Pharmacophore Modeling of Janus Kinase Inhibitors: Tools for Drug Discovery and Exposition Prediction
Abstract
1. Introduction
2. Results
2.1. Datasets
2.2. Pharmacophore Modeling
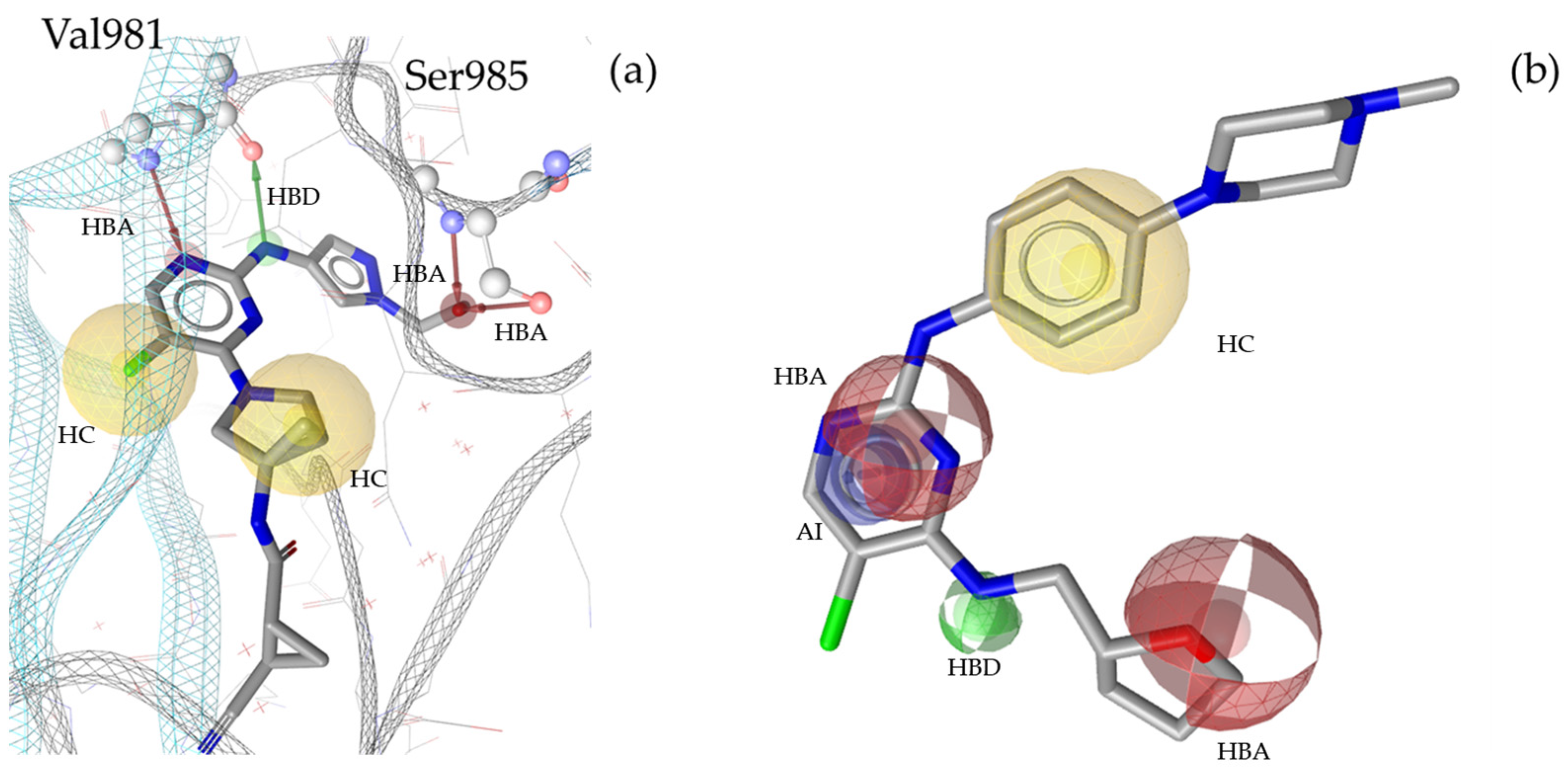
2.3. Theoretical Evaluation of the Generated Pharmacophore Models
2.4. Identified Pesticides During Virtual Screening Campaign
3. Discussion
4. Materials and Methods
4.1. Generation of Databases
4.2. Pharmacophore Model Generation
4.3. Theoretical Validation
- (a)
- Sensitivity = number of ACs identified by the model/number of ACs in the dataset
- (b)
- Specificity = number of ACs not identified by the model/number of IAs in the dataset
- (c)
- Accuracy = (number of TP/number of TN)/number of all the compounds in the database
- (d)
- YoA = number of TP/number of total hits
- (e)
- EF = YoA/(number of ACs in the database/number of all compounds in the database)
4.4. Virtual Screening
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macfarlane, E.; Carey, R.; Keegel, T.; El-Zaemay, S.; Fritschi, L. Dermal exposure associated with occupational end use of pesticides and the role of protective measures. Saf. Health Work 2013, 4, 136–141. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Yao, H.; Yang, Y.; Cui, M.; Tu, Z.; Stallones, L.; Xiang, H. Pesticide poisoning and neurobehavioral function among farm workers in Jiangsu, People’s Republic of China. Cortex 2016, 74, 396–404. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Zhang, J.; Wu, Y.; Sun, H. Chlorpyrifos exposure in farmers and urban adults: Metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ. Res. 2016, 149, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Jensen, B.H.; Petersen, A.; Petersen, P.B.; Christensen, T.; Fagt, S.; Trolle, E.; Poulsen, M.E.; Andersen, J.H. Cumulative dietary risk assessment of pesticides in food for the Danish population for the period 2012–2017. Food Chem. Toxicol. 2022, 168, 113359. [Google Scholar] [CrossRef]
- Fucic, A.; Duca, R.C.; Galea, K.S.; Maric, T.; Garcia, K.; Bloom, M.S.; Andersen, H.R.; Vena, J.E. Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides. Int. J. Environ. Res. Public Health 2021, 18, 6576. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Alavanja, M.C.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef]
- Purdue, M.P.; Hoppin, J.A.; Blair, A.; Dosemeci, M.; Alavanja, M.C. Occupational exposure to organochlorine insecticides and cancer incidence in the Agricultural Health Study. Int. J. Cancer 2007, 120, 642–649. [Google Scholar] [CrossRef]
- Mahajan, R.; Blair, A.; Lynch, C.F.; Schroeder, P.; Hoppin, J.A.; Sandler, D.P.; Alavanja, M.C. Fonofos exposure and cancer incidence in the agricultural health study. Environ. Health Perspect. 2006, 114, 1838–1842. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M. A comprehensive review of pesticides and the immune dysregulation: Mechanisms, evidence and consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef]
- Costa, C.; Rapisarda, V.; Catania, S.; Di Nola, C.; Ledda, C.; Fenga, C. Cytokine patterns in greenhouse workers occupationally exposed to α-cypermethrin: An observational study. Environ. Toxicol. Pharmacol. 2013, 36, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. Cytokines and cytokine receptors as targets of immune-mediated inflammatory diseases—RA as a role model. Inflamm. Regen. 2022, 42, 35. [Google Scholar] [CrossRef]
- Gadina, M.; Le, M.T.; Schwartz, D.M.; Silvennoinen, O.; Nakayamada, S.; Yamaoka, K.; O’shea, J.J. Janus kinases to jakinibs: From basic insights to clinical practice. Rheumatology 2019, 58, i4–i16. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, A.A.; Bao, K.; Lupardus, P.; Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug Discov. 2021, 20, 39–63. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving cognition of the JAK-STAT signaling pathway: Autoimmune disorders and cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Hoisnard, L.; Lebrun-Vignes, B.; Maury, S.; Mahevas, M.; El Karoui, K.; Roy, L.; Zarour, A.; Michel, M.; Cohen, J.L.; Amiot, A.; et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022, 12, 7140. [Google Scholar] [CrossRef]
- Giordano, D.; Biancaniello, C.; Argenio, M.A.; Facchiano, A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals 2022, 15, 646. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Ganellin, C.R.; Lindberg, P.; Mitscher, L.A. Glossary of terms used in medicinal chemistry (IUPAC Recommendations 1998). Pure Appl. Chem. 1998, 70, 1129–1143. [Google Scholar] [CrossRef]
- Kaserer, T.; Beck, K.R.; Akram, M.; Odermatt, A.; Schuster, D. Pharmacophore Models and Pharmacophore-Based Virtual Screening: Concepts and Applications Exemplified on Hydroxysteroid Dehydrogenases. Molecules 2015, 20, 22799–22832. [Google Scholar] [CrossRef]
- Schuster, D.; Wolber, G. Identification of bioactive natural products by pharmacophore-based virtual screening. Curr. Pharm. Des. 2010, 16, 1666–1681. [Google Scholar] [CrossRef] [PubMed]
- Simov, V.; Deshmukh, S.V.; Dinsmore, C.J.; Elwood, F.; Fernandez, R.B.; Garcia, Y.; Gibeau, C.; Gunaydin, H.; Jung, J.; Katz, J.D.; et al. Structure-based design and development of (benz)imidazole pyridones as JAK1-selective kinase inhibitors. Bioorganic Med. Chem. Lett. 2016, 26, 1803–1808. [Google Scholar] [CrossRef]
- Nakajima, Y.; Aoyama, N.; Takahashi, F.; Sasaki, H.; Hatanaka, K.; Moritomo, A.; Inami, M.; Ito, M.; Nakamura, K.; Nakamori, F.; et al. Design, synthesis, and evaluation of 4,6-diaminonicotinamide derivatives as novel and potent immunomodulators targeting JAK3. Bioorganic Med. Chem. 2016, 24, 4711–4722. [Google Scholar] [CrossRef] [PubMed]
- Spergel, S.H.; Mertzman, M.E.; Kempson, J.; Guo, J.; Stachura, S.; Haque, L.; Lippy, J.S.; Zhang, R.F.; Galella, M.; Pitt, S.; et al. Discovery of a JAK1/3 Inhibitor and Use of a Prodrug To Demonstrate Efficacy in a Model of Rheumatoid Arthritis. ACS Med. Chem. Lett. 2019, 10, 306–311. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Bharate, S.B. Recent Developments in the Use of Kinase Inhibitors for Management of Viral Infections. J. Med. Chem. 2022, 65, 893–921. [Google Scholar] [CrossRef]
- Davis, R.R.; Li, B.; Yun, S.Y.; Chan, A.; Nareddy, P.; Gunawan, S.; Ayaz, M.; Lawrence, H.R.; Reuther, G.W.; Lawrence, N.J.; et al. Structural Insights into JAK2 Inhibition by Ruxolitinib, Fedratinib, and Derivatives Thereof. J. Med. Chem. 2021, 64, 2228–2241. [Google Scholar] [CrossRef]
- Mesaros, E.F.; Dugan, B.J.; Dorsey, B.D.; Milkiewicz, K.L.; Curry, M.A.; Gingrich, D.E. Preparation and Uses of 1,2,4-triazolo [1,5a] Pyridine Derivatives. U.S. Patent 8633173-B2, 5 June 2009. [Google Scholar]
- Mathison, C.J.N.; Chianelli, D.; Rucker, P.V.; Nelson, J.; Roland, J.; Huang, Z.; Yang, Y.; Jiang, J.; Xie, Y.F.; Epple, R.; et al. Efficacy and Tolerability of Pyrazolo[1,5-a]pyrimidine RET Kinase Inhibitors for the Treatment of Lung Adenocarcinoma. ACS Med. Chem. Lett. 2020, 11, 558–565. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, C.J.; Yu, R.N.; Shu, L.; Zhang, T.T.; Zhang, D.Y. Discovery of novel selective Janus kinase 2 (JAK2) inhibitors bearing a 1H-pyrazolo[3,4-d]pyrimidin-4-amino scaffold. Bioorganic Med. Chem. 2019, 27, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Sloman, D.L.; Noucti, N.; Altman, M.D.; Chen, D.; Mislak, A.C.; Szewczak, A.; Hayashi, M.; Warren, L.; Dellovade, T.; Wu, Z.; et al. Optimization of microtubule affinity regulating kinase (MARK) inhibitors with improved physical properties. Bioorganic Med. Chem. Lett. 2016, 26, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Diao, Y.; Ge, H.; Xu, F.; Zhu, L.; Zhao, Z.; Li, H. Discovery and optimization of 2-aminopyridine derivatives as novel and selective JAK2 inhibitors. Bioorganic Med. Chem. Lett. 2020, 30, 127048. [Google Scholar] [CrossRef]
- Tan, L.; Akahane, K.; McNally, R.; Reyskens, K.M.S.E.; Ficarro, S.B.; Liu, S.; Herter-Sprie, G.S.; Koyama, S.; Pattison, M.J.; Labella, K.; et al. Development of Selective Covalent Janus Kinase 3 Inhibitors. J. Med. Chem. 2015, 58, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Bhide, R.S.; Keon, A.; Weigelt, C.; Sack, J.S.; Schmidt, R.J.; Lin, S.; Xiao, H.-Y.; Spergel, S.H.; Kempson, J.; Pitts, W.J.; et al. Discovery and structure-based design of 4,6-diaminonicotinamides as potent and selective IRAK4 inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 4908–4913. [Google Scholar] [CrossRef]
- Fensome, A.; Ambler, C.M.; Arnold, E.; Banker, M.E.; Clark, J.D.; Dowty, M.E.; Efremov, I.V.; Flick, A.; Gerstenberger, B.S.; Gifford, R.S.; et al. Design and optimization of a series of 4-(3-azabicyclo[3.1.0]hexan-3-yl)pyrimidin-2-amines: Dual inhibitors of TYK2 and JAK1. Bioorganic Med. Chem. 2020, 28, 115481. [Google Scholar] [CrossRef]
- Lawrence, H.R.; Mahajan, K.; Luo, Y.; Zhang, D.; Tindall, N.; Huseyin, M.; Gevariya, H.; Kazi, S.; Ozcan, S.; Mahajan, N.P.; et al. Development of novel ACK1/TNK2 inhibitors using a fragment-based approach. J. Med. Chem. 2015, 58, 2746–2763. [Google Scholar] [CrossRef]
- Menet, C.J.; Fletcher, S.R.; Van Lommen, G.; Geney, R.; Blanc, J.; Smits, K.; Jouannigot, N.; Deprez, P.; van der Aar, E.M.; Clement-Lacroix, P.; et al. Triazolopyridines as selective JAK1 inhibitors: From hit identification to GLPG0634. J. Med. Chem. 2014, 57, 9323–9342. [Google Scholar] [CrossRef]
- Bauer, S.M.; Pandey, A. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9868729-B2, Inhibitors of Protein Kinases. United States. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9868729-B2 (accessed on 16 December 2024).
- Krier, J.; Singh, R.R.; Kondić, T.; Lai, A.; Diderich, P.; Zhang, J.; Thiessen, P.A.; Bolton, E.E.; Schymanski, E.L. Discovering pesticides and their TPs in Luxembourg waters using open cheminformatics approaches. Environ. Int. 2022, 158, 106885. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Ghosh, S.; Nie, A.; An, J.; Huang, Z. Structure-based virtual screening of chemical libraries for drug discovery. Curr. Opin. Chem. Biol. 2006, 10, 194–202. [Google Scholar] [CrossRef]
- Sun, H. Pharmacophore-based virtual screening. Curr. Med. Chem. 2008, 15, 1018–1024. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Radominski, S.C.; Gomez-Reino, J.J.; Wang, L.; Krishnaswami, S.; Wood, S.P.; Soma, K.; Nduaka, C.I.; Kwok, K.; Valdez, H.; et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 2924–2937. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Stovin, C.; Alveyn, E.; Adeyemi, O.; Chan, C.K.D.; Patel, V.; Adas, M.A.; Atzeni, F.; Ng, K.K.H.; Rutherford, A.I.; et al. JAK inhibitors and the risk of malignancy: A meta-analysis across disease indications. Ann. Rheum. Dis. 2023, 82, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Gong, M.; Gu, Y.; Zhang, H.; Dong, B.; Guo, Q.; Pang, X.; Xiang, Q.; He, X.; et al. Risk of venous thromboembolism with janus kinase inhibitors in inflammatory immune diseases: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1189389. [Google Scholar] [CrossRef]
- Robinson, C.; Portier, C.J.; Čavoški, A.; Mesnage, R.; Roger, A.; Clausing, P.; Whaley, P.; Muilerman, H.; Lyssimachou, A. Achieving a High Level of Protection from Pesticides in Europe: Problems with the Current Risk Assessment Procedure and Solutions. Eur. J. Risk Regul. 2020, 11, 450–480. [Google Scholar] [CrossRef]
- Gerken, J.; Vincent, G.T.; Zapata, D.; Barron, I.G.; Zapata, I. Comprehensive assessment of pesticide use patterns and increased cancer risk. Front. Cancer Control Soc. 2024, 2, 1368086. [Google Scholar] [CrossRef]
- Yamazoe, Y.; Yamamoto, S.; Yoshida, M.; Kawanishi, T.; Kumagai, S. Mepanipyrim (Pesticides). Food Saf. 2016, 4, 28–29. [Google Scholar] [CrossRef][Green Version]
- Zweigle, J.; Schmidt, A.; Bugsel, B.; Vogel, C.; Simon, F.; Zwiener, C. Perfluoroalkyl acid precursor or weakly fluorinated organic compound? A proof of concept for oxidative fractionation of PFAS and organofluorines. Anal. Bioanal. Chem. 2024, 416, 6799–6808. [Google Scholar] [CrossRef]
- Weis, G.C.C.; Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.D.S.R.; de Oliveira Alves, A.; Machado, A.K.; Duarte, M.M.M.F.; da Cruz, I.B.M.; Costabeber, I.H. Immunomodulatory effect of mancozeb, chlorothalonil, and thiophanate methyl pesticides on macrophage cells. Ecotoxicol. Environ. Saf. 2019, 182, 109420. [Google Scholar] [CrossRef]
- Jabusch, T.W.; Tjeerdema, R.S. Partitioning of penoxsulam, a new sulfonamide herbicide. J. Agric. Food Chem. 2005, 53, 7179–7183. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Gyldenkærne, S.; Jones, R.R.; Olsen, S.F.; Tikellis, G.; Granström, C.; Dwyer, T.; Stayner, L.T.; Ward, M.H. Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environ. Int. 2020, 143, 105955. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Mgbudom-Okah, C.J.; Ndufeiya-Kumasi, L.C.; Monye, V.E.; Aruoren, O.; Ezim, O.E.; Omeodu, S.I.; Charles, I.A. Influence of triazines and lipopolysaccharide coexposure on inflammatory response and histopathological changes in the testis and liver of BalB/c mice. Heliyon 2024, 10, e24431. [Google Scholar] [CrossRef] [PubMed]
- Laetz, C.A.; Baldwin, D.H.; Collier, T.K.; Hebert, V.; Stark, J.D.; Scholz, N.L. The synergistic toxicity of pesticide mixtures: Implications for risk assessment and the conservation of endangered Pacific salmon. Environ. Health Perspect. 2009, 117, 348–353. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; A Shoemaker, B.; A Thiessen, P.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef]
- Labadie, S.; Dragovich, P.S.; Barrett, K.; Blair, W.S.; Bergeron, P.; Chang, C.; Deshmukh, G.; Eigenbrot, C.; Ghilardi, N.; Gibbons, P.; et al. Structure-based discovery of C-2 substituted imidazo-pyrrolopyridine JAK1 inhibitors with improved selectivity over JAK2. Bioorganic Med. Chem. Lett. 2012, 22, 7627–7633. [Google Scholar] [CrossRef]
- Su, Q.; Banks, E.; Bebernitz, G.; Bell, K.; Borenstein, C.F.; Chen, H.; Chuaqui, C.E.; Deng, N.; Ferguson, A.D.; Kawatkar, S.P.; et al. Discovery of (2R)-N-[3-[2-[(3-Methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide (AZD4205) as a Potent and Selective Janus Kinase 1 Inhibitor. J. Med. Chem. 2020, 63, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Siu, T.; Brubaker, J.; Fuller, P.; Torres, L.; Zeng, H.; Close, J.; Mampreian, D.M.; Shi, F.; Liu, D.; Fradera, X.; et al. The Discovery of 3-((4-Chloro-3-methoxyphenyl)amino)-1-((3R,4S)-4-cyanotetrahydro-2H-pyran-3-yl)-1H-pyrazole-4-carboxamide, a Highly Ligand Efficient and Efficacious Janus Kinase 1 Selective Inhibitor with Favorable Pharmacokinetic Properties. J. Med. Chem. 2017, 60, 9676–9690. [Google Scholar] [CrossRef] [PubMed]
- Arwood, M.L.; Liu, Y.; Harkins, S.K.; Weinstock, D.M.; Yang, L.; Stevenson, K.E.; Plana, O.D.; Dong, J.; Cirka, H.; Jones, K.L.; et al. New scaffolds for type II JAK2 inhibitors overcome the acquired G993A resistance mutation. Cell Chem. Biol. 2023, 30, 618–631.e12. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Figueroa, S.; De Vicente, J.; Hermann, J.; Jahangir, A.; Jin, S.; Kuglstatter, A.; Lynch, S.M.; Menke, J.; Niu, L.; Patel, V.; et al. Discovery of a series of novel 5H-pyrrolo[2,3-b]pyrazine-2-phenyl ethers, as potent JAK3 kinase inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 2522–2526. [Google Scholar] [CrossRef]
- Thorarensen, A.; Dowty, M.E.; Banker, M.E.; Juba, B.; Jussif, J.; Lin, T.; Vincent, F.; Czerwinski, R.M.; Casimiro-Garcia, A.; Unwalla, R.; et al. Design of a Janus Kinase 3 (JAK3) Specific Inhibitor 1-((2S,5R)-5-((7H-Pyrrolo[2,3-d]pyrimidin-4-yl)amino)-2-methylpiperidin-1-yl)prop-2-en-1-one (PF-06651600) Allowing for the Interrogation of JAK3 Signaling in Humans. J. Med. Chem. 2017, 60, 1971–1993. [Google Scholar] [CrossRef]
- Forster, M.; Chaikuad, A.; Bauer, S.M.; Holstein, J.; Robers, M.B.; Corona, C.R.; Gehringer, M.; Pfaffenrot, E.; Ghoreschi, K.; Knapp, S.; et al. Selective JAK3 Inhibitors with a Covalent Reversible Binding Mode Targeting a New Induced Fit Binding Pocket. Cell Chem. Biol. 2016, 23, 1335–1340. [Google Scholar] [CrossRef]
- Adams, C.; Aldous, D.J.; Amendola, S.; Bamborough, P.; Bright, C.; Crowe, S.; Eastwood, P.; Fenton, G.; Foster, M.; Harrison, T.K.P.; et al. Mapping the kinase domain of janus kinase 3. Bioorg. Med. Chem. Lett. 2003, 13, 3105–3110. [Google Scholar] [CrossRef]
- Burns, C.J.; Bourke, D.G.; Andrau, L.; Bu, X.; Charman, S.A.; Donohue, A.C.; Fantino, E.; Farrugia, M.; Feutrill, J.T.; Joffe, M.; et al. Phenylaminopyrimidines as inhibitors of Janus kinases (JAKs). Bioorg. Med. Chem. Lett. 2009, 19, 5887–5892. [Google Scholar] [CrossRef]
- Cole, A.G.; Bohnstedt, A.C.; Paradkar, V.; Kingsbury, C.; Quintero, J.G.; Park, H.; Lu, Y.; You, M.; Neagu, I.; Diller, D.J.; et al. 2-Benzimidazolyl-9-(chroman-4-yl)-purinone derivatives as JAK3 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6788–6792. [Google Scholar] [CrossRef]
- Gerspacher, M.; Furet, P.; Pissot-Soldermann, C.; Gaul, C.; Holzer, P.; Vangrevelinghe, E.; Lang, M.; Erdmann, D.; Radimerski, T.; Regnier, C.H.; et al. 2-Amino-aryl-7-aryl-benzoxazoles as potent, selective and orally available JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1724–1727. [Google Scholar] [CrossRef]
- Pissot-Soldermann, C.; Gerspacher, M.; Furet, P.; Gaul, C.; Holzer, P.; McCarthy, C.; Radimerski, T.; Regnier, C.H.; Baffert, F.; Drueckes, P.; et al. Discovery and SAR of potent, orally available 2,8-diaryl-quinoxalines as a new class of JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2609–2613. [Google Scholar] [CrossRef] [PubMed]
- Fidanze, S.D.; Erickson, S.A.; Wang, G.T.; Mantei, R.; Clark, R.F.; Sorensen, B.K.; Bamaung, N.Y.; Kovar, P.; Johnson, E.F.; Swinger, K.K.; et al. Imidazo[2,1-b]thiazoles: Multitargeted inhibitors of both the insulin-like growth factor receptor and members of the epidermal growth factor family of receptor tyrosine kinases. Bioorg. Med. Chem. Lett. 2010, 20, 2452–2455. [Google Scholar] [CrossRef]
- Dart, M.L.; Machleidt, T.; Jost, E.; Schwinn, M.K.; Robers, M.B.; Shi, C.; Kirkland, T.A.; Killoran, M.P.; Wilkinson, J.M.; Hartnett, J.R.; et al. Homogeneous Assay for Target Engagement Utilizing Bioluminescent Thermal Shift. ACS Med. Chem. Lett. 2018, 9, 546–551. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (2024). PubChem Bioassay Record for AID 1699, S.T.S.R.I.M.S.C.R.D., 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1699 (accessed on 25 February 2025).
- Xu, P.; Shen, P.; Yu, B.; Xu, X.; Ge, R.; Cheng, X.; Chen, Q.; Bian, J.; Li, Z.; Wang, J. Janus kinases (JAKs): The efficient therapeutic targets for autoimmune diseases and myeloproliferative disorders. Eur. J. Med. Chem. 2020, 192, 112155. [Google Scholar] [CrossRef]
- Belanger, D.B.; Williams, M.J.; Curran, P.J.; Mandal, A.K.; Meng, Z.; Rainka, M.P.; Yu, T.; Shih, N.Y.; Siddiqui, M.A.; Liu, M.; et al. Discovery of orally bioavailable imidazo[1,2-a]pyrazine-based Aurora kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6739–6743. [Google Scholar] [CrossRef]
- Siu, T.; Kozina, E.S.; Jung, J.; Rosenstein, C.; Mathur, A.; Altman, M.D.; Chan, G.; Xu, L.; Bachman, E.; Mo, J.R.; et al. The discovery of tricyclic pyridone JAK2 inhibitors. Part 1: Hit to lead. Bioorg. Med. Chem. Lett. 2010, 20, 7421–7425. [Google Scholar] [CrossRef] [PubMed]
- Wityak, J.; Das, J.; Moquin, R.V.; Shen, Z.; Lin, J.; Chen, P.; Doweyko, A.M.; Pitt, S.; Pang, S.; Shen, D.R.; et al. Discovery and initial SAR of 2-amino-5-carboxamidothiazoles as inhibitors of the Src-family kinase p56(Lck). Bioorg. Med. Chem. Lett. 2003, 13, 4007–4010. [Google Scholar] [CrossRef]
- Ma, H.; Filip, S.V.; Stent, M.A.H.; Dolan, J.A.; Dietrich, B. JAK3 Inhibitors for the Treatment of Autoimmune and Inflammatory Disorders. U.S. Patent 8592415-B2, 26 November 2013. [Google Scholar]
- Ren, X.; Duan, L.; He, Q.; Zhang, Z.; Zhou, Y.; Wu, D.; Pan, J.; Pei, D.; Ding, K. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med. Chem. Lett. 2010, 1, 454–459. [Google Scholar] [CrossRef]
- Flanagan, M.E.; Blumenkopf, T.A.; Brissette, W.H.; Brown, M.F.; Casavant, J.M.; Shang-Poa, C.; Doty, J.L.; Elliott, E.A.; Fisher, M.B.; Hines, M.; et al. Discovery of CP-690,550: A potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J. Med. Chem. 2010, 53, 8468–8484. [Google Scholar] [CrossRef]
- Ioannidis, S.; Lamb, M.L.; Wang, T.; Almeida, L.; Block, M.H.; Davies, A.M.; Peng, B.; Su, M.; Zhang, H.J.; Hoffmann, E.; et al. Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the Jak/Stat pathway. J. Med. Chem. 2011, 54, 262–276. [Google Scholar] [CrossRef]
- McDonnell, M.E.; Bian, H.; Wrobel, J.; Smith, G.R.; Liang, S.; Ma, H.; Reitz, A.B. Anilino-monoindolylmaleimides as potent and selective JAK3 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1116–1121. [Google Scholar] [CrossRef]
- Harikrishnan, L.S.; Kamau, M.G.; Wan, H.; Inghrim, J.A.; Zimmermann, K.; Sang, X.; Mastalerz, H.A.; Johnson, W.L.; Zhang, G.; Lombardo, L.J.; et al. Pyrrolo[1,2-f]triazines as JAK2 inhibitors: Achieving potency and selectivity for JAK2 over JAK3. Bioorg. Med. Chem. Lett. 2011, 21, 1425–1428. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, M.; Yu, H.; Kim, H.; Yoo, K.H.; Sim, T.; Hah, J.M. Structure based design and syntheses of amino-1H-pyrazole amide derivatives as selective Raf kinase inhibitors in melanoma cells. Bioorg. Med. Chem. 2011, 19, 1915–1923. [Google Scholar] [CrossRef]
- Medina, J.R.; Blackledge, C.W.; Heerding, D.A.; Campobasso, N.; Ward, P.; Briand, J.; Wright, L.; Axten, J.M. Aminoindazole PDK1 Inhibitors: A Case Study in Fragment-Based Drug Discovery. ACS Med. Chem. Lett. 2010, 1, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ioannidis, S.; Almeida, L.; Block, M.H.; Davies, A.M.; Lamb, M.L.; Scott, D.A.; Su, M.; Zhang, H.J.; Alimzhanov, M.; et al. In vitro and in vivo evaluation of 6-aminopyrazolyl-pyridine-3-carbonitriles as JAK2 kinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2958–2961. [Google Scholar] [CrossRef]
- Thomas, M.; Huang, W.S.; Wen, D.; Zhu, X.; Wang, Y.; Metcalf, C.A.; Liu, S.; Chen, I.; Romero, J.; Zou, D.; et al. Discovery of 5-(arenethynyl) hetero-monocyclic derivatives as potent inhibitors of BCR-ABL including the T315I gatekeeper mutant. Bioorg. Med. Chem. Lett. 2011, 21, 3743–3748. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Chen, C.; Huan, X.; Huang, H.; Wang, M.; Zhang, J.; Yan, Y.; Liu, J.; Zhang, T.; Zhang, D. Design, synthesis, and pharmacological evaluation of 4- or 6-phenyl-pyrimidine derivatives as novel and selective Janus kinase 3 inhibitors. Eur. J. Med. Chem. 2020, 191, 112148. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Chi, F.; Zhou, D.; Xie, Z.; Liu, Y.; Wu, H.; Yin, Z.; Shi, W.; Qian, H. Exploration of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Bispecific Inhibitors Based on the Moiety of Fedratinib for Treatment of Both Hematologic Malignancies and Solid Cancers. J. Med. Chem. 2023, 66, 5753–5773. [Google Scholar] [CrossRef]
- Lim, J.; Taoka, B.; Otte, R.D.; Spencer, K.; Dinsmore, C.J.; Altman, M.D.; Chan, G.; Rosenstein, C.; Sharma, S.; Su, H.P.; et al. Discovery of 1-amino-5H-pyrido[4,3-b]indol-4-carboxamide inhibitors of Janus kinase 2 (JAK2) for the treatment of myeloproliferative disorders. J. Med. Chem. 2011, 54, 7334–7349. [Google Scholar] [CrossRef]
- Giraud, F.; Marchand, P.; Carbonnelle, D.; Sartor, M.; Lang, F.; Duflos, M. Synthesis of N-aryl-3-(indol-3-yl)propanamides and their immunosuppressive activities. Bioorg. Med. Chem. Lett. 2010, 20, 5203–5206. [Google Scholar] [CrossRef]
- Gao, C.; Cahya, S.; Nicolaou, C.A.; Wang, J.; Watson, I.A.; Cummins, D.J.; Iversen, P.W.; Vieth, M. Selectivity data: Assessment, predictions, concordance, and implications. J. Med. Chem. 2013, 56, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- Liosi, M.E.; Krimmer, S.G.; Newton, A.S.; Dawson, T.K.; Puleo, D.E.; Cutrona, K.J.; Suzuki, Y.; Schlessinger, J.; Jorgensen, W.L. Selective Janus Kinase 2 (JAK2) Pseudokinase Ligands with a Diaminotriazole Core. J. Med. Chem. 2020, 63, 5324–5340. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.; Blanchard, S.; Soh, C.K.; Lee, C.; Williams, M.; Wang, H.; Dymock, B. Structure-based design of PDK1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Zificsak, C.A.; Gingrich, D.E.; Breslin, H.J.; Dunn, D.D.; Milkiewicz, K.L.; Theroff, J.P.; Thieu, T.V.; Underiner, T.L.; Weinberg, L.R.; Aimone, L.D.; et al. Optimization of a novel kinase inhibitor scaffold for the dual inhibition of JAK2 and FAK kinases. Bioorg. Med. Chem. Lett. 2012, 22, 133–137. [Google Scholar] [CrossRef]
- Schenkel, L.B.; Huang, X.; Cheng, A.; Deak, H.L.; Doherty, E.; Emkey, R.; Gu, Y.; Gunaydin, H.; Kim, J.L.; Lee, J.; et al. Discovery of potent and highly selective thienopyridine Janus kinase 2 inhibitors. J. Med. Chem. 2011, 54, 8440–8450. [Google Scholar] [CrossRef]
- Sonawane, Y.A.; Taylor, M.A.; Napoleon, J.V.; Rana, S.; Contreras, J.I.; Natarajan, A. Cyclin Dependent Kinase 9 Inhibitors for Cancer Therapy. J. Med. Chem. 2016, 59, 8667–8684. [Google Scholar] [CrossRef]
- William, A.D.; Lee, A.C.; Goh, K.C.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Lee, C.P.; Wang, H.; Williams, M.; et al. Discovery of kinase spectrum selective macrocycle (16E)-14-methyl-20-oxa-5,7,14,26-tetraazatetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8(27),9,11,16,21,23-decaene (SB1317/TG02), a potent inhibitor of cyclin dependent kinases (CDKs), Janus kinase 2 (JAK2), and fms-like tyrosine kinase-3 (FLT3) for the treatment of cancer. J. Med. Chem. 2012, 55, 169–196. [Google Scholar] [CrossRef]
- Menichincheri, M.; Ardini, E.; Magnaghi, P.; Avanzi, N.; Banfi, P.; Bossi, R.; Buffa, L.; Canevari, G.; Ceriani, L.; Colombo, M.; et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J. Med. Chem. 2016, 59, 3392–3408. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Chen, H.; Wold, E.A.; Tian, B.; Brasier, A.R.; Zhou, J. Drug Discovery Targeting Bromodomain-Containing Protein 4. J. Med. Chem. 2017, 60, 4533–4558. [Google Scholar] [CrossRef]
- Hoemann, M.; Wilson, N.; Argiriadi, M.; Banach, D.; Burchat, A.; Calderwood, D.; Clapham, B.; Cox, P.; Duignan, D.B.; Konopacki, D.; et al. Synthesis and optimization of furano[3,2-d]pyrimidines as selective spleen tyrosine kinase (Syk) inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 5562–5567. [Google Scholar] [CrossRef]
- Oza, V.; Ashwell, S.; Brassil, P.; Breed, J.; Ezhuthachan, J.; Deng, C.; Grondine, M.; Horn, C.; Liu, D.; Lyne, P.; et al. Synthesis and evaluation of triazolones as checkpoint kinase 1 inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.G.; Mustafa, N.; Tan, E.C.; Poulsen, A.; Ramanujulu, P.M.; Chng, W.J.; Yen, J.J.; Dymock, B.W. Design and Synthesis of Janus Kinase 2 (JAK2) and Histone Deacetlyase (HDAC) Bispecific Inhibitors Based on Pacritinib and Evidence of Dual Pathway Inhibition in Hematological Cell Lines. J. Med. Chem. 2016, 59, 8233–8262. [Google Scholar] [CrossRef]
- Dugan, B.J.; Gingrich, D.E.; Mesaros, E.F.; Milkiewicz, K.L.; Curry, M.A.; Zulli, A.L.; Dobrzanski, P.; Serdikoff, C.; Jan, M.; Angeles, T.S.; et al. A selective, orally bioavailable 1,2,4-triazolo[1,5-a]pyridine-based inhibitor of Janus kinase 2 for use in anticancer therapy: Discovery of CEP-33779. J. Med. Chem. 2012, 55, 5243–5254. [Google Scholar] [CrossRef]
- Mitton-Fry, M.J.; Berlinski, P.J.; Birchmeier, M.J.; Bowman, J.W.; Gonzales, A.J.; Kamerling, S.G.; Mann, D.W. Pyrrolo[2,3-d]pyrimidine Compounds. U.S. Patent US-9161939-B2, 20 October 2015. [Google Scholar]
- Yogo, T.; Nagamiya, H.; Seto, M.; Sasaki, S.; Shih-Chung, H.; Ohba, Y.; Tokunaga, N.; Lee, G.N.; Rhim, C.Y.; Yoon, C.H.; et al. Structure-Based Design and Synthesis of 3-Amino-1,5-dihydro-4H-pyrazolopyridin-4-one Derivatives as Tyrosine Kinase 2 Inhibitors. J. Med. Chem. 2016, 59, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Shin, H.; Park, K.S.; Kim, H.; Park, J.; Kim, K.; Nam, J.; Choo, H.; Chong, Y. Benzimidazole Derivatives as Potent JAK1-Selective Inhibitors. J. Med. Chem. 2015, 58, 7596–7602. [Google Scholar] [CrossRef]
- Chen, X.; Wilson, L.J.; Malaviya, R.; Argentieri, R.L.; Yang, S.M. Virtual screening to successfully identify novel janus kinase 3 inhibitors: A sequential focused screening approach. J. Med. Chem. 2008, 51, 7015–7019. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, O.; Marsden, B.; Pogacic, V.; Rellos, P.; Müller, S.; Bullock, A.N.; Schwaller, J.; Sundström, M.; Knapp, S. A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc. Natl. Acad. Sci. USA 2007, 104, 20523–20528. [Google Scholar] [CrossRef]
- DiMauro, E.F.; Newcomb, J.; Nunes, J.J.; Bemis, J.E.; Boucher, C.; Buchanan, J.L.; Buckner, W.H.; Cee, V.J.; Chai, L.; Deak, H.L.; et al. Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: Synthesis, SAR, and in vivo anti-inflammatory activity. J. Med. Chem. 2006, 49, 5671–5686. [Google Scholar] [CrossRef]
- Wang, T.; Lamb, M.L.; Block, M.H.; Davies, A.M.; Han, Y.; Hoffmann, E.; Ioannidis, S.; Josey, J.A.; Liu, Z.Y.; Lyne, P.D.; et al. Discovery of Disubstituted Imidazo[4,5-b]pyridines and Purines as Potent TrkA Inhibitors. ACS Med. Chem. Lett. 2012, 3, 705–709. [Google Scholar] [CrossRef]
- Liu, M.; Ju, X.; Zou, J.; Shi, J.; Jia, G. Recent researches for dual Aurora target inhibitors in antitumor field. Eur. J. Med. Chem. 2020, 203, 112498. [Google Scholar] [CrossRef]
- Lawrence, H.R.; Martin, M.P.; Luo, Y.; Pireddu, R.; Yang, H.; Gevariya, H.; Ozcan, S.; Zhu, J.Y.; Kendig, R.; Rodriguez, M.; et al. Development of o-chlorophenyl substituted pyrimidines as exceptionally potent aurora kinase inhibitors. J. Med. Chem. 2012, 55, 7392–7416. [Google Scholar] [CrossRef]
- Hanan, E.J.; van Abbema, A.; Barrett, K.; Blair, W.S.; Blaney, J.; Chang, C.; Eigenbrot, C.; Flynn, S.; Gibbons, P.; Hurley, C.A.; et al. Discovery of potent and selective pyrazolopyrimidine janus kinase 2 inhibitors. J. Med. Chem. 2012, 55, 10090–10107. [Google Scholar] [CrossRef] [PubMed]
- William, A.D.; Lee, A.C.; Blanchard, S.; Poulsen, A.; Teo, E.L.; Nagaraj, H.; Tan, E.; Chen, D.; Williams, M.; Sun, E.T.; et al. Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J. Med. Chem. 2011, 54, 4638–4658. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, T.; Kearney, P.C.; Kim, B.G.; Johnson, H.W.; Aay, N.; Arcalas, A.; Brown, D.S.; Chan, V.; Chen, J.; Du, H.; et al. SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 7653–7658. [Google Scholar] [CrossRef]
- Chen, J.J.; Thakur, K.D.; Clark, M.P.; Laughlin, S.K.; George, K.M.; Bookland, R.G.; Davis, J.R.; Cabrera, E.J.; Easwaran, V.; De, B.; et al. Development of pyrimidine-based inhibitors of Janus tyrosine kinase 3. Bioorg. Med. Chem. Lett. 2006, 16, 5633–5638. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Y.; Zang, J.; Gao, Q.; Wang, B.; Xu, W.; Zhang, Y. Design, synthesis and preliminary biological evaluation of 4-aminopyrazole derivatives as novel and potent JAKs inhibitors. Bioorg. Med. Chem. 2016, 24, 2660–2672. [Google Scholar] [CrossRef]
- Yu, R.N.; Chen, C.J.; Shu, L.; Yin, Y.; Wang, Z.J.; Zhang, T.T.; Zhang, D.Y. Structure-based design and synthesis of pyrimidine-4,6-diamine derivatives as Janus kinase 3 inhibitors. Bioorg. Med. Chem. 2019, 27, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.S.A.; Nielsen, J.J.; Park, S.; Park, J.; Liu, Q.; Kim, C.H.; Pommier, Y.; Agama, K.; Low, P.S.; Cushman, M. Application of Sequential Palladium Catalysis for the Discovery of Janus Kinase Inhibitors in the Benzo[ c]pyrrolo[2,3- h][1,6]naphthyridin-5-one (BPN) Series. J. Med. Chem. 2018, 61, 10440–10462. [Google Scholar] [CrossRef]
- Uckun, F.M.; Dibirdik, I.; Qazi, S.; Vassilev, A.; Ma, H.; Mao, C.; Benyumov, A.; Emami, K.H. Anti-breast cancer activity of LFM-A13, a potent inhibitor of Polo-like kinase (PLK). Bioorg. Med. Chem. 2007, 15, 800–814. [Google Scholar] [CrossRef]
- Van Epps, S.; Fiamengo, B.; Edmunds, J.; Ericsson, A.; Frank, K.; Friedman, M.; George, D.; George, J.; Goedken, E.; Kotecki, B.; et al. Design and synthesis of tricyclic cores for kinase inhibition. Bioorg. Med. Chem. Lett. 2013, 23, 693–698. [Google Scholar] [CrossRef]
- Soth, M.; Hermann, J.C.; Yee, C.; Alam, M.; Barnett, J.W.; Berry, P.; Browner, M.F.; Frank, K.; Frauchiger, S.; Harris, S.; et al. 3-Amido pyrrolopyrazine JAK kinase inhibitors: Development of a JAK3 vs JAK1 selective inhibitor and evaluation in cellular and in vivo models. J. Med. Chem. 2013, 56, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Malaviya, R.; Wilson, L.J.; Argentieri, R.; Chen, X.; Yang, C.; Wang, B.; Cavender, D.; Murray, W.V. Simplified staurosporine analogs as potent JAK3 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 326–331. [Google Scholar] [CrossRef]
- Lynch, S.M.; DeVicente, J.; Hermann, J.C.; Jaime-Figueroa, S.; Jin, S.; Kuglstatter, A.; Li, H.; Lovey, A.; Menke, J.; Niu, L.; et al. Strategic use of conformational bias and structure based design to identify potent JAK3 inhibitors with improved selectivity against the JAK family and the kinome. Bioorg. Med. Chem. Lett. 2013, 23, 2793–2800. [Google Scholar] [CrossRef]
- Guan, H.; Lamb, M.L.; Peng, B.; Huang, S.; Degrace, N.; Read, J.; Hussain, S.; Wu, J.; Rivard, C.; Alimzhanov, M.; et al. Discovery of novel Jak2-Stat pathway inhibitors with extended residence time on target. Bioorg. Med. Chem. Lett. 2013, 23, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- Marsilje, T.H.; Pei, W.; Chen, B.; Lu, W.; Uno, T.; Jin, Y.; Jiang, T.; Kim, S.; Li, N.; Warmuth, M.; et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J. Med. Chem. 2013, 56, 5675–5690. [Google Scholar] [CrossRef]
- Martin, M.W.; Newcomb, J.; Nunes, J.J.; Bemis, J.E.; McGowan, D.C.; White, R.D.; Buchanan, J.L.; DiMauro, E.F.; Boucher, C.; Faust, T.; et al. Discovery of novel 2,3-diarylfuro[2,3-b]pyridin-4-amines as potent and selective inhibitors of Lck: Synthesis, SAR, and pharmacokinetic properties. Bioorg. Med. Chem. Lett. 2007, 17, 2299–2304. [Google Scholar] [CrossRef]
- Siu, M.; Pastor, R.; Liu, W.; Barrett, K.; Berry, M.; Blair, W.S.; Chang, C.; Chen, J.Z.; Eigenbrot, C.; Ghilardi, N.; et al. 2-Amino-[1,2,4]triazolo[1,5-a]pyridines as JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 5014–5021. [Google Scholar] [CrossRef]
- Brasca, M.G.; Mantegani, S.; Amboldi, N.; Bindi, S.; Caronni, D.; Casale, E.; Ceccarelli, W.; Colombo, N.; De Ponti, A.; Donati, D.; et al. Discovery of NMS-E973 as novel, selective and potent inhibitor of heat shock protein 90 (Hsp90). Bioorg. Med. Chem. 2013, 21, 7047–7063. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, E.F.; Newcomb, J.; Nunes, J.J.; Bemis, J.E.; Boucher, C.; Buchanan, J.L.; Buckner, W.H.; Cheng, A.; Faust, T.; Hsieh, F.; et al. Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: Development of an expedient and divergent synthetic route and preliminary SAR. Bioorg. Med. Chem. Lett. 2007, 17, 2305–2309. [Google Scholar] [CrossRef]
- Côté, B.; Boulet, L.; Brideau, C.; Claveau, D.; Ethier, D.; Frenette, R.; Gagnon, M.; Giroux, A.; Guay, J.; Guiral, S.; et al. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6816–6820. [Google Scholar] [CrossRef]
- Seerden, J.P.; Leusink-Ionescu, G.; Woudenberg-Vrenken, T.; Dros, B.; Molema, G.; Kamps, J.A.; Kellogg, R.M. Synthesis and structure-activity relationships of 4-fluorophenyl-imidazole p38α MAPK, CK1δ and JAK2 kinase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3412–3418. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, R.Z.; Sun, S.L.; Zhao, C.; Fan, T.Y.; Chen, M.; Li, N.G.; Shi, Z.H. Small-Molecule Fms-like Tyrosine Kinase 3 Inhibitors: An Attractive and Efficient Method for the Treatment of Acute Myeloid Leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef]
- Zhao, H.; Caflisch, A. Discovery of ZAP70 inhibitors by high-throughput docking into a conformation of its kinase domain generated by molecular dynamics. Bioorg. Med. Chem. Lett. 2013, 23, 5721–5726. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Ioannidis, S.; Chuaqui, C.; Almeida, L.; Alimzhanov, M.; Bebernitz, G.; Bell, K.; Block, M.; Howard, T.; Huang, S.; et al. Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2 inhibitors. J. Med. Chem. 2014, 57, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Siu, T.; Kumarasinghe, S.E.; Altman, M.D.; Katcher, M.; Northrup, A.; White, C.; Rosenstein, C.; Mathur, A.; Xu, L.; Chan, G.; et al. The discovery of reverse tricyclic pyridone JAK2 inhibitors. Part 2: Lead optimization. Bioorg. Med. Chem. Lett. 2014, 24, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Johnson, T.W.; Bailey, S.; Brooun, A.; Bunker, K.D.; Burke, B.J.; Collins, M.R.; Cook, A.S.; Cui, J.J.; Dack, K.N.; et al. Design of potent and selective inhibitors to overcome clinical anaplastic lymphoma kinase mutations resistant to crizotinib. J. Med. Chem. 2014, 57, 1170–1187. [Google Scholar] [CrossRef]
- Shen, P.; Wang, Y.; Jia, X.; Xu, P.; Qin, L.; Feng, X.; Li, Z.; Qiu, Z. Dual-target Janus kinase (JAK) inhibitors: Comprehensive review on the JAK-based strategies for treating solid or hematological malignancies and immune-related diseases. Eur. J. Med. Chem. 2022, 239, 114551. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.H.; Zhang, H.; Wang, Y.C.; Yang, D.; Zhao, Y.L.; Yan, G.Y.; Xu, G.B.; Guan, H.Y.; Zhou, Y.H.; et al. Design, synthesis, and biological evaluation of 2,4-diamino pyrimidine derivatives as potent FAK inhibitors with anti-cancer and anti-angiogenesis activities. Eur. J. Med. Chem. 2021, 222, 113573. [Google Scholar] [CrossRef]
- Haidle, A.M.; Zabierek, A.A.; Childers, K.K.; Rosenstein, C.; Mathur, A.; Altman, M.D.; Chan, G.; Xu, L.; Bachman, E.; Mo, J.R.; et al. Thiophene carboxamide inhibitors of JAK2 as potential treatments for myleoproliferative neoplasms. Bioorg. Med. Chem. Lett. 2014, 24, 1968–1973. [Google Scholar] [CrossRef]
- Costales, A.; Mathur, M.; Ramurthy, S.; Lan, J.; Subramanian, S.; Jain, R.; Atallah, G.; Setti, L.; Lindvall, M.; Appleton, B.A.; et al. 2-Amino-7-substituted benzoxazole analogs as potent RSK2 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1592–1596. [Google Scholar] [CrossRef]
- Park, E.; Lee, S.J.; Moon, H.; Park, J.; Jeon, H.; Hwang, J.S.; Hwang, H.; Hong, K.B.; Han, S.H.; Choi, S.; et al. Discovery and Biological Evaluation of. J. Med. Chem. 2021, 64, 958–979. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Shi, W.; Zhao, D.; Wang, Q.; Chang, X.; He, X.; Wang, X.; Gao, Y.; Lu, P.; Zhang, X.; et al. Design and synthesis of boron-containing diphenylpyrimidines as potent BTK and JAK3 dual inhibitors. Bioorg. Med. Chem. 2020, 28, 115236. [Google Scholar] [CrossRef]
- Brasca, M.G.; Nesi, M.; Avanzi, N.; Ballinari, D.; Bandiera, T.; Bertrand, J.; Bindi, S.; Canevari, G.; Carenzi, D.; Casero, D.; et al. Pyrrole-3-carboxamides as potent and selective JAK2 inhibitors. Bioorg. Med. Chem. 2014, 22, 4998–5012. [Google Scholar] [CrossRef] [PubMed]
- Hanan, E.J.; Eigenbrot, C.; Bryan, M.C.; Burdick, D.J.; Chan, B.K.; Chen, Y.; Dotson, J.; Heald, R.A.; Jackson, P.S.; La, H.; et al. Discovery of selective and noncovalent diaminopyrimidine-based inhibitors of epidermal growth factor receptor containing the T790M resistance mutation. J. Med. Chem. 2014, 57, 10176–10191. [Google Scholar] [CrossRef]
- Goodwin, N.C.; Cianchetta, G.; Burgoon, H.A.; Healy, J.; Mabon, R.; Strobel, E.D.; Allen, J.; Wang, S.; Hamman, B.D.; Rawlins, D.B. Discovery of a Type III Inhibitor of LIM Kinase 2 That Binds in a DFG-Out Conformation. ACS Med. Chem. Lett. 2015, 6, 53–57. [Google Scholar] [CrossRef]
- Duan, J.J.; Lu, Z.; Jiang, B.; Yang, B.V.; Doweyko, L.M.; Nirschl, D.S.; Haque, L.E.; Lin, S.; Brown, G.; Hynes, J.; et al. Discovery of pyrrolo[1,2-b]pyridazine-3-carboxamides as Janus kinase (JAK) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 5721–5726. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.L.; Kormos, B.L.; Hayward, M.M.; Coffman, K.J.; Jasti, J.; Kurumbail, R.G.; Wager, T.T.; Verhoest, P.R.; Noell, G.S.; Chen, Y.; et al. Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J. Med. Chem. 2015, 58, 419–432. [Google Scholar] [CrossRef]
- Brasca, M.G.; Gnocchi, P.; Nesi, M.; Amboldi, N.; Avanzi, N.; Bertrand, J.; Bindi, S.; Canevari, G.; Casero, D.; Ciomei, M.; et al. Novel pyrrole carboxamide inhibitors of JAK2 as potential treatment of myeloproliferative disorders. Bioorg. Med. Chem. 2015, 23, 2387–2407. [Google Scholar] [CrossRef]
- Wan, H.; Schroeder, G.M.; Hart, A.C.; Inghrim, J.; Grebinski, J.; Tokarski, J.S.; Lorenzi, M.V.; You, D.; Mcdevitt, T.; Penhallow, B.; et al. Discovery of a Highly Selective JAK2 Inhibitor, BMS-911543, for the Treatment of Myeloproliferative Neoplasms. ACS Med. Chem. Lett. 2015, 6, 850–855. [Google Scholar] [CrossRef]
- Zimmermann, K.; Sang, X.; Mastalerz, H.A.; Johnson, W.L.; Zhang, G.; Liu, Q.; Batt, D.; Lombardo, L.J.; Vyas, D.; Trainor, G.L.; et al. 9H-Carbazole-1-carboxamides as potent and selective JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2809–2812. [Google Scholar] [CrossRef]
- Yamagishi, H.; Shirakami, S.; Nakajima, Y.; Tanaka, A.; Takahashi, F.; Hamaguchi, H.; Hatanaka, K.; Moritomo, A.; Inami, M.; Higashi, Y.; et al. Discovery of 3,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-2(1H)-one derivatives as novel JAK inhibitors. Bioorg. Med. Chem. 2015, 23, 4846–4859. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Inoue, T.; Nakai, K.; Mukoyoshi, K.; Hamaguchi, H.; Hatanaka, K.; Sasaki, H.; Tanaka, A.; Takahashi, F.; Kunikawa, S.; et al. Synthesis and evaluation of novel 1H-pyrrolo[2,3-b]pyridine-5-carboxamide derivatives as potent and orally efficacious immunomodulators targeting JAK3. Bioorg. Med. Chem. 2015, 23, 4871–4883. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Kim, J.T.; Son, H.Y.; Park, S.Y.; Cho, Y.S.; Koo, T.S.; Lee, H.; Kang, N.S. Discovery of Tyk2 inhibitors via the virtual site-directed fragment-based drug design. Bioorg. Med. Chem. Lett. 2015, 25, 3947–3952. [Google Scholar] [CrossRef]
- Liu, Q.; Batt, D.G.; Lippy, J.S.; Surti, N.; Tebben, A.J.; Muckelbauer, J.K.; Chen, L.; An, Y.; Chang, C.; Pokross, M.; et al. Design and synthesis of carbazole carboxamides as promising inhibitors of Bruton’s tyrosine kinase (BTK) and Janus kinase 2 (JAK2). Bioorg. Med. Chem. Lett. 2015, 25, 4265–4269. [Google Scholar] [CrossRef]
- Hart, A.C.; Schroeder, G.M.; Wan, H.; Grebinski, J.; Inghrim, J.; Kempson, J.; Guo, J.; Pitts, W.J.; Tokarski, J.S.; Sack, J.S.; et al. Structure-Based Design of Selective Janus Kinase 2 Imidazo[4,5-d]pyrrolo[2,3-b]pyridine Inhibitors. ACS Med. Chem. Lett. 2015, 6, 845–849. [Google Scholar] [CrossRef]
- Gehringer, M.; Laufer, S.A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62, 5673–5724. [Google Scholar] [CrossRef]
- ChEMBL Database, EMBL-EBI (2024). ChEMBL Document: Affinity Phenotypic Cellular Literature for EUbOPEN Chemogenomic Library (CHEMBL5444388)-ChEMBL(20 January 2022). Available online: https://www.ebi.ac.uk/chembl/explore/document/CHEMBL5444388 (accessed on 25 February 2025).
- Choi, J.S.; Hwang, H.J.; Kim, S.W.; Lee, B.I.; Lee, J.; Song, H.J.; Koh, J.S.; Kim, J.H.; Lee, P.H. Highly potent and selective pyrazolylpyrimidines as Syk kinase inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4441–4446. [Google Scholar] [CrossRef]
- Curry, M.A.; Dorsey, B.D.; Dugan, B.J.; Gingrich, D.E.; Mesaros, E.F.; Milkiewicz, K.L. Preparation and Uses of 1,2,4-triazolo[1,5a]pyridine Derivatives. U.S. Patent US-8633173-B2, 21 January 2014. [Google Scholar]
- Jones, P.; Storer, R.I.; Sabnis, Y.A.; Wakenhut, F.M.; Whitlock, G.A.; England, K.S.; Mukaiyama, T.; Dehnhardt, C.M.; Coe, J.W.; Kortum, S.W.; et al. Design and Synthesis of a Pan-Janus Kinase Inhibitor Clinical Candidate (PF-06263276) Suitable for Inhaled and Topical Delivery for the Treatment of Inflammatory Diseases of the Lungs and Skin. J. Med. Chem. 2017, 60, 767–786. [Google Scholar] [CrossRef]
- Casimiro-Garcia, A.; Trujillo, J.I.; Vajdos, F.; Juba, B.; Banker, M.E.; Aulabaugh, A.; Balbo, P.; Bauman, J.; Chrencik, J.; Coe, J.W.; et al. Identification of Cyanamide-Based Janus Kinase 3 (JAK3) Covalent Inhibitors. J. Med. Chem. 2018, 61, 10665–10699. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.M.; Jia, Z.J.; Mehrotra, M.; Song, Y.; Xu, Q.; Huang, W.; Venkataramani, C.; Rose, J.W.; Pandey, A. Inhibitors of Syk and/or JAK Kinase. U.S. Patent US-8501944-B2, 6 August 2013. [Google Scholar]
- Purandare, A.V.; Batt, D.G.; Liu, Q.; Johnson, W.L.; Mastalerz, H.; Zhang, G.; Zimmermann, K. Carbazole and Carboline Kinase Inhibitors. U.S. Patent US-8815840-B2, 26 August 2014. [Google Scholar]
- Woo, H.C.; Cash, B.; Ahearn, S.P.; Dinsmore, C.; Jung, J.; Pu, Q.; Rivkin, A.; Scott, M.E.; Witter, D.J.; Woo, H.C.; et al. Pyrrolopyrimidines as Janus Kinase Inhibitors. U.S. Patent US-8993756-B2, 31 March 2015. [Google Scholar]
- Forster, M.; Gehringer, M.; Laufer, S.A. Recent advances in JAK3 inhibition: Isoform selectivity by covalent cysteine targeting. Bioorg. Med. Chem. Lett. 2017, 27, 4229–4237. [Google Scholar] [CrossRef]
- Promo, M.A.; Xie, J.; Acker, B.A.; Hartmann, S.J.; Wolfson, S.G.; Huang, H.-C.; Jacobsen, E.J. Pyrrolo[2,3-d]pyrimidine Compounds as Janus Kinase Inhibitors. U.S. Patent US-8633206-B2, 21 January 2014. [Google Scholar]
- Brown, M.F.; Fenwick, A.E.; Flanagan, M.E.; Gonzales, A.; Johnson, T.A.; Kaila, N.; Mitton-Fry, M.J.; Strohbach, J.W.; TenBrink, R.E.; Trzupek, J.D.; et al. Pyrrolo[2,3-D]pyrimidine Derivatives. U.S. Patent US-9035074-B2, 19 May 2015. [Google Scholar]
- McAllister, A.; Murone, M.; Sengupta, S.; Shetty, S.J. Bicyclic Compounds and Their Uses as Dual c-SRC/JAK Inhibitors. U.S. Patent US-8440679-B2, 21 May 2013. [Google Scholar]
- Davies, A.; Ioannidis, S.; Lamb, M.; Su, M.; Wang, T.; Zhang, H. 9-(Pyrazol-3-yl)-9H-purine-2-amine and 3-(pyrazol-3-yl)-3H-imidazo[4,5-B] Pyridin-5-Amine Derivatives and Their Use for the Treatment of Cancer. U.S. Patent US8486966B2, 16 July 2013. [Google Scholar]
- De Vicente Fidalgo, J.; Hermann, J.C.; Lemoine, R.; Li, H.; Lovey, A.J. Inhibitors of JAK for the Treatment of Autoimmune and Inflammatory Diseases. U.S. Patent US-8618103-B2, 31 December 2013. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-8618103-B2 (accessed on 25 February 2025).
- Hynes, J.; Wu, H.; Kempson, J.; Duan, J.J.; Lu, Z.; Jiang, B.; Stachura, S.; Tokarski, J.S.; Sack, J.S.; Khan, J.A.; et al. Discovery of potent and efficacious pyrrolopyridazines as dual JAK1/3 inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, S.T.; Brown, G.D.; Doweyko, L.M.; Duan, J.; Guo, J. Pyrrolopyridazine JAK3 Inhibitors and Their Use for the Treatment of Inflammatory and Autoimmune Diseases. U.S. Patent US-8921368-B2, 30 December 2014. [Google Scholar]
- Allen, S.; Andrews, S.W.; Condroski, K.R.; Haas, J.; Huang, L. Substituted Pyrazolo[1,5-a]pyrimidine Compounds as Trk Kinase Inhibitors. U.S. Patent US-8791123-B2, 29 July 2014. [Google Scholar]
- Noji, S.; Shiozaki, M.; Miura, T.; Hara, Y.; Yamanaka, H.; Maeda, K.; Hori, A.; Inoue, M.; Hase, Y. Nitrogen-Containing Spirocyclic Compounds and Pharmaceutical Uses Thereof. U.S. Patent US-8609647-B2, 17 December 2013. [Google Scholar]
- Combs, A.P.; Sparks, R.B.; Yue, E.W.T.; Feng, H.; Bower, M.J. Macrocyclic Compounds and Their Use as Kinase Inhibitors. U.S. Patent US8765727B2, 1 July 2014. [Google Scholar]
- Brubaker, J.; Close, J.T.; Jung, J.; Martinez, M.; White, C. Acyclic Cyanoethylpyrazoles as Janus Kinase Inhibitors. U.S. Patent US9493441B2, 15 November 2016. [Google Scholar]
- Hansen, B.B.; Jepsen, T.H.; Larsen, M.; Sindet, R.; Vifian, T.; Burhardt, M.N.; Larsen, J.; Seitzberg, J.G.; Carnerup, M.A.; Jerre, A.; et al. Fragment-Based Discovery of Pyrazolopyridones as JAK1 Inhibitors with Excellent Subtype Selectivity. J. Med. Chem. 2020, 63, 7008–7032. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, M.M.; Alimzhanov, M.; Augustin, M.; Bebernitz, G.; Bell, K.; Chuaqui, C.; Deegan, T.; Ferguson, A.D.; Goodwin, K.; Huszar, D.; et al. Identification of azabenzimidazoles as potent JAK1 selective inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 60–67. [Google Scholar] [CrossRef]
- Stauffer, F.; Cowan-Jacob, S.W.; Scheufler, C.; Furet, P. Identification of a 5-[3-phenyl-(2-cyclic-ether)-methylether]-4-aminopyrrolo[2,3-d]pyrimidine series of IGF-1R inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2065–2067. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Hao, M.; Qiao, J.; Ju, C.; Xue, L.; Zhang, C. Design, synthesis and evaluation of pyrrolo[2,3-d]pyrimidine-phenylamide hybrids as potent Janus kinase 2 inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2936–2941. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.K.; Choo, H.; Chong, Y. Novel JAK1-selective benzimidazole inhibitors with enhanced membrane permeability. Bioorg. Med. Chem. Lett. 2016, 26, 3213–3215. [Google Scholar] [CrossRef]
- Cee, V.J.; Albrecht, B.K.; Geuns-Meyer, S.; Hughes, P.; Bellon, S.; Bready, J.; Caenepeel, S.; Chaffee, S.C.; Coxon, A.; Emery, M.; et al. Alkynylpyrimidine amide derivatives as potent, selective, and orally active inhibitors of Tie-2 kinase. J. Med. Chem. 2007, 50, 627–640. [Google Scholar] [CrossRef]
- Martin, M.W.; Newcomb, J.; Nunes, J.J.; McGowan, D.C.; Armistead, D.M.; Boucher, C.; Buchanan, J.L.; Buckner, W.; Chai, L.; Elbaum, D.; et al. Novel 2-aminopyrimidine carbamates as potent and orally active inhibitors of Lck: Synthesis, SAR, and in vivo antiinflammatory activity. J. Med. Chem. 2006, 49, 4981–4991. [Google Scholar] [CrossRef]
- Phuangsawai, O.; Beswick, P.; Ratanabunyong, S.; Tabtimmai, L.; Suphakun, P.; Obounchoey, P.; Srisook, P.; Horata, N.; Chuckowree, I.; Hannongbua, S.; et al. Evaluation of the anti-malarial activity and cytotoxicity of 2,4-diamino-pyrimidine-based kinase inhibitors. Eur. J. Med. Chem. 2016, 124, 896–905. [Google Scholar] [CrossRef]
- Hou, W.; Ren, Y.; Zhang, Z.; Sun, H.; Ma, Y.; Yan, B. Novel quinazoline derivatives bearing various 6-benzamide moieties as highly selective and potent EGFR inhibitors. Bioorg. Med. Chem. 2018, 26, 1740–1750. [Google Scholar] [CrossRef]
- Narayan, S.; Ramisetti, S.; Jaiswal, A.S.; Law, B.K.; Singh-Pillay, A.; Singh, P.; Amin, S.; Sharma, A.K. ASR352, A potent anticancer agent: Synthesis, preliminary SAR, and biological activities against colorectal cancer bulk, 5-fluorouracil/oxaliplatin resistant and stem cells. Eur. J. Med. Chem. 2019, 161, 456–467. [Google Scholar] [CrossRef]
- Vankayalapati, H.; Yerramreddy, V.K.; Gangireddy, P.; Appalaneni, R.P. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9206188-B2, Substituted Pyrrolo[2,3-b]pyridines as ITK and JAK Inhibitors. United States, 8 December 2015. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9206188-B2 (accessed on 25 February 2025).
- Wang, Y.; Huang, W.; Xin, M.; Chen, P.; Gui, L.; Zhao, X.; Tang, F.; Wang, J.; Liu, F. Identification of 4-(2-furanyl)pyrimidin-2-amines as Janus kinase 2 inhibitors. Bioorg. Med. Chem. 2017, 25, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Batt, D.G.; Bertrand, M.B.; Delucca, G.; Galella, M.A.; Ko, S.S. Substituted Tetrahydrocarbazole and Carbazole Carboxamide Compounds. United States Patent US-9334290-B2, 10 May 2016. Assignee: Bristol Myers Squibb Co. Available online: https://patents.google.com/patent/US9334290B2 (accessed on 25 February 2025).
- Hayashi, K.; Watanabe, T.; Toyama, K.; Kamon, J.; Minami, M. Substituted Pyrrolo[2,3-h][1,6]naphthyridines and Compositions Thereof as JAK Inhibitors. United States Patent US-9216999-B2, 22 December 2015. Available online: https://patents.google.com/patent/US9216999B2 (accessed on 25 February 2025).
- Yang, B.V.; Brown, G.D.; Gupta, A.K.; Pitts, W.J. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9428511-B2, Imidazopyridazine JAK3 Inhibitors and Their Use for the Treatment of Inflammatory and Autoimmune Diseases. United States. 30 August 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9428511-B2 (accessed on 25 February 2025).
- Czodrowski, P.; Mallinger, A.; Wienke, D.; Esdar, C.; Pöschke, O.; Busch, M.; Rohdich, F.; Eccles, S.A.; Ortiz-Ruiz, M.J.; Schneider, R.; et al. Structure-Based Optimization of Potent, Selective, and Orally Bioavailable CDK8 Inhibitors Discovered by High-Throughput Screening. J. Med. Chem. 2016, 59, 9337–9349. [Google Scholar] [CrossRef]
- Clark, M.P.; George, K.M.; Bookland, R.G.; Chen, J.; Laughlin, S.K.; Thakur, K.D.; Lee, W.; Davis, J.R.; Cabrera, E.J.; Brugel, T.A.; et al. Development of new pyrrolopyrimidine-based inhibitors of Janus kinase 3 (JAK3). Bioorg. Med. Chem. Lett. 2007, 17, 1250–1253. [Google Scholar] [CrossRef]
- Mitton-Fry, M.J.; Berlinski, P.J.; Birchmeier, M.J.; Bowman, J.W.; Gonzales, A.J. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9161939-B2, Pyrrolo[2,3-d]pyrimidine compounds. United States. 20 October 2015. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9161939-B2 (accessed on 25 February 2025).
- Smaill, J.B.; Gonzales, A.J.; Spicer, J.A.; Lee, H.; Reed, J.E.; Sexton, K.; Althaus, I.W.; Zhu, T.; Black, S.L.; Blaser, A.; et al. Tyrosine Kinase Inhibitors. 20. Optimization of Substituted Quinazoline and Pyrido[3,4-d]pyrimidine Derivatives as Orally Active, Irreversible Inhibitors of the Epidermal Growth Factor Receptor Family. J. Med. Chem. 2016, 59, 8103–8124. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xue, C.-B.; Li, H.-Y.; Li, Q. Piperidin-4-yl Azetidine Derivatives as JAK1 Inhibitors. U.S. Patent US-8765734-B2, 1 July 2014. Available online: https://patents.google.com/patent/US8765734B2 (accessed on 25 February 2025).
- Takahashi, K.; Watanabe, T.; Hayashi, K.; Kurihara, K.; Nakamura, T.; Yamamoto, A.; Nishimura, T.; Kamiyama, T.; Hidaka, Y. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9475813-B2, Tricyclic Pyrrolopyridine Compound, and JAK Inhibitor. United States. 25 October 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9475813-B2 (accessed on 25 February 2025).
- Xi, N.; Li, M.; Hu, H.; Dai, W. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9403801-B2, Substituted Heteroaryl Compounds and Methods of Use. United States. 2 August 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9403801-B2 (accessed on 25 February 2025).
- Goldstein, D.M.; Brameld, K.A.; Verner, E. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9187487-B2, Azaindole Derivatives as Tyrosine Kinase Inhibitors. United States. 17 November 2015. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9187487-B2 (accessed on 25 February 2025).
- Ge, Y.; Jin, Y.; Wang, C.; Zhang, J.; Tang, Z.; Peng, J.; Liu, K.; Li, Y.; Zhou, Y.; Ma, X. Discovery of Novel Bruton’s Tyrosine Kinase (BTK) Inhibitors Bearing a N,9-Diphenyl-9H-purin-2-amine Scaffold. ACS Med. Chem. Lett. 2016, 7, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Rodgers, J.D. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9216984-B2, 3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]octanenitrile and Heptanenitrile as JAK Inhibitors. United States. 22 December 2015. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9216984-B2 (accessed on 25 February 2025).
- Su, W.-G.; Deng, W.; Li, J.; Ji, J. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9346810-B2, Pyrrolopyrimidine Compounds and Uses Thereof. United States. 24 May 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9346810-B2 (accessed on 25 February 2025).
- Brubaker, J.; Childers, M.L.; Christopher, M.; Close, J.T.; Katz, J.D. National Center for Biotechnology Information (2024). PubChem Patent Summary for US-9328099-B2, Cyanomethylpyrazole Carboxamides as Janus kinase Inhibitors. United States. 3 May 2016. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-9328099-B2 (accessed on 25 February 2025).
- Reddy, E.P.; Reddy, M.V.R. Substituted Pyrido[2,3-d]pyrimidin-7(8H)-ones and Therapeutic Uses Thereof. US8889696B2, 18 November 2014. [Google Scholar]
- Lam, B.; Arikawa, Y.; Cramlett, J.; Dong, Q.; de Jong, R.; Feher, V.; Grimshaw, C.E.; Farrell, P.J.; Hoffman, I.D.; Jennings, A.; et al. Discovery of TAK-659 an orally available investigational inhibitor of Spleen Tyrosine Kinase (SYK). Bioorg. Med. Chem. Lett. 2016, 26, 5947–5950. [Google Scholar] [CrossRef]
- Blomgren, P.; Chandrasekhar, J.; Di Paolo, J.A.; Fung, W.; Geng, G.; Ip, C.; Jones, R.; Kropf, J.E.; Lansdon, E.B.; Lee, S.; et al. Discovery of Lanraplenib (GS-9876): A Once-Daily Spleen Tyrosine Kinase Inhibitor for Autoimmune Diseases. ACS Med. Chem. Lett. 2020, 11, 506–513. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F. Janus-Associated Kinase 1 (JAK1) Inhibitors as Potential Treatment for Immune Disorders. ACS Med. Chem. Lett. 2017, 8, 598–600. [Google Scholar] [CrossRef]
- Kempson, J.; Ovalle, D.; Guo, J.; Wrobleski, S.T.; Lin, S.; Spergel, S.H.; Duan, J.J.; Jiang, B.; Lu, Z.; Das, J.; et al. Discovery of highly potent, selective, covalent inhibitors of JAK3. Bioorg. Med. Chem. Lett. 2017, 27, 4622–4625. [Google Scholar] [CrossRef]
- Hanan, E.J.; Liang, J.; Wang, X.; Blake, R.A.; Blaquiere, N.; Staben, S.T. Monomeric Targeted Protein Degraders. J. Med. Chem. 2020, 63, 11330–11361. [Google Scholar] [CrossRef] [PubMed]
- Brameld, K.A.; Owens, T.D.; Verner, E.; Venetsanakos, E.; Bradshaw, J.M.; Phan, V.T.; Tam, D.; Leung, K.; Shu, J.; LaStant, J.; et al. Discovery of the Irreversible Covalent FGFR Inhibitor 8-(3-(4-Acryloylpiperazin-1-yl)propyl)-6-(2,6-dichloro-3,5-dimethoxyphenyl)-2-(methylamino)pyrido[2,3-d]pyrimidin-7(8H)-one (PRN1371) for the Treatment of Solid Tumors. J. Med. Chem. 2017, 60, 6516–6527. [Google Scholar] [CrossRef]
- Data for DCP Probe TP-030-2. Available online: https://www.ebi.ac.uk/chembl/explore/document/CHEMBL4507326 (accessed on 2 February 2023).
- Yao, L.; Mustafa, N.; Tan, E.C.; Poulsen, A.; Singh, P.; Duong-Thi, M.D.; Lee, J.X.T.; Ramanujulu, P.M.; Chng, W.J.; Yen, J.J.Y.; et al. Design and Synthesis of Ligand Efficient Dual Inhibitors of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Based on Ruxolitinib and Vorinostat. J. Med. Chem. 2017, 60, 8336–8357. [Google Scholar] [CrossRef]
- Fischer, T.; Krüger, T.; Najjar, A.; Totzke, F.; Schächtele, C.; Sippl, W.; Ritter, C.; Hilgeroth, A. Discovery of novel substituted benzo-anellated 4-benzylamino pyrrolopyrimidines as dual EGFR and VEGFR2 inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2708–2712. [Google Scholar] [CrossRef]
- Vazquez, M.L.; Kaila, N.; Strohbach, J.W.; Trzupek, J.D.; Brown, M.F.; Flanagan, M.E.; Mitton-Fry, M.J.; Johnson, T.A.; TenBrink, R.E.; Arnold, E.P.; et al. Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases. J. Med. Chem. 2018, 61, 1130–1152. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, J.; Close, J.; Siu, T.; Smith, G.F.; Torres, L.E. Pyrazole Carboxamides as Janus Kinase Inhibitors. U.S. Patent US9394282B2, 19 July 2016. [Google Scholar]
- Juillerat-Jeanneret, L.; Aubert, J.D.; Mikulic, J.; Golshayan, D. Fibrogenic Disorders in Human Diseases: From Inflammation to Organ Dysfunction. J. Med. Chem. 2018, 61, 9811–9840. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, G.; Li, H.; Liu, N.; Zhang, W.; Sheng, C. Discovery of Janus Kinase 2 (JAK2) and Histone Deacetylase (HDAC) Dual Inhibitors as a Novel Strategy for the Combinational Treatment of Leukemia and Invasive Fungal Infections. J. Med. Chem. 2018, 61, 6056–6074. [Google Scholar] [CrossRef]
- Grimster, N.P.; Anderson, E.; Alimzhanov, M.; Bebernitz, G.; Bell, K.; Chuaqui, C.; Deegan, T.; Ferguson, A.D.; Gero, T.; Harsch, A.; et al. Discovery and Optimization of a Novel Series of Highly Selective JAK1 Kinase Inhibitors. J. Med. Chem. 2018, 61, 5235–5244. [Google Scholar] [CrossRef]
- Chough, C.; Joung, M.; Lee, S.; Lee, J.; Kim, J.H.; Kim, B.M. Development of selective inhibitors for the treatment of rheumatoid arthritis: (R)-3-(3-(Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)-3-oxopropanenitrile as a JAK1-selective inhibitor. Bioorg. Med. Chem. 2018, 26, 1495–1510. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Y.; Kerekes, A.D.; Tagat, J.R.; Doll, R.J.; Xiao, Y.; Esposite, S.; Hruza, A.; Belanger, D.B.; Voss, M.; et al. Discovery of a highly potent orally bioavailable imidazo-[1, 2-a]pyrazine Aurora inhibitor. Bioorg. Med. Chem. Lett. 2018, 28, 1397–1403. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Al-Ameen, S.K.; Al-Koumi, D.M.; Hamad, M.G.; Jalal, N.A.; Oh, C.H. Recent Advances of Colony-Stimulating Factor-1 Receptor (CSF-1R) Kinase and Its Inhibitors. J. Med. Chem. 2018, 61, 5450–5466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, W.; Xin, M.; Chen, P.; Gui, L.; Zhao, X.; Zhu, X.; Luo, H.; Cong, X.; Wang, J.; et al. Discovery of potent anti-inflammatory 4-(4,5,6,7-tetrahydrofuro[3,2-c]pyridin-2-yl) pyrimidin-2-amines for use as Janus kinase inhibitors. Bioorg. Med. Chem. 2019, 27, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, J.; Ding, X.; Shen, H.C.; Zou, G. Small molecule approaches to treat autoimmune and inflammatory diseases (Part I): Kinase inhibitors. Bioorg. Med. Chem. Lett. 2021, 38, 127862. [Google Scholar] [CrossRef]
- Yao, L.; Ramanujulu, P.M.; Poulsen, A.; Ohlson, S.; Dymock, B.W. Merging of ruxolitinib and vorinostat leads to highly potent inhibitors of JAK2 and histone deacetylase 6 (HDAC6). Bioorg. Med. Chem. Lett. 2018, 28, 2636–2640. [Google Scholar] [CrossRef]
- Liu, Q.; Batt, D.G.; Chaudhry, C.; Lippy, J.S.; Pattoli, M.A.; Surti, N.; Xu, S.; Carter, P.H.; Burke, J.R.; Tino, J.A. Conversion of carbazole carboxamide based reversible inhibitors of Bruton’s tyrosine kinase (BTK) into potent, selective irreversible inhibitors in the carbazole, tetrahydrocarbazole, and a new 2,3-dimethylindole series. Bioorg. Med. Chem. Lett. 2018, 28, 3080–3084. [Google Scholar] [CrossRef] [PubMed]
- Pippione, A.C.; Sainas, S.; Federico, A.; Lupino, E.; Piccinini, M.; Kubbutat, M.; Contreras, J.M.; Morice, C.; Barge, A.; Ducime, A.; et al. N-Acetyl-3-aminopyrazoles block the non-canonical NF-kB cascade by selectively inhibiting NIK. Medchemcomm 2018, 9, 963–968. [Google Scholar] [CrossRef]
- Yin, L.; Li, H.; Liu, W.; Yao, Z.; Cheng, Z.; Zhang, H.; Zou, H. A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in gliobastoma therapy. Eur. J. Med. Chem. 2018, 144, 1–28. [Google Scholar] [CrossRef]
- Chough, C.; Lee, S.; Joung, M.; Lee, J.; Kim, J.H.; Kim, B.M. Design, synthesis and evaluation of (R)-3-(7-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-5-azaspiro[2.4]heptan-5-yl)-3-oxopropanenitrile as a JAK1-selective inhibitor. Medchemcomm 2018, 9, 477–489. [Google Scholar] [CrossRef]
- Chu-Farseeva, Y.Y.; Mustafa, N.; Poulsen, A.; Tan, E.C.; Yen, J.J.Y.; Chng, W.J.; Dymock, B.W. Design and synthesis of potent dual inhibitors of JAK2 and HDAC based on fusing the pharmacophores of XL019 and vorinostat. Eur. J. Med. Chem. 2018, 158, 593–619. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, K.; Tang, H.; Qi, Z.; Wang, T.; Mao, J.; Zhang, X.; Jiang, S. Development and Therapeutic Implications of Tyrosine Kinase 2 Inhibitors. J. Med. Chem. 2023, 66, 4378–4416. [Google Scholar] [CrossRef]
- Henry, S.P.; Jorgensen, W.L. Progress on the Pharmacological Targeting of Janus Pseudokinases. J. Med. Chem. 2023, 66, 10959–10990. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, C.; Song, S.; Huang, J.; Liu, Z.; Li, Y.; Meng, Q.; Zhang, J.; Yao, J.; Liu, K.; et al. Identification of highly potent BTK and JAK3 dual inhibitors with improved activity for the treatment of B-cell lymphoma. Eur. J. Med. Chem. 2018, 143, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Moslin, R.; Gardner, D.; Santella, J.; Zhang, Y.; Duncia, J.V.; Liu, C.; Lin, J.; Tokarski, J.S.; Strnad, J.; Pedicord, D.; et al. Identification of imidazo[1,2-. Medchemcomm 2017, 8, 700–712. [Google Scholar] [CrossRef]
- Noji, S.; Hara, Y.; Miura, T.; Yamanaka, H.; Maeda, K.; Hori, A.; Yamamoto, H.; Obika, S.; Inoue, M.; Hase, Y.; et al. Discovery of a Janus Kinase Inhibitor Bearing a Highly Three-Dimensional Spiro Scaffold: JTE-052 (Delgocitinib) as a New Dermatological Agent to Treat Inflammatory Skin Disorders. J. Med. Chem. 2020, 63, 7163–7185. [Google Scholar] [CrossRef]
- Li, Y.; Ye, T.; Xu, L.; Dong, Y.; Luo, Y.; Wang, C.; Han, Y.; Chen, K.; Qin, M.; Liu, Y.; et al. Discovery of 4-piperazinyl-2-aminopyrimidine derivatives as dual inhibitors of JAK2 and FLT3. Eur. J. Med. Chem. 2019, 181, 111590. [Google Scholar] [CrossRef]
- EUbOPEN. Selectivity Literature for EUbOPEN Chemogenomic Library. Available online: https://www.ebi.ac.uk/chembl/explore/document/CHEMBL5465560 (accessed on 2 July 2023).
- Miao, Q.; Ma, K.; Chen, D.; Wu, X.; Jiang, S. Targeting tropomyosin receptor kinase for cancer therapy. Eur. J. Med. Chem. 2019, 175, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Moslin, R.; Zhang, Y.; Wrobleski, S.T.; Lin, S.; Mertzman, M.; Spergel, S.; Tokarski, J.S.; Strnad, J.; Gillooly, K.; McIntyre, K.W.; et al. Identification of N-Methyl Nicotinamide and N-Methyl Pyridazine-3-Carboxamide Pseudokinase Domain Ligands as Highly Selective Allosteric Inhibitors of Tyrosine Kinase 2 (TYK2). J. Med. Chem. 2019, 62, 8953–8972. [Google Scholar] [CrossRef]
- Bryan, M.C.; Rajapaksa, N.S. Kinase Inhibitors for the Treatment of Immunological Disorders: Recent Advances. J. Med. Chem. 2018, 61, 9030–9058. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, W.; Li, Y.; Tang, M.; Yang, T.; Liu, K.; Chen, Y.; Deng, D.; Xiang, M.; Chen, L. Discovery of 3-(4-(2-((1H-Indol-5-yl)amino)-5-fluoropyrimidin-4-yl)-1H-pyrazol-1-yl)propanenitrile Derivatives as Selective TYK2 Inhibitors for the Treatment of Inflammatory Bowel Disease. J. Med. Chem. 2021, 64, 1966–1988. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Q.; Liao, X.; Ge, H.; Huo, G.; Zhang, L.; Chen, N.; Zhai, X.; Hong, Y.; Wang, L.; et al. Discovery of 6-(2-(dimethylamino)ethyl)-N-(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole-6-yl)pyrimidin-2-yl)-5,6,7,8-tetrahydro-1,6-naphthyridin-2-amine as a highly potent cyclin-dependent kinase 4/6 inhibitor for treatment of cancer. Eur. J. Med. Chem. 2019, 178, 352–364. [Google Scholar] [CrossRef]
- Liang, X.; Zang, J.; Li, X.; Tang, S.; Huang, M.; Geng, M.; Chou, C.J.; Li, C.; Cao, Y.; Xu, W.; et al. Discovery of Novel Janus Kinase (JAK) and Histone Deacetylase (HDAC) Dual Inhibitors for the Treatment of Hematological Malignancies. J. Med. Chem. 2019, 62, 3898–3923. [Google Scholar] [CrossRef]
- Liang, X.; Zang, J.; Zhu, M.; Gao, Q.; Wang, B.; Xu, W.; Zhang, Y. Design, Synthesis, and Antitumor Evaluation of 4-Amino-(1H)-pyrazole Derivatives as JAKs Inhibitors. ACS Med. Chem. Lett. 2016, 7, 950–955. [Google Scholar] [CrossRef]
- Bach, J.; Eastwood, P.; González, J.; Gómez, E.; Alonso, J.A.; Fonquerna, S.; Lozoya, E.; Orellana, A.; Maldonado, M.; Calaf, E.; et al. Identification of 2-Imidazopyridine and 2-Aminopyridone Purinones as Potent Pan-Janus Kinase (JAK) Inhibitors for the Inhaled Treatment of Respiratory Diseases. J. Med. Chem. 2019, 62, 9045–9060. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, L.; Wang, X.; Pan, Z. Discovery of 7H-pyrrolo[2,3-d]pyrimidine derivatives as selective covalent irreversible inhibitors of interleukin-2-inducible T-cell kinase (Itk). Eur. J. Med. Chem. 2019, 173, 167–183. [Google Scholar] [CrossRef]
- CoE, J.W.; Dehnhardt, C.M.; Jones, P.; Korturn, S.W.; Sabnis, Y.A.; Wakenhut, F.M.; Whitlock, G.A. JAK Inhibitors for the Treatment of Inflammatory Diseases. U.S. Patent US8895544B2, 25 November 2014. [Google Scholar]
- Garton, N.S.; Barker, M.D.; Davis, R.P.; Douault, C.; Hooper-Greenhill, E.; Jones, E.; Lewis, H.D.; Liddle, J.; Lugo, D.; McCleary, S.; et al. Optimisation of a novel series of potent and orally bioavailable azanaphthyridine SYK inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Leonard, K.A.; Madge, L.A.; Krawczuk, P.J.; Wang, A.; Kreutter, K.D.; Bacani, G.M.; Chai, W.; Smith, R.C.; Tichenor, M.S.; Harris, M.C.; et al. Discovery of a Gut-Restricted JAK Inhibitor for the Treatment of Inflammatory Bowel Disease. J. Med. Chem. 2020, 63, 2915–2929. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhong, Z.; Li, X.; Zhou, Y.; Pan, Z. Discovery of an Orally Available Janus Kinase 3 Selective Covalent Inhibitor. J. Med. Chem. 2019, 62, 1054–1066. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Substance Record for SID 395763535, S., Source: PATENTSCOPE (WIPO). 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/395763535 (accessed on 18 December 2024).
- Yuan, X.; Wu, H.; Bu, H.; Zhou, J.; Zhang, H. Targeting the immunity protein kinases for immuno-oncology. Eur. J. Med. Chem. 2019, 163, 413–427. [Google Scholar] [CrossRef]
- Ritzén, A.; Sørensen, M.D.; Dack, K.N.; Greve, D.R.; Jerre, A.; Carnerup, M.A.; Rytved, K.A.; Bagger-Bahnsen, J. Fragment-Based Discovery of 6-Arylindazole JAK Inhibitors. ACS Med. Chem. Lett. 2016, 7, 641–646. [Google Scholar] [CrossRef]
- Fensome, A.; Ambler, C.M.; Arnold, E.; Banker, M.E.; Brown, M.F.; Chrencik, J.; Clark, J.D.; Dowty, M.E.; Efremov, I.V.; Flick, A.; et al. Dual Inhibition of TYK2 and JAK1 for the Treatment of Autoimmune Diseases: Discovery of (( S)-2,2-Difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J. Med. Chem. 2018, 61, 8597–8612. [Google Scholar] [CrossRef]
- Chen, P.; Norris, D.; Das, J.; Spergel, S.H.; Wityak, J.; Leith, L.; Zhao, R.; Chen, B.C.; Pitt, S.; Pang, S.; et al. Discovery of novel 2-(aminoheteroaryl)-thiazole-5-carboxamides as potent and orally active Src-family kinase p56(Lck) inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 6061–6066. [Google Scholar] [CrossRef]
- Yang, T.; Hu, M.; Qi, W.; Yang, Z.; Tang, M.; He, J.; Chen, Y.; Bai, P.; Yuan, X.; Zhang, C.; et al. Discovery of Potent and Orally Effective Dual Janus Kinase 2/FLT3 Inhibitors for the Treatment of Acute Myelogenous Leukemia and Myeloproliferative Neoplasms. J. Med. Chem. 2019, 62, 10305–10320. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Bioassay Record for AID 1791642, N.S.F.J., Source: ChEMBL. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1791642 (accessed on 25 December 2024).
- Lee, S.M.; Yoon, K.B.; Lee, H.J.; Kim, J.; Chung, Y.K.; Cho, W.J.; Mukai, C.; Choi, S.; Kang, K.W.; Han, S.Y.; et al. The discovery of 2,5-isomers of triazole-pyrrolopyrimidine as selective Janus kinase 2 (JAK2) inhibitors versus JAK1 and JAK3. Bioorg. Med. Chem. 2016, 24, 5036–5046. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, L.; Fu, Z.; Shuai, B.; Luo, M.; Hu, G.; Chen, J.; Sun, J.; Wang, J.; Li, J.; et al. Discovery and optimization of selective RET inhibitors via scaffold hopping. Bioorg. Med. Chem. Lett. 2021, 47, 128149. [Google Scholar] [CrossRef] [PubMed]
- Egyed, A.; Bajusz, D.; Keserű, G.M. The impact of binding site waters on the activity/selectivity trade-off of Janus kinase 2 (JAK2) inhibitors. Bioorg. Med. Chem. 2019, 27, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Yen, R.; Heckrodt, T.J.; McMurtrie, D.; Singh, R.; Taylor, V.; Masuda, E.S.; Park, G.; Payan, D.G. Optimization of Pyrimidine Compounds as Potent JAK1 Inhibitors and the Discovery of R507 as a Clinical Candidate. ACS Med. Chem. Lett. 2022, 13, 1805–1811. [Google Scholar] [CrossRef]
- Liu, L.; Norman, M.H.; Lee, M.; Xi, N.; Siegmund, A.; Boezio, A.A.; Booker, S.; Choquette, D.; D’Angelo, N.D.; Germain, J.; et al. Structure-based design of novel class II c-Met inhibitors: 2. SAR and kinase selectivity profiles of the pyrazolone series. J. Med. Chem. 2012, 55, 1868–1897. [Google Scholar] [CrossRef]
- Schlapbach, A.; Feifel, R.; Hawtin, S.; Heng, R.; Koch, G.; Moebitz, H.; Revesz, L.; Scheufler, C.; Velcicky, J.; Waelchli, R.; et al. Pyrrolo-pyrimidones: A novel class of MK2 inhibitors with potent cellular activity. Bioorg. Med. Chem. Lett. 2008, 18, 6142–6146. [Google Scholar] [CrossRef]
- Bauer, D.; Whittington, D.A.; Coxon, A.; Bready, J.; Harriman, S.P.; Patel, V.F.; Polverino, A.; Harmange, J.C. Evaluation of indazole-based compounds as a new class of potent KDR/VEGFR-2 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4844–4848. [Google Scholar] [CrossRef]
- Zhou, H.; McGowan, M.A.; Lipford, K.; Christopher, M.; Fradera, X.; Witter, D.; Lesburg, C.A.; Li, C.; Methot, J.L.; Lampe, J.; et al. Discovery and optimization of heteroaryl piperazines as potent and selective PI3Kδ inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126715. [Google Scholar] [CrossRef]
- Shah, R.R.; Redmond, J.M.; Mihut, A.; Menon, M.; Evans, J.P.; Murphy, J.A.; Bartholomew, M.A.; Coe, D.M. Hi-JAK-ing the ubiquitin system: The design and physicochemical optimisation of JAK PROTACs. Bioorg. Med. Chem. 2020, 28, 115326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, X.; Wang, C.; Sun, X.; Sun, H.; Ma, T.; Li, Y.; Liu, K.; Chen, L.; Ma, X. Synthesis and biological activity of thieno[3,2-d]pyrimidines as potent JAK3 inhibitors for the treatment of idiopathic pulmonary fibrosis. Bioorg. Med. Chem. 2020, 28, 115254. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tokuhara, H.; Ohba, Y.; Okabe, A.; Nakayama, M.; Nakagawa, H.; Skene, R.; Hoffman, I.; Zou, H.; Yoshida, M. Efficient synthesis of tert-butyl 3-cyano-3-cyclopropyl-2-oxopyrrolidine-4-carboxylates: Highly functionalized 2-pyrrolidinone enabling access to novel macrocyclic Tyk2 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126963. [Google Scholar] [CrossRef]
- Lu, K.; Wu, W.; Zhang, C.; Liu, Z.; Xiao, B.; Yuan, Z.; Li, A.; Chen, D.; Zhai, X.; Jiang, Y. Discovery of triazolo [1,5-a] pyridine derivatives as novel JAK1/2 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127225. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Zu, W.; Song, J.; Wang, H.; Zhong, Y.; Li, H.; Zhang, Y.; Gao, Q.; Kong, B.; et al. Identification of. J. Med. Chem. 2020, 63, 6748–6773. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xue, G.; Pan, Z. Design, synthesis and structure-activity relationship of indolylindazoles as potent and selective covalent inhibitors of interleukin-2 inducible T-cell kinase (ITK). Eur. J. Med. Chem. 2020, 187, 111918. [Google Scholar] [CrossRef]
- Gerstenberger, B.S.; Ambler, C.; Arnold, E.P.; Banker, M.E.; Brown, M.F.; Clark, J.D.; Dermenci, A.; Dowty, M.E.; Fensome, A.; Fish, S.; et al. Discovery of Tyrosine Kinase 2 (TYK2) Inhibitor (PF-06826647) for the Treatment of Autoimmune Diseases. J. Med. Chem. 2020, 63, 13561–13577. [Google Scholar] [CrossRef] [PubMed]
- Zak, M.; Hanan, E.J.; Lupardus, P.; Brown, D.G.; Robinson, C.; Siu, M.; Lyssikatos, J.P.; Romero, F.A.; Zhao, G.; Kellar, T.; et al. Discovery of a class of highly potent Janus Kinase 1/2 (JAK1/2) inhibitors demonstrating effective cell-based blockade of IL-13 signaling. Bioorg. Med. Chem. Lett. 2019, 29, 1522–1531. [Google Scholar] [CrossRef]
- Baillache, D.J.; Unciti-Broceta, A. Recent developments in anticancer kinase inhibitors based on the pyrazolo[3,4-d]pyrimidine scaffold. RSC Med. Chem. 2020, 11, 1112–1135. [Google Scholar] [CrossRef]
- Thoma, G.; Blanz, J.; Bühlmayer, P.; Drückes, P.; Kittelmann, M.; Smith, A.B.; van Eis, M.; Vangrevelinghe, E.; Zerwes, H.G.; Che, J.J.; et al. Syk inhibitors with high potency in presence of blood. Bioorg. Med. Chem. Lett. 2014, 24, 2278–2282. [Google Scholar] [CrossRef]
- Luo, L.; Jia, J.J.; Zhong, Q.; Zhong, X.; Zheng, S.; Wang, G.; He, L. Synthesis and anticancer activity evaluation of naphthalene-substituted triazole spirodienones. Eur. J. Med. Chem. 2021, 213, 113039. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hu, M.; Chen, Y.; Xiang, M.; Tang, M.; Qi, W.; Shi, M.; He, J.; Yuan, X.; Zhang, C.; et al. N-(Pyrimidin-2-yl)-1,2,3,4-tetrahydroisoquinolin-6-amine Derivatives as Selective Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms. J. Med. Chem. 2020, 63, 14921–14936. [Google Scholar] [CrossRef] [PubMed]
- Matheson, C.J.; Coxon, C.R.; Bayliss, R.; Boxall, K.; Carbain, B.; Fry, A.M.; Hardcastle, I.R.; Harnor, S.J.; Mas-Droux, C.; Newell, D.R.; et al. 2-Arylamino-6-ethynylpurines are cysteine-targeting irreversible inhibitors of Nek2 kinase. RSC Med. Chem. 2020, 11, 707–731. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Langevine, C.; Smith, D.; Li, J.; Tokarski, J.S.; Khan, J.; Ruzanov, M.; Strnad, J.; Zupa-Fernandez, A.; et al. Discovery of BMS-986202: A Clinical Tyk2 Inhibitor that Binds to Tyk2 JH2. J. Med. Chem. 2021, 64, 677–694. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Wang, J.A.; Meng, L.; Pei, X.; Zhang, L.; An, L.; Li, C.L.; Miao, Y.L. Design, synthesis, biological activity evaluation of 3-(4-phenyl-1H-imidazol-2-yl)-1H-pyrazole derivatives as potent JAK 2/3 and aurora A/B kinases multi-targeted inhibitors. Eur. J. Med. Chem. 2021, 209, 112934. [Google Scholar] [CrossRef]
- Sanachai, K.; Aiebchun, T.; Mahalapbutr, P.; Seetaha, S.; Tabtimmai, L.; Maitarad, P.; Xenikakis, I.; Geronikaki, A.; Choowongkomon, K.; Rungrotmongkol, T. Discovery of novel JAK2 and EGFR inhibitors from a series of thiazole-based chalcone derivatives. RSC Med. Chem. 2021, 12, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, C.; He, C.; Qian, D.; Lu, L.; Sun, Y.; Xu, M.; Zhuo, J.; Liu, P.C.C.; Klabe, R.; et al. Discovery of Pemigatinib: A Potent and Selective Fibroblast Growth Factor Receptor (FGFR) Inhibitor. J. Med. Chem. 2021, 64, 10666–10679. [Google Scholar] [CrossRef]
- Xu, P.; Shen, P.; Wang, H.; Qin, L.; Ren, J.; Sun, Q.; Ge, R.; Bian, J.; Zhong, Y.; Li, Z.; et al. Discovery of imidazopyrrolopyridines derivatives as novel and selective inhibitors of JAK2. Eur. J. Med. Chem. 2021, 218, 113394. [Google Scholar] [CrossRef]
- Rao, D.; Li, H.; Ren, X.; Sun, Y.; Wen, C.; Zheng, M.; Huang, H.; Tang, W.; Xu, S. Discovery of a potent, selective, and covalent ZAP-70 kinase inhibitor. Eur. J. Med. Chem. 2021, 219, 113393. [Google Scholar] [CrossRef]
- Howard, S.; Berdini, V.; Boulstridge, J.A.; Carr, M.G.; Cross, D.M.; Curry, J.; Devine, L.A.; Early, T.R.; Fazal, L.; Gill, A.L.; et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J. Med. Chem. 2009, 52, 379–388. [Google Scholar] [CrossRef]
- Pollard, J.R.; Mortimore, M. Discovery and development of aurora kinase inhibitors as anticancer agents. J. Med. Chem. 2009, 52, 2629–2651. [Google Scholar] [CrossRef]
- Yang, T.; Cui, X.; Tang, M.; Qi, W.; Zhu, Z.; Shi, M.; Yang, L.; Pei, H.; Zhang, W.; Xie, L.; et al. Identification of a Novel 2,8-Diazaspiro[4.5]decan-1-one Derivative as a Potent and Selective Dual TYK2/JAK1 Inhibitor for the Treatment of Inflammatory Bowel Disease. J. Med. Chem. 2022, 65, 3151–3172. [Google Scholar] [CrossRef]
- Liang, X.; Tang, S.; Liu, X.; Liu, Y.; Xu, Q.; Wang, X.; Saidahmatov, A.; Li, C.; Wang, J.; Zhou, Y.; et al. Discovery of Novel Pyrrolo[2,3-. J. Med. Chem. 2022, 65, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Wellaway, C.R.; Baldwin, I.R.; Bamborough, P.; Barker, D.; Bartholomew, M.A.; Chung, C.W.; Dümpelfeld, B.; Evans, J.P.; Fazakerley, N.J.; Homes, P.; et al. Investigation of Janus Kinase (JAK) Inhibitors for Lung Delivery and the Importance of Aldehyde Oxidase Metabolism. J. Med. Chem. 2022, 65, 633–664. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liao, M.; Li, M.; Sun, M.; Xi, N.; Zeng, Y. Structure-based discovery of potent inhibitors of Axl: Design, synthesis, and biological evaluation. RSC Med. Chem. 2022, 13, 1246–1264. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wu, H.; Guo, Q.; Zheng, X.; Wei, C.; Liao, Y.; Shen, L.; Mi, J.; Li, J.; Chen, S.; et al. Synthesis and evaluation of hydrazinyl-containing pyrrolo[2,3-d]pyrimidine series as potent, selective and oral JAK1 inhibitors for the treatment of rheumatoid arthritis. Bioorg. Med. Chem. Lett. 2022, 74, 128905. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds. Eur. J. Med. Chem. 2017, 142, 328–375. [Google Scholar] [CrossRef]
- Asati, V.; Anant, A.; Patel, P.; Kaur, K.; Gupta, G.D. Pyrazolopyrimidines as anticancer agents: A review on structural and target-based approaches. Eur. J. Med. Chem. 2021, 225, 113781. [Google Scholar] [CrossRef]
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef]
- Yamagishi, H.; Inoue, T.; Nakajima, Y.; Maeda, J.; Tominaga, H.; Usuda, H.; Hondo, T.; Moritomo, A.; Nakamori, F.; Ito, M.; et al. Discovery of tricyclic dipyrrolopyridine derivatives as novel JAK inhibitors. Bioorg. Med. Chem. 2017, 25, 5311–5326. [Google Scholar] [CrossRef]
- Kiss, R.; Polgár, T.; Kirabo, A.; Sayyah, J.; Figueroa, N.C.; List, A.F.; Sokol, L.; Zuckerman, K.S.; Gali, M.; Bisht, K.S.; et al. Identification of a novel inhibitor of JAK2 tyrosine kinase by structure-based virtual screening. Bioorg. Med. Chem. Lett. 2009, 19, 3598–3601. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Malaviya, R.; Yang, C.; Argentieri, R.; Wang, B.; Chen, X.; Murray, W.V.; Cavender, D. Synthetic staurosporines via a ring closing metathesis strategy as potent JAK3 inhibitors and modulators of allergic responses. Bioorg. Med. Chem. Lett. 2009, 19, 3333–3338. [Google Scholar] [CrossRef]
- Ioannidis, S.; Lamb, M.L.; Davies, A.M.; Almeida, L.; Su, M.; Bebernitz, G.; Ye, M.; Bell, K.; Alimzhanov, M.; Zinda, M. Discovery of pyrazol-3-ylamino pyrazines as novel JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6524–6528. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, M.W.; Pierce, A.C.; Duffy, J.P.; Gao, H.; Messersmith, D.; Salituro, F.G.; Nanthakumar, S.; Come, J.; Zuccola, H.J.; Swenson, L.; et al. 2-Aminopyrazolo[1,5-a]pyrimidines as potent and selective inhibitors of JAK2. Bioorg. Med. Chem. Lett. 2009, 19, 6529–6533. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ledeboer, M.W.; Duffy, J.P.; Salituro, F.G.; Pierce, A.C.; Zuccola, H.J.; Block, E.; Shlyakter, D.; Hogan, J.K.; Bennani, Y.L. A novel chemotype of kinase inhibitors: Discovery of 3,4-ring fused 7-azaindoles and deazapurines as potent JAK2 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 153–156. [Google Scholar] [CrossRef]
- Wei, H.; Myers, M.R.; Hanney, B.; Spada, A.P.; Bilder, G.; Galzcinski, H.; Amin, D.; Needle, S.; Page, K.; Jayyosi, Z.; et al. Potent Quinoxaline-Based Inhibitors of PDGF Receptor Tyrosine Kinase Activity. Part 2: The Synthesis and Biological Activities of RPR127963, an Orally Bioavailable Inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3097–3100. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Sorrell, F.J.; Berger, B.T.; Collins, R.J.; Vasconcelos, S.N.S.; Massirer, K.B.; Knapp, S.; Bennett, J.; Fedorov, O.; Patel, H.; et al. Discovery of a Potent Dual SLK/STK10 Inhibitor Based on a Maleimide Scaffold. J. Med. Chem. 2021, 64, 13259–13278. [Google Scholar] [CrossRef]
- Ruel, R.; Thibeault, C.; L’Heureux, A.; Martel, A.; Cai, Z.W.; Wei, D.; Qian, L.; Barrish, J.C.; Mathur, A.; D’Arienzo, C.; et al. Discovery and preclinical studies of 5-isopropyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(2-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine (BMS-645737), an in vivo active potent VEGFR-2 inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 2985–2989. [Google Scholar] [CrossRef]
- Das, J.; Moquin, R.V.; Pitt, S.; Zhang, R.; Shen, D.R.; McIntyre, K.W.; Gillooly, K.; Doweyko, A.M.; Sack, J.S.; Zhang, H.; et al. Pyrazolo-pyrimidines: A novel heterocyclic scaffold for potent and selective p38 alpha inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2652–2657. [Google Scholar] [CrossRef]
- Bhide, R.S.; Cai, Z.W.; Zhang, Y.Z.; Qian, L.; Wei, D.; Barbosa, S.; Lombardo, L.J.; Borzilleri, R.M.; Zheng, X.; Wu, L.I.; et al. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J. Med. Chem. 2006, 49, 2143–2146. [Google Scholar] [CrossRef]
- Borzilleri, R.M.; Bhide, R.S.; Barrish, J.C.; D’Arienzo, C.J.; Derbin, G.M.; Fargnoli, J.; Hunt, J.T.; Jeyaseelan, R.; Kamath, A.; Kukral, D.W.; et al. Discovery and evaluation of N-cyclopropyl- 2,4-difluoro-5-((2-(pyridin-2-ylamino)thiazol-5-ylmethyl)amino)benzamide (BMS-605541), a selective and orally efficacious inhibitor of vascular endothelial growth factor receptor-2. J. Med. Chem. 2006, 49, 3766–3769. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wrobleski, S.T.; Lin, J.; Ahmed, G.; Metzger, A.; Wityak, J.; Gillooly, K.M.; Shuster, D.J.; McIntyre, K.W.; Pitt, S.; et al. 5-Cyanopyrimidine derivatives as a novel class of potent, selective, and orally active inhibitors of p38alpha MAP kinase. J. Med. Chem. 2005, 48, 6261–6270. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Sawant, D.B.; Fisk, H.A.; Mao, L.; Li, C.; Chettiar, S.; Li, P.K.; Darby, M.V.; Brueggemeier, R.W. Novel pyrrolopyrimidines as Mps1/TTK kinase inhibitors for breast cancer. Bioorg. Med. Chem. 2017, 25, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Seidel, T.; Langer, T. Conformational Sampling of Small Molecules With iCon: Performance Assessment in Comparison With OMEGA. Front. Chem. 2018, 6, 229. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]
- Chrencik, J.E.; Patny, A.; Leung, I.K.; Korniski, B.; Emmons, T.L.; Hall, T.; Weinberg, R.A.; Gormley, J.A.; Williams, J.M.; Day, J.E.; et al. Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J. Mol. Biol. 2010, 400, 413–433. [Google Scholar] [CrossRef]
- Tsui, V.; Gibbons, P.; Ultsch, M.; Mortara, K.; Chang, C.; Blair, W.; Pulk, R.; Stanley, M.; Starovasnik, M.; Williams, D.; et al. A new regulatory switch in a JAK protein kinase. Proteins 2011, 79, 393–401. [Google Scholar] [CrossRef]
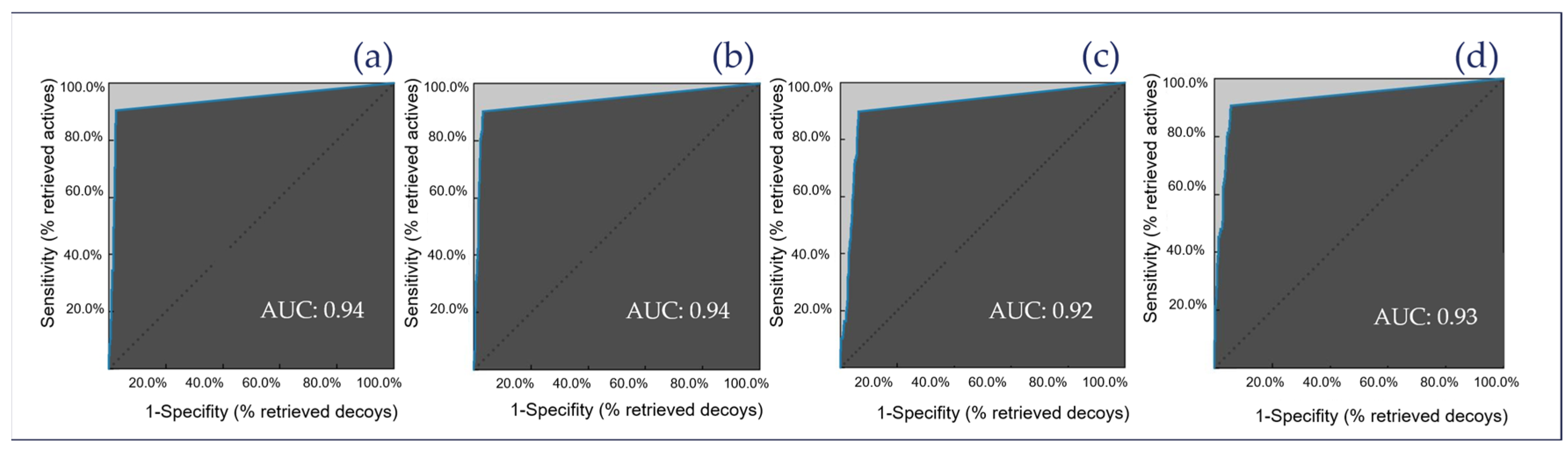
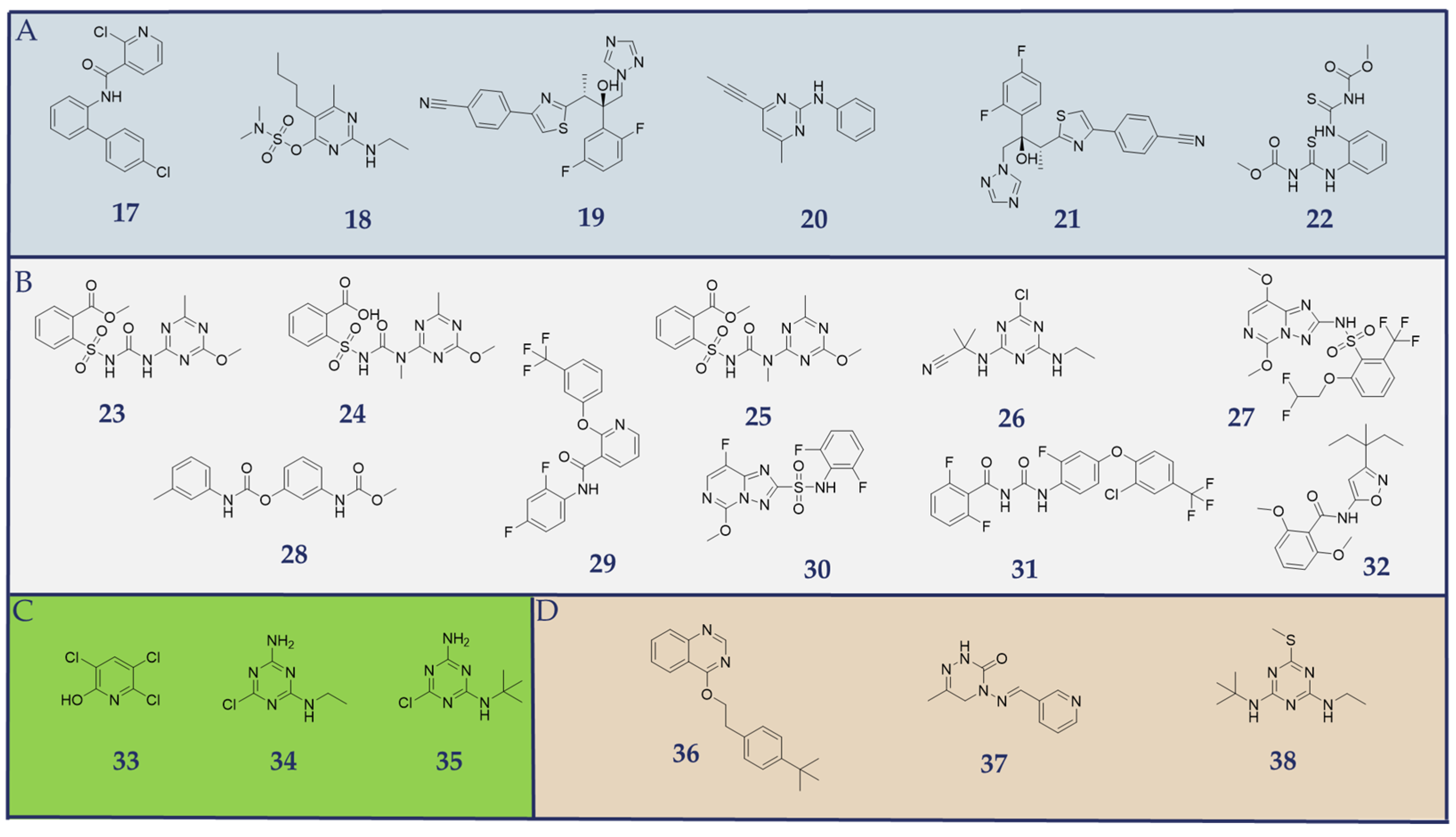
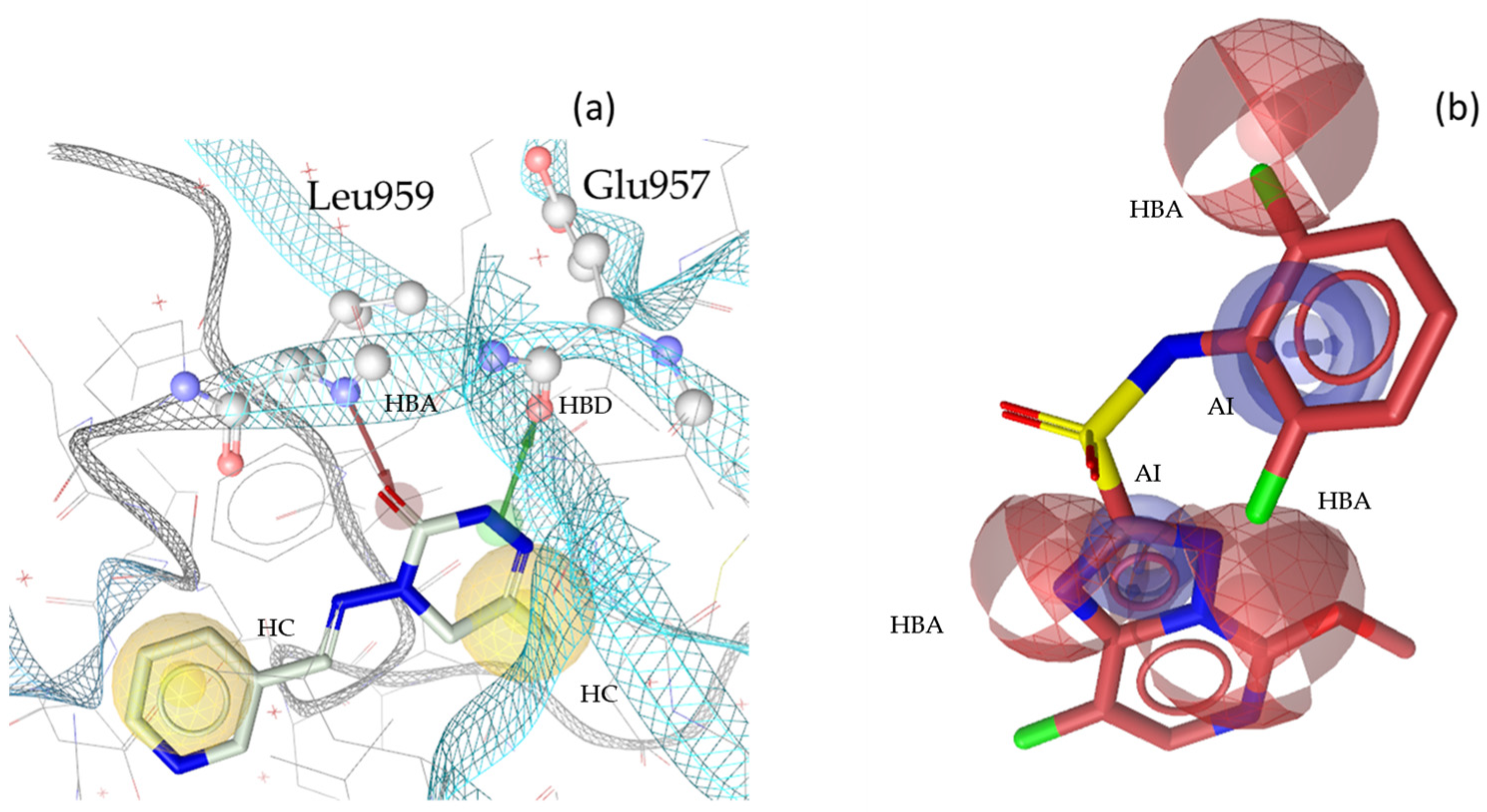

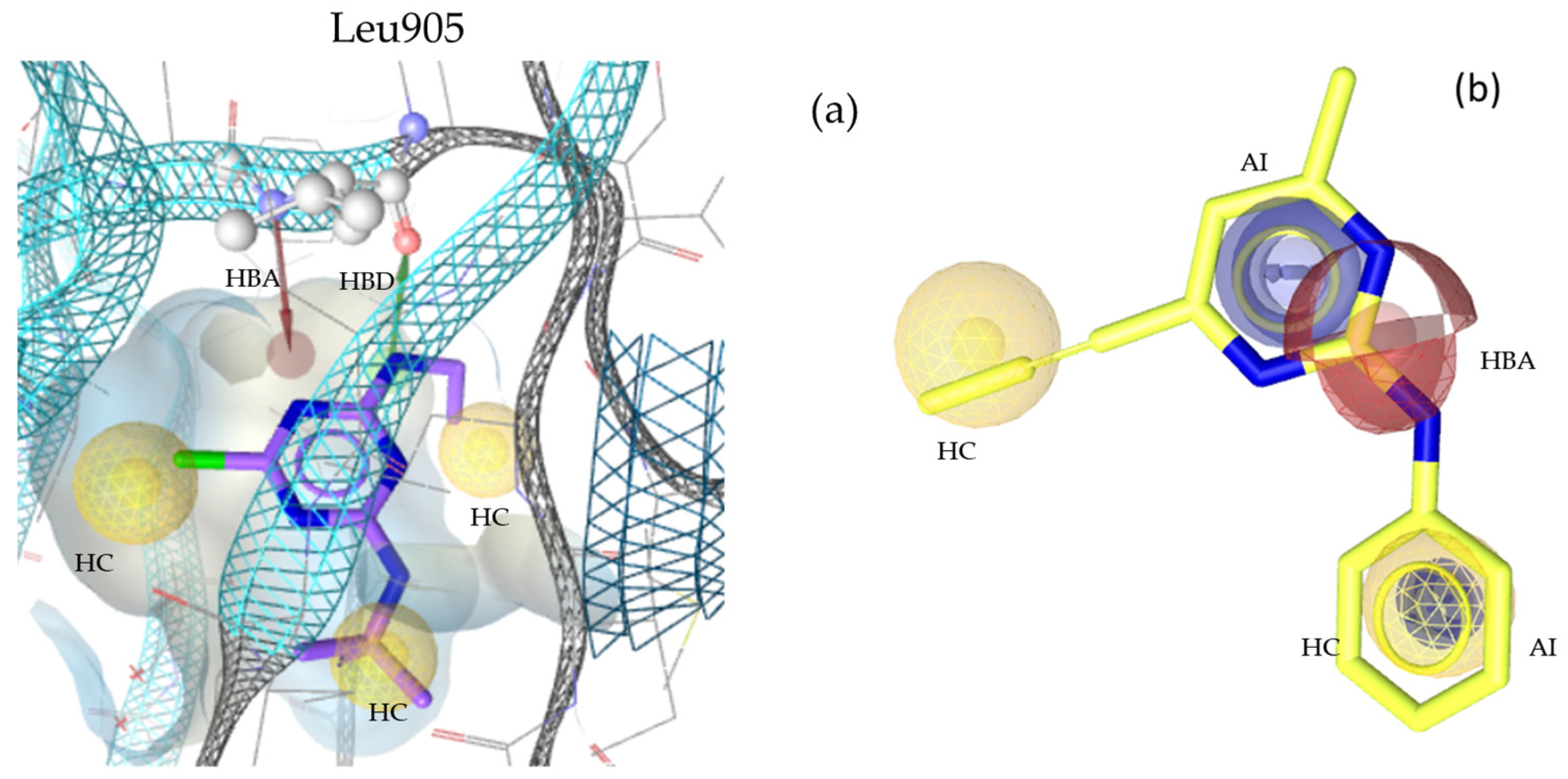
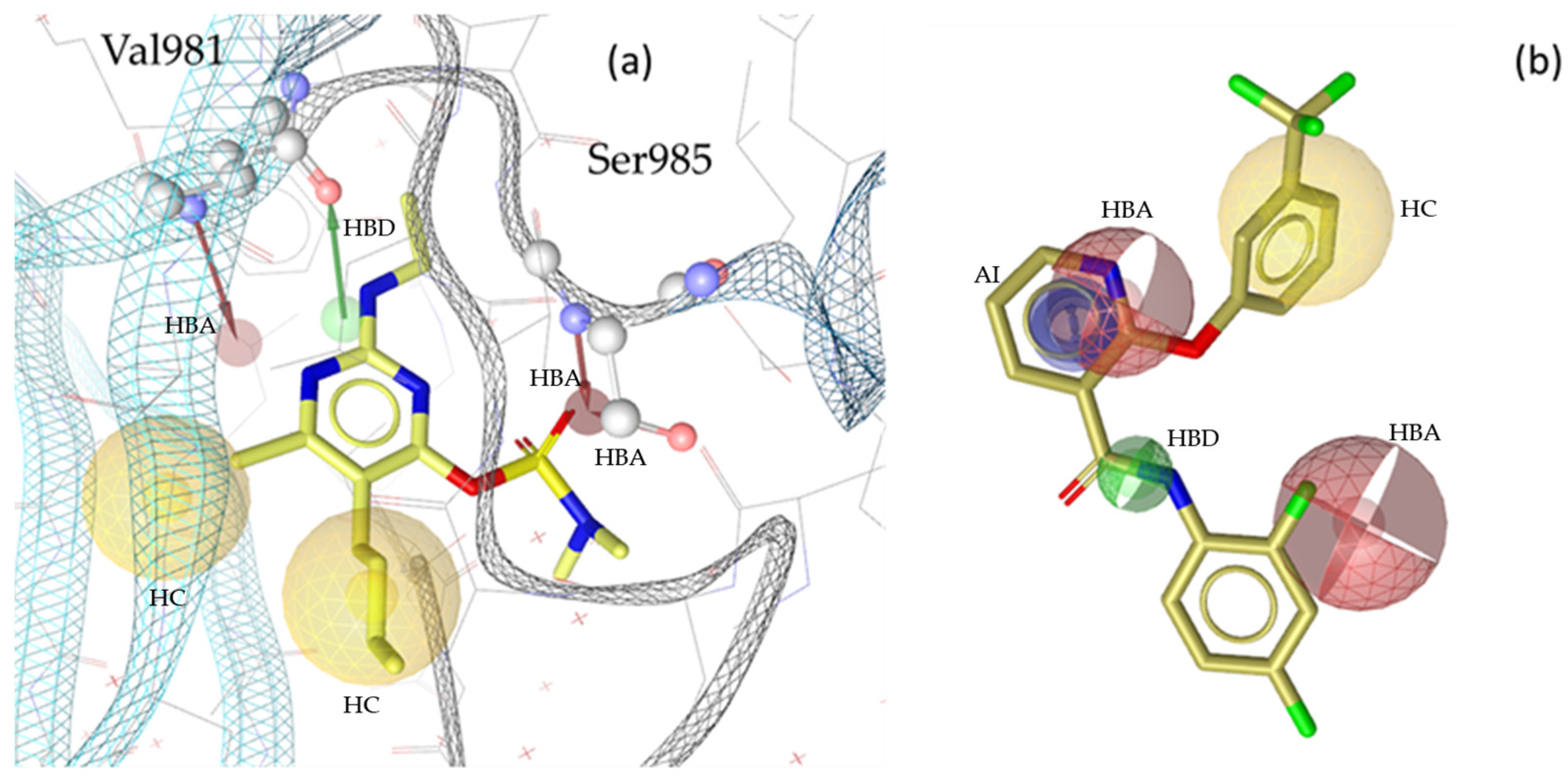
| Overall Evaluation | JAK1 TOTAL (8 Models) | JAK2 TOTAL (10 Models) | JAK3 TOTAL (10 Models) | TYK2 TOTAL (9 Models) |
|---|---|---|---|---|
| active hits/TPs | 95 | 167 | 116 | 68 |
| inactive hits | 0 | 15 | 2 | 40 |
| decoy hits | 79 | 75 | 292 | 136 |
| FPs | 79 | 90 | 294 | 176 |
| TNs | 3232 | 2799 | 4247 | 2935 |
| number of ACs in database | 105 | 185 | 129 | 75 |
| number of IAs in database | 48 | 49 | 42 | 61 |
| number of DCs in database | 3263 | 2840 | 4499 | 3050 |
| Model Accuracy | 0.97 | 0.96 | 0.93 | 0.94 |
| YoA | 0.55 | 0.65 | 0.28 | 0.28 |
| EF | 17.76 | 10.80 | 10.24 | 11.84 |
| sensitivity | 0.90 | 0.90 | 0.86 | 0.91 |
| specificity | 1.00 | 0.99 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, F.; Temml, V.; Schuster, D. Pharmacophore Modeling of Janus Kinase Inhibitors: Tools for Drug Discovery and Exposition Prediction. Molecules 2025, 30, 2183. https://doi.org/10.3390/molecules30102183
Fischer F, Temml V, Schuster D. Pharmacophore Modeling of Janus Kinase Inhibitors: Tools for Drug Discovery and Exposition Prediction. Molecules. 2025; 30(10):2183. https://doi.org/10.3390/molecules30102183
Chicago/Turabian StyleFischer, Florian, Veronika Temml, and Daniela Schuster. 2025. "Pharmacophore Modeling of Janus Kinase Inhibitors: Tools for Drug Discovery and Exposition Prediction" Molecules 30, no. 10: 2183. https://doi.org/10.3390/molecules30102183
APA StyleFischer, F., Temml, V., & Schuster, D. (2025). Pharmacophore Modeling of Janus Kinase Inhibitors: Tools for Drug Discovery and Exposition Prediction. Molecules, 30(10), 2183. https://doi.org/10.3390/molecules30102183






