Efficiently Degrading RhB Using Bimetallic Co3O4/ZnO Oxides: Ultra-Fast and Persistent Activation of Permonosulfate
Abstract
1. Introduction
2. Results and Discussion
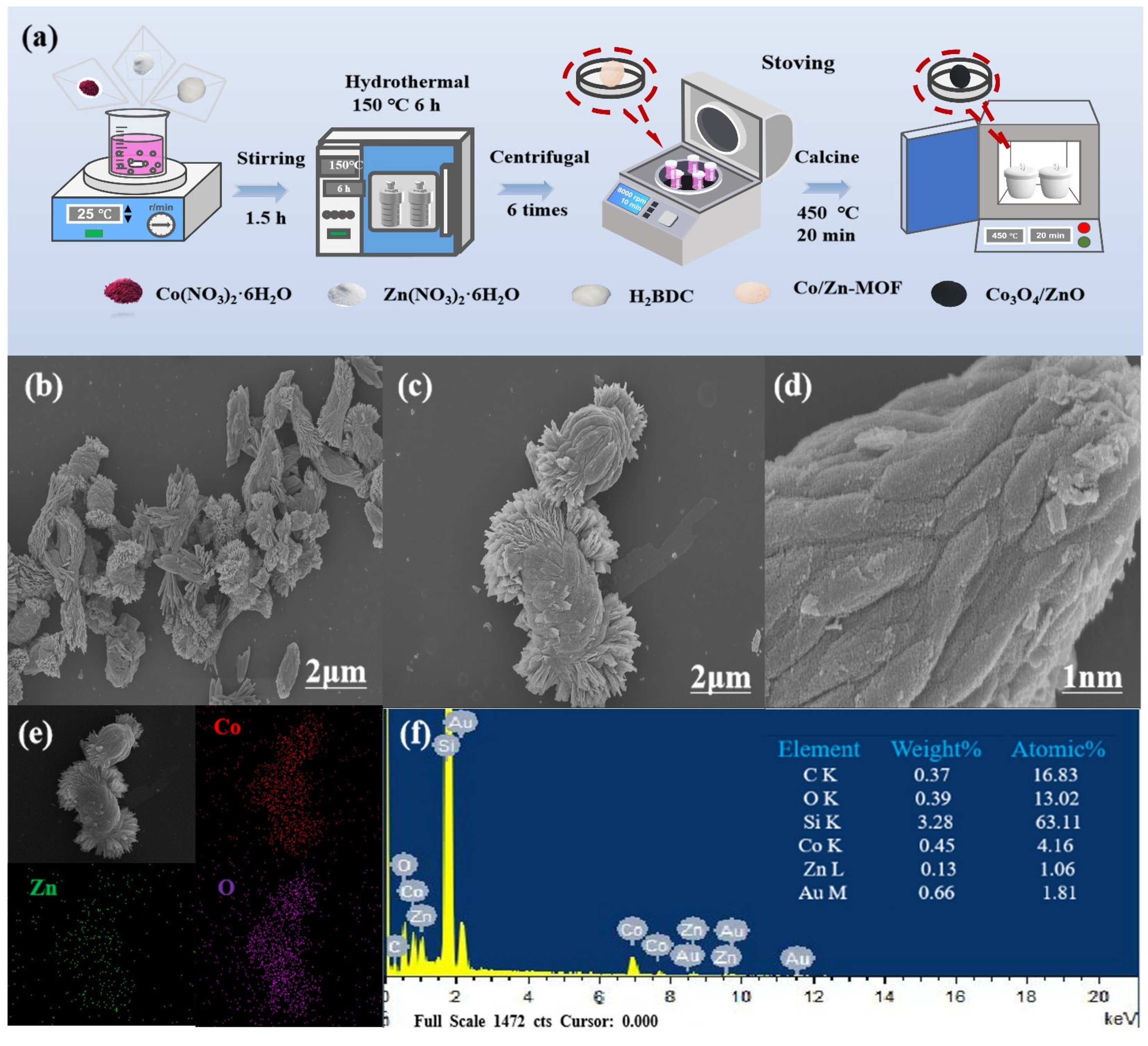
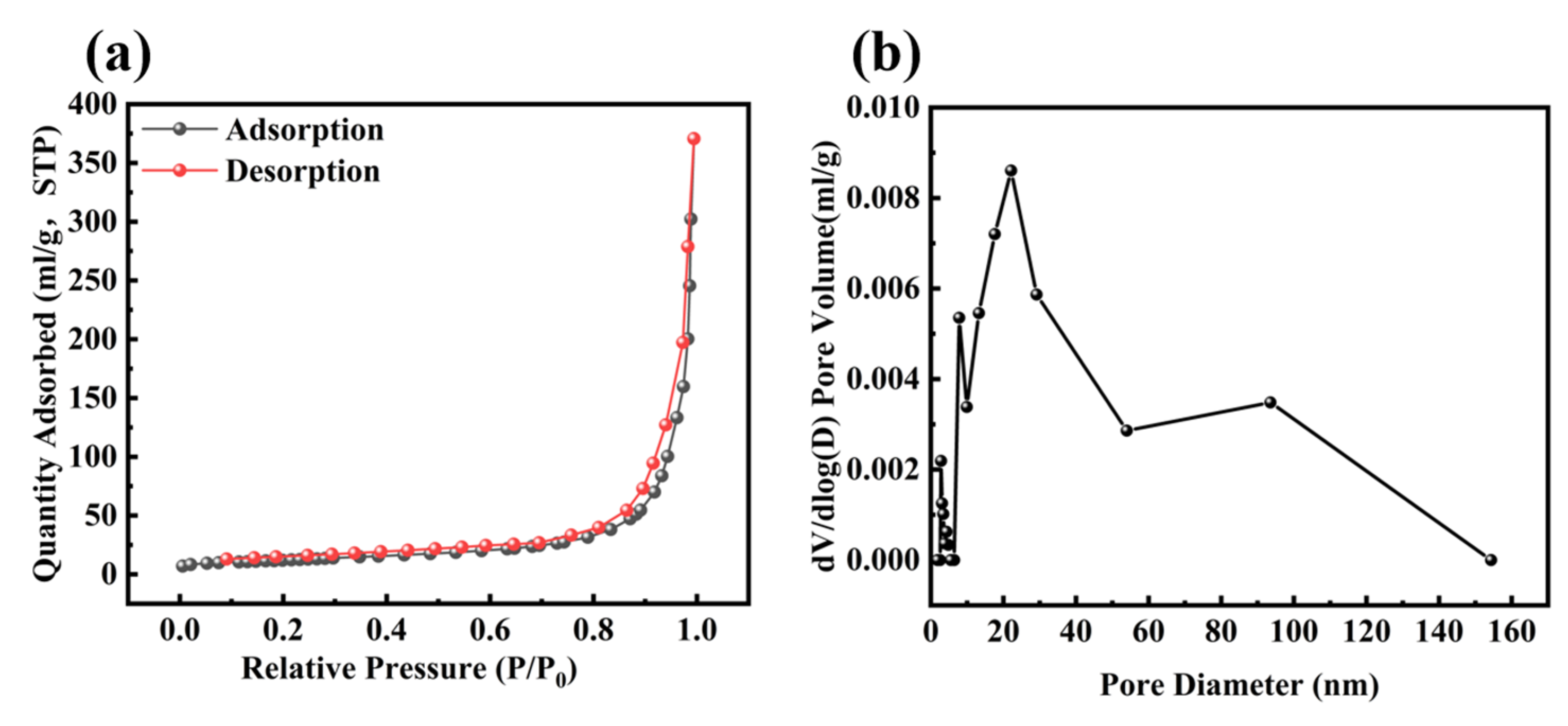
2.1. Synthesis and Characterization of Material
2.2. Catalytic Activity of Co3O4/ZnO
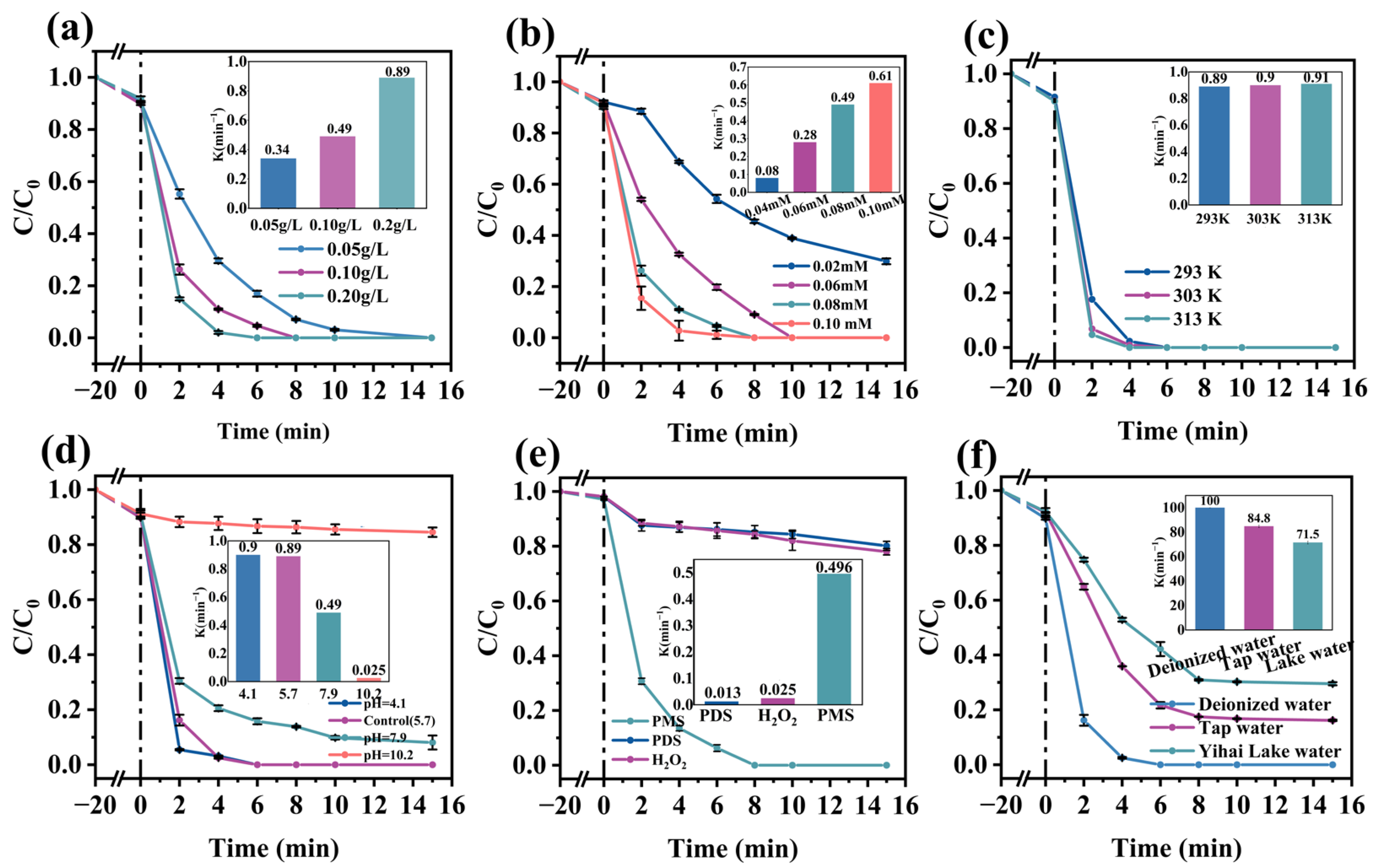
2.3. Effect of Different Parameters on RhB Degradation Efficiency
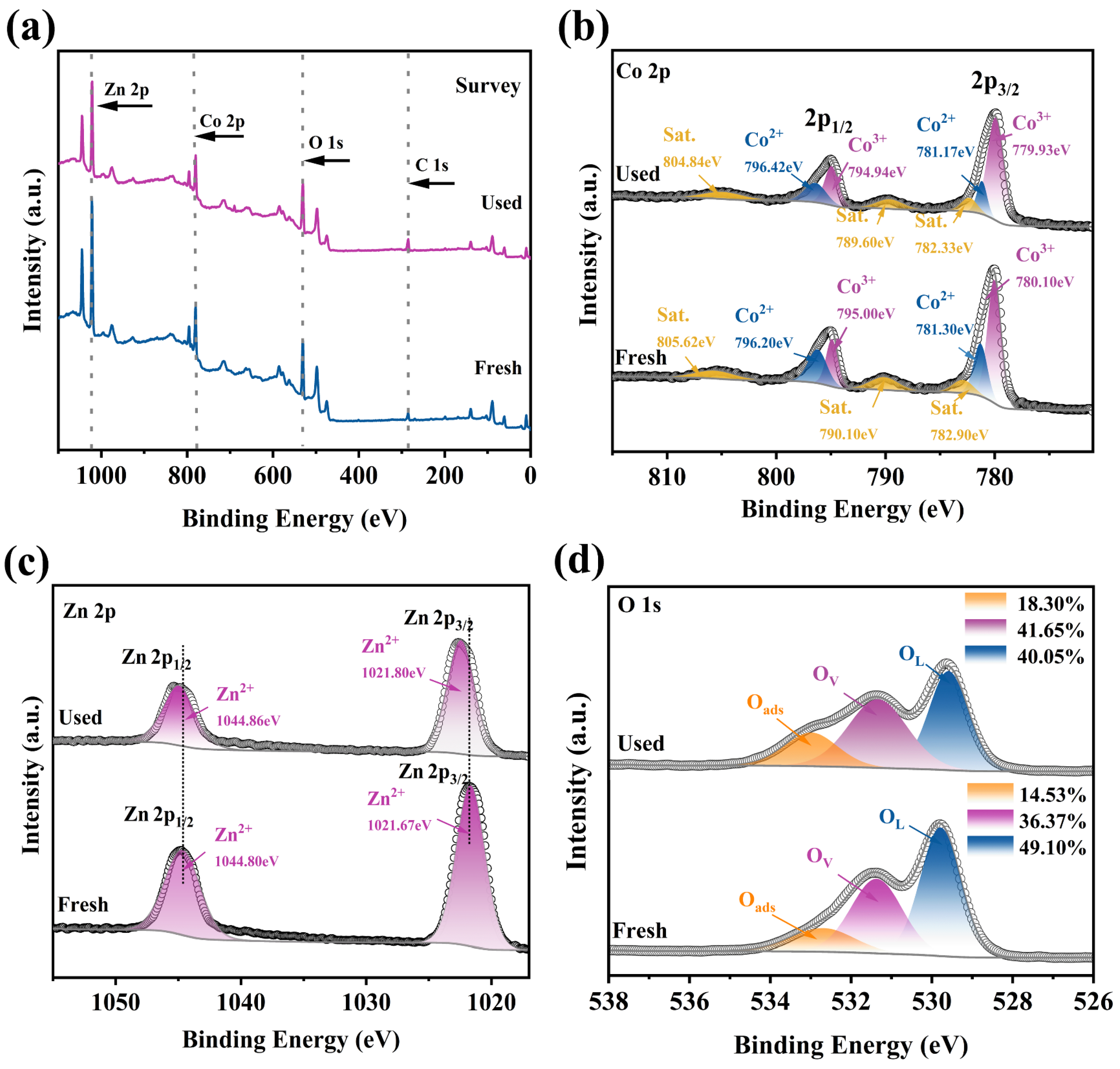
2.4. Reusability and Stability of Co3O4/ZnO Composite
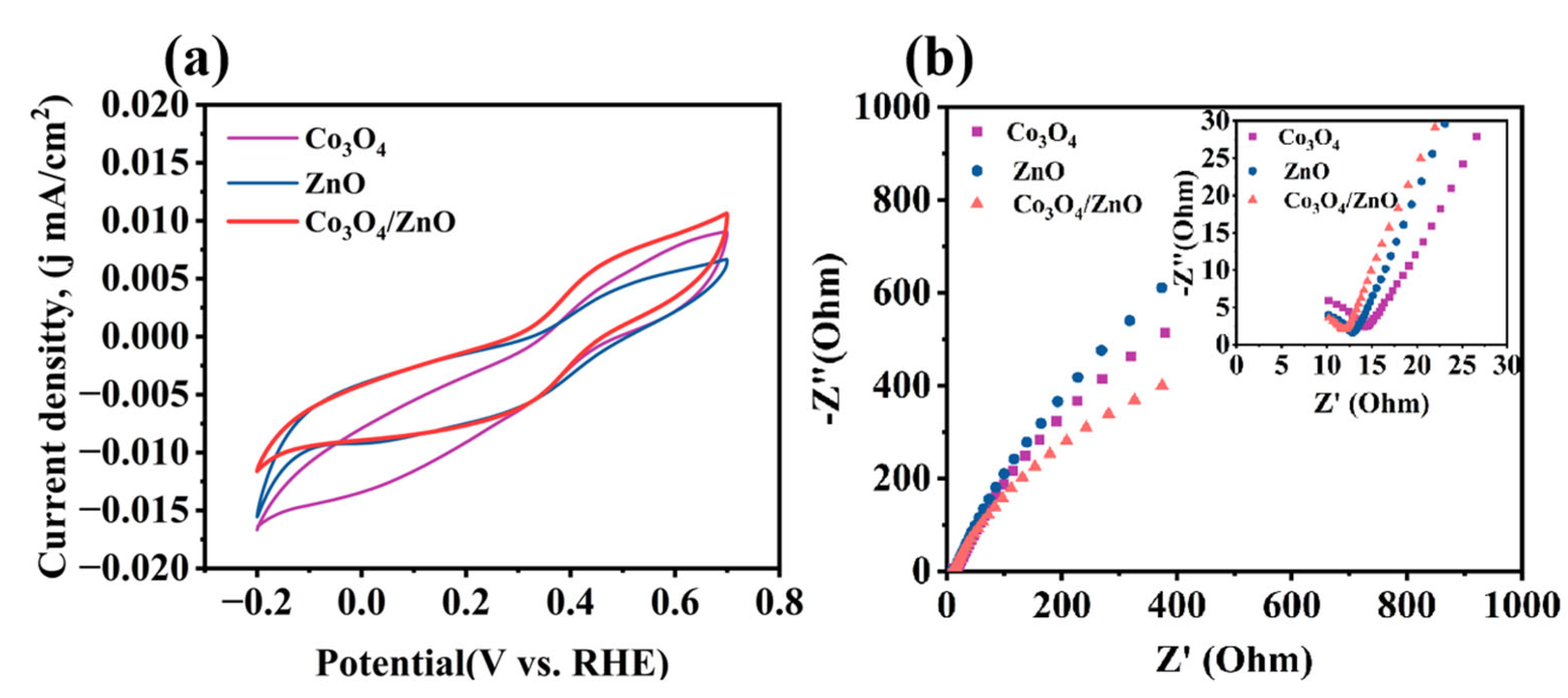
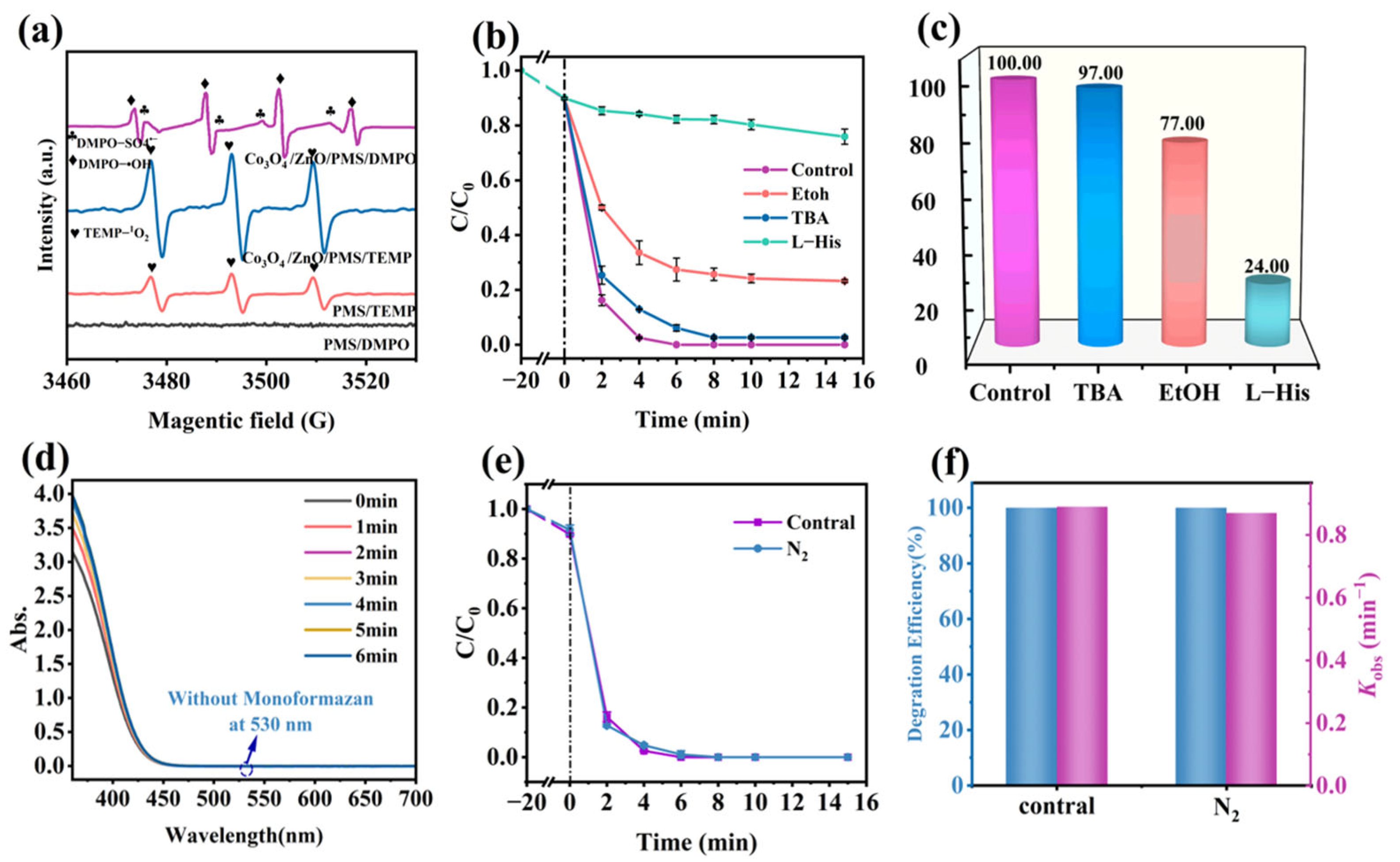
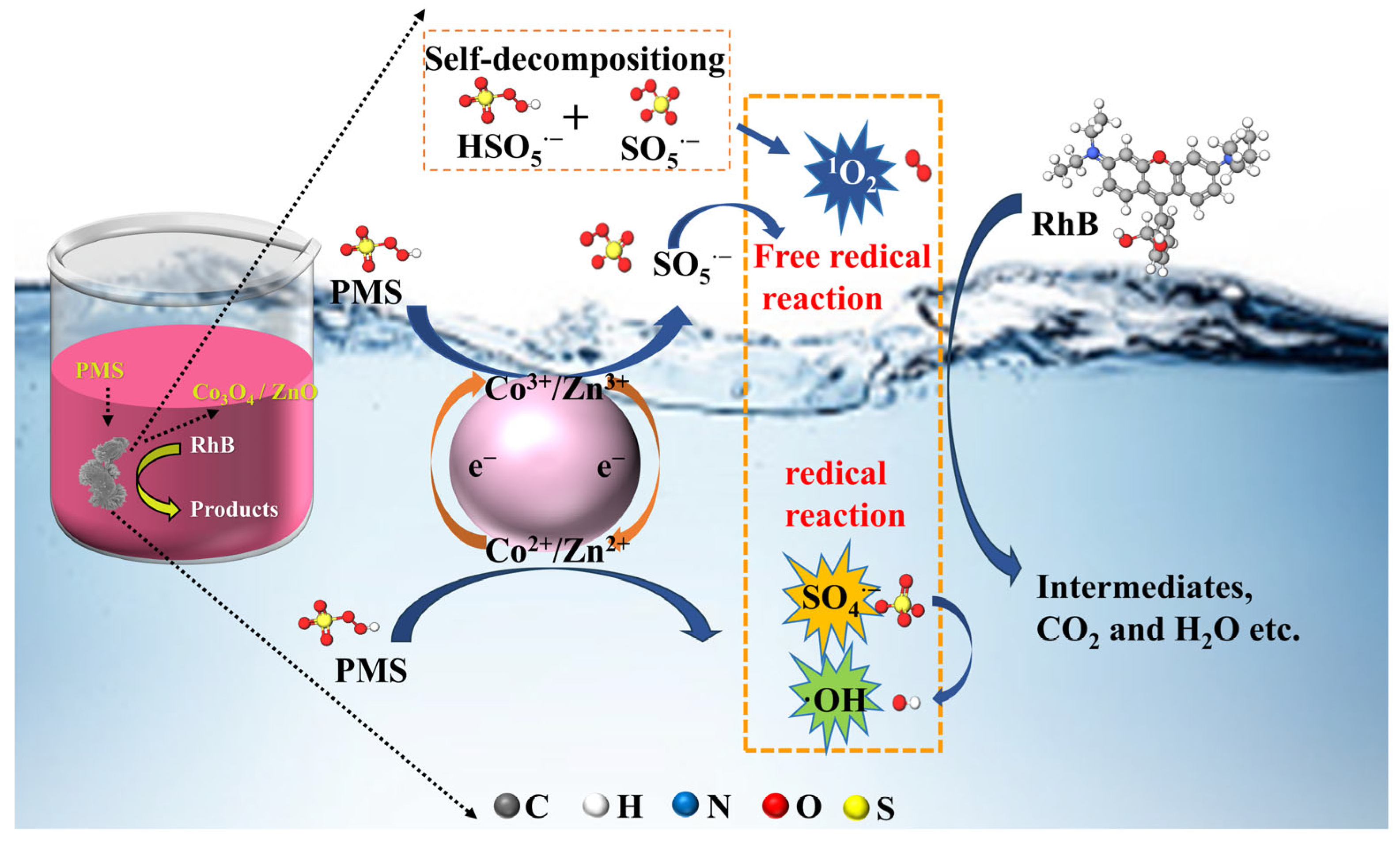
2.5. Mechanism Study
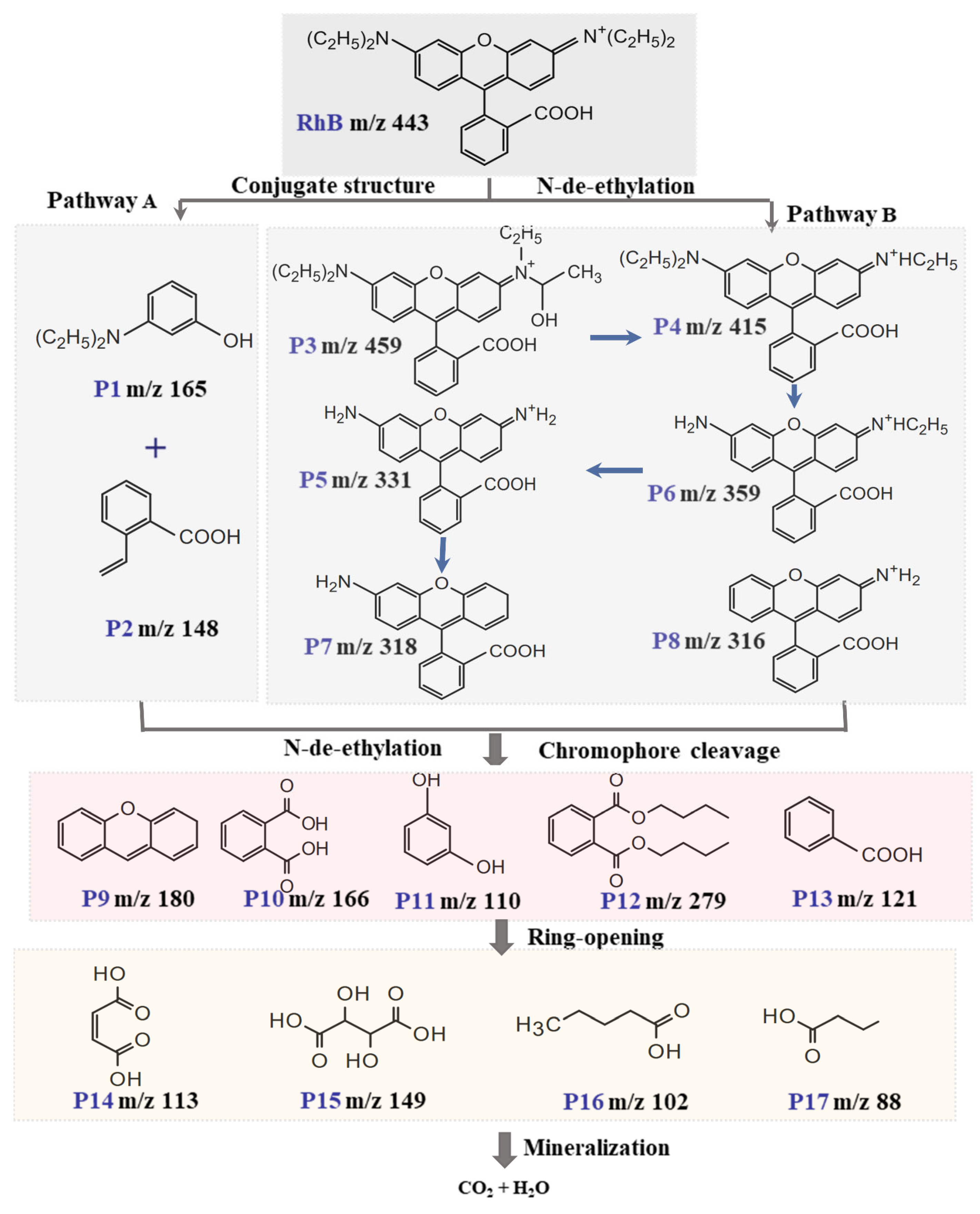
2.6. Possible Degradation Intermediates and Pathways
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Co3O4/ZnO Composite
3.3. Experiments on the Catalytic Activity of RhB
3.4. Characterization of Composites
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Zhang, Y.; Liang, J.; Luo, Y.; Liu, Q.; Yang, Y.; Sun, X. Facile hydrothermal synthesis of co-glycerate as an efficient peroxymonosulfate activator for rhodamine B degradation. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 648, 129239. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zhang, Z.; Cao, S.; Liu, H.; Shen, S.; Wang, W. Ultrathin Cu-Fe oxide nanosheets boosting persulfate activation to remove organic pollutants with coupling and transformation between radical and nonradical mechanism. Sep. Purif. Technol. 2022, 281, 119978. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Y.; Jia, X.; Yang, Y.; Li, J.; Liu, H. Acetic acid mediated fabrication of highly exposed Fe(III)/Fe(II) sites in Fe2O3/MA for enhanced peroxymonosulfate activation. J. Alloys Compd. 2023, 960, 170649. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Li, C.; Yin, S.; Yan, Y.; Liang, C.; Ma, Q.; Guo, R.; Zhang, Y.; Deng, J.; Sun, Z. Efficient benzo(a)pyrene degradation by coal gangue-based catalytic material for peroxymonosulfate activation. J. Environ. Manag. 2024, 351, 119645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S.; Ran, L.; Yu, H. Enhanced PMS activation by surface electronic reconstruction at Co3O4/ZnO heterointerface: Performance and mechanism. Process Saf. Environ. Prot. 2023, 171, 330–340. [Google Scholar] [CrossRef]
- Mohamed Reda, G.; Fan, H.; Tian, H. Room-temperature solid state synthesis of Co3O4/ZnO p–n heterostructure and its photocatalytic activity. Adv. Powder Technol. 2017, 28, 953–963. [Google Scholar] [CrossRef]
- Fu, W.-X.; Qin, M.-Z.; Niu, H.-Y.; Hu, Q.-Y.; Guo, D.; Chen, M.-M.; Niu, C.-G.; Huang, D.-W. Insights into the high performance of Co–Mn oxide supported by carbon fiber frameworks for ciprofloxacin oxidation process. J. Clean. Prod. 2023, 392, 136062. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Y.; Zeng, G.; Gu, Y.; Shi, Y.; Yi, K.; Ouyang, Y.; Hu, J.; Shi, L. Membrane layers intensifying quorum quenching alginate cores and its potential for membrane biofouling control. Bioresour. Technol. 2019, 279, 195–201. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- Wang, M.; Cui, Y.; Cao, H.; Wei, P.; Chen, C.; Li, X.; Xu, J.; Sheng, G. Activating peroxydisulfate with Co3O4/NiCo2O4 double-shelled nanocages to selectively degrade bisphenol A—A nonradical oxidation process. Appl. Catal. B-Environ. 2021, 282, 119585. [Google Scholar] [CrossRef]
- Cui, J.; Gao, X.; He, B.; Yao, Y.; Zhao, Y.; Wang, T.; Yin, X. ZIFs material-derived bimetallic oxide heterojunctions activate perodisulfate to degrade chlortetracycline rapidly: The synergistic effects of oxygen vacancies and rapid electron transfer. Chem. Eng. J. 2024, 483, 149426. [Google Scholar] [CrossRef]
- Banik, A.; Biswas, K. Synthetic Nanosheets of Natural van der Waals Heterostructures. Angew. Chem-Int. Ed. 2017, 56, 14561–14566. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Cao, Y.; Chen, Y.; Du, X.; Yang, Y.; Wang, Z. Two-dimensional ultrathin perforated Co3O4 nanosheets enhanced PMS-Activated selective oxidation of organic micropollutants in environmental remediation. Chem. Eng. J. 2022, 427, 131953. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Wang, S.; Zhu, H.; Xiong, J. Efficient catalytic ozonation over CuO/ZnO for ofloxacin degradation: The synergistic effect between CuO and ZnO promotes the catalytic effect. Desalin. Water Treat. 2023, 286, 136–149. [Google Scholar] [CrossRef]
- Rawat, A.; Panwar, S.; Purohit, L.P. Hollow cylindrical ternary ZnO/Co3O4/CuO nanocomposite thick film on inter-digitated electrodes for selective ammonia gas sensing. Surf. Interfaces 2023, 42, 103404. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2/Co3O4/reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Wang, T.; Lu, J.; Lei, J.; Zhou, Y.; Zhao, H.; Chen, X.; Faysal Hossain, M.; Zhou, Y. Highly efficient activation of peroxymonosulfate for rapid sulfadiazine degradation by Fe3O4 @Co3S4. Sep. Purif. Technol. 2023, 307, 122755. [Google Scholar] [CrossRef]
- Jiang, R.; Zhong, D.; Xu, Y.; Chang, H.; He, Y.; Zhang, J.; Liao, P. Chitosan derived N-doped carbon anchored Co3O4-doped MoS2 nanosheets as an efficient peroxymonosulfate activator for degradation of dyes. Int. J. Biol. Macromol. 2024, 265, 130519. [Google Scholar] [CrossRef]
- Anh Tran, V.; Khoa Phung, T.; Thuan Le, V.; Ky Vo, T.; Tai Nguyen, T.; Anh Nga Nguyen, T.; Quoc Viet, D.; Quang Hieu, V.; Thi Vo, T.-T. Solar-light-driven photocatalytic degradation of methyl orange dye over Co3O4-ZnO nanoparticles. Mater. Lett. 2021, 284, 128902. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Hong, P.; Li, Y.; Xie, C.; Wu, Z.; Li, M.; Kong, L. Oxygen Vacancies of Mn/CeOx-H induced non-radical activation of peroxymonosulfate through electron mediation for bisphenol A degradation. J. Environ. Chem. Eng. 2023, 11, 111078. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Zhou, Q.; Hong, P.; Shi, X.; Li, Y.; Yao, K.; Zhang, W.; Wang, C.; He, J.; Zhang, K.; Kong, L. Efficient degradation of tetracycline by a novel nanoconfinement structure Cu2O/Cu@MXene composite. J. Hazard. Mater. 2023, 448, 130995. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Wei, J.; Hong, P.; Li, Y.; Xie, C.; Wu, Z.; Zhang, K.; Li, M.; He, J.; Kong, L. Mechanistic insight into active species formation during Fenton-like processes by regulating dissimilar charged groups on Fe3O4 nanospheres. Sep. Purif. Technol. 2023, 319, 124082. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, B.; Yang, Y.; Song, Y.; Yang, Z.; Li, H. Purification of high-arsenic groundwater by magnetic bimetallic MOFs coupled with PMS: Balance of catalysis and adsorption and promotion mechanism of PMS. Chem. Eng. J. 2022, 432, 134417. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Xie, T.; Xu, Q.; Chen, H.; Xiang, B. Switching the adsorption sites of PMS on SrCoO2.52 to enhance catalytic performance. J. Mater. Chem. A 2024, 12, 1274–1283. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Ling, L.; Zhou, Y. Enhanced activation of PMS by a novel Fenton-like composite Fe3O4/S-WO3 for rapid chloroxylenol degradation. Chem. Eng. J. 2022, 446, 137067. [Google Scholar] [CrossRef]
- Yuan, R.; Hu, L.; Yu, P.; Wang, H.; Wang, Z.; Fang, J. Nanostructured Co3O4 grown on nickel foam: An efficient and readily recyclable 3D catalyst for heterogeneous peroxymonosulfate activation. Chemosphere 2018, 198, 204–215. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Wang, J.; Zhang, R.; Ma, X.; Tao, H. Bimetal-organic framework MIL-53(Fe,Ni) stimulates peroxydisulfate to degrade rhodamine B: Properties and degradation mechanism. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 664, 131208. [Google Scholar] [CrossRef]
- Xu, X.; Zong, S.; Chen, W.; Liu, D. Comparative study of Bisphenol A degradation via heterogeneously catalyzed H2O2 and persulfate: Reactivity, products, stability and mechanism. Chem. Eng. J. 2019, 369, 470–479. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; He, S.; Shi, L.; Guo, K.; Fang, J. Reinvestigation on High-Valent Cobalt for the Degradation of Micropollutants in the Co(II)/Peroxymonosulfate System: Roles of Co(III). Environ. Sci. Technol. 2024, 58, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Song, L.; Zhao, Z.; Liu, W.; Zhou, Y.; Shang, J.; Cheng, X. CuFe2O4/CuO magnetic nano-composite activates PMS to remove ciprofloxacin: Ecotoxicity and DFT calculation. Chem. Eng. J. 2022, 446, 137183. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, S.; Zhang, Y.; Song, Y.; Wu, D.; Jiang, K. Cellulose nanocrystals mediated spontaneous weaving of ZIF-67 hybrid networks: Enhanced peroxymonosulfate activation and rapid removal of dye. Surf. Interfaces 2024, 50, 104539. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, F.; Xia, C.; Zhu, D.; Chen, J.; Liu, N.; Zhao, Y.; Qi, R.; Guo, W.; You, B.; et al. Asymmetric CO-CHO Coupling over Pr Single-Atom Alloy Enables Industrial-Level Electrosynthesis of Ethylene. J. Am. Chem. Soc. 2025, 147, 15654–15665. [Google Scholar] [CrossRef]
- Shilpa, R.; Anand, A.C.; Sibi, K.S.; Kumar, S.R.S.; Rakhi, R.B. MnO2/MoS2 heterostructured self-standing bifunctional electrodes for efficient alkaline simulated seawater electrolysis. Int. J. Hydrogen Energy 2025, 117, 73–85. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Qin, W.; Wu, X.; Yang, G. Laser modification-induced NiCo2O4-δ with high exterior Ni3+/Ni2+ ratio and substantial oxygen vacancies for electrocatalysis. Electrochim. Acta 2019, 297, 623–632. [Google Scholar] [CrossRef]
- Morales-Rodríguez, H.D.; Nguyen-Ba, K.; Chen, F.; Shen, Q.; Tu, R.; Zhang, L.M.; Castillo-Alvarado, F.L.; Rodríguez-Hernández, J.I.; Vargas-Garcia, J.R. Modulating the electronic structure of MoS2 nanosheets by Mn doping for improving hydrogen evolution reaction: An experimental and theoretical DFT-QTAIM study. Mater. Today Commun. 2024, 38, 107786. [Google Scholar] [CrossRef]
- He, W.-X.; Wang, X.-W.; Yu, C.; Zhang, W.-H.; Li, J.; Li, X. The optimized oxygen evolution reaction of FeCoNi layer double hydroxide by the adhesion of Ag nanoparticles. Int. J. Hydrogen Energy 2024, 61, 603–610. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, F.; Zhang, L.; Guo, X.; Wang, D.; Niu, R.; Yang, H.; Li, J.; Ma, G.; Lei, Z. Iron-modulated Ni3S2 derived from a Ni-MOF-based Prussian blue analogue for a highly efficient oxygen evolution reaction. Dalton Trans. 2022, 51, 17283–17291. [Google Scholar] [CrossRef]
- Guo, R.; Xi, B.; Hu, Y.; Yuan, Y.; Chen, H.; Gong, Y.; Ge, X.; Lv, N. Enhancing persulfate activation by carbon − based Mn − Fe nanoparticles for groundwater ISCO treatment: Efficiency, stability and mechanism. Chem. Eng. J. 2023, 476, 146827. [Google Scholar] [CrossRef]
- Yang, D.; Hu, Y.; Hong, P.; Shen, G.; Li, Y.; He, J.; Zhang, K.; Wu, Z.; Xie, C.; Liu, J.; et al. Preassembly strategy to anchor single atoms on carbon nitride layers achieving versatile Fenton-like catalysis. Sep. Purif. Technol. 2023, 308, 122955. [Google Scholar] [CrossRef]
- Zhao, D.; Armutlulu, A.; Chen, Q.; Xie, R. Enhanced ciprofloxacin degradation by electrochemical activation of persulfate using iron decorated carbon membrane cathode: Promoting direct single electron transfer to produce 1O2. Chem. Eng. J. 2022, 437, 135264. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.P.; Vallejos, S. AACVD Synthesis and Characterization of Iron and Copper Oxides Modified ZnO Structured Films. Nanomater. 2020, 10, 471. [Google Scholar] [CrossRef]
- Qiu, X.; Zhao, Y.; Jia, Z.; Li, C.; Jin, R.; Mutabazi, E. Fe and Zn co-doped carbon nanoparticles as peroxymonosulfate activator for efficient 2,4-dichorophenol degradation. Environ. Res. 2024, 240, 117313. [Google Scholar] [CrossRef]
- Asif, A.H.; Rafique, N.; Hirani, R.A.K.; Shi, L.; Wang, Y.; Duan, X.; Yin, Y.; Sun, H. MIL-53(Fe) derived magnetic CuFe2O4/Fe2O3 composite for catalytic oxidation of sulfamethoxazole via peroxymonsulfate activation. Chem. Eng. J. 2023, 469, 143915. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Lan, M.-Y.; Wang, F.; Yi, X.-H.; Wang, C.-C. ZIF-67-based catalysts in persulfate advanced oxidation processes (PS-AOPs) for water remediation. J. Environ. Chem. Eng. 2022, 10, 107997. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Tu, Y.; Gu, S.; Su, Z.; Peng, Z. Synthesis and evaluation of iron-carbon composites derived from coal gasification fine slag for peroxydisulfate activation in rhodamine B removal. J. Environ. Chem. Eng. 2024, 12, 113805. [Google Scholar] [CrossRef]
- Pang, Y.; Kong, L.; Chen, D.; Yuvaraja, G.; Mehmood, S. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B. J. Hazard. Mater. 2020, 384, 121447. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Zhou, M.; Liang, L.; Li, K. Oxidation of Rhodamine B by persulfate activated with porous carbon aerogel through a non-radical mechanism. J. Hazard. Mater. 2018, 358, 53–61. [Google Scholar] [CrossRef]
- Li, F.; Wei, J.; Wang, D.; Han, Y.; Han, D.; Gong, J. Ce-doped CuCoO2 delafossite with switchable PMS activation pathway for tetracycline degradation. Chem. Eng. J. 2024, 481, 148633. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, A.; Xian, L.; Wang, Y.; Long, Y.; Fan, G. Facile fabrication of surface vulcanized Co-Fe spinel oxide nanoparticles toward efficient 4-nitrophenol destruction. J. Hazard. Mater. 2022, 430, 128433. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, G.; Li, W.; Fang, Z.; Liu, H.; Lv, W.; Liu, G. Magnetic nitrogen-doped carbon nanotubes as activators of peroxymonosulfate and their application in non-radical degradation of sulfonamide antibiotics. J. Clean. Prod. 2022, 380, 135064. [Google Scholar] [CrossRef]
- Chang, L.; Xue, X.; Deng, Q.; Xie, X.; Zhang, X.; Cheng, C.; Chai, H.; Huang, Y. Modulating the electronic structure of Co center via MgO@C co-doping for PMS activation to remove levofloxacin. Sep. Purif. Technol. 2023, 321, 124151. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, M.; Chen, Z.; Dong, L.; Li, B.; Tao, L.; Wang, H.; Li, D. Fabrication of novel direct Z-scheme + isotype heterojunction photocatalyst g-C3N4/TiO2 with peroxymonosulfate (PMS) activation synergy and 2D/0D structure. Catal. Sci. Technol. 2022, 12, 7199–7207. [Google Scholar] [CrossRef]
- Xu, M.; Lu, M.; Yang, Y.; Ai, L.; Fan, H.; Guo, N.; Wang, L. Efficient degradation of pollutants by Bi2WO6/BiOCl heterojunction activated peroxymonosulfate: Performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 112156. [Google Scholar] [CrossRef]
- Chang, L.; Xie, X.; Zhang, X.; Chai, H.; Huang, Y. Overlooked key role of Mo(VI) in Fe2(MoO4)3 for peroxymonosulfate activation with 1O2 dominated degradation pathway. Sep. Purif. Technol. 2023, 322, 124360. [Google Scholar] [CrossRef]
- Li, S.; Ning, X.; Hao, P.; Cao, Y.; Xie, J.; Hu, J.; Lu, Z.; Hao, A. Defect-rich MoS2 piezocatalyst: Efficient boosting piezocatalytic activation of PMS activity towards degradation organic pollutant. Dye. Pigm. 2022, 206, 110678. [Google Scholar] [CrossRef]
- Chen, X.; Peng, C.; Luo, F.; Du, G.; Zhang, Y.; Zhao, J.; Jiang, L.; Su, H.; Shan, S.; Hu, T. Novel MIL-68(Fe)/MoS2 composites promote peroxymonosulfate activation for efficiently removing Rhodamine B. J. Alloys Compd. 2024, 976, 173369. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao, X.; Zhang, M.; Li, Y.; Guo, J.; Lan, Y.; Chen, C. Waste self-heating bag derived CoFe2O4 composite enhances peroxymonosulfate activation: Performance, mechanism, and adaptability under high-salinity conditions. J. Water Process. Eng. 2024, 60, 105221. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, R.; Shu, T.; Wang, Y.; Li, L. Synthesis of Co-doped Fe metal–organic framework MIL-101(Fe,Co) and efficient degradation of organic dyes in water. Sep. Purif. Technol. 2023, 304, 122300. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, L.; Shi, X.; Wei, M.; Cao, J.; Long, Y. Core-shell Prussian blue analogues@ poly(m-phenylenediamine) as efficient peroxymonosulfate activators for degradation of Rhodamine B with reduced metal leaching. J. Colloid Interface Sci. 2019, 534, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, M.; You, L.; Cheng, G.; Li, J.; Zhou, K. Oxygen vacancy induced peroxymonosulfate activation by Mg-doped Fe2O3 composites for advanced oxidation of organic pollutants. Chemosphere 2021, 279, 130482. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Cui, Y.-K.; Wen, J.-T.; Wang, Y.-S.; Jia, M.-H.; He, S.-Z.; Wang, W.-K.; Xu, J. Flexible regulation of persulfate activation mechanisms through tuning Cu valence in CuBTC-derived copper oxide catalysts for improved pollutant degradation. Chem. Eng. J. 2023, 476, 146565. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Liu, R.; Zhao, F.; He, S.; Wang, Y.; Wang, X.; Huang, H.; Yi, M.; Zhu, S. Efficiently Degrading RhB Using Bimetallic Co3O4/ZnO Oxides: Ultra-Fast and Persistent Activation of Permonosulfate. Molecules 2025, 30, 2237. https://doi.org/10.3390/molecules30102237
Sun B, Liu R, Zhao F, He S, Wang Y, Wang X, Huang H, Yi M, Zhu S. Efficiently Degrading RhB Using Bimetallic Co3O4/ZnO Oxides: Ultra-Fast and Persistent Activation of Permonosulfate. Molecules. 2025; 30(10):2237. https://doi.org/10.3390/molecules30102237
Chicago/Turabian StyleSun, Bai, Rui Liu, Fengshou Zhao, Shengnan He, Yun Wang, Xiangxiang Wang, Hao Huang, Mingjian Yi, and Shuguang Zhu. 2025. "Efficiently Degrading RhB Using Bimetallic Co3O4/ZnO Oxides: Ultra-Fast and Persistent Activation of Permonosulfate" Molecules 30, no. 10: 2237. https://doi.org/10.3390/molecules30102237
APA StyleSun, B., Liu, R., Zhao, F., He, S., Wang, Y., Wang, X., Huang, H., Yi, M., & Zhu, S. (2025). Efficiently Degrading RhB Using Bimetallic Co3O4/ZnO Oxides: Ultra-Fast and Persistent Activation of Permonosulfate. Molecules, 30(10), 2237. https://doi.org/10.3390/molecules30102237




