Recent Advancements in the Synthesis of Functional Polyolefins by Non-Bridged Half-Titanocenes
Abstract
1. Introduction
2. Synthesis of Functional Polyolefins by Direct Copolymerization of Olefin with Functional Comonomers
2.1. Synthesis of Functional Polyethylene
2.1.1. Synthesis of Functional Polyethylene Containing SiR3 (R = Me, iPr) Groups
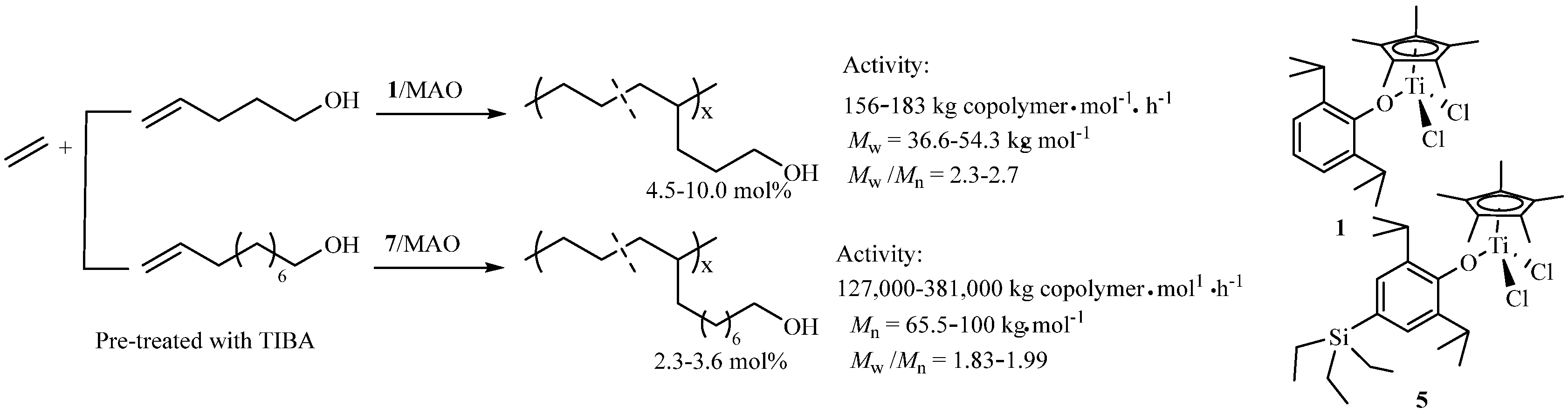
2.1.2. Synthesis of Functional Polyethylene Containing OH Groups
2.2. Synthesis of Functional Polyolefin Elastomer
3. Synthesis of Functional Polyolefins by Post-Functionalization of Polyolefin Containing Reactive Groups
3.1. Synthesis of Functional Polyethylene by Post-Functionalization Method
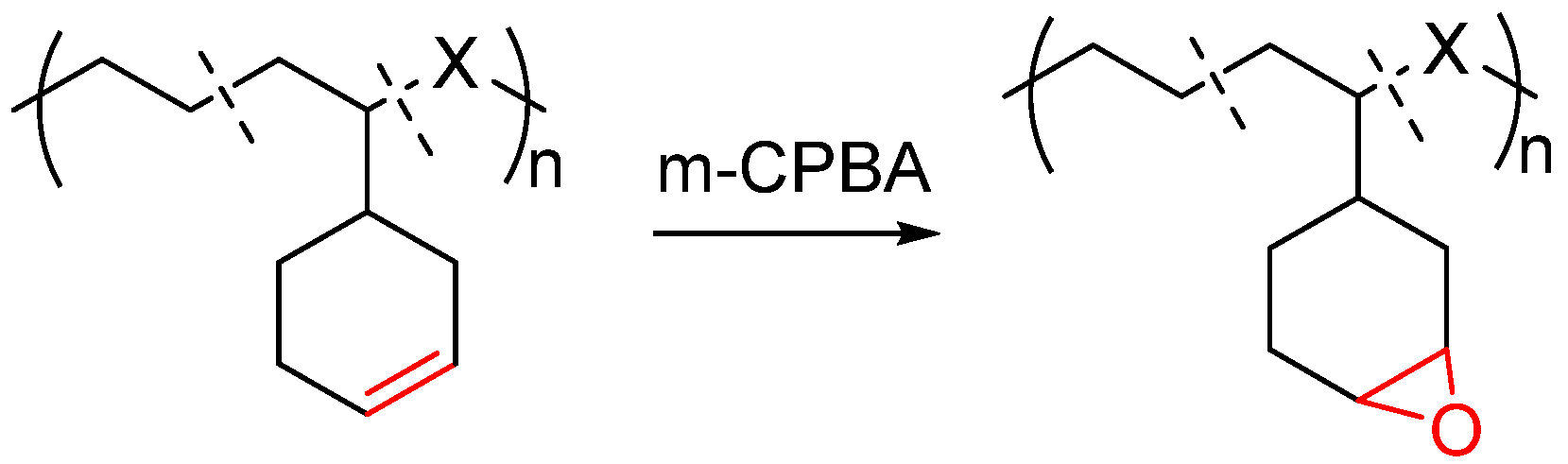
3.2. Synthesis of Functional Poly(α-Olefin) by Post-Functionalization Method
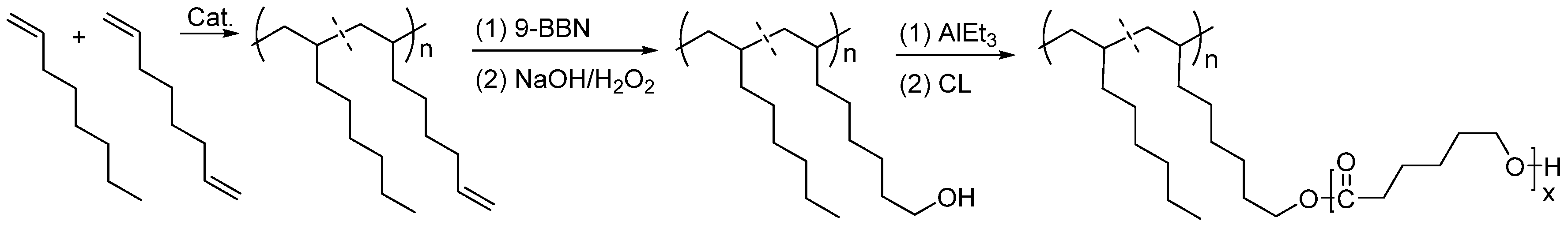
3.3. Synthesis of Functional Ethylene-Propylene Elastomer by Post-Functionalization Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.V.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.W.S.; Teo, J.Y.Q.; Loh, X.J.; Lim, J.Y.C. Polyolefins and polystyrene as chemical resources for a sustainable future: Challenges, advances, and prospects. ACS Mater. Lett. 2021, 3, 1660–1676. [Google Scholar] [CrossRef]
- Stuerzel, M.; Mihan, S.; Muelhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.W.; Yan, S.G.; Yong, T. Sustainable developments in polyolefin chemistry: Progress, challenges, and outlook. Prog. Polym. Sci. 2023, 143, e101713. [Google Scholar]
- Wang, Y.; Wang, Q.; Tan, C.; Chen, C.L. Synthesis of Polar-functionalized Isotactic Polypropylenes Using Commercial Heterogeneous Ziegler–Natta Catalyst. J. Am. Chem. Soc. 2024, 146, 6837–6845. [Google Scholar] [CrossRef]
- Williamson, J.B.; Na, C.G.; Johnson, R.R.; Daniel, W.F.M.; Alexanian, E.J.; Leibfarth, F.A. Chemo- and Regioselective Functionalization of Isotactic Polypropylene: A Mechanistic and Structure–Property Study. J. Am. Chem. Soc. 2019, 141, 12815–12823. [Google Scholar] [CrossRef]
- D’Anania, O.; Stefano, F.D.; Rosa, C.D.; Talarico, G.; Girolamo, R.D. Closing the Loop of Cyclopolymerization of Nonconjugated α,ω-Diolefin Diasteroselectivity and α-Olefin Polymerization Enantioselectivity. ACS Catal. 2024, 14, 16649–16662. [Google Scholar] [CrossRef]
- Williamson, B.J.; Czaplyski, W.L.; Alexanian, J.E.; Leibfarth, A.F. C-H Functionalization of Commodity Polymers. Angew. Chem. Int. Ed. 2018, 57, 6261–6265. [Google Scholar] [CrossRef]
- Kay, J.C.; Goring, D.P.; Burnett, A.C.; Hornby, B.; Lewtas, K.; Morris, S.; Morton, C.; McNally, T.; Theaker, W.G.; Waterson, C.; et al. Polyolefin–Polar Block Copolymers from Versatile New Macromonomers. J. Am. Chem. Soc. 2018, 140, 13921–13934. [Google Scholar] [CrossRef]
- Bunescu, A.; Lee, S.; Li, Q.; Hartwig, F.J. Catalytic Hydroxylation of Polyethylenes. ACS Cent. Sci. 2017, 3, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kermagoret, A.; Debuigne, A.; Jerome, C.; Detrembleur, C. Precision design of ethylene- and polar-monomer-based copolymers by organometallic-mediated radical polymerization. Nat. Chem. 2014, 6, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Franssen, G.M.N.; Reek, H.N.J.; Bruin de, B. Synthesis of functional ‘polyolefins’: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Birajdar, R.S.; Chikkali, S.H. Insertion copolymerization of functional ethylene: Quo Vadis? Eur. Polym. J. 2021, 143, e110183. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, G.; Hu, X.; Jian, Z.B. Recent advances in nickel mediated copolymerization of olefin with polar monomers. Coord. Chem. Rev. 2021, 435, e213802. [Google Scholar] [CrossRef]
- Liu, G.X.; Huang, Z. Recent advances in coordination insertion copolymerization of ethylene with polar functionalized comonomers. Chin. J. Chem. 2020, 38, 1445–1448. [Google Scholar] [CrossRef]
- Keyes, A.; Basbug Alhan, H.E.; Ordonez, E.; Ha, U.; Beezer, D.B.; Dau, H.; Liu, Y.S.; Tsogtgerel, E.; Jones, G.R.; Harth, E. Ethylene and vinyl polar monomers: Bridging the gap for next generation materials. Angew. Chem. Int. Ed. 2019, 58, 12370–12391. [Google Scholar] [CrossRef]
- Chen, C.L. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Chem. Rev. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Bialek, M.; Fryga, J. Copolymerization of Ethylene with Selected Vinyl Monomers Catalyzed by Group 4 Metal and Vanadium Complexes with Multidentate Ligands: A Short Review. Polymers 2021, 13, 4456. [Google Scholar] [CrossRef]
- Okuda, J. Molecular olefin polymerization catalysts: From metallocenes to half-sandwich complexes with functionalized cyclopentadienyl ligands. J. Organomet. Chem. 2020, 1000, 122833. [Google Scholar] [CrossRef]
- Chen, J.Z.; Gao, Y.S.; Marks, T.J. Early Transition Metal Catalysis for Olefin-Polar Monomer Copolymerization. Angew. Chem. Int. Ed. 2020, 59, 14726–14735. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Liu, W.J.; Chen, C.L. Late transition metal catalyzed α-olefin polymerization and copolymerization with polar monomers. Mater. Chem. Front. 2017, 1, 2487–2494. [Google Scholar] [CrossRef]
- Chung, T.C.M. Functional Polyolefins for Energy Applications. Macromolecules 2013, 46, 6671–6698. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Bouyahyi, M.; Duchateau, R. Potential of Functionalized Polyolefins in a Sustainable Polymer Economy: Synthetic Strategies and Applications. Acc. Chem. Res. 2022, 55, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, F.; Villaccio, S.; D’Anania, O.; Talarico, G.; De Rosa, C.; Scoti, M. Combining Cyclic Units and Unsaturated Pendant Groups by Propene/1,5-Hexadiene Copolymerization Toward Functional Isotactic Polypropylene. ACS Macro Lett. 2024, 13, 407–414. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.Y. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823. [Google Scholar] [CrossRef]
- Doremaele, v.G.H.J.; Duin, v.M.; Valla, M.; Berthoud, A. On the Development of Titanium κ1-Amidinate Complexes, Commercialized as Keltan ACE™ Technology, Enabling the Production of an Unprecedented Large Variety of EPDM Polymer Structures. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, Y.K.; Zhang, J.B.; Liu, S.F.; Li, Z.B. Dinuclear Half-Titanocene Complex Bearing an Anthracene-Bridged Bifunctional Alkoxide Ligand: Unprecedented Cooperativity toward Copolymerization of Ethylene with 1-Octene or Norbornene. Macromolecules 2023, 57, 162–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, J.; Zhang, S.; Miao, Q.; Wu, Y. Ethylene/propylene copolymerization catalyzed by half-titanocenes containing monodentate anionic nitrogen ligands: Effect of ligands on catalytic behaviour and structure of copolymer. Polym. Chem. 2018, 9, 48–59. [Google Scholar] [CrossRef]
- Amin, S.B.; Marks, T.J. Alkenylsilane Structure Effects on Mononuclear and Binuclear Organotitanium-Mediated Ethylene Polymerization: Scope and Mechanism of Simultaneous Polyolefin Branch and Functional Group Introduction. J. Am. Chem. Soc. 2007, 129, 2938–2953. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Marks, T.J. Alkenylsilane Effects on Organotitanium-Catalyzed Ethylene Polymerization. Toward Simultaneous Polyolefin Branch and Functional Group Introduction. J. Am. Chem. Soc. 2006, 128, 4506–4507. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Shin, S.M.; Han, C.J.; Kim, S.Y. Chain transfer reaction in metallocene catalyzed ethylene copolymerization with allyltrimethylsilane. Polym. Bull. 1999, 43, 333–340. [Google Scholar] [CrossRef]
- Nomura, K.; Kakinuki, K.; Fujiki, M.; Itagaki, K. Direct Precise Functional Group Introduction into Polyolefins: Efficient Incorporation of Vinyltrialkylsilanes in Ethylene Copolymerizations by Nonbridged Half-Titanocenes. Macromolecules 2008, 41, 8974–8976. [Google Scholar] [CrossRef]
- Liu, J.; Nomura, K. Efficient Functional Group Introduction into Polyolefins by Copolymerization of Ethylene with Allyltrialkylsilane Using Nonbridged Half-Titanocenes. Macromolecules 2008, 41, 1070–1072. [Google Scholar] [CrossRef]
- Wang, W.; Hou, L.; Luo, S.; Zheng, G.; Wang, H. Synthesis and 13C NMR Spectroscopy Analysis of Ethylene Copolymer with High Content of 4-Penten-1-ol. Macromol. Chem. Phys. 2013, 214, 2245–2249. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Yan, Q.; Nomura, K. The Effect of SiMe3 and SiEt3 Para Substituents for High Activity and Introduction of a Hydroxy Group in Ethylene Copolymerization Catalyzed by Phenoxide-Modified Half-Titanocenes. Angew. Chem. Int. Ed. 2020, 59, 23072–23076. [Google Scholar] [CrossRef]
- Jiang, Y.; Shimoyama, D.; Gao, J.; Nomura, K. Synthesis of ethylene copolymers with 2-allylphenol by using half-titanocene catalysts containing SiEt3-, SiiPr3-substituted phenoxide ligands, Cp*TiCl2(O-2,6-iPr2-4-SiR3-C6H2) (R = Et, iPr). Catal. Sci. Technol. 2024, 14, 3800–3806. [Google Scholar] [CrossRef]
- Zanchin, G.; Leone, G. Polyolefin Thermoplastic Elastomers from Polymerization Catalysis: Advantages, Pitfalls and Future Challenges. Prog. Polym. Sci. 2021, 113, 101342. [Google Scholar] [CrossRef]
- Wei, C.Z.; Guo, L.L.; Zhu, C.; Cui, C.M. Boryloxy Titanium Complex-Enabled High Polar Monomer Contents in Catalytic Copolymerization of Olefins. Angew. Chem. Int. Ed. 2024, e202414464. [Google Scholar] [CrossRef]
- Itagaki, K.; Nomura, K. Efficient Synthesis of Functionalized Polyolefin by Incorporation of 4-Vinylcyclohexene in Ethylene Copolymerization Using Half-Titanocene Catalysts. Macromolecules 2009, 42, 5097–5103. [Google Scholar] [CrossRef]
- Kitphaitun, S.; Chaimongkolkunasin, S.; Manit, J.; Makino, R.; Kadota, J.; Hirano, H.; Nomura, K. Ethylene/Myrcene Copolymers as New Bio-Based Elastomers Prepared by Coordination Polymerization Using Titanium Catalysts. Macromolecules 2021, 54, 10049–10058. [Google Scholar] [CrossRef]
- Kawamura, K.; Nomura, K. Ethylene Copolymerization with Limonene and β-Pinene: New Bio Based Polyolefins Prepared by Coordination Polymerization. Macromolecules 2021, 54, 4693–4703. [Google Scholar] [CrossRef]
- Ren, X.; Guo, F.; Fu, H.; Song, Y.; Li, Y.; Hou, Z. Scandium catalyzed copolymerization of myrcene with ethylene and propylene: Convenient syntheses of versatile functionalized polyolefins. Polym. Chem. 2018, 9, 1223–1233. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.; Fujiki, M.; Takemoto, A. Facile, Efficient Functionalization of Polyolefins via Controlled Incorporation of Terminal Olefins by Repeated 1,7-Octadiene Insertion. J. Am. Chrm. Soc. 2007, 129, 14170–14171. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chen, X.Z.; Zhang, Y.X. Epoxidation of ethylene propylene diene rubber by t-butyl hydroperoxide in the presence of molybdenum oxide. React. Funct. Polym. 2001, 47, 93–99. [Google Scholar] [CrossRef]
- Kim, I.; Kang, P.S.; Ha, C. Efficient graft-from functionalization of ethylene-propylene-diene rubber (EPDM) dissolved in hexane. React. Funct. Polym. 2005, 64, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zha, H.; Cheng, P.Y.; Zhang, S.; Liu, X.W.; Wu, Y.X. Vanadium(V) Complexes Containing Unsymmetrical N-Heterocyclic Carbene Ligands: Highly Efficient Synthesis and Catalytic Behavior towards Ethylene/Propylene Copolymerization. Chin. J. Polym. Sci. 2024, 42, 32–41. [Google Scholar] [CrossRef]
- Thakur, V.; Shan, C.L.P.; Li, G.; Han, T.; Doelder, J.D. Sponge EPDM by design. Plast. Rubber Compos. 2019, 48, 32–41. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.L.; Xiang, B.; Liu, X.W.; Zhang, S.; Wu, Y.X. Imidazolidin-2-iminato vanadium complexes for the synthesis of ethylene/propylene/5-ethylidene 2-norbornene (ENB) terpolymers with high ENB incorporation and ultra-high molecular weight. Polym. Chem. 2024, 15, 2148–2156. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, P.; Zhang, Z.; Fan, K.; Lu, R.; Zhang, S.; Wu, Y. Highly Efficient Terpolymerization of Ethylene/Propylene/ENB with Half-Titanocene Cayalytic System. Polym. Chem. 2021, 12, 6417–6425. [Google Scholar] [CrossRef]
- Windmuller, H.J.P.; van Doremaele, J.H.G. Process for the Production of a Polymer Comprising Monomeric Units of Ethylene, an A-Olefin and a Vinyl Norbornene. WO 2005005496 A2, 20 January 2005. [Google Scholar]
- von Haken, E.R.S.; Stephan, W.D.; Brown, J.S.; Jeremin, D.; Wang, Y.Q. Cyclopentadienyl/Phosphinimine Catalyst with One and Only One Activatable Ligand. WO 0005236, 3 February 2000. [Google Scholar]
- Stephan, D.W.; Stewart, J.C.; Guérin, F.; Courtenay, S.; Kickham, J.; Hollink, E.; Beddie, C.; Hoskin, A.; Graham, T.; Wei, P.R.; et al. An Approach to Catalyst Design: Cyclopentadienyl-Titanium Phosphinimide Complexes in Ethylene Polymerization. Organometallics 2003, 22, 1937–1947. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dong, H. Recent Advancements in the Synthesis of Functional Polyolefins by Non-Bridged Half-Titanocenes. Molecules 2025, 30, 39. https://doi.org/10.3390/molecules30010039
Chen Y, Dong H. Recent Advancements in the Synthesis of Functional Polyolefins by Non-Bridged Half-Titanocenes. Molecules. 2025; 30(1):39. https://doi.org/10.3390/molecules30010039
Chicago/Turabian StyleChen, Yanjun, and Haiqian Dong. 2025. "Recent Advancements in the Synthesis of Functional Polyolefins by Non-Bridged Half-Titanocenes" Molecules 30, no. 1: 39. https://doi.org/10.3390/molecules30010039
APA StyleChen, Y., & Dong, H. (2025). Recent Advancements in the Synthesis of Functional Polyolefins by Non-Bridged Half-Titanocenes. Molecules, 30(1), 39. https://doi.org/10.3390/molecules30010039





