Electrochemical α-C(sp3)–H/N–H Cross-Coupling of Isochromans and Azoles
Abstract
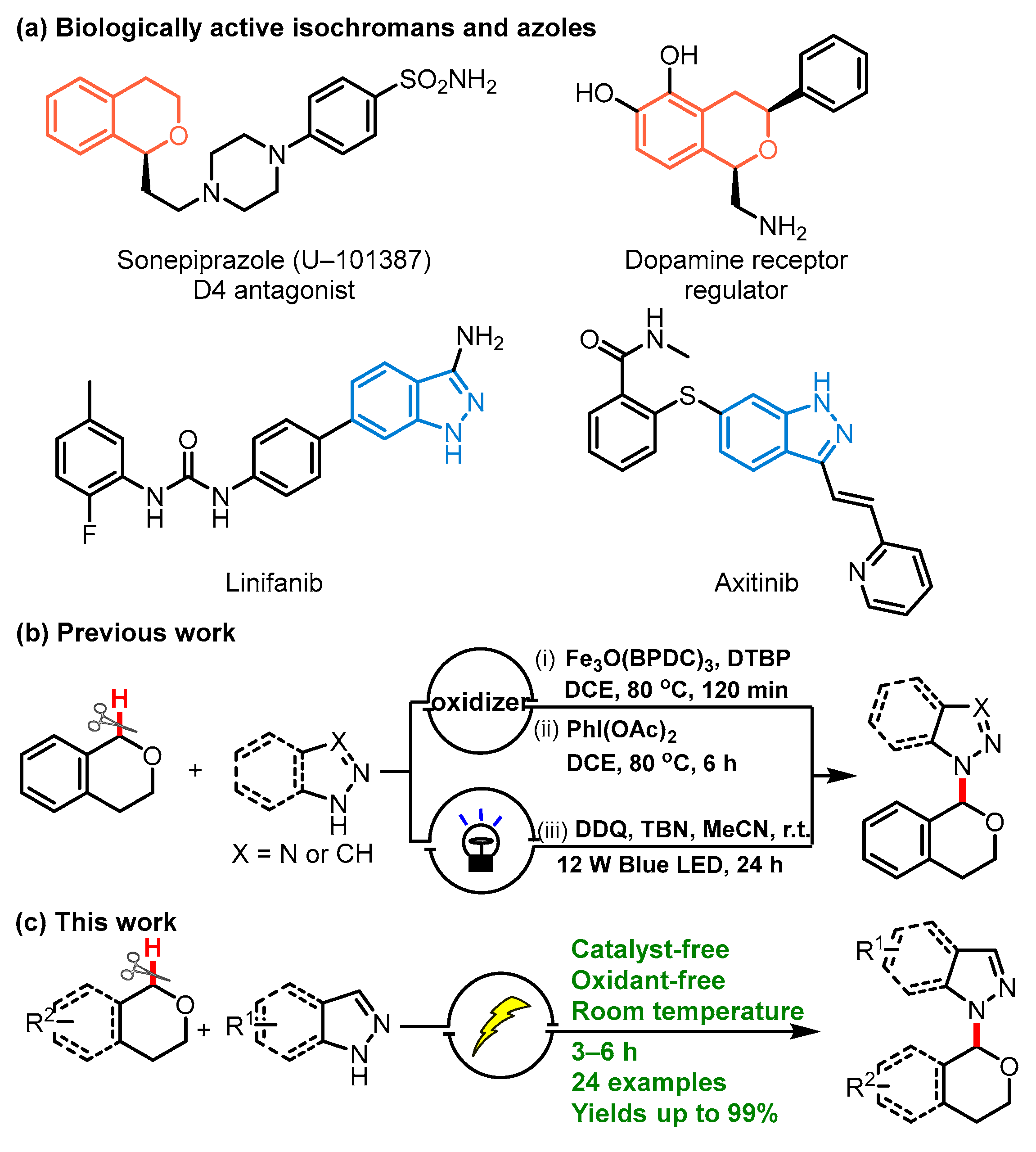
1. Introduction
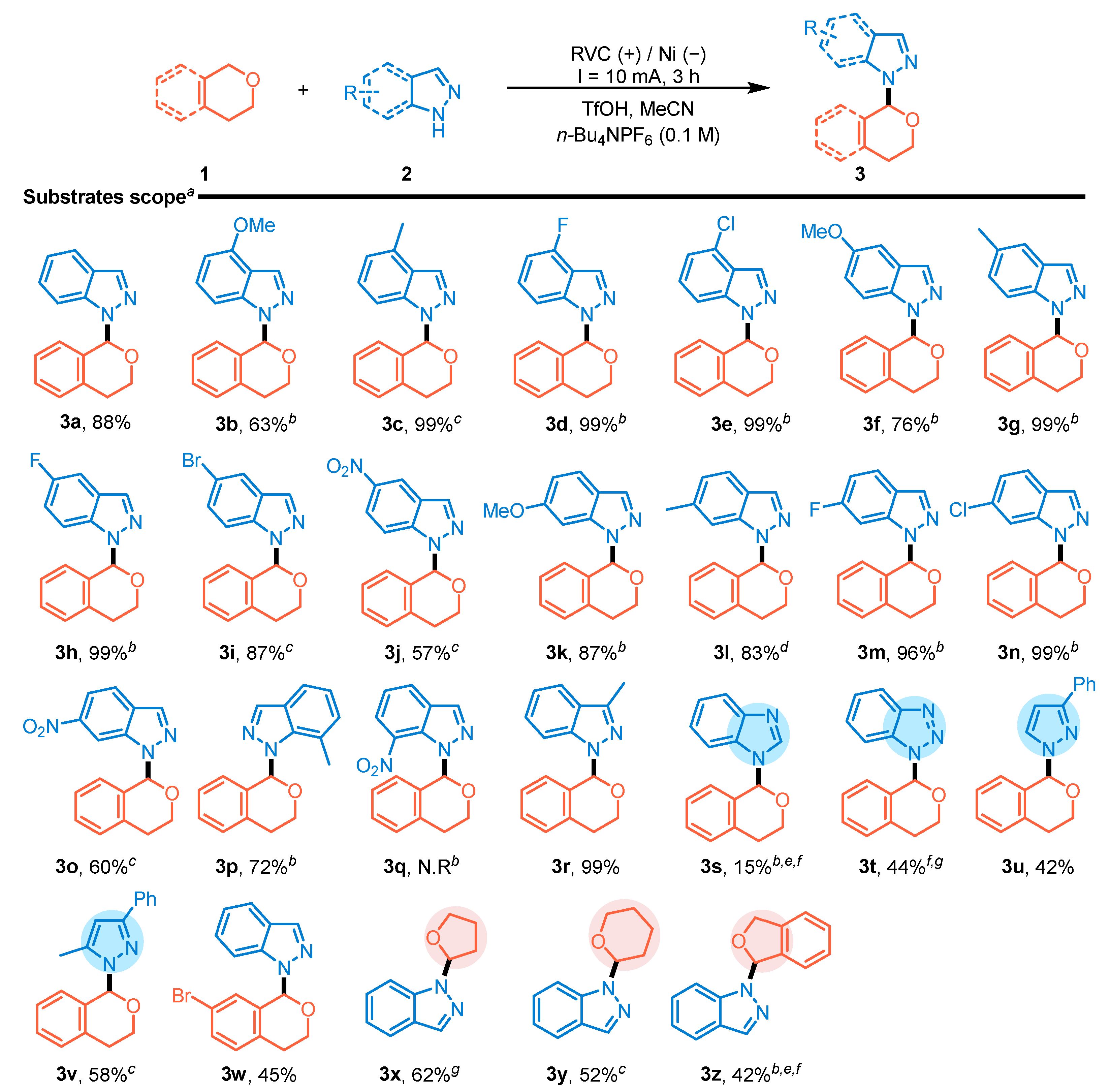
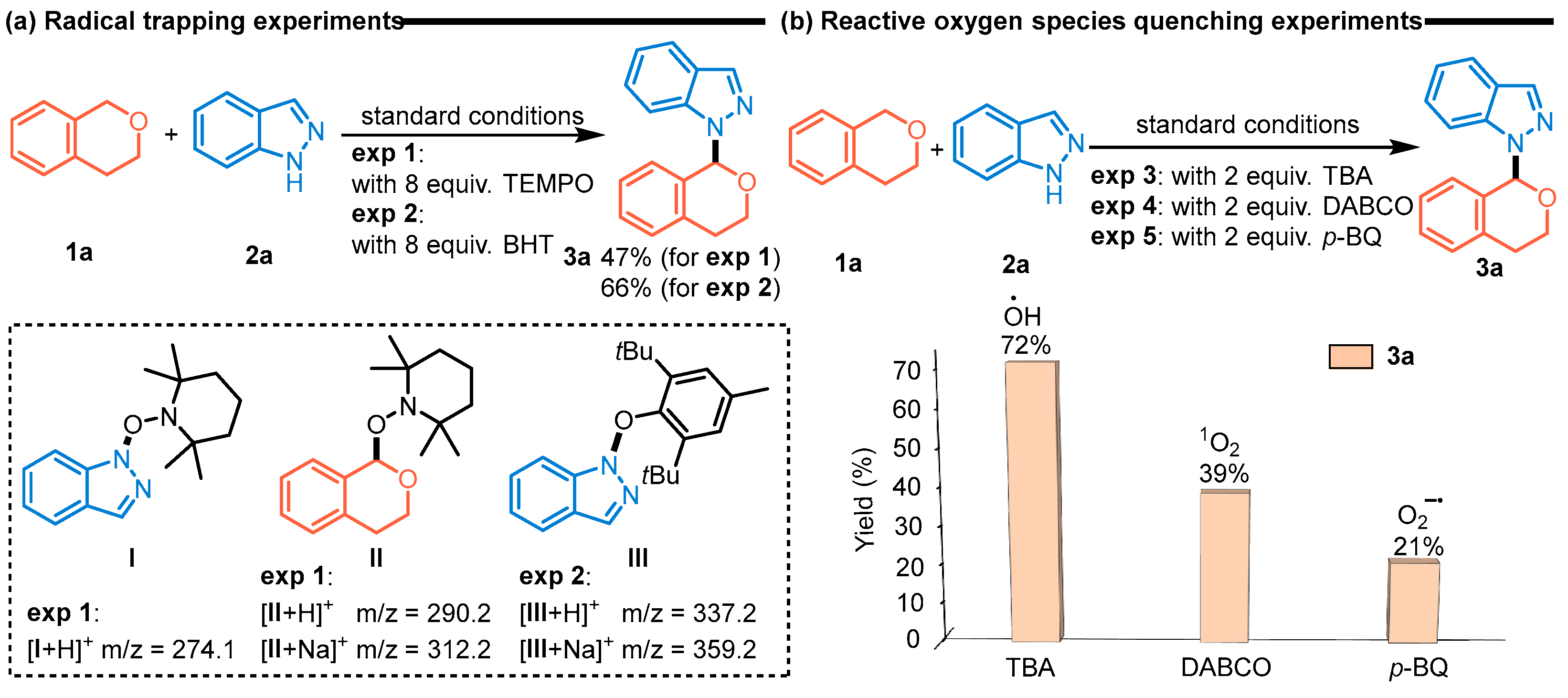
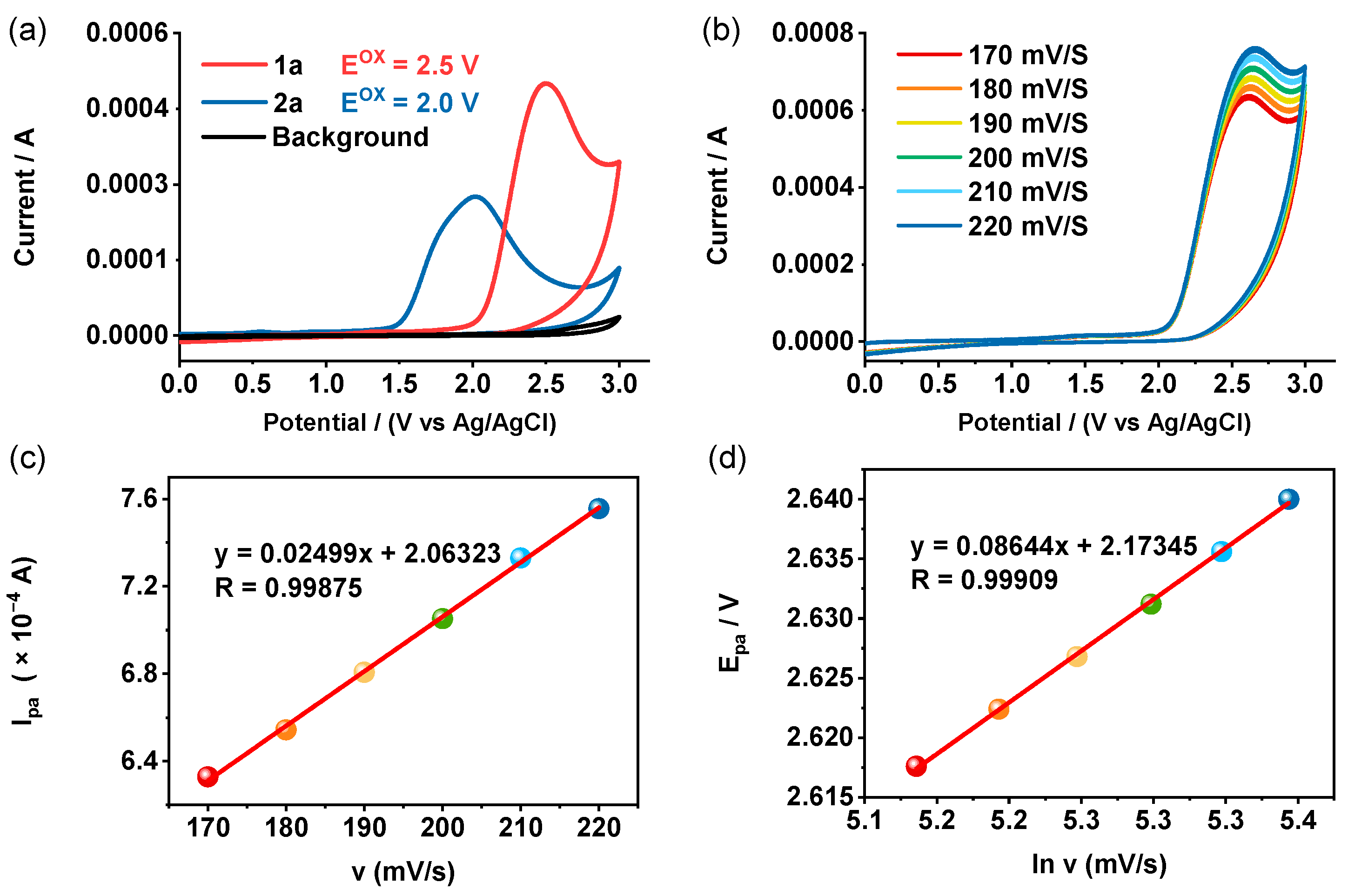
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Cyclic Voltammetry Experiments
3.3. General Procedure for the Electrochemical Synthesis of 1-(Isochroman-1-yl)-1H-Indazole
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girad, S.A.; Knauber, T.; Li, C.-J. The Cross-Dehydrogenative Coupling of Csp3-H Bonds: A Versatile Strategy for C-C Bond Formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef]
- Tang, S.; Lei, A. Oxidative R1–H/R2–H Cross-Coupling with Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 13128–13135. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kang, H.; Li, J.; Li, C.-J. En Route to Intermolecular Cross-Dehydrogenative Coupling Reactions. J. Org. Chem. 2019, 84, 12705–12721. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J. Cross-Dehydrogenative Coupling (CDC): Exploring C−C Bond Formations beyond Functional Group Transformations. Acc. Chem. Res. 2009, 42, 335–344. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, D.; Lei, A. Recent Advances of Transition-Metal Catalyzed Radical Oxidative Cross-Couplings. Acc. Chem. Res. 2014, 47, 3459–3470. [Google Scholar] [CrossRef]
- Yuan, Y.; Lei, A. Electrochemical Oxidative Cross-Coupling with Hydrogen Evolution Reactions. Acc. Chem. Res. 2019, 52, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Recent Advances in Oxidative R1-H/R2-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef]
- Röckl, J.L.; Pollok, D.; Franke, R.; Waldvogel, S.R. A Decade of Electrochemical Dehydrogenative C,C-Coupling of Aryls. Acc. Chem. Res. 2020, 53, 45–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Z.; Warratz, S.; Ma, C.; Ackermann, L. Recent advances in electrooxidative radical transformations of alkynes. Sci. China Chem. 2023, 66, 703–724. [Google Scholar] [CrossRef]
- Roy, S.; Karmakar, S.; Mondal, I.; Naskar, K.; Deb, I. Electrochemical C(sp3)–C(sp3) cross-dehydrogenative coupling: Enabling access to 9-substituted fluorescent acridanes. Chem. Commun. 2023, 59, 9074–9077. [Google Scholar] [CrossRef]
- Kong, Y.; Kim, J.K.; Li, Y.; Zhang, J.; Huang, M.; Wu, Y. An oxidant- and catalyst-free electrooxidative cross-coupling approach to 3-tetrahydroisoquinoline substituted coumarins. Green Chem. 2021, 23, 1274–1279. [Google Scholar] [CrossRef]
- Cao, H.; Long, C.-J.; Yang, D.; Guan, Z.; He, Y.-H. Electrochemical Cross-Dehydrogenative Coupling of Isochroman and Unactivated Ketones. J. Org. Chem. 2023, 88, 4145–4154. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Song, H.; Wang, Q. Phosphorous acid–assisted electrochemical α-tetrahydrofuranylation of sulfonamides and amides. Green Chem. 2023, 25, 1970–1974. [Google Scholar] [CrossRef]
- Zhu, Q.-R.; Zhang, P.-Z.; Sun, X.; Gao, H.; Wang, P.-L.; Li, H. Electrochemical N(sp2)–H/C(sp3)–H cross-coupling reaction between sulfoximines and alkylarenes. Green Chem. 2024, 26, 5824–5831. [Google Scholar] [CrossRef]
- Kong, Y.; Huang, M.; Li, Y.; Kim, J.K.; Gong, M.; Wu, Y. Convergent Paired Electrolysis for the Synthesis of Pyrazolyl-Substituted Tetrahydroisoquinolines. Adv. Synth. Catal. 2023, 365, 4198–4204. [Google Scholar] [CrossRef]
- Fang, S.; Zhong, K.; Zeng, S.; Hu, X.; Sun, P.; Ruan, Z. The electrochemically enabled α-C(sp3)–H azolation of ketones. Chem. Commun. 2023, 59, 11425–11428. [Google Scholar] [CrossRef]
- Hou, Z.-W.; Liu, D.-J.; Xiong, P.; Lai, X.-L.; Song, J.; Xu, H.-C. Site-Selective Electrochemical Benzylic C−H Amination. Angew. Chem. Int. Ed. 2021, 60, 2943–2947. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Song, H.; Wang, Q. Electrochemical direct α-amidation and α-pyrazolation of N-alkoxy- and N-aryloxycarbonyl pyrrolidines. Green Chem. 2024, 26, 7419–7423. [Google Scholar] [CrossRef]
- Hou, Z.-W.; Li, L.; Wang, L. Organocatalytic electrochemical amination of benzylic C–H bonds. Org. Chem. Front. 2021, 8, 4700–4705. [Google Scholar] [CrossRef]
- Wu, J.; Shi, H.; Liu, J.; Wang, R.; Zhou, J.; Xu, X.-L.; Xu, H.-J. Electrochemical oxidative C(sp3)–H/O–H cross-coupling for the synthesis of α-acyloxyketones. Org. Chem. Front. 2023, 10, 2459–2464. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Li, Y.; Zhang, H.; Yang, D.; Nagasaka, M.; Lv, Z.; Guan, Z.; Cao, Y.; Gong, F.; et al. Electrochemical Oxidation Enables Regioselective and Scalable α-C(sp3)-H Acyloxylation of Sulfides. J. Am. Chem. Soc. 2021, 143, 3628–3637. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, K.; Liu, Y.; Song, H.; Wang, Q. Electrochemical α-C(sp3)–H/O–H cross-coupling of isochromans and alcohols assisted by benzoic acid. Chem. Commun. 2022, 58, 10949–10952. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Pajkert, R.; Wang, L.; Li, Z.; Röschenthaler, G.-V.; Han, J. Chemistry of electrochemical oxidative reactions of sulfinate salts. Green Chem. 2020, 22, 3028–3059. [Google Scholar] [CrossRef]
- Martins, D.G.M.; Meirinho, A.G.; Ahmed, D.N.; Braga, D.A.L.; Mendes, D.S.R. Recent Advances in Electrochemical Chalcogen (S/Se)-Functionalization of Organic Molecules. ChemElectroChem 2019, 6, 5928–5940. [Google Scholar] [CrossRef]
- Wang, J.-H.; Li, X.-B.; Li, J.; Lei, T.; Wu, H.-L.; Nan, X.-L.; Tung, C.-H.; Wu, L.-Z. Photoelectrochemical cell for P–H/C–H cross-coupling with hydrogen evolution. Chem. Commun. 2019, 55, 10376–10379. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Fang, P.; Wu, J.; Wang, F.; Liu, Z.-Q. Electrochemical chlorination of least hindered tertiary and benzylic C(sp3)–H bonds. Green Chem. 2024, 26, 507–512. [Google Scholar] [CrossRef]
- Fuchigami, T.; Inagi, S. Recent Advances in Electrochemical Systems for Selective Fluorination of Organic Compounds. Acc. Chem. Res. 2020, 53, 322–334. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, H.; Lei, A. Oxidation-Induced C-H Functionalization: A Formal Way for C-H Activation. Chin. J. Chem. 2018, 36, 692–697. [Google Scholar] [CrossRef]
- Zhao, Z.; Kang, K.; Yue, J.; Ji, X.; Qiao, H.; Fan, P.; Zheng, X. Research progress in biological activities of isochroman derivatives. Eur. J. Med. Chem. 2021, 210, 113073. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Aderibigbe, B.A. Antifungal Activities of Natural Products and Their Hybrid Molecules. Pharmaceutics 2023, 15, 2673. [Google Scholar] [CrossRef]
- Deng, Y.; Hu, Z.; Xue, J.; Yin, J.; Zhu, T.; Liu, S. Visible-Light-Promoted α-C(sp3)–H Amination of Ethers with Azoles and Amides. Org. Lett. 2024, 26, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Doan, S.H.; Ngo, A.N.V.; Nguyen, T.T.; Phan, N.T.S. Direct C–N coupling of azoles with ethers via oxidative C–H activation under metal–organic framework catalysis. J. Ind. Eng. Chem. 2016, 44, 136–145. [Google Scholar] [CrossRef]
- Sun, B.; Yan, Z.; Jin, C.; Su, W. (Diacetoxyiodo)benzene-Mediated Transition-Metal-Free Amination of C(sp3)–H Bonds Adjacent to Heteroatoms with Azoles: Synthesis of N-Alkylated Azoles. Synlett 2018, 29, 2432–2436. [Google Scholar]
- Gong, M.; Wu, Q.; Kim, J.K.; Huang, M.; Li, Y.; Wu, Y.; Kim, J.S. Electric-field-controlled highly regioselective thiocyanation of N-containing heterocycles. Sci. China Chem. 2024, 67, 1263–1269. [Google Scholar] [CrossRef]
- Alkorta, I.; Claramunt, R.M.; Elguero, J.; Gutiérrez-Puebla, E.; Monge, M.Á.; Reviriego, F.; Roussel, C. Study of the Addition Mechanism of 1H-Indazole and Its 4-, 5-, 6-, and 7-Nitro Derivatives to Formaldehyde in Aqueous Hydrochloric Acid Solutions. J. Org. Chem. 2022, 87, 5866–5881. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Zhou, Y.; Chiang, C.-W.; Lei, A. Electro-Oxidative C(sp3)-H Amination of Azoles via Intermolecular Oxidative C(sp3)-H/N-H Cross-Coupling. ACS Catal. 2017, 7, 8320–8323. [Google Scholar] [CrossRef]



| Entry | Anode/Cathode | Electrolyte | Additive | Yield (%) b |
|---|---|---|---|---|
| 1 | RVC/Ni | n-Bu4NBF4 | - | trace |
| 2 | RVC/Ni | n-Bu4NBF4 | TfOH | 39 |
| 3 | RVC/Ni | n-Bu4NBF4 | AcOH | 2 |
| 4 c | RVC/Ni | n-Bu4NBF4 | TfOH | 73 |
| 5 c | RVC/Ni | n-Bu4NClO4 | TfOH | 54 |
| 6 c | RVC/Ni | n-Bu4NPF6 | TfOH | 88 |
| 7 c | RVC/Ni | n-Bu4NOAc | TfOH | 39 |
| 8 c | C/Ni | n-Bu4NPF6 | TfOH | 73 |
| 9 c | RVC/Pt | n-Bu4NPF6 | TfOH | 71 |
| 10 c,d | RVC/Ni | n-Bu4NPF6 | TfOH | 50 |
| 11 c,e,f | RVC/Ni | n-Bu4NPF6 | TfOH | 72 |
| 12 c,g | RVC/Ni | n-Bu4NPF6 | TfOH | 88 |
| 13 c,h | RVC/Ni | n-Bu4NPF6 | TfOH | 69 |
| 14 c,f | RVC/Ni | n-Bu4NPF6 | TfOH | 84 |
| 15 c | RVC/Ni | n-Bu4NPF6 | - | 70 |
| 16 c,i | RVC/Ni | n-Bu4NPF6 | TfOH | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Yan, B.; Wu, L.; Li, Y.; Hao, X.; Gong, M.; Wu, Y. Electrochemical α-C(sp3)–H/N–H Cross-Coupling of Isochromans and Azoles. Molecules 2025, 30, 4. https://doi.org/10.3390/molecules30010004
Li G, Yan B, Wu L, Li Y, Hao X, Gong M, Wu Y. Electrochemical α-C(sp3)–H/N–H Cross-Coupling of Isochromans and Azoles. Molecules. 2025; 30(1):4. https://doi.org/10.3390/molecules30010004
Chicago/Turabian StyleLi, Guoping, Bing Yan, Liangliang Wu, Yabo Li, Xinqi Hao, Ming Gong, and Yangjie Wu. 2025. "Electrochemical α-C(sp3)–H/N–H Cross-Coupling of Isochromans and Azoles" Molecules 30, no. 1: 4. https://doi.org/10.3390/molecules30010004
APA StyleLi, G., Yan, B., Wu, L., Li, Y., Hao, X., Gong, M., & Wu, Y. (2025). Electrochemical α-C(sp3)–H/N–H Cross-Coupling of Isochromans and Azoles. Molecules, 30(1), 4. https://doi.org/10.3390/molecules30010004







