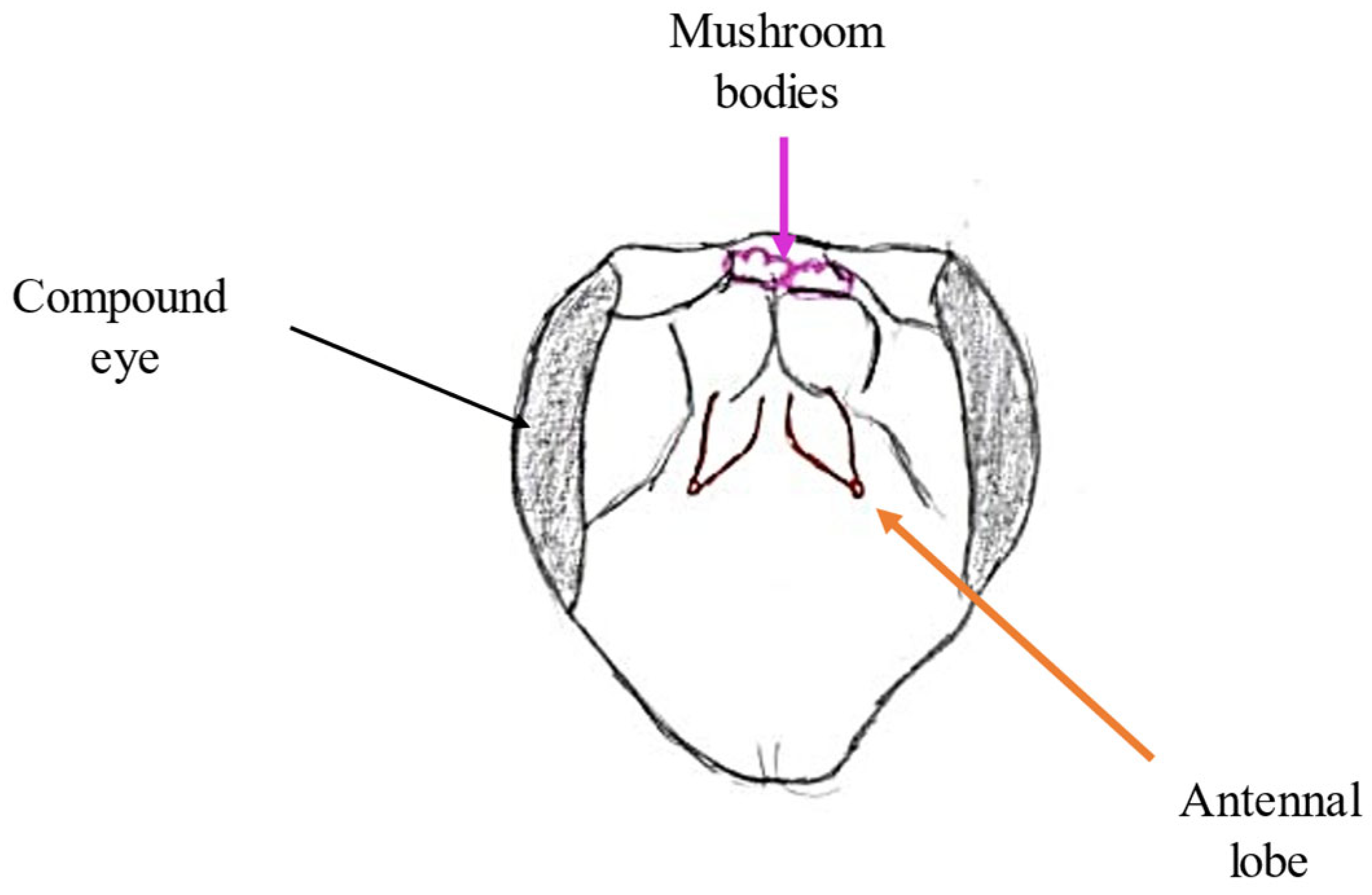
2.5. Antennal Lobe
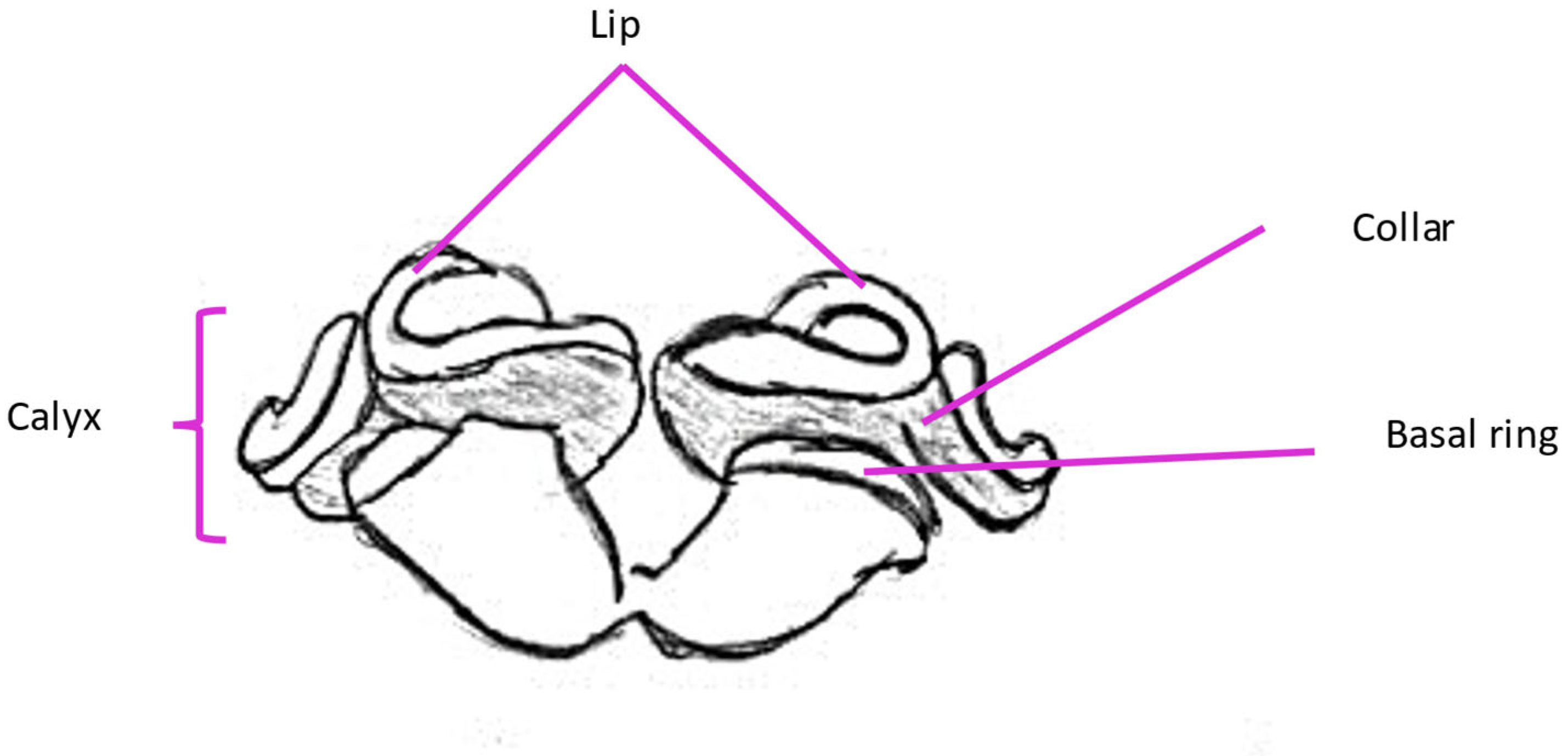
2.6. Mushroom Bodies and Higher Structures
3. Pheromones in the Life of the Honeybee
3.1. QMP
3.1.1. Site of Synthesis
3.1.2. Chemical Composition
3.1.3. Juvenile Hormone and QMP
3.1.4. Inhibition of Ovarian Development
3.1.5. Copulatory Behavior
3.1.6. Recognizing Nestmates
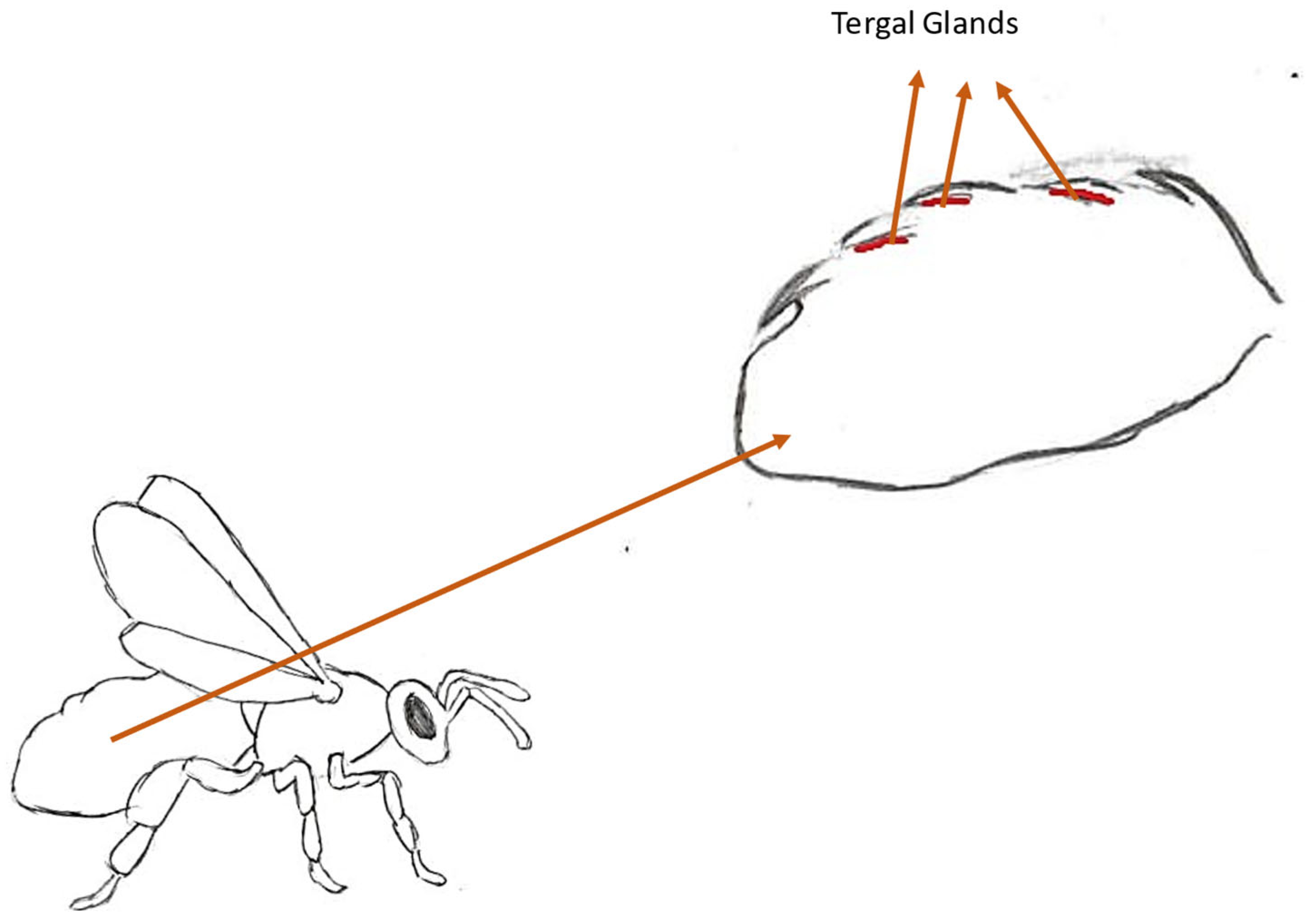
3.2. Tergal Gland Pheromones
3.2.1. Location and Structure of the Gland
3.2.2. Chemical Composition
3.2.3. Support Pheromone
3.2.4. Dependence of Activity on Age
3.2.5. An Exception to the Rule—The Rebels
3.3. Dufour’s Gland Pheromone
3.3.1. Location and Structure of the Gland
3.3.2. Chemical Composition
3.3.3. Queen’s Secretion
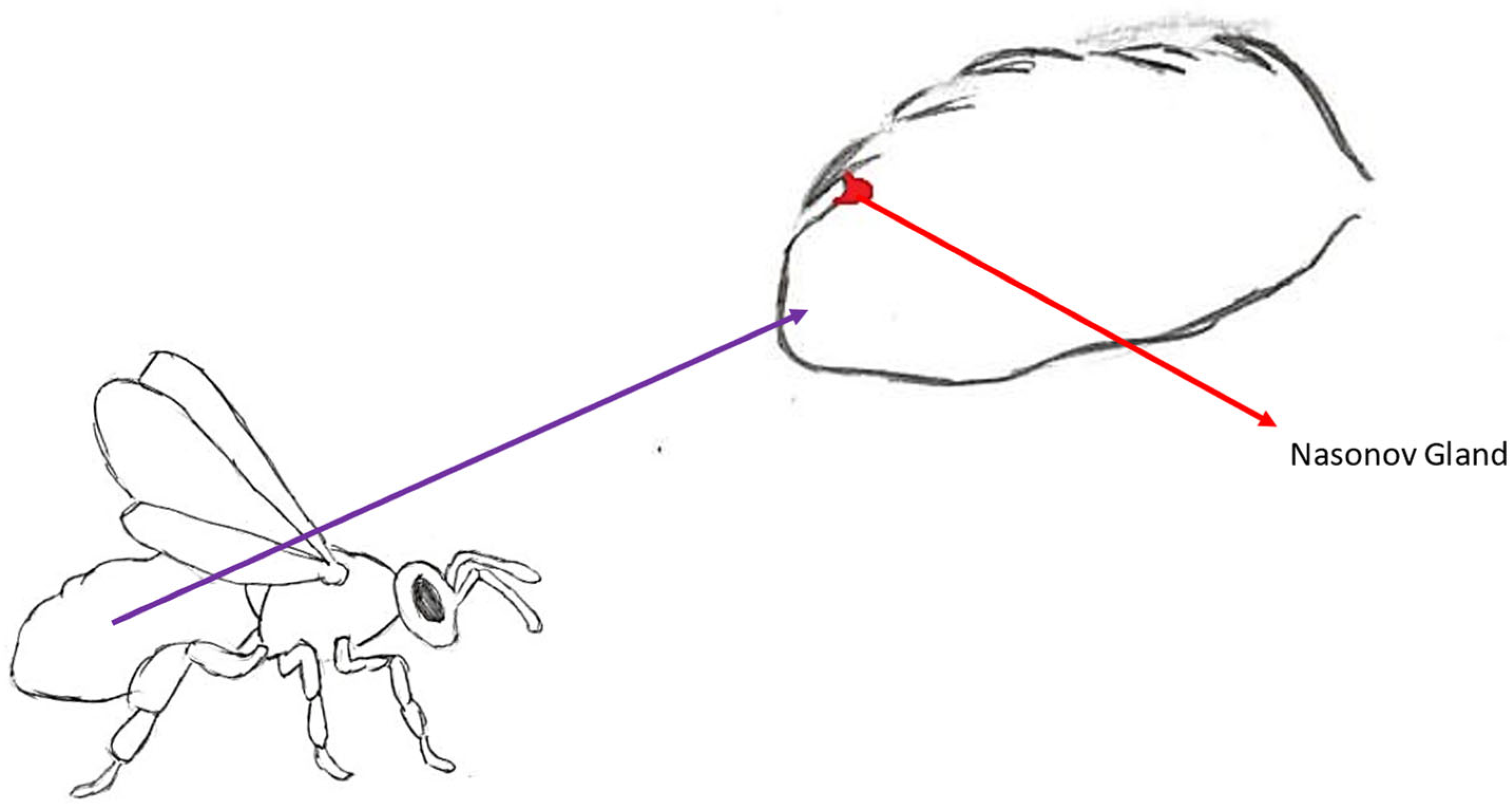
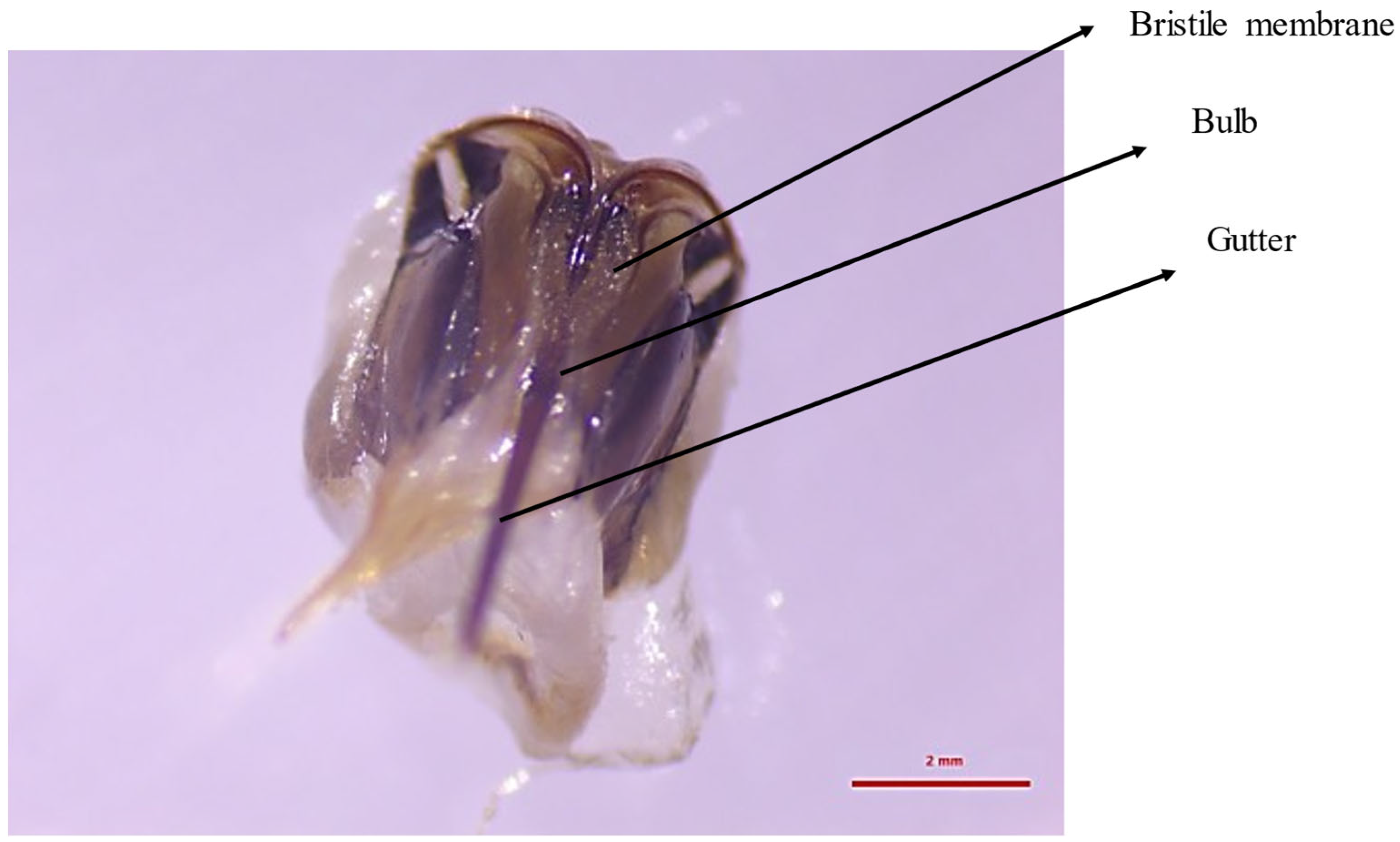
3.4. Nasonov’s Pheromone
3.4.1. Place of Synthesis and Method of Exposure
3.4.2. Chemical Composition
3.4.3. Luring to a New Home
3.4.4. Back to the Hive
3.4.5. Nasonov for the Rebels
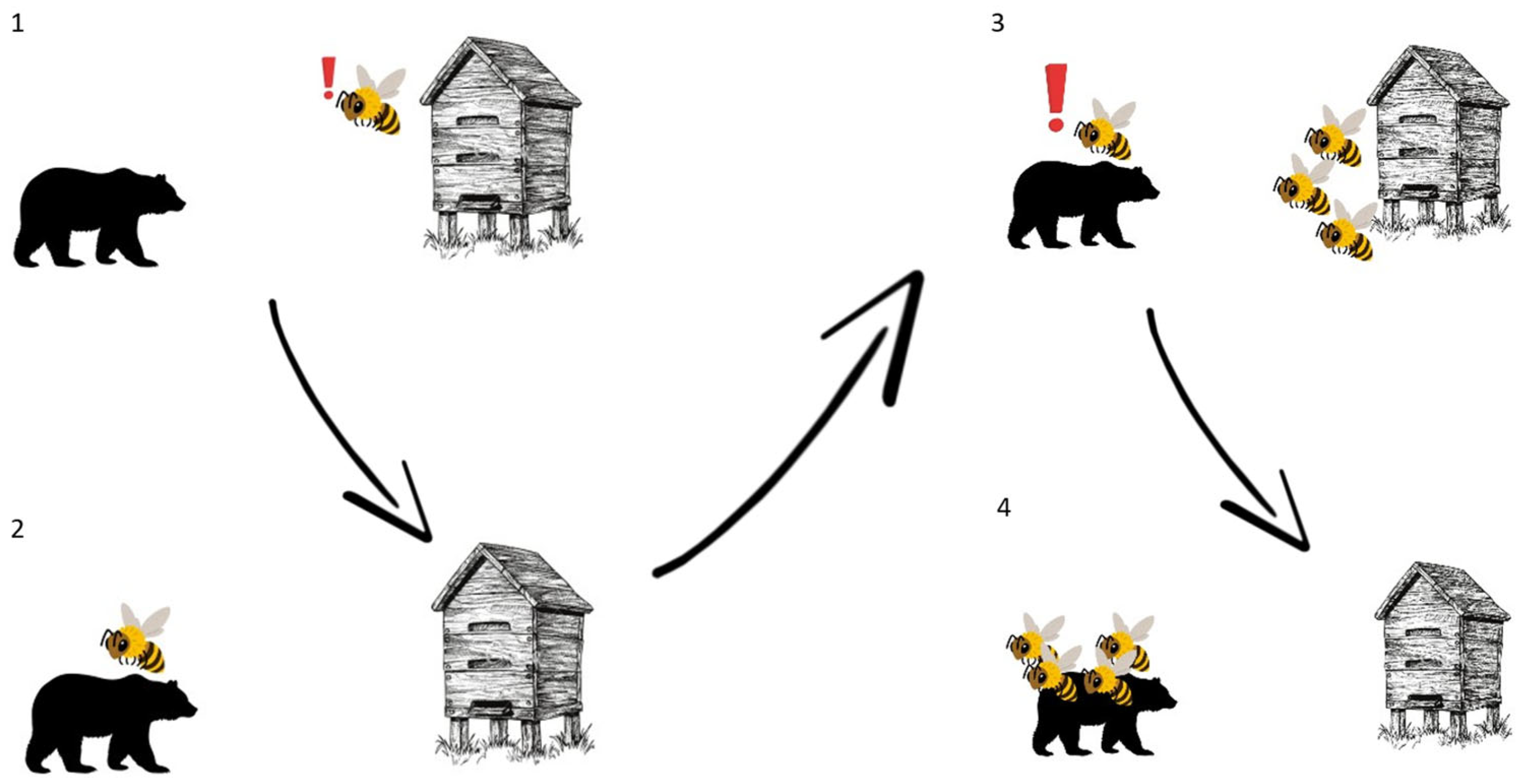
3.5. Alarm Pheromone
3.5.1. Place of Synthesis
3.5.2. Chemical Composition
3.5.3. Aggressive Behavior
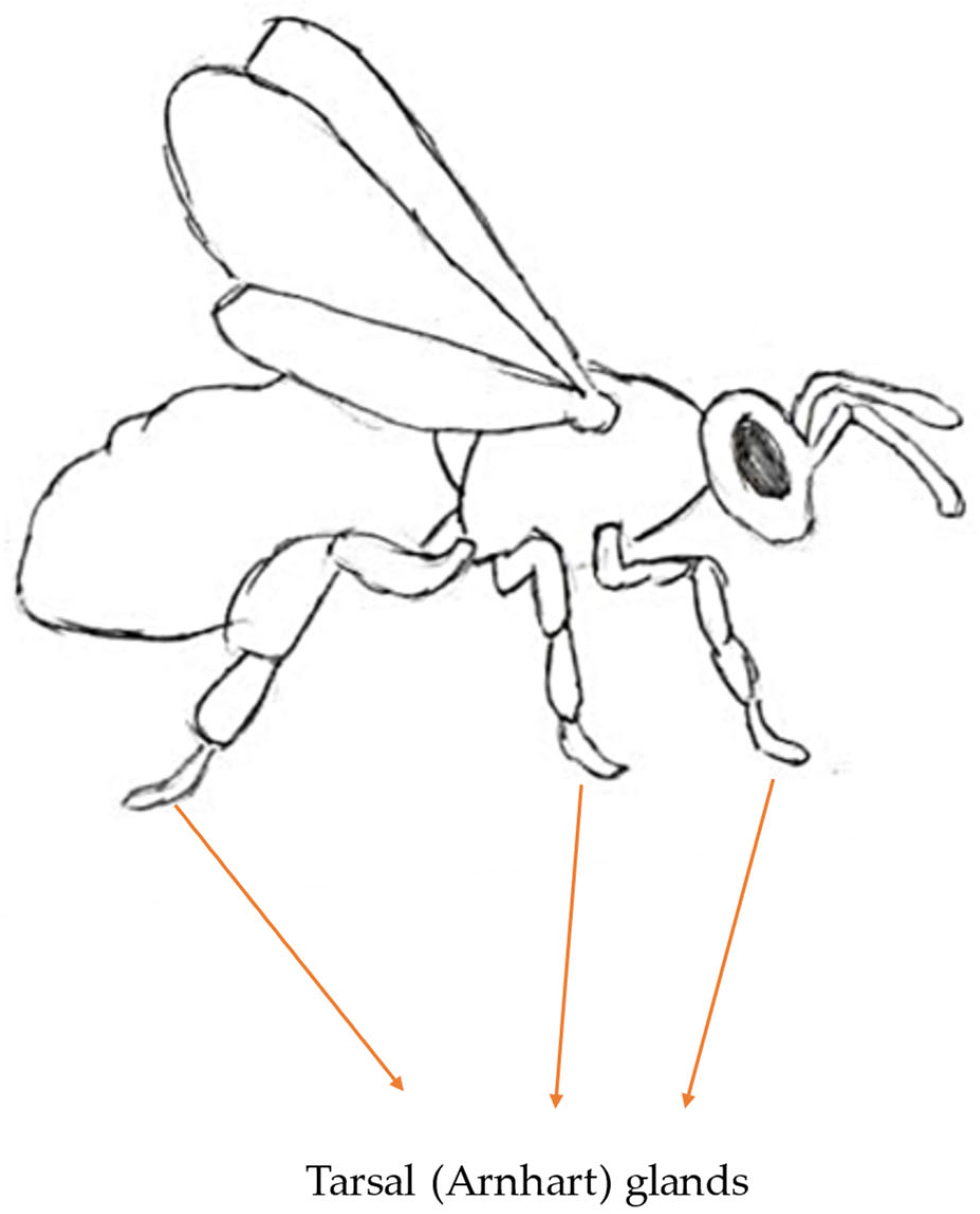
3.6. Tarsal Gland Pheromone
3.6.1. Secretion Site
3.6.2. Chemical Composition
3.6.3. Queen’s Regin
3.6.4. Worker’s Mark
4. Conclusions
- Antennae are an organ specialized in receiving environmental signals, including pheromones.
- Sensilla occurring on the antennae flagellum show significant differences, resulting from the type of stimuli received.
- Stimuli received by some of the sensilla types remain unknown.
- Honeybees possess specialized brain structures that process pheromone information (antennal lobe; mushroom bodies).
- Even though extensive testing has reached the cellular level, the reason for pairs of mushroom bodies occurring is yet to be solved.
- Castes show differences in the occurrence of sensilla types, as well as in the morphology of special brain structures.
- The presence of the QMP in a colony is essential for its proper functioning.
- The specification of QMP components affecting the inhibition of ovarian development remains a topic to be explored.
- HDA enantiomers show a possible supporting effect for 9-ODA during sexual attraction of drones.
- The secretion of tergite glands is supportive of the QMP.
- Dufour gland function in the queen remains a contentious issue.
- The role previously assigned in egg marking has been called into question and replaced by a theoretical signaling of the queen’s reproductive capabilities.
- Nasonov’s pheromone enables the worker to orientate herself when leaving to forage and returning to the nest.
- The sting alarm pheromone enables more guard bees to be involved in nest protection.
- The tarsal gland pheromone has been thoroughly researched, although there is a lack of recent research focusing on its composition and properties.
- Its function for drones remains a mystery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, B.R. Division of Labor in Honeybees: Form, Function, and Proximate Mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef]
- Yan, H.; Simola, D.F.; Bonasio, R.; Liebig, J.; Berger, S.L.; Reinberg, D. Eusocial Insects as Emerging Models for Behavioural Epigenetics. Nat. Rev. Genet. 2014, 15, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Howse, P.; Stevens, I.; Jones, O. Insect Pheromones and Their Use in Pest Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Reddy, G.V.P.; Guerrero, A. New Pheromones and Insect Control Strategies. Vitam. Horm. 2010, 83, 493–519. [Google Scholar] [PubMed]
- Yew, J.Y.; Chung, H. Insect Pheromones: An Overview of Function, Form, and Discovery. Prog. Lipid Res. 2015, 59, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Cholé, H.; Merlin, A.; Henderson, N.; Paupy, E.; Mahé, P.; Arnold, G.; Sandoz, J.C. Antenna Movements as a Function of Odorants’ Biological Value in Honeybees (Apis mellifera L.). Sci. Rep. 2022, 12, 11674. [Google Scholar] [CrossRef]
- Dürr, V.; Berendes, V.; Strube-Bloss, M. Sensorimotor Ecology of the Insect Antenna: Active Sampling by a Multimodal Sensory Organ. Adv. Insect Physiol. 2022, 63, 1–105. [Google Scholar] [CrossRef]
- Halvaci, E.; Kozak, T.; Gül, M.; Kars, H.; Şen, F. Bee Anatomy: A Comprehensive Overview of Bee Morphology and Physiology. J. Sci. Rep.-B 2023, 8, 1–19. [Google Scholar]
- Kloppenburg, P. Anatomy of the Antennal Motoneurons in the Brain of the Honeybee (Apis mellifera). J. Comp. Neurol. 1995, 363, 333–343. [Google Scholar] [CrossRef]
- Strachecka, A.; Martyna, W. Anatomia i Fizjologia Pszczoły Miodnej, 2nd ed.; Bee & Honey: Kęty, Poland, 2024; ISBN 978-83-961646-4-3. [Google Scholar]
- Frasnelli, E.; Anfora, G.; Trona, F.; Tessarolo, F.; Vallortigara, G. Morpho-Functional Asymmetry of the Olfactory Receptors of the Honeybee (Apis mellifera). Behav. Brain Res. 2010, 209, 221–225. [Google Scholar] [CrossRef]
- de Meira, O.M.; Gonçalves, R.B. Comparative Morphology and Evolution of the Cranial Musculature in Bees (Hymenoptera: Apoidea). Arthropod Struct. Dev. 2021, 65, 101112. [Google Scholar] [CrossRef]
- Suwannapong, G.; Noiphrom, J.; Benbow, M.E. Ultramorphology of Antennal Sensilla in Thai Single Open Nest Honeybees (Hymenoptera: Apidae). J. Trop. Asian Entomol. 2012, 1, 1–12. [Google Scholar]
- Ahmed, K.-A.S.; El-Bermawy, S.M.; El-Gohary, H.Z.; Bayomy, A.M. Electron Microscope Study on Workers Antennae and Sting Lancets of Three Subspecies of Honey Bee Apis mellifera L. (Hymenoptera: Apidae) and Its Bearing on Their Phylogeny. Egypt. Acad. J. Biol. Sci. A Entomol. 2015, 8, 105–124. [Google Scholar] [CrossRef]
- Abdelmegeed, S.M.; Sawires, S.G.; History, A. Comparative Studies on the Antennal Sense Organs of Queen and Worker Honey Bees, Apis mellifera L. Egypt. Acad. J. Biol. Sci. A Entomol. 2015, 8, 65–75. [Google Scholar] [CrossRef]
- Kropf, J.; Kelber, C.; Bieringer, K.; Rössler, W. Olfactory Subsystems in the Honeybee: Sensory Supply and Sex Specificity. Cell Tissue Res. 2014, 357, 583. [Google Scholar] [CrossRef]
- de Brito Sanchez, M.G. Taste Perception in Honey Bees. Chem. Senses 2011, 36, 675–692. [Google Scholar] [CrossRef]
- Awad, A.A.; Moustafa, A.M.; Abdel-Rahman, M.F.; Sayed, R.Q. Influence of Different Statuses of Honey Bee Queens, Apis mellifera L. on the Ultrastructure of the Flagella on (3-Day Old) Workers. AACE Clin. Case Rep. 2021, 7, 22–36. [Google Scholar] [CrossRef]
- Groh, C.; Brockmann, A.; Altwein, M.; Tautz, J. Selective Blocking of Contact Chemosensilla in Apis mellifera. Apidologie 2002, 33, 33–40. [Google Scholar] [CrossRef]
- Bortolotti, L.; Costa, C. Chemical Communication in the Honey Bee Society. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014; Chapter 5. [Google Scholar]
- Sandoz, J.-C. Olfaction in Honey Bees: From Molecules to Behavior. In Honeybee Neurobiology and Behavior; Springer: Dordrecht, The Netherlands, 2012; pp. 235–252. [Google Scholar]
- Murithi, M.K. Gene Expression Profiling of Odorant Binding Proteins in the Tsetse Fly Glossina brevipalpis. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 20 June 2016. [Google Scholar]
- Paoli, M.; Galizia, G.C. Olfactory Coding in Honeybees. Cell Tissue Res. 2021, 383, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Rigosi, E.; Frasnelli, E.; Vinegoni, C.; Antolini, R.; Anfora, G.; Vallortigara, G.; Haase, A. Searching for Anatomical Correlates of Olfactory Lateralization in the Honeybee Antennal Lobes: A Morphological and Behavioural Study. Behav. Brain Res. 2011, 221, 290–294. [Google Scholar] [CrossRef]
- Tiraboschi, E.; Leonardelli, L.; Segata, G.; Haase, A. Parallel Processing of Olfactory and Mechanosensory Information in the Honey Bee Antennal Lobe. Front. Physiol. 2021, 12, 790453. [Google Scholar] [CrossRef]
- Cabirol, A.; Brooks, R.; Groh, C.; Barron, A.B.; Devaud, J.M. Experience during Early Adulthood Shapes the Learning Capacities and the Number of Synaptic Boutons in the Mushroom Bodies of Honey Bees (Apis mellifera). Learn. Mem. 2017, 24, 557–562. [Google Scholar] [CrossRef][Green Version]
- Suenami, S.; Oya, S.; Kohno, H.; Kubo, T. Kenyon Cell Subtypes/Populations in the Honeybee Mushroom Bodies: Possible Function Based on Their Gene Expression Profiles, Differentiation, Possible Evolution, and Application of Genome Editing. Front. Psychol. 2018, 9, 376105. [Google Scholar] [CrossRef]
- Strube-Bloss, M.F.; Rössler, W. Multimodal Integration and Stimulus Categorization in Putative Mushroom Body Output Neurons of the Honeybee. R. Soc. Open Sci. 2018, 5, 171785. [Google Scholar] [CrossRef] [PubMed]
- Scholl, C.; Kübert, N.; Muenz, T.S.; Rössler, W. CaMKII Knockdown Affects Both Early and Late Phases of Olfactory Long-Term Memory in the Honeybee. J. Exp. Biol. 2015, 218, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Privitt, J.J.; Van Nest, B.N.; Fahrbach, S.E. Altered Synaptic Organization in the Mushroom Bodies of Honey Bees Exposed as Foragers to the Pesticide Fipronil. Front. Bee Sci. 2023, 1, 1219991. [Google Scholar] [CrossRef]
- Traynor, K.S.; Le Conte, Y.; Page, R.E. Queen and Young Larval Pheromones Impact Nursing and Reproductive Physiology of Honey Bee (Apis mellifera) Workers. Behav. Ecol. Sociobiol. 2014, 68, 2059–2073. [Google Scholar] [CrossRef]
- Elias Santos, D.; Amaral de Souza, E.; Ueira Vieira, C.; Cola Zanuncio, J.; Eduardo Serrão, J. Morphology of Mandibular and Intramandibular Glands in Workers and Virgin Queens of Melipona scutellaris. Apidologie 2015, 46, 23–34. [Google Scholar] [CrossRef]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.M.; Gharibyan, V.; Su, M.B.; Zhou, Y.Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.P.J.; et al. Histone Deacetylase Inhibitor Activity in Royal Jelly Might Facilitate Caste Switching in Bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef]
- Alhosin, M. Epigenetics Mechanisms of Honeybees: Secrets of Royal Jelly. Epigenet. Insights 2023, 16, 25168657231213717. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Principles of Insect Morphology; Cornell University Press: Ithaca, NY, USA, 1935. [Google Scholar]
- Walsh, E.; Rangel, J. Queen Pheromones and Mandibular Gland Dissection. Bee World 2018, 95, 3–5. [Google Scholar] [CrossRef]
- Mumoki, F.N.; Pirk, C.W.W.; Yusuf, A.A.; Crewe, R.M. Reproductive Parasitism by Worker Honey Bees Suppressed by Queens through Regulation of Worker Mandibular Secretions. Sci. Rep. 2018, 8, 7701. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Slessor, K.N.; Higo, H.A.; Winston, M.L. New Components of the Honey Bee (Apis mellifera L.) Queen Retinue Pheromone. Proc. Natl. Acad. Sci. USA 2003, 100, 4486–4491. [Google Scholar] [CrossRef] [PubMed]
- Rangel, J.; Böröczky, K.; Schal, C.; Tarpy, D.R. Honey Bee (Apis mellifera) Queen Reproductive Potential Affects Queen Mandibular Gland Pheromone Composition and Worker Retinue Response. PLoS ONE 2016, 11, e0156027. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Fischer, P.; Hampton, J.E. Uncoupling Primer and Releaser Responses to Pheromone in Honey Bees. Naturwissenschaften 2007, 94, 375–379. [Google Scholar] [CrossRef]
- Jarriault, D.; Barrozo, R.B.; de Carvalho Pinto, C.J.; Greiner, B.; Dufour, M.C.; Masante-Roca, I.; Gramsbergen, J.B.; Anton, S.; Gadenne, C. Age-Dependent Plasticity of Sex Pheromone Response in the Moth, Agrotis ipsilon: Combined Effects of Octopamine and Juvenile Hormone. Horm. Behav. 2009, 56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, H.J.; Nakagawa, S.; Mercer, A.R. Juvenile Hormone Enhances Aversive Learning Performance in 2-Day Old Worker Honey Bees While Reducing Their Attraction to Queen Mandibular Pheromone. PLoS ONE 2014, 9, e112740. [Google Scholar] [CrossRef]
- Cardoen, D.; Ernst, U.R.; Boerjan, B.; Bogaerts, A.; Formesyn, E.; De Graaf, D.C.; Wenseleers, T.; Schoofs, L.; Verleyen, P. Worker Honeybee Sterility: A Proteomic Analysis of Suppressed Ovary Activation. J. Proteome Res. 2012, 11, 2838–2850. [Google Scholar] [CrossRef][Green Version]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Paleolog, J.; Woyciechowski, M. Reproductive Potential Accelerates Preimaginal Development of Rebel Workers in Apis mellifera. Animals 2021, 11, 3245. [Google Scholar] [CrossRef]
- Langlands, Z.; Du Rand, E.E.; Crailsheim, K.; Yusuf, A.A.; Pirk, C.W.W. Prisoners Receive Food Fit for a Queen: Honeybees Feed Small Hive Beetles Protein-Rich Glandular Secretions through Trophallaxis. J. Exp. Biol. 2021, 224, jeb234807. [Google Scholar] [CrossRef]
- Castillo, C.; Chen, H.; Graves, C.; Maisonnasse, A.; Le Conte, Y.; Plettner, E. Biosynthesis of Ethyl Oleate, a Primer Pheromone, in the Honey Bee (Apis mellifera L.). Insect Biochem. Mol. Biol. 2012, 42, 404–416. [Google Scholar] [CrossRef]
- Ronai, I.; Oldroyd, B.P.; Vergoz, V. Queen Pheromone Regulates Programmed Cell Death in the Honey Bee Worker Ovary. Insect Mol. Biol. 2016, 25, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Jarriault, D.; Mercer, A.R. Queen Mandibular Pheromone: Questions That Remain to Be Resolved. Apidologie 2012, 43, 292–307. [Google Scholar] [CrossRef][Green Version]
- Brockmann, A.; Dietz, D.; Spaethe, J.; Tautz, J. Beyond 9-ODA: Sex Pheromone Communication in the European Honey Bee Apis mellifera L. J. Chem. Ecol. 2006, 32, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Drijfhout, F.P.; Kather, R.; Martin, S.J. The Role of Cuticular Hydrocarbons in Insects. In Behavioral and Chemical Ecology; Zhang, W., Liu, H., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 91–114. ISBN 978-1-60741-099-7. [Google Scholar]
- Khidr, S.K.; Linforth, R.S.T.; Hardy, I.C.W. Genetic and Environmental Influences on the Cuticular Hydrocarbon Profiles of Goniozus Wasps. Entomol. Exp. Appl. 2013, 147, 175–185. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H. Behavioral and Chemical Ecology; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; ISBN 9781607410997. [Google Scholar]
- Otte, T.; Hilker, M.; Geiselhardt, S. Phenotypic Plasticity of Cuticular Hydrocarbon Profiles in Insects. J. Chem. Ecol. 2018, 44, 235–247. [Google Scholar] [CrossRef]
- Châline, N.; Sandoz, J.C.; Martin, S.J.; Ratnieks, F.L.W.; Jones, G.R. Learning and Discrimination of Individual Cuticular Hydrocarbons by Honeybees (Apis mellifera). Chem. Senses 2005, 30, 327–335. [Google Scholar] [CrossRef]
- Kather, R.; Martin, S.J. Evolution of Cuticular Hydrocarbons in the Hymenoptera: A Meta-Analysis. J. Chem. Ecol. 2015, 41, 871–883. [Google Scholar] [CrossRef]
- Fan, Y.; Richard, F.J.; Rouf, N.; Grozinger, C.M. Effects of Queen Mandibular Pheromone on Nestmate Recognition in Worker Honeybees, Apis mellifera. Anim. Behav. 2010, 79, 649–656. [Google Scholar] [CrossRef]
- Vernier, C.L.; Krupp, J.J.; Marcus, K.; Hefetz, A.; Levine, J.D.; Ben-Shahar, Y. The Cuticular Hydrocarbon Profiles of Honey Bee Workers Develop via a Socially-Modulated Innate Process. eLife 2019, 8, e41855. [Google Scholar] [CrossRef]
- Cassier’, P.; Lensky, Y. The Exocrine Glands of The Honey Bees Their Structure and Secretory Products. In Bee Products: Properties, Applications, and Apitherapy; Springer: Boston, MA, USA, 1997; Volume 1, pp. 137–150. [Google Scholar]
- Jaremek, M.; Olszewski, K.; Chobotow, J.; Strachecka, A. The Morphological Image of Fat Body and Tergal Gland Cells in Uninseminated Apis mellifera Queen Bees. Insects 2024, 15, 244. [Google Scholar] [CrossRef]
- Okosun, O.O.; Yusuf, A.A.; Crewe, R.M.; Pirk, C.W.W. Effects of Age and Reproductive Status on Tergal Gland Secretions in Queenless Honey Bee Workers, Apis mellifera scutellata and A. m. capensis. J. Chem. Ecol. 2015, 41, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Wossler, T.C.; Veale, R.B.; Crewe, R.M. How Queen-like Are the Tergal Glands in Workers of Apis mellifera capensis and Apis mellifera scutellata? Apidologie 2000, 31, 55–66. [Google Scholar] [CrossRef]
- Okosun, O.O.; Yusuf, A.A.; Crewe, R.M.; Pirk, C.W.W. Tergal Gland Components of Reproductively Dominant Honey Bee Workers Have Both Primer and Releaser Effects on Subordinate Workers. Apidologie 2019, 50, 173–182. [Google Scholar] [CrossRef]
- Wossler, T.C.; Crewe, R.M. The Releaser Effects of the Tergal Gland Secretion of Queen Honeybees (Apis mellifera). J. Insect Behav. 1999, 12, 343–351. [Google Scholar] [CrossRef]
- Princen, S.A.; Oliveira, R.C.; Ernst, U.R.; Millar, J.G.; Van Zweden, J.S.; Wenseleers, T. Honeybees Possess a Structurally Diverse and Functionally Redundant Set of Queen Pheromones. Proc. R. Soc. B 2019, 286, 20190517. [Google Scholar] [CrossRef]
- De Oliveira Azevedo, D.; Weinstein Teixeira, É.; Teles, M.L.; Florêncio Alves, M.; Camargo, A.C.; Moreti, C.; Blochtein, B.; Zanuncio, J.C.; Serrão, J.E. Comparative Analyses of the Abdominal Tergal Glands in Apis mellifera (Hymenoptera: Apidae) Queens. Anim. Biol. 2007, 57, 329–338. [Google Scholar] [CrossRef]
- Wilde, J. Chów i Hodowla Pszczół; PWRiL: Warszawa, Poland, 2024; ISBN 978-83-09-01180-4. [Google Scholar]
- Martin, S.J.; Dils, V.; Billen, J. Morphology of the Dufour Gland within the Honey Bee Sting Gland Complex. Apidologie 2005, 36, 543–546. [Google Scholar] [CrossRef]
- Mitra, A. Function of the Dufour’s Gland in Solitary and Social Hymenoptera. J. Hymenopt. Res. 2013, 35, 33–58. [Google Scholar] [CrossRef]
- Katzav-Gozansky, T.; Soroker, V.; Hefetz, A. Honeybees Dufour’s Gland—Idiosyncrasy of a New Queen Signal. Apidologie 2002, 33, 525–537. [Google Scholar] [CrossRef]
- Katzav-Gozansky, T.; Soroker, V.; Hefetz, A.; Cojocaru, M.; Erdmann, D.H.; Francke, W. Plasticity of Caste-Specific Dufour’s Gland Secretion in the Honey Bee (Apis mellifera L.). Naturwissenschaften 1997, 84, 238–241. [Google Scholar] [CrossRef]
- Strachecka, A.; Chobotow, J.; Kuszewska, K.; Olszewski, K.; Skowronek, P.; Bryś, M.; Paleolog, J.; Woyciechowski, M. Morphology of Nasonov and Tergal Glands in Apis mellifera Rebels. Insects 2022, 13, 401. [Google Scholar] [CrossRef]
- Srinivasan, M.; Reinhard, J. The Role of Scents in Honey Bee Foraging and Recruitment. In Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches; CRC Press: Boca Raton, FL, USA, 2009; pp. 165–182. [Google Scholar]
- Resh, V.; Cardé, R. (Eds.) Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Schmidt, J.O. Attractant or Pheromone: The Case of Nasonov Secretion and Honeybee Swarms. J. Chem. Ecol. 1999, 25, 2051–2056. [Google Scholar] [CrossRef]
- Drum, N.H.; Rothenbuhler, W.C. Non-Stinging Aggressive Responses of Worker Honeybees to Hivemates, Intruder Bees and Bees Affected with Chronic Bee Paralysis. J. Apic. Res. 1983, 22, 256–260. [Google Scholar] [CrossRef]
- Weyda, F.; Kodrík, D. New Functionally Ultrastructural Details of the Honey Bee Stinger Tip: Serrated Edge and Pitted Surface. J. Apic. Res. 2021, 60, 875–878. [Google Scholar] [CrossRef]
- Ramirez-Esquivel, F.; Ravi, S. Functional Anatomy of the Worker Honeybee Stinger (Apis mellifera). iScience 2023, 26, 107103. [Google Scholar] [CrossRef]
- Kannan, K.; Galizia, C.G.; Nouvian, M. Olfactory Strategies in the Defensive Behaviour of Insects. Insects 2022, 13, 470. [Google Scholar] [CrossRef]
- Nouvian, M.; Hotier, L.; Claudianos, C.; Giurfa, M.; Reinhard, J. Appetitive Floral Odours Prevent Aggression in Honeybees. Nat. Commun. 2015, 6, 10247. [Google Scholar] [CrossRef]
- Ramirez-Moreno, D.; Galizia, C.G.; Nouvian, M. Division of Labour during Honeybee Colony Defence: Poetic and Scientific Views. Philos. Trans. R. Soc. B Biol. Sci. 2025, 380, 20230272. [Google Scholar] [CrossRef]
- Goodman, L. Form and Function in the Honey Bee; International Bee Research Association: Cardiff, UK, 2003; pp. xii, 220. [Google Scholar]
- Lensky, Y.; Cassier, P.; Finkel, A.; Delorme-Joulie, C.; Levinsohn, M. The Fine Structure of the Tarsal Glands of the Honeybee Apis mellifera L. (Hymenoptera) Cell Tissue Res. 1985, 240, 153–158. [Google Scholar] [CrossRef]
- Lensky, Y.; Cassier, P.; Finkel, A.; Teeshbee, A.; Schlesinger, R.; Delorme-Joulie, C.; Levinsohn, M. Les Glandes Tarsales de l’abeille Meillifique (Apis mellifera L.) Reines, Ouvrières et Faux-Bourdons (Hymenoptera, Apidae). II: Rôle Biologique. Ann. Sci. Nat. Zool. Biol. Anim. 1984, 6, 167–175. [Google Scholar]
- Tew, J.; Evans, J.; Conrad, R.; Wahl, R.; Candles, R. A Closer Look: Tarsal Glands/Footprint Pheromone. Bee Culture, 23 October 2015. [Google Scholar]
- Lensky, Y.; Slabezki, Y. The inhibiting effect of the queen bee (Apis mellifera L.) foot-print pheromone on the construction of swarming queen cups. J. Insect Physiol. 1981, 27, 313–323. [Google Scholar] [CrossRef]
- Butler, C.G.; Fletcher, D.J.C.; Watler, D. Nest-entrance marking with pheromones by the honeybee—Apis mellifera L., and by a wasp, Vespula vulgaris L. Anim. Behav. 1969, 17, 142–147. [Google Scholar] [CrossRef]
- Ribbands, C.R. Communication between honeybees. I: The response of crop-attached bees to the scent of their crop. Proc. R. Entomol. Soc. Lond. A 1954, 29, 141–144. [Google Scholar] [CrossRef]
- Ferguson, A.W.; Free, J.B. Production of a Forage-Marking Pheromone by the Honeybee. J. Apic. Res. 1979, 18, 128–135. [Google Scholar] [CrossRef]
- Giurfa, M.; Núñez, J.A. Honeybees Mark with Scent and Reject Recently Visited Flowers. Oecologia 1992, 89, 113–117. [Google Scholar] [CrossRef]


| Type of Sensilla | Feature | ||
|---|---|---|---|
| Location on antennae | Shape | Type of stimulus | |
| Trichodea | Every annuli of the flagellum. | Hair-like. Subtype A: thin and straight. Sybtype B: tapered and slightly bent. Subtype C: thin and arched. Subtype D: thick and strongly bent. | Mostly mechanoreceprors, with subtype A desribed as olfactory. |
| Placodea | 3rd to 10th segments. | Oval pores in the cuticule, 9 × 6 µm thin. | Olfactoryreceptors. |
| Basiconica | 4th to 10th flagelomers on the dorsal side and 8th, 9th and 10th on the ventral side; not present in drones. | Peg-like with nearly flat ends and numerous pores. | Gustatory and olfactory receptors. |
| Coleoconica | 6th to 10th flagellomeres. | Externally grooved projection in a wide pit with a circular shape. | Hygroreceptor. |
| Coleocapitular | Tip of each antennae. | Located in a cavity in the cuticle. In the hole with a diameter of about 1.4 µm, there is a mushroom-shaped protrusion. | Hygro- and thermoreceptor. |
| Campaniform | Dorsal side of the 9th through 10th segments. | Disks with a flat and oval design. The cross-section resembles a bun with a dome. | Mechanoreceptors (register external skeleton stretch), presumably hygro- and thermoreceptors. |
| Ampullacea | 6th to 10th flagellomeres, disregarding the ventral side. Often occur near Sensilla Coleoconica. | Holding a small peg with a sculpted surface inside a tiny aperture. | Presumably detect carbon dioxide levels. |
| Chaetica | 3rd, 8th, and 10th flagellomeres for drones; presumably on the entire flagellum surface for workers. | Similar to Basiconica but with only one pore on the apex of the peg. | Gustatoryreceptors with the ability to detect aminoacids. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryboś, A.; Staniszewska, P.; Bryś, M.S.; Strachecka, A. The Pheromone Landscape of Apis mellifera: Caste-Determined Chemical Signals and Their Influence on Social Dynamics. Molecules 2025, 30, 2369. https://doi.org/10.3390/molecules30112369
Gryboś A, Staniszewska P, Bryś MS, Strachecka A. The Pheromone Landscape of Apis mellifera: Caste-Determined Chemical Signals and Their Influence on Social Dynamics. Molecules. 2025; 30(11):2369. https://doi.org/10.3390/molecules30112369
Chicago/Turabian StyleGryboś, Anna, Patrycja Staniszewska, Maciej Sylwester Bryś, and Aneta Strachecka. 2025. "The Pheromone Landscape of Apis mellifera: Caste-Determined Chemical Signals and Their Influence on Social Dynamics" Molecules 30, no. 11: 2369. https://doi.org/10.3390/molecules30112369
APA StyleGryboś, A., Staniszewska, P., Bryś, M. S., & Strachecka, A. (2025). The Pheromone Landscape of Apis mellifera: Caste-Determined Chemical Signals and Their Influence on Social Dynamics. Molecules, 30(11), 2369. https://doi.org/10.3390/molecules30112369







