Abstract
Efficient access to pyranoisoquinoline derivatives via rhodium-catalyzed double C-H functionalization of phenyl oxadiazoles and diazo compounds has been developed. Two C-C bonds and one C-O and C-N bond formation was realized by this tandem reaction, along with the formation of two heterocycles, affording diversified pyran-fused isoquinolines in moderate to good yields with broad functional group tolerance under mild reaction conditions.
1. Introduction
As a representative fused heterocyclic scaffold, pyranoisoquinoline derivatives are ubiquitous in bioactive compounds and natural products. For instance (Figure 1), Pranoprofen (I) and Amlexanox (II) are marketed anti-inflammatory drugs [1,2]. Compound III inhibits mitogen-activated protein kinase 2 (MK-2) and suppresses expression of TNFa in U937 cells [3]. Compound IV has potential activity for the treatment of Alzheimer’s disease [4].
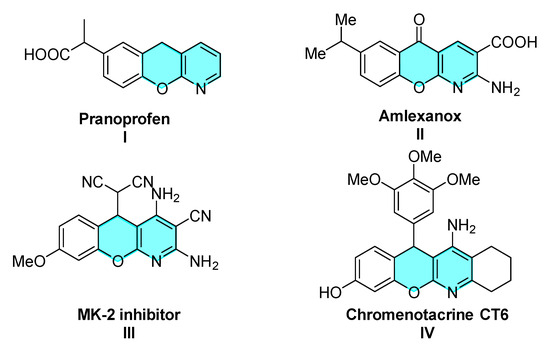
Figure 1.
Representative molecules.
Thus, the development of efficient methods for accessing such frameworks has attracted much more attention from the synthetic community. For example, in 2018, Cheng’s group developed a rhodium-catalyzed functionalization of aromatic C-H bonds to obtain a series of pyrano and isoquinoline derivatives with good optical properties [Scheme 1a] [5]. The group also developed an asymmetric double six-membered ring compound of heteroaryl and alkynes [Scheme 1b] [6]. You et al. reported a strategy for the synthesis of bicycles via Rh-catalyzed activation of double C-H bonds of benzamides [Scheme 1c] [7]. Maheswari developed a cascade strategy for the assembly of 3,4-dihydrophenanthridines using high-valent iodonium ylides as a carbene precursor [Scheme 1d] [8]. The yield of pyran-fused isoquinolines obtained via the reported literature methods is low and requires nitrogen protection, leading to the complex synthesis of pyran-fused isoquinolines. In 2022, Shang’s group reported the formation of a pyrano [4,3,2-ij]isoquinoline skeleton using bis-orthorhombic-C-H activation of methyl benzimidazolate and subsequent N,O-bicycloaddition with a-carbonyl diazides. However, AcCl, the raw material for the synthesis of methyl benzimidazolate, is highly toxic. The yields of pyran-fused isoquinolines synthesized by this reaction are generally low and the atom economy is poor [Scheme 1e] [9]. Therefore, it is necessary to further explore the low-toxicity and high-efficiency method to prepare pyran-fused isoquinolines. The unique physicochemical properties of pyranoisoquinolines have inspired more synthetic workers to develop new synthetic strategies and obtain molecules with diverse structures.
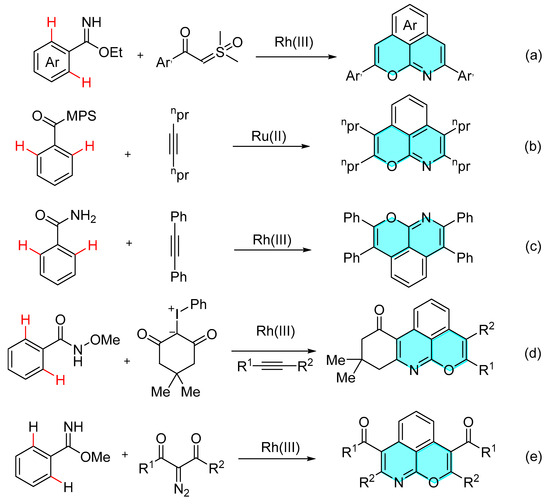
Scheme 1.
Different raw materials and catalysts of reported construction for chromopyridine skeleton. (a) Ethyl benzoate and α-aryl sulfoximines, Rh-catalyst. (b) Sulfoximines and alkyne compounds, Ru- catalyst. (c) Benzamide and arylacetylene, Rh-catalyst. (d) N-Methoxybenzamide, hypervalent iodonium ylides and alkyne, Rh-catalyst. (e) Methyl benzimidazolate and diazo compounds, Rh-catalyst.
Due to their unique structure, oxadiazoles are not only used as the cores of bioactive compounds as well as functional materials but are also applied as directing groups in the field of catalytic C-H bond activation. For example, in 2017, Zhu’s group used cobalt(III) to catalyze the construction of 1-aminoisoquinolines by oxadiazole [Scheme 2a] [10]. Later on, they reported a Rh(III)-catalyzed coupling of alkenyl oxadiazoles with alkynes for the synthesis of 2-acylamino acids and 2-aminopyridines, which are important heterocyclic backbones in a variety of natural products and synthetic drugs with readily reactive functional groups [Scheme 2b] [11]. In 2018, Dong and colleagues developed an efficient Rh(III)-catalyzed one-pot synthesis of indene derivatives from oxadiazole and allyl alcohols [Scheme 2c] [12]. In 2023, Dong’s group developed access to isoquinoline compounds with good chemo- and regioselectivity via Rh(III)-catalyzed [4+2] cycloaddition reactions of oxadiazoles and difluoromethylene alkynes [Scheme 2d] [13]. The reaction type of oxadiazole involved in the above report is a simple ortho-C-H activation reaction, which generally retains the unexpected structure and does not conform to the principle of atomic economy. Herein, we report a Rh-catalyzed double C-H activation of aryl oxadiazoles with diazo compounds. The reaction method is simple, the raw materials are easy to obtain, and it does not require N2 conditions. The yields of the prepared product are high. This reaction provides efficient access to obtain the pyranoisoquinoline skeleton [Scheme 2e].
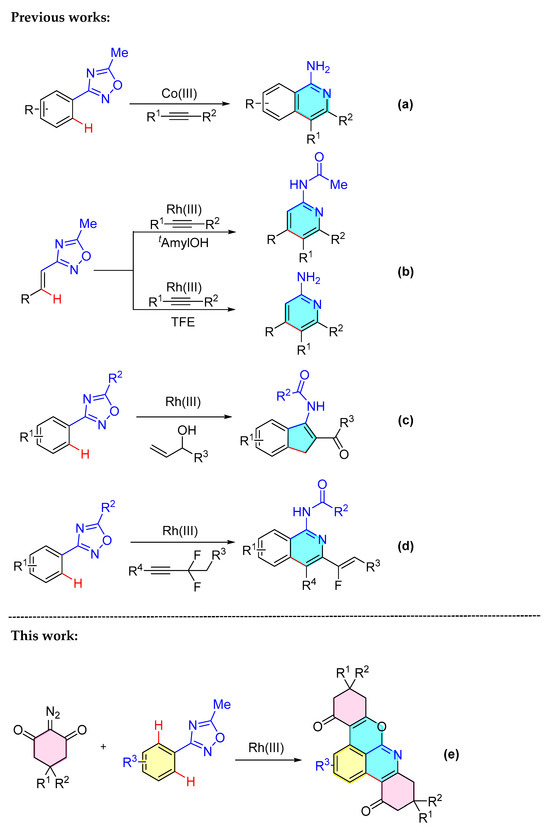
Scheme 2.
Transition-metal-catalyzed oxadiazole-directed C–H bond functionalization reactions. (a) Co-catalyzed reaction of oxadiazoles with alkynes. (b) Rh-catalyzed reaction of oxadiazoles with alkynes. (c) Rh-catalyzed reactions of oxadiazoles with allylic alcohols. (d) Rh-catalyzed reaction of oxadiazoles with difluoromethylene alkynes. (e) Rh-catalyzed bicyclisation of oxadiazoles with diazides.
2. Results and Discussion
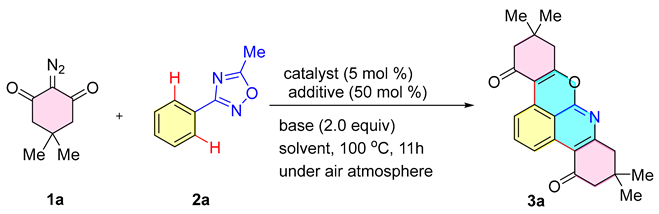
This research was started with 2-diazo-5,5-dimethylcyclohexane-1,3-dione (1a) and 5-methyl-3-phenyl-1,2,4-oxadiazole (2a) as the model substrates (Table 1). A screening of the catalysts was carried out. First, the expected reaction to generate the chromopyridine product 3a could be in [Cp*RhCl2]2 (5 mol %) as the catalyst (Table 1, entries 1–6). Other additives, including AgSbF6, AgOAc, AgBF4, and AgOTf, failed to promote the reaction, resulting in a significant decrease in reaction yield (Table 1, entries 7–10). Satisfactory yields of 3a can also be obtained by reducing or increasing the content of additives (Table 1, entries 17 and 18). Common solvents such as toluene, acetonitrile, and DCE failed to improve product yields (Table 1, entries 12–14). For reaction temperatures of 90 °C and 110 °C, the isolated yields of product 3a were 77% and 75%, respectively (Table 1, entries 19 and 20). No obvious change was revealed in the reaction after further optimization (Table 1, entries 21 and 22). The reaction yield was 39% when AgOAc was used as a base. The NaOAc and KOAc did not promote the reaction when used as bases (Table 1, entries 23–25). The reaction yield gradually increased with an increasing amount of Cu(OAc)2·H2O (Table 1, entries 26–28). The reaction yield was highest when the amount of base reached 2.0 equiv (entry 6), after which the reaction yield remained constant with an increasing amount (entry 29). The reaction yields gradually increased with the amount of AgNTf2 additive increasing (Table 1, entries 30–33). When the amount of AgNTf2 reached a certain amount, the reaction yield declined instead of increasing (Table 1, entry 34).

Table 1.
Optimization of the reaction conditions a.
The interaction range of oxadiazole 2 with cyclic diazo compounds was examined under optimal conditions (Scheme 3). Typically, substrate 2 with both electron-donating (Me, Bu, OMe, and SMe) and electron-absorbing groups (F, Cl, and Br) was successfully converted to the desired chromopyridine products 3a–3h in good yields (75–95%). When R3 was CF3 or Ph, the corresponding yields of products 3i and 3j were 68% and 70%, respectively. In addition, the reaction of oxadiazoles with cyclic diazonium compounds yielded 3k and 3l in 85% and 83% yields when the oxadiazole interstitials were linked to the substituted OMe and F. The reaction of different types of diazonium compounds with oxadiazole 2 also proceeded well, giving the target products 3m–3t in 80–95% yields. Single-crystal characterization determined the structure of 3a [14]. However, 3-phenyl-1,2,4-oxadiazole with other substituents could not give the desired product in this reaction. Due to the steric exclusion of the 3-phenyl-1,2,4-oxadiazole substituent group, the insertion of the diazo compound in the neighboring position was prevented, which is not conducive to the formation of Rh-carbene.
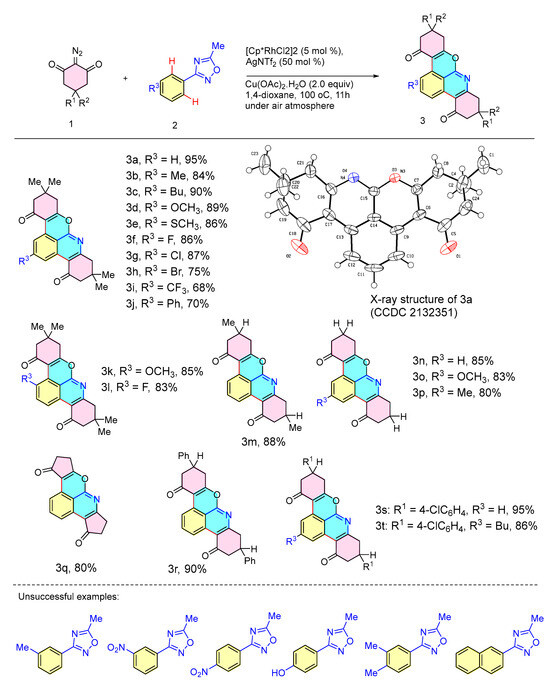
Scheme 3.
Substrate scope of cyclic 2-diazo-1,3-diketones with oxadiazole a,b. a Reagents and conditions: cyclic 2-diazo compound 1 (0.35 mmol), oxadiazole 2 (0.1 mmol), [Cp*RhCl2]2 (5 mol %), AgNTf2 (50 mol %), and Cu(OAc)2·H2O (2.0 equiv) in 1,4-dioxane (3 mL) at 100 °C under air atmosphere for 11 h. b Isolated yields.
The reaction was scaled up to 4 mmol (scaled up to 40 times) to yield the desired product 3a in 82% yield (Scheme 4). To demonstrate the utility of product 3, its reactivity was then investigated in palladium-catalyzed cross-coupling reactions. As shown in the results in Scheme 5, the generated chromium pyridine compound 3h reacted smoothly with styrene, phenylacetylene, phenylboronic acid, diphenylamine, and bis(pinacolato)diboron to obtain the corresponding products 4, 5, 6, 7, and 8 in 88%, 91%, 93%, 89%, and 89% yields, respectively. The generated chromium pyridine compound 3h could also be reduced by NaBH4 to obtain product 9 in 95% yields.
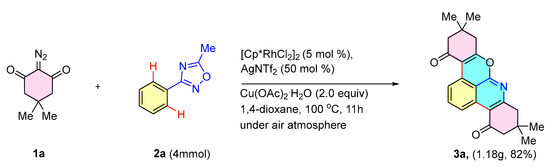
Scheme 4.
Gram-scale reaction.
Scheme 5.
Examples of further synthetic transformations.
Some of the products we prepared are partially similar in structure to those prepared by the previous Shang group, for example, products 3a and 3b [9]. Other products that they did not obtain include 3q, 3r, and 3s. We were able to synthesis pyran-fused isoquinolines using cyclic diazides with different substituents. We report a Rh-catalyzed double C-H activation reaction of an aryl oxadiazole with a diazo compound. Compared with previous reports, our works have the advantages of easy availability of feedstock, no need to be carried out under nitrogen atmosphere, and high yield. Most of the synthesized compounds have bright fluorescence. The fluorescence emission range of these pyran-fused isoquinoline compounds is very wide, which indicates that they have a wide range of applications in biological imaging, organic light-emitting diodes, optoelectronic devices, fluorescent sensors, and biomedical imaging [5,6,9].
Based on the experimental results and literature reports, a possible reaction mechanism was proposed in Scheme 6. Initially, [Cp*RhCl2]2 undergoes anion exchange in the presence of AgNTf2 and Cu(OAc)2·H2O to generate a Rh(III)-active catalyst. The active catalyst and substrate 2a were activated by the C-H bond to form a five-membered rhodium ring as intermediate A. Subsequently, the cyclic 2-diazo-1,3-diketone 1a coordinates with intermediate A to form intermediate B by releasing N2. Then, migratory insertion of carbine into Rh-C bond completes the C-C coupling and affords the six-membered intermediate C, which can be protonated with acetic acid to generate intermediate D and release the Rh(III)-catalyst. Intermediate E is an isomer of intermediate D. Repeating the above steps, intermediate G was obtained. Intermediate F is an isomer of intermediate G. Intermediate G coordinates with the Rh(III)-active substance to generate intermediate H. Intermediate H undergoes an elimination reaction to eliminate H2O and obtain intermediate I. The Rh remains trivalent throughout and does not undergo a valence state change. Cu does not participate in the reaction in this process. Subsequently, intermediate I undergoes electron transfer to generate intermediate J [11,12,13]. In addition, complex J undergoes reductive elimination to form the C-N bond to generate complex K and generate Rh(I). Rh(I) is reoxidized to Rh(III) in the next catalytic cycle using the N-O bond cleavage as the internal oxidant [10,11,15]. Intermediate K undergoes oxidation by Cu(OAc)2·H2O followed by an intramolecular nucleophilic addition reaction to produce the desired product 3a [16].
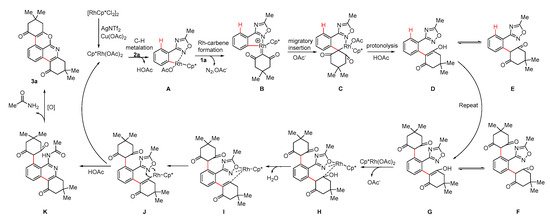
Scheme 6.
Proposed reaction mechanism. (A–C) Rhodium ring intermediate. (D) Unimolecular insertion product intermediate. (E) An isomer of D. (G) Bimolecular insertion intermediate. (F) An isomer of G. (H–J) Rhodium ring intermediates. (K) Unimolecular cyclisation and insertion intermediate.
3. Materials and Methods
3.1. General Methods (Chemistry)
The general methods are described in the Supplementary Materials. The 500 MHz NMR spectrometer (AVANCE IIITM HD 500), the 400 MHz NMR spectrometer (AVANCENEO 400) and the X-ray single crystal diffractometer (D8 VENTURE) are from Bruker, Germany. The company is located in Wurzbach in the Berlin region of eastern Germany. NMR analysis was carried out at 298 K. High Resolution Mass measurement was performed on Agilent QTOF 6520 mass spectrometer with electron spray ionization (ESI or APCI) as the ion source (Agilent Technologies, Santa Clara, CA, USA). The melting points were measured using SGWX-4 melting point apparatus and were not corrected (Zhejiang NADE Scientific Instrument Co., Ltd., Hangzhou, China). The software is ChemBioDraw Ultra (ACS Document 1996) and MestReNova (15.0).
3.2. General Procedures for the Preparation of Compounds 3
A Schlenk tube was equipped with a magnetic stir bar and charged with substituted 2-diazo-1,3-dione 1 (0.35 mmol), 2 (0.1 mmol), [Cp*RhCl2]2 (5 mol %), Cu(OAc)2·H2O (2.0 equiv), and AgNTf2 (0.5 equiv.), which were stirred in 1,4-dioxane (3.0 mL) under atmosphere at 100 °C for 11 h. After completion, the reaction mixture was purified by flash chromatography, eluting with ethyl acetate and petroleum ether to give product 3 as a yellow solid.
- 6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3a), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 34.4 mg, yield: 95%; m.p 259–260 °C. 1H NMR (500 MHz, CDCl3) δ 9.20 (dd, J = 8.7, 0.9 Hz, 1H), 8.80 (dd, J = 7.7, 0.9 Hz, 1H), 7.86 (dd, J = 8.7, 7.7 Hz, 1H), 3.03 (s, 2H), 2.80 (s, 2H), 2.60 (s, 2H), 2.53 (s, 2H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 199.4, 197.0, 168.0, 161.4, 159.8, 136.9, 136.0, 127.8, 123.5, 120.8, 117.6, 116.2, 112.2, 54.3, 53.1, 47.9, 42.7, 33.0, 32.3, 28.6, 28.5. HRMS (ESI) m/z: [M + H]+ calcd for C23H24NO3+ 362.1751; found 362.1753.
- 2,6,6,11,11-pentamethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3b), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 31.5 mg, yield: 84%; m.p 256–257 °C. 1H NMR (500 MHz, CDCl3) δ 9.03 (s, 1H), 8.65 (s, 1H), 3.04 (s, 2H), 2.78 (s, 2H), 2.59–2.58 (m, 5H), 2.52 (s, 2H), 1.18 (s, 6H), 1.13 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 199.6, 197.1, 168.2, 161.7, 159.7, 147.5, 137.1, 127.6, 123.1, 122.3, 117.2, 114.4, 112.1, 54.3, 53.2, 47.9, 42.7, 32.9, 32.3, 28.6, 28.5, 23.8. HRMS (ESI) m/z: [M + H]+ calcd for C24H26NO3+ 376.1907; found 376.1906.
- 2-butyl-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3c), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 37.5 mg, yield: 90%; m.p 196–197 °C. 1H NMR (500 MHz, CDCl3) δ 9.02 (d, J = 1.5 Hz, 1H), 8.66 (d, J = 1.4 Hz, 1H), 2.99 (s, 2H), 2.86–2.78 (m, 2H), 2.77 (s, 2H), 2.58 (s, 2H), 2.51 (s, 2H), 1.74–1.59 (m, 2H), 1.43–1.35 (m, 2H), 1.18 (s, 6H), 1.13 (s, 6H), 0.93 (t, J = 7.4 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 199.6, 197.2, 168.1, 161.7, 159.7, 152.4, 137.1, 127.6, 122.6, 121.8, 117.3, 114.6, 112.2, 54.3, 53.2, 48.0, 42.7, 37.8, 34.0, 33.0, 32.3, 28.6, 28.5, 23.0, 14.4. HRMS (ESI) m/z: [M + H]+ calcd for C27H32NO3+ 418.2377; found 418.2368.
- 2-methoxy-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3d), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 34.8 mg, yield: 89%; m.p 260–261 °C. 1H NMR (500 MHz, CDCl3) δ 8.70 (d, J = 2.4 Hz, 1H), 8.41 (d, J = 2.4 Hz, 1H), 3.96 (s, 3H), 2.98 (s, 2H), 2.77 (s, 2H), 2.58 (s, 2H), 2.51 (s, 2H), 1.18 (s, 6H), 1.12 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 199.6, 196.9, 168.5, 166.1, 162.6, 159.2, 139.3, 129.4, 116.8, 111.7, 111.5, 111.3, 104.0, 56.1, 54.3, 53.0, 48.0, 42.7, 32.9, 32.3, 28.6, 28.5. HRMS (ESI) m/z: [M + H]+ calcd for C24H26NO4+ 392.1856; found 392.1853.
- 6,6,11,11-tetramethyl-2-(methylthio)-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3e), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 35.1 mg, yield: 86%; m.p 305–306 °C. 1H NMR (400 MHz, CDCl3) δ 9.04 (d, J = 1.5 Hz, 1H), 8.72 (d, J = 1.5 Hz, 1H), 3.00 (s, 2H), 2.79 (s, 2H), 2.62 (s, 3H), 2.59 (s, 2H), 2.52 (s, 2H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 199.1, 196.5, 168.3, 162.0, 150.5, 136.6, 127.0, 117.8, 116.6, 115.9, 112.9, 111.1, 53.9, 52.7, 47.6, 42.4, 32.5, 31.9, 28.1, 28.0, 14.7. HRMS (ESI) m/z: [M + H]+ calcd for C24H26NO3S+ 408.1628; found 408.1625.
- 2-fluoro-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3f), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 32.6 mg, yield: 86%; m.p 235–236 °C. 1H NMR (500 MHz, CDCl3) δ 8.94 (dd, J = 11.9, 2.5 Hz, 1H), 8.62 (dd, J = 10.6, 2.5 Hz, 1H), 3.03 (s, 2H), 2.82 (s, 2H), 2.60 (s, 2H), 2.54 (s, 2H), 1.20 (s, 6H), 1.14 (s, 6H). 13C NMR (100 MHz, CDCl3) δ198.8, 196.2, 168.7 (d, JC,F = 27.0 Hz), 166.4, 162.3, 138.8, 130.5 (d, JC,F = 13.7 Hz), 117.1, 113.1, 110.2 (d, JC,F = 29.4 Hz), 108.7, 108.4, 53.7, 52.5, 47.5, 42.3, 32.6, 32.0, 28.2, 28.1. HRMS (ESI) m/z: [M + H]+ calcd for C23H23FNO3+ 380.1656; found 380.1665.
- 2-chloro-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3g), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 34.4 mg, yield: 87%; m.p 234–235 °C. 1H NMR (500 MHz, CDCl3) δ 9.27 (d, J = 1.9 Hz, 1H), 8.84 (d, J = 1.9 Hz, 1H), 3.03 (s, 2H), 2.81 (s, 2H), 2.60 (s, 2H), 2.53 (s, 2H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 199.1, 196.6, 168.8, 162.5, 159.5, 143.6, 137.6, 129.2, 122.9, 121.5, 116.8, 114.4, 111.3, 54.1, 52.9, 47.9, 42.7, 32.9, 32.3, 28.6, 28.5. HRMS (ESI) m/z: [M + H]+ calcd for C23H23ClNO3+ 396.1361; found 396.1369.
- 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3h), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 32.9 mg, yield: 75%; m.p 259–260 °C. 1H NMR (500 MHz, CDCl3) δ 9.43 (s, 1H), 8.97 (s, 1H), 3.03 (s, 2H), 2.81 (s, 2H), 2.60 (s, 2H), 2.53 (s, 2H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 198.7, 196.2, 168.5, 166.3, 162.2, 159.0, 130.4, 117.0, 113.0, 111.2, 110.2, 110.0, 108.7, 108.4, 53.7, 52.5, 47.5, 42.3, 32.5, 31.9, 28.2, 28.1. HRMS (ESI) m/z: [M + H]+ calcd for C23H23BrNO3+ 440.0856; found 440.0853.
- 6,6,11,11-tetramethyl-2-(trifluoromethyl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3i), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 29.2 mg, yield: 68%; m.p 240–241 °C. 1H NMR (400 MHz, CDCl3) δ 9.56 (dd, J = 1.7, 0.8 Hz, 1H), 9.08 (d, J = 1.6 Hz, 1H), 3.07 (s, 2H), 2.83 (s, 2H), 2.63 (s, 2H), 2.56 (s, 2H), 1.21 (s, 6H), 1.15 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 198.7, 196.1, 168.5, 162.0, 159.0, 136.2, 128.7, 127.8, 120.3 (q, JC,F = 5.6 Hz), 117.5, 116.5 (q, JC,F = 3.8 Hz), 111.2, 53.7, 52.5, 47.4, 42.3, 32.6, 31.9, 28.1, 28.0. HRMS (ESI) m/z: [M + H]+ calcd for C24H23F3NO3+ 430.1625; found 430.1624.
- 6,6,11,11-tetramethyl-2-phenyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3j), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 30.6 mg, yield: 70%; m.p 280–281 °C. 1H NMR (400 MHz, CDCl3) δ 9.51 (d, J = 1.6 Hz, 1H), 9.14 (d, J = 1.6 Hz, 1H), 7.82–7.79 (m, 2H), 7.51–7.47 (m, 2H), 7.44–7.40 (m, 1H), 3.04 (s, 2H), 2.81 (s, 2H), 2.62 (s, 2H), 2.54 (s, 2H), 1.20 (s, 6H), 1.16 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 199.0, 196.6, 167.9, 161.5, 159.3, 148.1, 140.5, 136.9, 128.9, 128.5, 128.0, 127.8, 121.2, 119.6, 117.3, 114.7, 111.8, 53.9, 52.7, 47.6, 42.3, 32.6, 31.9, 28.2, 28.1. HRMS (ESI) m/z: [M + H]+ calcd for C29H28NO3+ 438.2064; found 438.2066.
- 3-methoxy-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3k), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 33.3 mg, yield: 85%; m.p 258–259 °C. 1H NMR (500 MHz, CDCl3) δ 8.78 (d, J = 8.6 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 3.96 (s, 3H), 2.96 (s, 2H), 2.75 (s, 2H), 2.69 (s, 2H), 2.50 (s, 2H), 1.18 (s, 6H), 1.16 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 197.2, 197.0, 166.4, 159.3, 158.2, 153.3, 126.6, 122.0, 121.5, 120.2, 118.1, 115.7, 112.0, 56.5, 54.0, 53.0, 47.4, 42.5, 34.2, 32.4, 29.2, 28.6. HRMS (ESI) m/z: [M + H]+ calcd for C24H26NO4+ 392.1856; found 392.1857.
- 3-fluoro-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3l), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 31.5 mg, yield: 83%; m.p 234–235 °C. 1H NMR (500 MHz, CDCl3) δ 9.43 (s, 1H), 9.97 (s, 1H), 3.03 (s, 2H), 2.81 (s, 2H), 2.60 (s, 2H), 2.53 (s, 2H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 196.8, 196.3, 167.4, 159.5 (d, JC,F = 58.8 Hz), 156.6, 154.6, 126.2, 124.6 (t, JC,F = 12.5 Hz), 122.1 (d, JC,F = 7.8 Hz), 121.5 (d, JC,F = 22.5 Hz), 119.4, 118.3, 117.6, 111.8, 53.7, 53.0, 47.6, 42.5, 34.0, 32.3, 28.9, 28.6. HRMS (ESI) m/z: [M + H]+ calcd for C23H23FNO3+ 380.1656; found 380.1658.
- 6,11-dimethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3m), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 29.3 mg, yield: 88%; m.p 241–242 °C. 1H NMR (500 MHz, CDCl3) δ 9.19 (d, J = 8.6 Hz, 1H), 8.81 (d, J = 7.4 Hz, 1H), 7.85 (t, J = 8.2 Hz, 1H), 3.27–3.08 (m, 1H), 3.02–2.91 (m, 1H), 2.92–2.77 (m, 2H), 2.76–2.60 (m, 2H), 2.55–2.27 (m, 4H), 1.21–1.18 (m, 6H). 13C NMR (125 MHz, CDCl3) δ 199.4, 197.0, 169.0, 162.4, 159.4, 137.0, 136.0, 127.9, 123.6, 120.9, 118.2, 116.2, 112.8, 48.8, 47.4, 42.3, 37.0, 29.3, 28.0, 21.5, 21.2. HRMS (ESI) m/z: [M + H]+ calcd for C21H20NO3+ 334.1438; found 334.1447.
- 6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3n), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 25.9 mg, yield: 85%; m.p 239–240 °C. 1H NMR (500 MHz, CDCl3) δ 9.19 (d, J = 8.7 Hz, 1H), 8.81 (d, J = 7.7 Hz, 1H), 7.85 (dd, J = 8.6, 7.8 Hz, 1H), 3.15 (t, J = 6.2 Hz, 2H), 2.95 (t, J = 6.3 Hz, 2H), 2.83–2.71 (m, 2H), 2.70–2.57 (m, 2H), 2.37–1.99 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 199.4, 197.0, 169.5, 163.0, 159.2, 137.7, 136.0, 128.0, 123.7, 121.1, 118.6, 116.2, 113.2, 40.7, 39.2, 34.1, 29.2, 22.0, 20.4. HRMS (ESI) m/z: [M + H]+ calcd for C19H16NO3+ 306.1125; found 306.1134.
- 2-methoxy-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3o), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 27.8 mg, yield: 83%; m.p 309–310 °C. 1H NMR (400 MHz, CDCl3) δ 8.74 (d, J = 2.4 Hz, 1H), 8.46 (d, J = 2.4 Hz, 1H), 3.98 (s, 3H), 3.12 (t, J = 6.2 Hz, 2H), 2.94 (t, J = 6.3 Hz, 2H), 2.75–2.72 (m, 2H), 2.68–2.64 (m, 2H), 2.21–2.16 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 199.2, 196.5, 169.6, 165.7, 163.8, 158.3, 139.2, 129.2, 117.4, 112.3, 111.4, 111.0, 104.0, 55.7, 40.3, 38.8, 33.9, 28.8, 21.7, 19.9. HRMS (ESI) m/z: [M + H]+ calcd for C20H18NO4+ 336.1230; found 336.1232.
- 2-methyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3p), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 25.5 mg, yield: 80%; m.p 328–329 °C. 1H NMR (400 MHz, CDCl3) δ 8.99 (s, 1H), 8.63 (s, 1H), 3.12 (t, J = 6.4 Hz, 2H), 2.93 (t, J = 6.4 Hz, 2H), 2.73 (t, J = 6.4 Hz, 2H), 2.66 (t, J = 6.4 Hz, 2H), 2.57 (s, 3H), 2.20–2.17 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 199.1, 196.7, 169.2, 162.9, 158.7, 147.0, 136.9, 127.2, 122.8, 122.1, 117.7, 114.0, 112.6, 40.3, 38.9, 33.7, 28.8, 23.4, 21.6, 19.9. HRMS (ESI) m/z: [M + H]+ calcd for C20H18NO3+ 320.1281; found 320.1286.
- 5,6,9,10-tetrahydrocyclopenta[5,6]pyrano[4,3,2-ij]cyclopenta[c]isoquinoline-4,11-Dione (3q), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 22.2 mg, yield: 80%; m.p 214–215 °C. 1H NMR (500 MHz, CDCl3) δ 8.62 (d, J = 8.3 Hz, 1H), 8.19 (d, J = 7.3 Hz, 1H), 7.86 (t, J = 7.9 Hz, 1H), 3.44–3.14 (m, 2H), 3.17–2.90 (m, 2H), 2.85–2.80 (m, 2H), 2.80–2.75 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 204.7, 200.5, 180.9, 174.6, 163.3, 136.0, 134.9, 126.8, 121.7, 121.4, 118.8, 117.0, 115.5, 36.5, 34.9, 29.2, 26.0. HRMS (ESI) m/z: [M + H]+ calcd for C17H12NO3+ 278.0812; found 278.0816.
- 6,11-diphenyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3r), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 41.1 mg, yield: 90%; m.p 263–264 °C. 1H NMR (500 MHz, CDCl3) δ 9.26 (d, J = 8.6 Hz, 1H), 8.88 (d, J = 7.7 Hz, 1H), 7.91 (t, J = 8.2 Hz, 1H), 7.42–7.38 (m, 4H), 7.36–7.29 (m, 6H), 3.59 (d, J = 14.8 Hz, 2H), 3.51–3.28 (m, 2H), 3.19 (d, J = 8.0 Hz, 2H), 3.11–3.02 (m, 1H), 3.02–2.76 (m, 3H). 13C NMR (125 MHz, CDCl3) δ 198.1, 195.7, 168.2, 161.8, 159.2, 142.6, 141.4, 136.6, 135.8, 129.1, 128.9, 127.5, 127.4, 127.1, 126.7, 126.6, 123.4, 120.8, 117.9, 112.6, 47.1, 45.8, 41.0, 39.2, 37.9, 36.0. HRMS (ESI) m/z: [M + H]+ calcd for C31H24NO3+ 458.1751; found 458.1744.
- 6,11-bis(4-chlorophenyl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3s), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 49.9 mg, yield: 95%; m.p 329–330 °C. 1H NMR (500 MHz, CDCl3) δ 9.24 (d, J = 8.7 Hz, 1H), 8.86 (d, J = 7.7 Hz, 1H), 7.90 (t, J = 8.2 Hz, 1H), 7.38–7.34 (m, 4H), 7.27–7.24 (m, 4H), 3.58–3.55 (m, 2H), 3.41–3.33 (m, 2H), 3.16–3.13 (m, 2H), 3.03 (d, J = 13.5 Hz, 1H), 2.96–2.85 (m, 3H). 13C NMR (125 MHz, CDCl3) δ 198.0, 195.6, 168.3, 161.8, 159.5, 141.5, 140.2, 137.0, 136.3, 133.8, 133.2, 129.7, 129.4, 128.5, 128.4, 127.7, 123.8, 121.3, 118.3, 116.3, 113.1, 47.3, 46.0, 41.3, 39.0, 37.7, 36.4. HRMS (ESI) m/z: [M + H]+ calcd for C31H22ClNO3+ 526.0971; found 526.0978.
- 2-butyl-6,11-bis(4-chlorophenyl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione (3t), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 50.0 mg, yield: 86%; m.p 230–231 °C. 1H NMR (500 MHz, CDCl3) δ 9.08 (s, 1H), 8.73 (d, J = 1.4 Hz, 1H), 7.35 (dd, J = 14.3, 8.5 Hz, 4H), 7.26 (s, 1H), 7.25–7.23 (m, 3H), 3.66–3.50 (m, 2H), 3.43–3.24 (m, 2H), 3.21–3.06 (m, 2H), 3.08–2.98 (m, 1H), 2.97–2.73 (m, 5H), 1.74–1.68 (m, 2H), 1.43–1.39 (m, 2H), 0.95 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 197.8, 195.4, 168.0, 161.6, 159.1, 152.4, 141.2, 139.8, 136.9, 133.4, 132.8, 129.3, 129.0, 128.1, 128.0, 127.1, 122.7, 122.0, 117.6, 114.4, 112.7, 47.0, 45.7, 41.0, 38.7, 37.4, 37.4, 36.0, 33.6, 22.6, 14.0. HRMS (ESI) m/z: [M + H]+ calcd for C35H30ClNO3+ 582.1597; found 582.1604.
3.3. General Procedures for the Preparation of Compounds 4
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), styrene (0.1 mmol), Pd(OAc)2 (4 mol %), K3PO4 (3.0 equiv), and DMAc (2 mL). Then, the flask was sealed under Ar and stirred at 110 °C for 12 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 4.
- (E)-6,6,11,11-tetramethyl-2-styryl-6,7,11,12-tetrahydrochromeno[2,3,4-gh] phenanthridine-4,13(5H,10H)-dione (4), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 40.7 mg, yield: 88%; m.p 186–187 °C. 1H NMR (400 MHz, CDCl3) δ 9.12 (s, 1H), 8.70 (d, J = 1.2 Hz, 1H), 7.25–7.13 (m, 5H), 6.88–6.71 (m, 2H), 2.99 (s, 2H), 2.75 (s, 2H), 2.55 (s, 2H), 2.44 (s, 2H), 1.15 (s, 6H), 1.12 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 198.6, 195.9, 167.4, 161.2, 159.2, 145.1, 136.6, 136.5, 133.3, 130.2, 129.0, 128.2, 127.5, 127.0, 123.3, 121.2, 117.1, 114.6, 111.7, 53.8, 52.6, 47.5, 42.3, 32.5, 31.8, 28.1, 28.0. HRMS (ESI) m/z: [M + H]+ calcd for C31H30NO3+ 464.2220; found 464.2213.
3.4. General Procedures for the Preparation of Compounds 5
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), ethynylbenzene (0.1 mmol), PdCl2 (5 mol %), PPh3 (15 mol %), CuI (5 mol %), and Et3N (2 mL). Then, the flask was sealed under Ar and stirred at 80 °C for 12 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 5.
- 6,6,11,11-tetramethyl-2-(phenylethynyl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh] phenanthridine-4,13(5H,10H)-dione (5), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 41.9 mg, yield: 91%; m.p 222–223 °C. 1H NMR (500 MHz, CDCl3) δ 9.40 (s, 1H), 8.94 (s, 1H), 7.74–7.53 (m, 2H), 7.50–7.30 (m, 3H), 3.03 (s, 2H), 2.80 (s, 2H), 2.62 (s, 2H), 2.55 (s, 2H), 1.20 (s, 6H), 1.15 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 198. 8, 196.4, 168.0, 161.7, 159.1, 136.3, 132.0, 130.9, 128.8, 128.4, 127.4, 126.2, 122.9, 122.7, 116.7, 114.7, 111.3, 93.1, 89.8, 53.8, 52.6, 47.5, 42.3, 32.6, 31.9, 28.2, 28.1. HRMS (ESI) m/z: [M + H]+ calcd for C31H28NO3+ 462.2064; found 462.2067.
3.5. General Procedures for the Preparation of Compounds 6
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), p-tolylboronic acid (0.1 mmol), Pd(PPh3)4 (5 mol %), K2CO3 (2.0 equiv), and EtOH:Toluene (V:V = 1:2.5, 3.5 mL). Then, the flask was sealed under Ar and stirred at 100 °C for 3 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 6.
- 6,6,11,11-tetramethyl-2-(p-tolyl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh] phenanthridine-4,13(5H,10H)-dione (6), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 41.9 mg, yield: 93%; m.p 143–144 °C. 1H NMR (500 MHz, CDCl3) δ 9.51 (s, 1H), 9.15 (s, 1H), 7.73 (d, J = 7.5 Hz, 2H), 7.30 (d, J = 7.5 Hz, 2H), 3.04 (s, 2H), 2.81 (s, 2H), 2.62 (s, 2H), 2.55 (s, 2H), 2.43 (s, 3H), 1.21 (s, 6H), 1.16 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 199.1, 196.6, 167.9, 161.5, 159.4, 148.1, 138.6, 137.5, 137.0, 134.7, 129.6, 127.9, 120.8, 119.5, 117.3, 114.6, 111.9, 53.9, 52.8, 47.6, 42.4, 32.6, 31.9, 28.2, 28.1, 21.2. HRMS (ESI) m/z: [M + H]+ calcd for C30H30NO3+ 452.2220; found 452.2227.
3.6. General Procedures for the Preparation of Compounds 7
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), 4-methyl-N-phenylaniline (0.1 mmol), Pd(OAc)2 (10 mol %), RuPhos (20 mol %), NaOtBu (1.2 equiv), and 1,4-Dioxane (2 mL). Then, the flask was sealed under Ar and stirred at 110 °C for 12 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 7.
- 6,6,11,11-tetramethyl-2-(phenyl(p-tolyl)amino)-6,7,11,12-tetrahydrochromeno[2,3,4-gh] phenanthridine-4,13(5H,10H)-dione (7), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 48.2 mg, yield: 89%; m.p 199–200 °C. 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 1H), 8.46 (s, 1H), 7.37 (t, J = 7.5 Hz, 2H), 7.24–7.16 (m, 5H), 7.12 (d, J = 8.0 Hz, 2H), 2.92 (s, 2H), 2.74 (s, 2H), 2.46 (s, 2H), 2.41 (s, 2H), 2.36 (s, 3H), 1.14 (s, 6H), 1.09 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 198.7, 196.5, 167.9, 162.2, 158.7, 154.7, 146.2, 143.4, 138.0, 135.3, 130.4, 129.7, 128.3, 126.6, 125.4, 115.9, 112.9, 111.5, 110.2, 110.0, 53.8, 52.6, 47.7, 42.4, 32.4, 31.9, 28.2, 28.1, 21.1. HRMS (ESI) m/z: [M + H]+ calcd for C36H35N2O3+ 543.2642; found 543.2648.
3.7. General Procedures for the Preparation of Compounds 8
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), (Bpin)2 (4.0 equiv), Pd2(dba)3 (10 mol %), XPhos (40 mol %), KOAc (6.0 equiv), and 1,4-Dioxane (2 mL). Then, the flask was sealed under Ar and stirred at 110 °C for 12 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 8.
- 6,6,11,11-tetramethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-6,7,11,12-tetrahydrochromeno[2,3,4-gh] phenanthridine-4,13(5H,10H)-dione (8), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 43.3 mg, yield: 89%; m.p 265–266 °C. 1H NMR (500 MHz, CDCl3) δ 9.61 (s, 1H), 9.14 (s, 1H), 3.03 (s, 2H), 2.79 (s, 2H), 2.60 (s, 2H), 2.53 (s, 2H), 1.37 (s, 12H), 1.19 (s, 6H), 1.14 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 198.8, 196.3, 167.3, 160.7, 159.5, 135.6, 129.9, 126.3, 125.2, 117.5, 116.9, 112.0, 84.4, 53.8, 52.7, 47.5, 42.3, 32.6, 31.9, 28.2, 28.1, 24.9. HRMS (ESI) m/z: [M + H]+ calcd for C29H35BNO5+ 488.2603; found 488.2604.
3.8. General Procedures for the Preparation of Compounds 9
A Schlenk tube was equipped with a magnetic stir bar and charged with 2-bromo-6,6,11,11-tetramethyl-6,7,11,12-tetrahydrochromeno[2,3,4-gh]phenanthridine-4,13(5H,10H)-dione 3h (0.1 mmol), NaBH4 (1.0 equiv), CeCl3·7H2O (1.0 equiv), and MeOH (2 mL). Then, the flask was sealed under Ar and stirred at rt for 3 h. After the reaction was quenched by the addition of water, the mixture was extracted with dichloromethane, and the combined organic layer was dried over sodium sulfate. The concentration in vacuo followed by silica gel column purification with petroleum ether/ethyl acetate eluent gave the desired product 9.
- 2-bromo-6,6,11,11-tetramethyl-4,5,6,7,10,11,12,13-octahydrochromeno[2,3,4-gh] phenanthridine-4,13-diol (9), a yellow solid after purification by flash column chromatography (petroleum ether/ethyl acetate = 10/1), 42.1 mg, yield: 95%; m.p 174–175 °C. 1H NMR (500 MHz, CDCl3) δ 8.03 (s, 1H), 7.55 (s, 1H), 5.39–4.99 (m, 1H), 4.84–4.82 (m, 1H), 2.78 (d, J = 17.2 Hz, 1H), 2.62 (d, J = 17.3 Hz, 1H), 2.46 (d, J = 17.6 Hz, 1H), 2.35 (d, J = 17.5 Hz, 1H), 2.11 (dd, J = 13.7, 6.1 Hz, 1H), 2.01 (dd, J = 13.9, 5.8 Hz, 1H), 1.82 (dd, J = 13.8, 5.7 Hz, 1H), 1.76 (dd, J = 13.7, 5.2 Hz, 1H), 1.19 (s, 3H), 1.15 (s, 3H), 1.06 (s, 3H), 1.01 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 157.9, 154.3, 150.7, 139.4, 133.3, 128.3, 122.3, 119.1, 114.6, 110.5, 65.6, 65.1, 46.8, 45.9, 45.3, 41.4, 30.4, 30.3, 29.0, 28.6, 28.3. HRMS (ESI) m/z: [M + H]+ calcd for C23H27BrNO3+ 444.1169; found 444.1171.
4. Conclusions
In conclusion, we have developed an efficient double C-H activation/cyclization reaction for the construction of pyranoisoquinoline derivatives from aryl oxadiazoles and diazo compounds. This reaction features a broad substrate scope and mild conditions. In addition, large-scale preparation and further transformations of the products were carried out, revealing the practicality of the annulation method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30010149/s1. The characterization data for products 3, 4, 5, 6, 7, 8, and 9, including the 1H-NMR, 13C-NMR, and 19F-NMR spectroscopies, are available online. CCDC 2132351 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif [14] or by emailing data_request@ccdc.cam.ac.uk or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax:+44-1223-336033.
Author Contributions
Writing—original draft preparation, E.Z.; conceptualization, E.Z.; resources, E.Z.; data curation, M.S.; writing—review and editing, L.G.; funding acquisition, E.Z., M.S. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financed by the Key Project of the Scientific Research Foundation of Anhui Province Education Department (2023AH051554 and 2024AH051730), Huainan City Guiding Science and Technology Program Projects (2023028 and 2023037), and the Science and Technology Plan Project of Huainan City (2023A319).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Makino, H.; Saijo, T.; Ashida, Y.; Kuriki, H.; Maki, Y. Mechanism of action of an antiallergic agent, Amlexanox (AA-673), in inhibiting histamine release from mast cells: Acceleration of cAMP Generation and Inhibition of Phosphodiesterase. Int. Arch. Allergy Immunol. 1987, 82, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Akyol-Salman, I.; Leçe-Sertöz, D.; Baykal, O. Topical pranoprofen 0.1% is as effective anti-inflammatory and analgesic agent as diclofenac sodium 0.1% after strabismus surgery. J. Ocul. Pharmacol. Ther. 2007, 23, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Hegde, S.; Reinhard, E.; Gomez, L.; Vernier, W.F.; Lee, L.; Liu, S.; Sambandam, A.; Snider, P.A.; Masih, L. Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2005, 15, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Oset-Gasque, M.J.; González, M.P.; Pérez-Peña, J.; García-Font, N.; Romero, A.; Del Pino, J.; Ramos, E.; Hadjipavlou-Litina, D.; Soriano, E.; Chioua, M.; et al. Toxicological and pharmacolo-gical evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines 11-amino-12-aryl-8, 9, 10, 12-tetrahydro-7H-chromeno [2, 3-b] quinolin-3-ols for the potential prevention and treatment of alzheimer’s disease. Eur. J. Med. Chem. 2014, 74, 491–501. [Google Scholar]
- Wu, X.; Xiong, H.; Sun, S.; Cheng, J. Rhodium-catalyzed relay carbenoid functionalization of aromatic C−H bonds toward fused heteroarenes. Org. Lett. 2018, 20, 1396–1399. [Google Scholar]
- Shankar, M.; Ghosh, K.; Mukherjee, K.; Rit, R.K.; Sahoo, A.K. One-pot unsymmetrical {[4 + 2] and [4 + 2]} double annulations of o/o′-C−H bonds of arenes: Access to unusual pyranoisoquinolines. Org. Lett. 2018, 20, 5144–5148. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Yin, J.; Zhang, Y.; Han, W.; Lan, J.; Wu, D.; Bin, Z.; You, J. Double ortho-C−H activation/annulation of benzamides with aryl alkynes: A route to double-helical polycyclic heteroaromatics. J. Org. Chem. 2019, 84, 15697–15705. [Google Scholar] [CrossRef] [PubMed]
- Mayakrishnan, S.; Tamizmani, M.; Maheswari, N.U. Harnessing hypervalent iodonium ylides as carbene precursors: C–H activation of N-methoxybenzamides with a Rh (III)-catalyst. Chem. Commun. 2020, 56, 15462–15465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, E.; Duan, J.; Xu, K.; He, X.; Shang, Y. Assembly of Pyran-Fused Isoquinolines via Rhodium-Catalyzed Double Annulations of Methyl Benzimidates with Diazo Compounds. Synthesis 2022, 54, 4583–4591. [Google Scholar] [CrossRef]
- Yang, F.; Yu, J.; Liu, Y.; Zhu, J. Cobalt (III)-catalyzed oxadiazole-directed C−H activation for the synthesis of 1-aminoisoquinolines. Org. Lett. 2017, 19, 2885–2888. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, J.; Liu, Y.; Zhu, J. Rhodium (III)-catalyzed oxadiazole-directed alkenyl C–H activation for synthetic access to 2-acylamino and 2-amino pyridines. J. Org. Chem. 2017, 82, 9978–9987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.-S.; Xia, Y.-Q.; Dong, L. Efficient synthesis of functionalized indene derivatives via Rh (III)-catalyzed cascade reaction between oxadiazoles and allylic alcohols. Adv. Synth. Catal. 2019, 361, 2037–2041. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, Y.-H.; Dong, L. Direct assembly of vinyl fluorinated isoquinolines via Rh (III)-catalyzed [4 + 2] annulation. J. Org. Chem. 2023, 88, 10789–10800. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ccdc.cam.ac.uk/structures (accessed on 23 December 2024).
- Nishii, Y.; Bachon, A.; Moon, S.; Bolm, C.; Miura, M. Rhodium-catalyzed Synthesis of 1-(Acylamino)isoquinolines through Direct Annulative Coupling of 3-Aryl-1,2,4-oxadiazoles with Alkynes. Chem. Lett. 2017, 46, 1347–1349. [Google Scholar] [CrossRef]
- He, Y.; Zheng, J.; Dong, L. Rh(III)-Catalyzed cascade annulation to produce an N-acetyl chain of spiropyrroloisoquinoline derivatives. Org. Biomol. Chem. 2022, 20, 2293–2299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).