Abstract
Six new triterpenoids, heritieras C–H (1–6), along with thirteen known triterpenoids (7–19), were isolated from the leaves of Heritiera littoralis. Their structures were identified by spectroscopic analysis, including 1D and 2D nuclear magnetic resonance (NMR), high-resolution electrospray ionization mass spectrometry (HRESIMS), and by comparison with the literature. Anti-inflammatory activity of the isolates was evaluated using the lipopolysaccharide (LPS) stimulated RAW 264.7 cell model. Among the isolated triterpenoid, compounds 1, 12, 16, 17, and 18 demonstrated inhibitory activity against nitric oxide (NO) release, in which compound 18 exhibited the best activity with an IC50 value of 18.13 μM. The potential anti-inflammatory mechanism was investigated using molecular docking. The triterpenoids from H. littoralis could be served as potential candidates for the development of new anti-inflammatory agents.
1. Introduction
Malvaceae is a family consisting of herbs, subshrubs, shrubs, or trees, with about 245 genera and 4300 species, of which about 59 genera and 264 species are found in China [1,2]. The genus Heritiera belongs to the family Malvaceae growing in tropical and subtropical regions of Asia. It consists of 35 species, of which 3 species can be found in provinces of Guangdong, Guangxi, Hainan, and Yunnan in China [3].
The genus Heritiera has a long history for medicinal use in China, especially for folk use [4,5]. Heritiera littoralis Dryand, a semi-mangrove plant, has been used as a traditional medicine by the Jing people [6]. This plant has been reported for its wide ranges of activities. The water extract of the bark from H. littoralis can be used to treat hematuria, diarrhea, and dysentery. The seeds are used to treat diarrhea. The twigs are served as toothbrushes [7,8]. Previous phytochemical investigations of H. littoralis revealed a few chemical compositions, such as flavonoids, terpenoids, phenylpropanoids, lignans, steroids, and triterpenoid saponins [9,10,11,12,13,14]. The constituents from H. littoralis have shown an impressive range of biological activities, including antibacterial, antioxidant [15], anti-inflammatory [16,17], and toxicant effects [11].
To further explore the anti-inflammatory ingredients from the leaves of H. littoralis, the chemical constitutions and pharmacological activities of H. littoralis were investigated. Nineteen compounds, including six new triterpenoids, heritieras C–H (1–6), and thirteen known triterpenoids (7–19) (Figure 1), were isolated from the extract of the leaves of H. littoralis. Furthermore, the isolates were evaluated for their anti-inflammatory activity against the production of the NO in LPS induced RAW 264.7 macrophage cells. The potential anti-inflammatory mechanism was further investigated using molecular docking. Herein, the isolation, purification, and determination of the isolates and their anti-inflammatory activity and molecular docking assay are described.
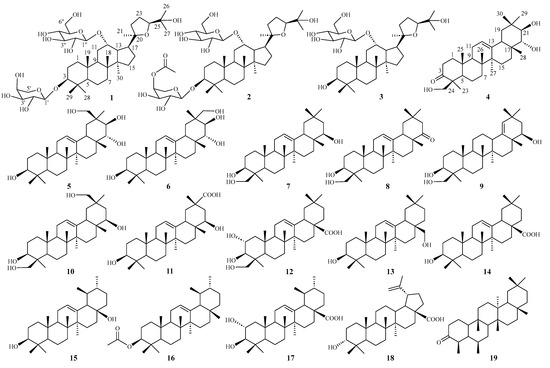
Figure 1.
Structures of 1–19 from H. littoralis.
2. Results and Discussion
2.1. Elucidation of the Chemical Structures of Heritieras C–H (1–6)
Compound 1, a white amorphous powder, had the molecular formula of C41H70O13 determined by HRESIMS m/z 793.4706 [M + Na]+ (calcd. m/z 793.4714), indicating seven unsaturated degrees. The 1H-NMR spectrum (Table 1) of 1 exhibited eight methyl singlets at δH 0.63 (3H, s, H-30), 0.95 (3H, s, H-28), 1.03 (3H, s, H-18), 1.16 (3H, s, H-21), 1.26 (3H, s, H-29), 1.41 (6H, s, H-19, 26), 1.48 (3H, s, H-27), and two anomeric protons, δH 4.72 (1H, d, J = 6.4 Hz) and δH 5.14 (1H, d, J = 8.0 Hz), as well as overlapping signals belonging to sugar moieties around δH 3.76−4.54. The 13C-NMR, DEPT, and HSQC spectra of 1 displayed 41 carbon signals, including 30 carbons attributed to a triterpenoid skeleton [three oxygenated methines (δC 78.0, 81.8, 84.7), and two oxygenated quaternary carbon (δC 71.7, 86.9), four methines (δC 41.6, 50.0, 51.4, 54.4), four quaternary carbon (δC 38.5, 41.7, 41.9, 50.5), nine methylenes (δC 18.8, 22.3, 26.7, 27.1, 32.0, 34.6, 35.0, 36.1, 36.8), eight methyls (δC 17.2, 17.4×2, 23.6, 25.0, 26.7, 28.1, 30.5)] and the other 11 carbons assigned to two sugar moieties [one L-arabinopyranose (δC 66.8, 69.6, 72.9, 75.2, 102.3) and one D-glucopyranose (δC 63.9, 72.9, 75.8, 78.3, 79.1, 102.7)]. The 1H−1H COSY data revealed the partial structures –CH2(1)−CH2(2)−CH(3)−, –CH2(6)−CH2(7)−, −CH(9)−CH2(11)−CH(12)−CH(13)−CH(17)−CH2(16)−CH2(15)− and -CH2(22)−CH2(23)−CH3(24)− (Figure 2A). Detailed analysis of the abovementioned NMR data showed that the data of 1 was highly similar to those of ocotillol-II [18], indicating that 1 has the same basic dammarane triterpenoid skeleton as ocotillol-II. The differences from ocotillol-II are that 1 has an L-arabinopyranose connected at position 3 and a D-glucopyranose connected at position 12. Furthermore, the HMBC correlations from the anomeric protons δH 4.72 (H-1′) to δC 81.8 (C-3) and δH 5.14 (H-1″) to δC 78.0 (C-12) suggested that the two sugars are situated at C-3 and C-12, respectively (Figure 2A). After acid hydrolysis of 1 using 1 M HCl, one set of carbon signals for an α-L-arabinopyranose and another set of carbon signals for a β-D-glucopyranose were identified by comparison on HPLC with authentic samples [19] (Figure S51). In addition, the relative stereochemistry was deduced from ROESY spectrum (Figure 2B). In the ROESY spectrum, the correlations between H-3/H-5/H3-30/H-17 and H-12/H3-18/H3-19 revealed that H-3, 5, 30, and 17 were located on one side, whereas H-12, 18, and 19 were on the other side. According to biosynthetic characteristics of dammarane trierpenoid, H-18 and 19 are β-orientation, whereas H-30 is α-orientation [20]. Consequently, it can be deduced that H-3 is α-orientation and H-12 is β-orientation. In addition, the absolute configurations at C-20 and C-24 were determined to be R and S, respectively, by comparing with the 13C NMR chemical shift in analogous epoxydammaranes [21,22]. Thus, the structure of 1 was deduced as (20R,24S)-20,24-epoxy-3β,12α,25-trihydoxy-12-O-β-D-glucopyranosyldammarane-3-O-α-L-arabinopyranoside (1), named heritiera C (Figure 1).

Table 1.
1H NMR (600 MHz) and 13C NMR (150 MHz) spectral data of compounds 1–3 (pyridine-d5, δ in ppm, J in Hz).
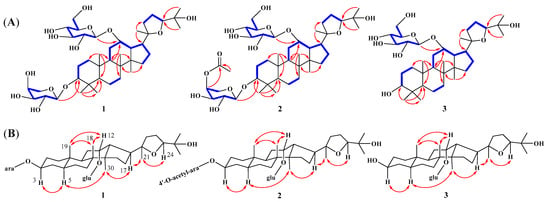
Figure 2.
(A) 1H-1H COSY (blue bold bonds) and key HMBC (red arrows) correlations of 1–3. (B) ROESY (double dashed arrows) correlations of 1–3.
Compound 2 was isolated as white amorphous powder. Its molecular formula was established as C43H72O14 by HRESIMS m/z 835.4820 [M + Na]+ (calcd. m/z 835.4820), which is 42 Da more than that of 1. Extensive analysis of the NMR data (Table 1) revealed that 2 shares almost the same structure as 1, the difference is one more acetyl group (δC 170.7, 20.9) at position 4′ in 2. The location of the acetyl group was confirmed by the HMBC correlations from δH 5.41 (H-4′) to δC 170.7 (-OOCCH3) (Figure 2A). The sugar units were determined to be α-L-arabinopyranose and β-D-glucopyranose based on the hydrolysis of 2 with 1 M HCl and the assays of HPLC (Figure S51). Moreover, the diagnostic ROESY correlations of H-3/H-5/H-30/H-17 suggested that H-3 is α-oriented, while the ROESY correlations of H-12/H-18/H-19 indicated that H-12 is β-oriented (Figure 2B). In addition, to determine the absolute configuration of the chiral centers C-20 and C-24 in compound 2, the carbon signals at δC 86.9 (C-20) and 84.6 (C-24) were compared with those of analogous dammaranes [21,22], suggesting that the chiral centers are 20R and 24S, respectively. Therefore, the structure of 2 was determined as (20R,24S)-20,24-epoxy-3β,12α,trihydoxy-12-O-β-D-glucopyranosyldammarane-3-O-[4′-O-acetyl]-α-L-arabinopyran-oside (2), named heritiera D (Figure 1).
Compound 3 was isolated as white amorphous powder. Its molecular formula was established as C36H62O9 by HRESIMS m/z 661.4291 [M + Na]+ (calcd. m/z 661.4292), which is 132 Da less than that of 1. Extensive analysis of the NMR data (Table 1) revealed that 3 shares almost the same structure as 1, the only difference is that compound 3 is one less arabinopyranose group at position 3 than 1. Moreover, the HMBC correlations from δH δH 5.17 (H-1″) to δC 77.2 (C-12) suggested that the D-glucopyranose are situated at C-12 (Figure 2A). The sugar unit was determined to be β-D-glucopyranose based on the hydrolysis of 3 with 1 M HCl and the assays of HPLC (Figure S49). Furthermore, the β-orientations of H-12 were then verified by the ROESY peaks of H-12/H-18/H-19 (Figure 2B). In addition, to determine the absolute configuration for chiral centers C-20/24 of 3, the comparisons of the carbon signals of compound 3 at δC 86.9 (C-20) and 84.6 (C-24) with those of analogous dammaranes suggested its chiral centers to be 20R and 24S [21,22]. Consequently, the structure of 3 was identified as (20R,24S)-20,24-epoxy-3β,12α,25-trihydoxy −12-O-β-D-glucopyranosyldammarane (3), named heritiera E (Figure 1).
Compound 4 was isolated as white amorphous powder. Its molecular formula was established as C30H48O4 by HRESIMS m/z 473.3633 [M + H]+ (calcd. m/z 473.3631), suggesting seven unsaturated degrees. The 1H-NMR spectrum (Table 2) of 4 exhibited seven methyl singlets at δH 1.06 (3H, s, H-26), 1.17 (3H, s, H-25), 1.22 (3H, s, H-27), 1.25 (3H, s, H-30), 1.29 (3H, s, H-28), 1.45 (3H, s, H-29), and 1.50 (3H, s, H-23), and one olefinic proton δH 5.35 (1H, t, J = 3.6 Hz, H-12). Its 13C NMR spectrum (Table 2) revealed the presence of 30 carbon resonances, which were classified by DEPT and HSQC spectra, including one carbonyl carbon (δC 214.9), one double bond (δC 122.6, 145.0), three oxygenated carbons (δC 65.6, 75.0, 80.0), and seven methyl carbons (δC 16.1, 17.4, 21.2, 21.7, 22.7, 27.0, 31.9). Extensive analysis of the NMR data (Table 2) revealed that 4 shares almost the same structure as soyasapogenol A [23]. The difference between these two compounds is that soyasapogenol A has four oxygenated carbon signals, while 4 has three oxygenated carbon signals and a carbonyl carbon signal (δC 214.9), suggesting that one oxygenated carbon in soyasapogenol A has been oxidated to a carbonyl carbon in 4. In the 1H−1H COSY spectrum of 4, the following partial structures were verified as –CH2(1)−CH2(2)−, –CH(5)−CH2(6)−CH2(7)−, –CH(9)−CH2(11)−CH(12)−, –CH2(15)−CH2(16)−, –CH(18)−CH2(19)− and –CH(21)−CH(22)− (Figure 3A). Furthermore, the key HMBC correlations from δH 2.37 and 2.83 (H-2), 1.50 (H3-23), as well as 3.89 and 4.37 (H-24) to δC 214.9 (C-3) confirmed that the carbonyl carbon was situated at C-3 (Figure 3A). In addition, the relative configurations of 4 were determined by NOESY spectrum (Figure 3B). The key correlations between H-5/H-9/H-21/H3-23/H3-27/H3-29 indicate that H-5, H-9, H-21, H3-23, H3-27, and H3-29 are on the same side, suggesting that H-5, H-9, H-21, H3-23, H3-27, and H3-29 are α-orientation. The correlations between H-18/H-22/H2-24/H3-25/H3-26/H3-28/H3-30 suggest that H-18, H-22, H2-24, H3-25, H3-26, H3-28, and H3-30 are on the same side, indicating that H-18, H-22, H2-24, H3-25, H3-26, H3-28, and H3-30 are β-orientation. Consequently, according to the above analysis and the biosynthetic characteristics of oleanane triterpenoid [20], the absolute configuration of 4 can be determined as 21β,22α,24-trihydroxy-3-oxo-olean-12-ene (4), named heritiera F (Figure 1).

Table 2.
1H NMR and 13C NMR spectral data of compounds 4–6 (pyridine-d5, δ in ppm, J in Hz).
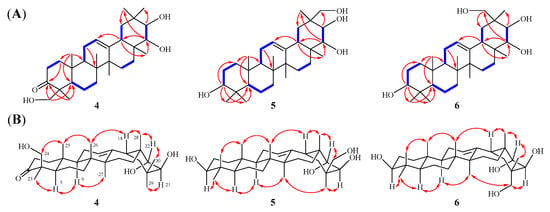
Figure 3.
(A) 1H-1H COSY (blue bold bonds) and key HMBC (red arrows) correlations of 4–6. (B) NOESY (double dashed arrows) correlations of 4–6.
Compound 5 was isolated as white amorphous powder. Its molecular formula was established as C30H50O4 by HRESIMS m/z 497.3606 [M + Na]+ (calcd. m/z 497.3607), suggesting six unsaturated degrees. The 1H-NMR spectrum (Table 2) of 5 exhibited seven methyl singlets at δH 0.96 (3H, s, H-25), 1.06 (3H, s, H-27), 1.08 (3H, s, H-23), 1.26 (3H, s, H-24), 1.31 (3H, s, H-26), 1.34 (3H, s, H-28) and 1.44 (3H, s, H-29), and one olefinic proton δH 5.36 (1H, t, J = 3.4 Hz, H-12). Its 13C NMR spectrum (Table 2) revealed the presence of 30 carbon resonances, which were classified by DEPT and HSQC spectra, including one double bond (δC 123.4, 144.8), four oxygenated carbons (δC 67.4, 76.6, 78.4, 79.8), and seven methyl carbons (δC 16.1, 16.9, 17.4, 22.8, 27.1, 27.4, 29.1). Extensive analysis of the NMR data (Table 2) revealed that 5 shares almost the same structure as abrisapogenol L [24], the difference being in that 5 possesses a methyl group at C-24 instead of a CH2OH at C-24 in abrisapogenol L. In the 1H−1H COSY spectrum of 5, the partial structures revealed are as follows: –CH2(1)−CH2(2)−CH(3)−, –CH(5)−CH2(6)−CH2(7)−, –CH(9)−CH2(11)−CH(12)−, –CH2(15)−CH2(16)−, –CH(18)−CH2(19)− and –CH(21)−CH(22)− (Figure 3A). In addition, the key HMBC correlations from δH 3.46 (H-3) and 0.88 (H-5) to δC 16.9 (C-23) and 29.1 (C-24) confirmed that two methyl groups of C-23 and C-24 were linked to C-4 in 5. (Figure 3A). Furthermore, the relative configurations of 5 were determined by NOESY spectrum (Figure 3B). In the NOESY spectrum of 5, the correlations of H-3/H-5/H-9/H-21/H3-27/H3-29 suggest that H-3, H-5, H-9, H-21, H3-27 and H3-29 are on the same side, indicating H-3, H-5, H-9, H-21, H3-27 and H3-29 are α-orientation. The NOESY correlations of H-18/H-22/H3-28/H2-30 suggest that H-18, H-22, H3-28 and H2-30 are on the same side, indicating H-18, H-22, H3-28 and H2-30 are β-orientation. According to the above analysis, the structure of 5 was deduced as 3β,21β,22α,30-tetrahydroxy-olean-12-ene (5), named heritiera G (Figure 1).
Compound 6 was isolated as white amorphous powder. Its molecular formula was established as C30H50O4 by HRESIMS m/z 497.3596 [M + Na]+ (calcd. m/z 497.3607), which is the same as that of 5. The 1D NMR data (Table 1) for 5 and 6 are highly similar, the difference being in C-21 (δH 4.51, δC 70.8), C-29 (δH 3.66 and 4.07, δC 72.0) and C-30 (δH 1.54, δC 17.9) in 6 instead of C-21 (δH 4.15, δC 76.6), C-29 (δH 1.44, δC 27.4), and C-30 (δH 4.33 and 4.42, δC 67.4) in 5. From the above analysis, it can be inferred that 6 possesses a methyl group at C-29 and a CH2OH at C-30 instead of a CH2OH at C-29 and a methyl group at C-30. In the NOESY spectrum of 6, the correlations of H-3/H-5/H-9/Hα-16/H-21/H3-27/H2-29 suggest that H-3, H-5, H-9, Hα-16, H-21, H3-27, and H2-29 are on the same side, indicating H-3, H-5, H-9, Hα-16, H-21, H3-27, and H2-29 are α-orientation. The NOESY correlations of H-18/H-22/H3-28/H3-30 suggest that H-18, H-22, H3-28, and H3-30 are on the same side, indicating H-18, H-22, H3-28, and H3-30 are β-orientation (Figure 3B). Thus, the structure of 6 was deduced as 3β,21β,22α,29-tetrahydroxyl-olean-12-ene (6), named heritiera H (Figure 1).
By comparing the measured NMR (1H and 13C) and MS data to those reported in the literature, the known triterpenoids were identified as soyasapogenol B (7) [25], soyasapogenol E (8) [26], soyasapogenol H (9) [27], wistariasapogenol B (10) [28], triptotriterpenic acid B (11) [29], 2α,3β,24-trihydroxy olea-12-en-28-oic acid (12) [30], erythrodiol (13) [31], oleanolic acid (14) [32], uvaol (15) [33], β-amyrin acetate (16) [34], 2α-hydroxy ursolic acid (17) [35], betulinic acid (18) [13], and friedelin (19) [36] (Figure 1).
2.2. Anti-Inflammatory Assay of the Isolates
Cell viability was measured by an MTT assay. The RAW 264.7 cell viability assays showed that the survival rate remained greater than 90% after treatment with all isolates at different concentrations from 0 to 50 μM. All isolates were evaluated for their anti-inflammatory effects on the production of NO in the RAW 264.7 macrophage cell line exposed to the inflammatory stimulus by LPS. Dexamethasone was used as a positive control. The effects of active compounds (IC50 < 50 μM) on the production of NO by LPS-induced RAW 264.7 cells are shown in Table 3.

Table 3.
Anti-inflammatory effects of active compounds (IC50 < 50 μM) on the production of the NO in LPS-stimulated RAW 264.7 Cells a.
The results showed that compound 18 substantially inhibited the release of NO, with IC50 value of 18.13 μM. The value is slightly lower than that of positive control, dexamethasone, with IC50 value of 16.37 μM. Compounds 1, 12, 16, and 17 showed moderate effects with IC50 values of 47.12, 45.31, 39.98, and 25.23 μM, respectively. Compounds 2–11, 15, and 19 showed no significant effects against LPS-induced nitric oxide production in RAW 264.7 macrophages.
2.3. Predicted Binding Modes of Compounds and iNOS, COX-2 Using Molecular Docking Analysis
NO serves as an indicator of inflammatory response, and elevated levels of NO within tissues signify the overexpression of critical proteins, iNOS and COX-2 [37,38]. Specifically, iNOS plays a role in catalyzing the production of NO within the inflammatory signaling pathway [39]. Studies have shown that the interaction between iNOS/COX-2 and certain small molecules can halt the inflammatory process and prevent the overproduction of downstream inflammatory mediators [40]. In an effort to elucidate the potential mechanisms behind NO inhibition, the binding interactions between bioactive compounds and iNOS/COX-2 were examined.
The compounds (1, 12, 16, 17, 18, and dexamethasone) were subjected to molecular docking. Results from molecular docking study revealed that compounds 1, 12, 16, 17, 18, and dexamethasone had strong interactions with the iNOS/COX-2 protein (Figure 4, Figure 5 and Figures S53–S62) and the binding residues and the logarithms of free binding energies were collated in Tables S1 and S2. The results show that 18 docks effectively into the active sites of iNOS and COX-2, with binding energies of −9.0 kcal/mol, which is comparable to those of dexamethasone (positive control, with binding energies of −9.5 and −9.6 kcal/mol, respectively). Further simulations were conducted to model the docking sites of compound 18 with iNOS and COX-2. The results show that compound 18 binds to the amino acid residues TRP-457 and GLY-365 of the iNOS protein through hydrogen bonds, and to the amino acid residues ARG-1044 and GLN-1370 of the COX-2 protein through hydrogen bonds as well.
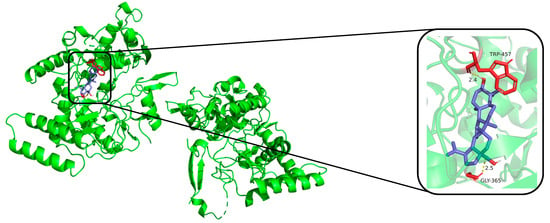
Figure 4.
Molecular docking result of compound 18 with iNOS protein. Molecular docking simulation obtained at lowest energy conformation, highlighting potential hydrogen contacts of compound 18 (iNOS protein is shown in green, compound 18 are in purple). For clarity, only interacting residues are labeled. Hydrogen bonds between amino acid residues and compounds are represented by yellow dashed lines. These figures were created by PyMOL (3.0.0).
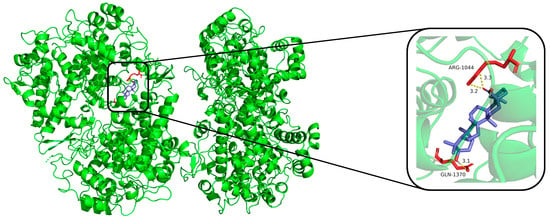
Figure 5.
Molecular docking result of compound 18 with COX-2 protein. Molecular docking simulation obtained at lowest energy conformation, highlighting potential hydrogen contacts of compound 18 (COX-2 protein is shown in green, compound 18 are in purple). For clarity, only interacting residues are labeled. Hydrogen bonds between amino acid residues and compounds are represented by yellow dashed lines. These figures were created by PyMOL.
Comparison their IC50 values with the results of molecular docking, it was discovered that the IC50 values of the active compounds correlate with the trends observed in molecular docking study. Compounds with higher activity have lower Logarithms of free binding energies (FBE). For example, compound 18 and dexamethasone form hydrogen bonds with the amino acid residue GLY-365 in the iNOS protein to show lower IC50 values and free binding energies. Meanwhile, compounds 12, 17, and 18 all feature an α-oriented hydroxyl group (OH) on ring A and demonstrate excellent activities. They are likely the critical factor contributing to their activities.
3. Experimental
3.1. General Experimental Procedures
UV spectra were acquired with a TU-1901 spectrophotometer. NMR experiments were conducted on Bruker Advance 600 or 500 MHz spectrometers. Optical rotations were acquired based on a JASCO P-2000 polarimeter. HRESIMS were recorded on Agilent 6545 Q-TOF LC-MS mass spectrometer. Semi-preparative HPLC separations were conducted on an Aglient 1260 instrument equipped with a DAD detector and a C18 column; analytical HPLC was conducted on an Aglient 1260 instrument equipped with a DAD detector and an Aglient C18 column. OD values of 96-well were measured with an imark Bio-Rad plate microplate reader.
3.2. Plant Material
The dried leave (5.0 kg) of H. littoralis was obtained from Beilun Estuary in Guangxi Province, China, in September 2018 and identified by professor Qiuping Zhong (Guangxi Institute of Botany). A voucher specimen (No. ID-20180910) is deposited at the Guangxi key laboratory of green chemical materials and safety technology, Beibu Gulf University, China.
3.3. Extraction and Isolation
Dried leaves (5.0 kg) of H. littoralis were extracted with H2O-EtOH (20 L each, 25:75) under the extraction tank (4 h each), The filtrates were combined and dried under reduced pressure to yield a residue (0.52 kg). The concentrates were suspended in H2O (1 L) and partitioned with EtOAc (5 × 1 L) and n-BuOH fraction (5 × 1 L). The EtOAc extract (233 g) was eluted by silica gel CC (200~300 mesh) using petroleum ether (PE) and ethyl acetate (95:5 to 50:50 v/v) as eluents and six fractions (Frs. 1–6) were obtained based on TLC assay. Fr. 2 (43.1 g) was subjected to silica gel CC (200~300 mesh) and eluted sequentially with PE-CH2Cl2 (100:0, 90:10, 80:20, 50:50, and 0:100, v/v) to yield five fractions (Fr. 2.1−Fr. 2.5). Fr. 2.3 was purified by recrystallization to yield 19 (12.6 mg). Fr. 2.4 was further separated using Sephadex LH-20 (PE:CH2Cl2:MeOH = 1:2:1, v/v/v) to obtain compound 16 (5.2 mg). Six fractions (Fr. 3.1−Fr. 3.6) were obtained by further separation of Fr. 3 (24.3 g) on an ODS column eluted with H2O-MeOH (40:60 to 0:100, v/v). Fr. 3.3 was subjected to silica gel CC (200~300 mesh) and eluted sequentially with CH2Cl2-MeOH (100:0, 98:2, 96:4, 94:6, 90:10, and 1:1, v/v) to yield five fractions (Fr. 3.3.1−Fr. 3.3.5), according to TLC monitoring. Fr. 3.3.2 was purified by semipreparative HPLC (5 μm, 9.3 × 250 mm, 2.5 mL/min, 197 nm), eluted with H2O-MeCN (27:73, v/v) to yield 11 (tR 14.2 min, 7.3 mg), 13 (tR 23.8 min, 9.4 mg), and 14 (tR 32.9 min, 12.3 mg). After purification over Sephadex LH-20 CC eluted with MeOH, Fr. 3.3.3 was purified by semipreparative HPLC (5 μm, 9.3 × 250 mm, 2.5 mL/min, 197 nm), to obtain compounds 4 (tR 16.7 min, 5.6 mg), 7 (tR 20.1 min, 39.1 mg), and 9 (tR 28.6 min, 4.6 mg). Fr. 3.4 was purified by semipreparative HPLC (5 μm, 9.3 × 250 mm, 2.5 mL/min, 210 nm), eluted with H2O-MeOH (15:85, v/v) to yield 18 (tR 36.7 min, 8.6 mg). Fr.4 (20.7 g) was further separated using Sephadex LH-20 (MeOH) to yield five fractions (Fr. 4.1−Fr. 4.4). Fr. 4.1 (6.1 g) was submitted to ODS column eluted with H2O-MeOH (40:60 to 10:90, v/v) to give 12 (tR 15.4 min, 7.2 mg), 15 (tR 18.6 min, 6.5 mg), and 17 (tR 26.1 min, 12.1 mg). Fr. 4.2 (3.5 g) was submitted to Sephadex LH-20 column eluted with MeOH to give 8 (20.1 mg). Compounds 5 (tR 16.7 min, 4.8 mg), 6 (tR 25.2 min, 13.7 mg), and 10 (tR 15.4 min, 54.0 mg) were afforded by semipreparative HPLC purification of Fr. 4.3 (5 μm, 9.3 × 250 mm, 2.5 mL/min, 196 nm), eluted with H2O-MeCN (23:77, v/v). Fr. 4.4 purified by semipreparative HPLC (H2O:MeOH = 40:60, v/v) separations to yield 3 (tR 23.5 min, 5.2 mg). Fr. 5 (23.1 g) was purified successively by ODS column eluted with H2O-MeOH (50:50 to 10:90, v/v) and a Sephadex LH-20 column (H2O:MeOH = 80:20, v/v) to give 1 (8.2 g) and 2 (4.5 mg).
3.4. Characterization of the Isolates
Heritiera C (1): white amorphous powder; [α − 10.7 (c 0.10, MeOH); HRESIMS m/z 793.4914 [M + Na]+, calcd for C41H70O13Na). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 1; all significant data are given in the electronic Supplementary Materials (Figures S1−S8).
Heritiera D (2): white amorphous powder; [α − 23.5 (c 0.10, MeOH); HRESIMS m/z 835.4820 ([M + Na]+, calcd for C43H72O14Na). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 1; all significant data are given in the Supplementary Materials (Figures S9−S16).
Heritiera E (3): white amorphous powder; [α − 17.2 (c 0.10, MeOH); HRESIMS m/z 661.4291 ([M + Na]+, calcd for C36H62O9Na). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 1; all significant data are given in the Supplementary Materials (Figures S17−S24).
Heritiera F (4): white amorphous powder; [α + 15.4 (c 0.10, MeOH); HRESIMS m/z 473.3633 [M + H]+, calcd for C30H48O4). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 2; all significant data are given in the electronic Supplementary Materials (Figures S25−S32).
Heritiera G (5): white amorphous powder; [α + 27.4 (c 0.10, MeOH); HRESIMS m/z 497.3607 ([M + Na]+, calcd for C30H50O4Na). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 2; all significant data are given in the Supplementary Materials (Figures S33−S40).
Heritiera H (6): white amorphous powder; [α + 20.2 (c 0.10, MeOH); HRESIMS m/z 497.3596 ([M + Na]+, calcd for C30H50O4Na). For 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, see Table 2; all significant data are given in the Supplementary Materials (Figures S41−S48).
Soyasapogenol B (7): colorless crystals; 1H NMR (400 MHz, CDCl3) δ: 5.14 (1H, t, J = 3.7 Hz, H-12), 4.10 (1H, d, J = 11.0 Hz, H-3), 3.49 (1H, s, H-22), 1.13 (3H, s, H-23), 1.00 (3H, s, H-25), 0.92 (3H, s, H-26), 0.84 (3H, s, H-27), 0.82 (3H, s, H-28), 0.80 (3H, s, H-29), 0.75 (3H, s, H-30); 13C NMR (100 MHz, CDCl3) δ: 38.3 (C-1), 25.7 (C-2), 80.4 (C-3), 42.0 (C-4), 55.8 (C-5), 18.4 (C-6), 33.1 (C-7), 39.5 (C-8), 47.7 (C-9), 37.3 (C-10), 23.6 (C-11), 122.3 (C-12), 143.7 (C-13), 42.3 (C-14), 28.3 (C-15), 27.2 (C-16), 36.6 (C-17), 45.0 (C-18), 46.0 (C-19), 30.4 (C-20), 41.1 (C-21), 76.3 (C-22), 22.4 (C-23), 64.5 (C-24), 16.1 (C-25), 16.8 (C-26), 25.0 (C-27), 28.4 (C-28), 32.2 (C-29), 19.6 (C-30).
Soyasapogenol E (8): white amorphous powder; 1H NMR (600 MHz, CDCl3) δ: 5.29 (1H, t, J = 3.7 Hz, H-12), 4.20 (1H, d, J = 11.1 Hz, H-24), 3.44 (1H, dd, J = 11.8, 4.5 Hz, H-24), 3.34 (1H, d, J = 11.1 Hz, H-3), 1.25 (3H, s, H-27), 1.22 (3H, s, H-23), 0.99 (6H, s, H-26, 29), 0.93 (3H, s, H-30), 0.89 (3H, s, H-25), 0.85 (3H, s, H-28); 13C NMR (150 MHz, CDCl3) δ: 38.5 (C-1), 27.7 (C-2), 80.9 (C-3), 42.9 (C-4), 55.9 (C-5), 18.5 (C-6), 33.0 (C-7), 39.8 (C-8), 47.7 (C-9), 36.8 (C-10), 23.9 (C-11), 123.7 (C-12), 141.7 (C-13), 41.9 (C-14), 25.4 (C-15), 27.3 (C-16), 47.7 (C-17), 47.7 (C-18), 46.8 (C-19), 34.3 (C-20), 51.0 (C-21), 217.3 (C-22), 25.5 (C-23), 22.5 (C-23), 64.6 (C-24), 16.2 (C-25), 16.8 (C-26), 25.2 (C-28), 32.1 (C-29), 20.7 (C-30).
Soyasapogenol H (9): colorless crystals; 1H NMR (600 MHz, pyridine-d5) δ: 4.98 (1H, s, H-19), 4.52 (1H, d, J = 11.0 Hz, H-24), 4.00 (1H, dd, J = 12.3, 3.8 Hz, H-22), 3.69 (1H, dd, J = 11.0, 4.3 Hz, H-3), 2.39 (1H, d, J = 11.6 Hz, H-24), 1.54 (3H, s, H-23), 1.34 (3H, s, H-30), 1.14 (3H, s, H-29), 1.08 (6H, s, 25, 26), 0.92 (3H, s, H-28), 0.87 (3H, s, 27); 13C NMR (150 MHz, pyridine-d5) δ: 39.6 (C-1), 28.1 (C-2), 80.5 (C-3), 43.7 (C-4), 56.9 (C-5), 19.5 (C-6), 35.5 (C-7), 40.7 (C-8), 52 (C-9), 37.7 (C-10), 22.0 (C-11), 27.3 (C-12), 39.1 (C-13), 43.3 (C-14), 29.0 (C-15), 35.7 (C-16), 41.3 (C-17), 143.3 (C-18), 129.7 (C-19), 34.1 (C-20), 42.7 (C-21), 75.8 (C-22), 23.9 (C-23), 64.9 (C-24), 18.9 (C-25), 17.8 (C-26), 15.4 (C-27), 16.6 (C-28), 32.5 (C-29), 30.5 (C-30).
Wistariasapogenol B (10): white amorphous powder; 1H NMR (600 MHz, pyridine-d5) δ: 3.95 (2H, m, H-24, 29), 3.81 (4H, m, H-24, 29), 3.73 (1H, m, H-22), 3.69 (1H, dd, 1.5, 5.9, H-3), 1.58 (3H, s, H-30), 1.29 (3H, s, H-28), 1.25 (3H, s, H-27), 1.09 (3H, s, H-23), 1.01 (3H, s, H-26), 0.94 (3H, s, H-25); 13C NMR (150 MHz, pyridine-d5) δC 39.3 (C-1), 28.8 (C-2), 80.5 (C-3), 43.6 (C-4), 56.7 (C-5), 19.5 (C-6), 33.9 (C-7), 40.4 (C-8), 48.5 (C-9), 37.4 (C-10), 24.4 (C-11), 123.1 (C-12), 145.0 (C-13), 42.7 (C-14), 26.8 (C-15), 28.8 (C-16), 38.5 (C-17), 45.6 (C-18), 42.4 (C-19), 36.3 (C-20), 39.3 (C-21), 75.6 (C-22), 24.4 (C-23), 65.0 (C-24), 16.6 (C-25), 17.4 (C-26), 26.2 (C-27), 21.7 (C-28), 70.6 (C-29), 28.9 (C-30).
Triptotriterpenic acid B (11): white amorphous powder; 1H NMR (600 MHz, pyridine-d5) δ: 5.44 (1H, t, J = 3.6 Hz, H-12), 4.04 (1H, dd, J = 7.4, 3.2 Hz, H-22), 3.46 (1H, dd, J = 11.2, 4.9 Hz, H-3), 1.30 (3H, s, H-30), 1.27 (3H, s, H-28), 1.26 (3H, s, H-27), 1.08 (3H, s, H-23), 1.08 (3H, s, H-24), 1.00 (3H, s, H-26), 0.88 (1H, s, H-25); 13C NMR (150 MHz, pyridine-d5) δ: 38.4 (C-1), 29.1 (C-2), 78.4 (C-3), 40.0 (C-4), 56.1 (C-5), 19.2 (C-6), 33.6 (C-7), 40.4 (C-8), 48.4 (C-9), 37.7 (C-10), 25.4 (C-11), 123.6 (C-12), 144.7 (C-13), 42.8 (C-14), 26.7 (C-15), 21.4 (C-16), 39.5 (C-17), 45.1 (C-18), 41.9 (C-19), 43.0 (C-20), 38.2 (C-21), 75.7 (C-22), 29.3 (C-23), 17.0 (C-24), 16.2 (C-25), 17.6 (C-26), 28.5 (C-27), 25.9 (C-28), 181.92 (C-29), 24.3 (C-30).
2α,3β,24-trihydroxy olea-12-en-28-oic acid (12): white amorphous powder; 1H NMR (500 MHz, DMSO-d6) δ: 5.15 (1H, brs, H-12), 4.24 (1H, m, H-2), 3.90 (1H, brs, H-3), 3.71 (1H, d, J = 10.7 Hz, H-24), 3.20 (1H, d, J = 10.7 Hz, H-24),1.09 (3H, s, H-27), 0.93 (3H, s, H-23), 0.87 (6H, s, H-25, 30), 0.85 (3H, s, H-29), 0.67 (3H, s, H-26); 13C NMR (125 MHz, DMSO-d6) δ: 41.3 (C-1), 64.7 (C-2), 72.6 (C-3), 41.8 (C-4), 48.3 (C-5), 18.0 (C-6), 32.8 (C-7), 47.1 (C-9), 37.7 (C-10), 23.2 (C-11), 121.5 (C-12), 143.9 (C-13), 43.8 (C-14), 27.2 (C-15), 22.9 (C-16), 45.7 (C-17), 40.8 (C-18), 45.5 (C-19), 30.4 (C-20), 33.4 (C-21), 32.2 (C-22), 22.6 (C-23), 63.8 (C-24), 16.8 (C-25), 16.5 (C-26), 25.7 (C-27), 178.6 (C-28), 32.9 (C-29), 23.4 (C-30).
Erythrodiol (13): white amorphous powder; 1H NMR (600 MHz, CDCl3) δ: 5.19 (1H, d, J = 3.3 Hz, H-12), 3.56 (1H, d, J = 11.0 Hz, H-28), 3.22 (1H, d, J = 11.0 Hz, H-28), 3.12 (1H, m, H-3), 1.16 (3H, s, H-27), 0.99 (3H, s, H-23), 0.94 (3H, s, H-24), 0.93 (3H, s, H-30), 0.88 (3H, s, H-29), 0.86 (3H, s, H-26), 0.78 (3H, s, H-25); 13C NMR (150 MHz, CDCl3) δ: 38.7 (C-1), 26.1 (C-2),79.1 (C-3), 38.9 (C-4), 55.3 (C-5), 18.5 (C-6), 32.7 (C-7), 39.9 (C-8), 47.7 (C-9), 37.0 (C-10), 22.1 (C-11), 122.5 (C-12), 144.3 (C-13), 41.8 (C-14), 28.2 (C-15), 23.7 (C-16), 37.0 (C-17), 42.4 (C-18), 46.6 (C-19), 31.1 (C-20), 34.2 (C-21), 31.2 (C-22), 27.3 (C-23), 15.7 (C-24), 15.6 (C-25), 16.8 (C-26), 25.7 (C-27), 69.8 (C-28), 33.3 (C-29), 23.6 (C-30).
Oleanolic acid (14): white amorphous powder; 1H NMR (400 MHz, CDCl3) δ: 5.27 (1H, d, J = 3.5 Hz, H-12), 3.12 (1H, m, H-3), 1.16 (3H, s, H-27), 0.99 (3H, s, H-23), 0.93 (3H, s, H-30), 0.90 (3H, s, H-29), 0.91 (3H, s, H-25), 0.77 (3H, s, H-24), 0.74 (3H, s, H-26); 13C NMR (100 MHz, CDCl3) δ: 38.5 (C-1), 27.3 (C-2), 79.1 (C-3), 38.9 (C-4), 55.3 (C-5), 18.5 (C-6), 32.7 (C-7), 39.4 (C-8), 47.7 (C-9), 37.0 (C-10), 23.1 (C-11), 122.8 (C-12), 143.7 (C-13), 41.8 (C-14), 27.8 (C-15), 23.7 (C-16), 46.7 (C-17), 41.1 (C-18), 46.0 (C-19), 30.8 (C-20), 33.9 (C-21), 32.6 (C-22), 28.2 (C-23), 15.7 (C-24), 15.6 (C-25), 17.3 (C-26), 26.1 (C-27), 183.6 (C-28), 33.3 (C-29), 23.6 (C-30).
Uvaol (15): white amorphous powder; 1H NMR (500 MHz, CDCl3) δ: 5.14 (1H, t, J = 3.6 Hz, H-12), 3.52 (1H, t, J = 8.2 Hz, H-28a), 2.89 (d, J = 15.5 Hz, 1H), 2.78 (1H, d, J = 15.5 Hz, H-28b), 1.25 (3H, s, H-27), 1.10 (s, 3H, H-25), 1.00 (s, 3H, H-23), 0.99 (s, 3H, H-26), 0.95 (s, 3H, H-29), 0.93 (s, 3H, H-30), 0.79 (s, 3H, H-24); 13C NMR (125 MHz, CDCl3) δ: 38.9 (C-1), 27.4 (C-2), 79.2 (C-3), 38.2 (C-4), 55.3 (C-5), 18.5 (C-6), 32.9 (C-7), 39.5 (C-8), 47.8 (C-9), 43.5 (C-10), 23.4 (C-11), 125.1 (C-12), 138.7 (C-13), 42.2 (C-14), 26.1 (C-15), 23.5 (C-16), 37.0 (C-17), 54.2 (C-18), 40.2 (C-19), 39.6 (C-20), 35.3 (C-21), 30.8 (C-22), 28.3 (C-23), 15.8 (C-24), 15.8 (C-25), 16.9 (C-26), 23.5 (C-27), 70.2 (C-28), 17.5 (C-29), 21.5 (C-30).
β-amyrin acetate (16): white amorphous powder; 1H NMR (500 MHz, CDCl3) δ: 4.50 (1H, m, H-3), 5.25 (1H, t, J = 3.6 Hz, H-12), 1.10 (3H, s, H-24), 0.90 (3H, s, H-25), 0.85 (3H, s, H-26), 0.89 (3H, s, H-27), 0.99 (3H, s, H-28), 1.05 (3H, s, H-29), 0.91 (3H, s, H-30), 0.93 (3H, s, H-31), 2.03 (3H, s, H-32). 13C NMR (125 MHz, CDCl3) δ: 38.4 (C-1), 23.7 (C-2), 81.1 (C-3), 37.8 (C-4), 55.4 (C-5), 18.4 (C-6), 32.7 (C-7), 39.8 (C-8), 47.6 (C-9), 37.0 (C-10), 23.6 (C-11), 121.8 (C-12), 145.4 (C-13), 41.9 (C-14), 26.3 (C-15), 27.1 (C-16), 32.6 (C-17), 47.4 (C-18), 46.9 (C-19), 31.2 (C-20), 34.8 (C-21), 37.3 (C-22), 16.9 (C-23), 28.2 (C-24), 15.7 (C-25), 16.9 (C-26), 26.1 (C-27), 23.8 (C-28), 33.4 (C-29), 28.5 (C-30), 171.2 (C=O), 21.4 (CH3-C=O).
2α-hydroxy ursolic acid (17): white amorphous powder; 1H NMR (500 MHz, DMSO-d6) δ: 5.16 (1H, dd, J = 15.2, 5.6 Hz, H-12), 1.08 (3H, s, H-27), 0.92 (9H, m, H-23, 24, 26), 0.88 (1H, s, H-25), 0.74 (3H, s, H-29), 0.70 (3H, s, H-30); 13C NMR (125 MHz, DMSO-d6) δ: 47.1 (C-1), 67.5 (C-2), 82.3 (C-3), 39.5 (C-4), 54.8 (C-5), 18.2 (C-6), 32.6 (C-7), 38.9 (C-8), 46.8 (C-9), 36.3 (C-10), 23.0 (C-11), 124.5 (C-12), 138.2 (C-13), 41.7 (C-14), 27.5 (C-15), 23.8 (C-16), 47.0 (C-17), 52.4 (C-18), 38.4 (C-19), 37.6 (C-20), 30.2 (C-21), 36.3 (C-22), 28.8 (C-23), 17.1 (C-24), 17.0 (C-25), 17.2 (C-26), 23.3 (C-27), 178.3 (C-28), 16.4 (C-29), 21.5 (C-30).
Betulinic acid (18): white amorphous powder; 1H NMR (500 MHz, CDCl3) δ: 4.78 (1H, brs, H-29a), 4.61 (1H, brs, H-29b), 3.19 (1H, dd, J = 11.2, 4.9 Hz, H-3), 1.69 (3H, s, H-30), 0.97 (3H, s, H-23), 0.96 (3H, s, H-26), 0.93 (3H, s, H-25), 0.82 (3H, s, H-24), 0.75 (3H, s, H-27); 13C NMR (125 MHz, CDCl3) δ: 38.7 (C-1), 27.4 (C-2), 79.0 (C-3), 38.9 (C-4), 55.6 (C-5), 18.3 (C-6), 34.3 (C-7), 40.7 (C-8), 50.5 (C-9), 37.2 (C-10), 20.8 (C-11), 25.5 (C-12), 38.4 (C-13), 42.4 (C-14), 29.5 (C-15), 32.1 (C-16), 56.3 (C-17), 46.9 (C-18), 49.2 (C-19), 150.5 (C-20), 29.7 (C-21), 37.0 (C-22), 28.0 (C-23), 15.4 (C-24), 16.0 (C-25), 16.1 (C-26), 14.7 (C-27), 77.0 (C-28), 109.7 (C-29), 19.4 (C-30).
Friedelin (19): white amorphous powder; 1H-NMR (500 MHz, CDCl3) δ: 0.72 (3H, s, H-3), 0.87 (3H, s), 0.90 (3H, s), 0.95 (3H, s), 0.98 (3H, s), 1.01 (3H, s), 1.05 (3H, s), 1.18 (3H, s); 13C NMR (125 MHz, CDCl3) δ: 22.2 (C-1), 41.7 (C-2), 213.1 (C-3), 58.2 (C-4), 42.2 (C-5), 41.4 (C-6), 18.2 (C-7), 53.2 (C-8), 37.6 (C-9), 59.5 (C-10), 36.1 (C-11), 30.5 (C-12), 39.8 (C-13), 38.4 (C-14), 35.2 (C-15), 32.6 (C-16), 30.0 (C-17), 42.9 (C-18), 35.3 (C-19), 28.1 (C-20), 32.9 (C-21), 39.4 (C-22), 7.0 (C-23), 14.7 (C-24) 18.0 (C-25), 20.2 (C-26), 18.6 (C-27), 32.2(C-28), 35.5 (C-29), 31.9 (C-30).
3.5. Enzymatic Hydrolysis of Compounds 1–3
Acidic hydrolysis of compounds 1–3 were carried out according to the method described previously [18]. The configuration of the sugar moiety was determined by comparing their retention times with the derivatives of authentic samples. D-glucose and L-arabinose were confirmed by comparison with those of authentic samples tR: 6.5 min (D-glucose); 6.8 min (L-arabinose) (Figures S49−S51).
3.6. Anti-Inflammatory Assay
All compounds showed no cytotoxicity on RAW 264.7 cells at the concentrations from 0 to 50. All isolates from the leaves of H. littoralis were subjected to MTT assays to assess the cell viability of LPS stimulated RAW 264.7 cell model. Anti-inflammatory activity of terpenoids were evaluated using LPS stimulated RAW 264.7 cell model. The productions of NO were determined by Griess analysis [41,42].
3.7. Molecular Docking Studies
Molecular docking simulations were performed using the software AutoDock Vina along with AutoDock Tools (ADT 1.5.6) using the hybrid Lamarckian Genetic Algorithm (LGA) [43]. The three-dimensional (3D) crystal structures of iNOS (PDB code, 3E6T) and COX-2 (PDB code, 1PXX) were obtained from the RCSB Protein Data Bank, which resolution was 2.5 Å [44]. The standard 3D structures (PDB format) of selected compounds for molecular docking were constructed by chem3D Pro 22.0 software, whose configurations were determined by their NOESY spectra and Chem3D modeling. The cubic grid box of 20 Å size (x, y, z) with a spacing of 1.000 Å and grid maps were built. All of the other parameters were used according to default settings of AutoDock Vina. Results differing by less than 2.0 Å in positional root mean-square deviation (RMSD) were clustered together, and the results of the most favorable free energy of binding were chosen as the resultant complex structures.
4. Conclusions
Six new compounds, heritieras C–H (1–6), and thirteen known triterpenoids (7–19) were isolated from the leaves of H. littoralis. Five triterpenoids decreased secretions of NO on LPS stimulated RAW 264.7 cells. Among these compounds, compound 18 substantially inhibit the release of NO. The results from molecular docking revealed the preliminary anti-inflammatory mechanism due to the bindings of iNOS/COX-2 with bioactive triterpenoids. Our research disclosed that H. littoralis is potentially useful as a medicine for the treatment of inflammation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30010131/s1, Experimental details, including figures and tables of this manuscript, are provided as Supplementary Material.
Author Contributions
J.L. conceived, designed and wrote the article. Y.Z. and C.R. contributed the samples/reagents/materials/analysis tools. X.L., R.P., J.X., B.Y., Q.Z. and Y.L. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
The author (J. Li and Y. J. Zhang) acknowledges the following grants for funding this project: The Natural Science Foundation of Guangxi Province (2021GXNSFAA220100); Research project of Beibu Gulf University (2021KYQD05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of the article can be obtained from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The Biodiversity Committee of Chinese Academy of Sciences. Catalogue of Life China: 2024 Annual Checklist; The Biodiversity Committee of Chinese Academy of Sciences: Beijing, China, 2024. [Google Scholar]
- Calixto-Júnior, J.T.; Morais, S.M.; Colares, A.V.; Coutinho, H.D.M. The Genus Luehea (Malvaceae-Tiliaceae): Review about chemical and pharmacological aspects. J. Pharm. 2016, 2016, 1368971. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China. Flora of China. Science Press: Beijing, China, 1984; Volume 49, pp. 39–40. [Google Scholar]
- Lin, P. Medicinal plants of mangrove in China. J. Mar. Drugs 1984, 12, 45–51. [Google Scholar]
- Shao, C.L.; Fu, X.M.; Wang, C.Y.; Han, L.; Fang, Y.C.; Li, G.Q.; Zeng, X.Q.; Liu, G.X.; Guan, H.S. Investigation on the status of mangrove resources and medicinal research in China III. status of folk medicinal usage and medicinal research. J. Ocean Univ. Chin. 2009, 39, 712–718. [Google Scholar]
- Du, Q.; Wei, W.M.; Mi, D.Q. Knowledge and existing status of medicinal ethnobotany of mangrove among Jing People in Guangxi. Guihaia 2016, 36, 405–412. [Google Scholar]
- Ning, X.Q.; Li, J.F.; Huang, Y.; Tan, Y.F.; Deng, J.G. Study on the species of medicinal mangroves and their folk medicinal efficacy in Guangxi. Guide Chin. Med. 2013, 11, 73–75. [Google Scholar]
- Fan, H.Q.; Liang, S.C. (Eds.) Research and Management on China Mangroves; Science Press: Beijing, China, 1995; pp. 164–172. [Google Scholar]
- Cui, J.G.; Lu, Y.; Huang, Y.M. Review on bioactive substances from mangrove. Nat. Prod. Res. Dev. 2017, 29, 1626–1633. [Google Scholar]
- Ge, L.; Li, Y.J.; Yang, K.D. Chemical constituents of the leaves of Heritiera littoralis. Chem. Nat. Compd. 2016, 52, 603–604. [Google Scholar] [CrossRef]
- Miles, D.H.; Vallapa, C.W. Toxicants from mangrove plants, VII. Vallapin and vallapianin, novel sesquiterpene lactones from the mangrove plant Heritiera littoralis. J. Nat. Prod. 1991, 54, 286–289. [Google Scholar] [CrossRef]
- Takeda, Y.; Miyazaki, K.; Shimizu, H.; Masuda, T.; Otsuka, H. A new phenylpropanoid-glycerol conjugate from Heritiera littoralis Dryand. Nat. Med. 2000, 54, 22–25. [Google Scholar]
- Tian, Y.; Wu, J.; Xi, S.H.; Zhang, D.J.; Xu, L.R.; Zhang, S. Studies on the triterpenoid components of Heritiera littoralis. Chin. Trad. Herb. Drugs 2007, 3, 35–36. [Google Scholar]
- Tian, Y.; Wu, J.; Zhang, S. Flavonoids from leaves of Heritiera littoralis. J. Chin. Pharm. Sci. 2004, 13, 214–216. [Google Scholar]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; De Koning, C.B. A new cinnamoylgly-coflavonid antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef]
- Liang, X.Q.; Niu, P.; Li, J.; Guan, X.L.; Zhang, Y.J.; Li, J. Discovery of anti-inflammatory triterpenoid glucosides from the Heritiera littoralis Dryand. Molecules 2023, 28, 1658. [Google Scholar] [CrossRef] [PubMed]
- Tewtrakul, S.; Tansakul, P.; Daengrot, C.; Ponglimanont, C.; Karalai, C. Anti-inflammatory principles from Heritiera littoralis bark. Phytomedicine 2010, 17, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Tanaka, N.; Ichikawa, S.; Tanaka, O. Triterpenes of betula platyphylla sukatchev var. Japonica hara and the configuration at C-24 of ocotillol-II and its related compounds. Tetrahedron Lett. 1968, 9, 4239–4242. [Google Scholar]
- Liang, X.Q.; Deng, S.P.; Huang, Y.; Pan, L.W.; Chang, Y.L.; Hou, P.; Ren, C.Y.; Xu, F.W.; Yang, R.Y.; Li, K.Y.; et al. Triterpenoids from the leaves of Cyclocarya paliurus and their glucose uptake activity in 3T3-L1 adipocytes. Molecules 2023, 28, 3294. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.H.; Lou, H.X. Natural Medicinal Chemistry, 7th ed.; People’s Publishing Agence: Beijing, China, 2016. [Google Scholar]
- Seger, C.; Pointinger, S.; Greger, H.; Hofer, O. Isoeichlerianic acid from Aglaia silvestris and revision of the stereochemistry of foveolin B. Tetrahedron Lett. 2008, 49, 4313–4315. [Google Scholar] [CrossRef]
- Cui, B.S.; Li, S. New triterpenoid saponins from the leaves of Cyclocarya paliurus. Chin. Chem. Lett. 2015, 26, 585–589. [Google Scholar] [CrossRef]
- Arao, T.; Kinjo, J.; Nohara, T.; Isobe, R. Oleanene-type triterpene glycosides from Puerariae Radix. II. Isolation of saponins and the application of tandem mass spectrometry to their structure determination. Chem. Pharm. Bull. 1995, 43, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Miyao, H.; Sakai, Y.; Takeshita, T.; Hinjo, J.; Nohara, T. Triterpene saponins from Abrus cantoniensis (Leguminosae). I. Isolatioin and characterization of four new saponins and a new sapogenol. Chem. Pharm. Bull. 1996, 44, 1222–1227. [Google Scholar] [CrossRef]
- Ding, P.L.; Hou, A.J.; Chen, D.F. Three new isoprenylated flavonoids from the roots of Sophora flavescens. J. Asian Nat. Prod. Res. 2005, 7, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Simonet, A.M.; Galindo, J.C.G.; Pacheco, P.C.; Sánchez, J.A. Bioactive polar triterpenoids from Melilotus messanensis. Phytochemistry 1998, 49, 709–717. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Mella, M.; Quadrelli, P.; Avato, P. Artefact formation during acid hydrolysis of saponins from Medicago spp. Phytochemistry 2017, 138, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Fujishima, Y.; Saino, K.; Tian, R.H.; Nohara, T. Five new triterpene glycosides from Wisteria branchybotrys (Leguminosae). Chem. Pharm. Bull. 1995, 43, 636–640. [Google Scholar] [CrossRef]
- Chen, Y. Triterpenoids from Tripterygium willfordii. J. Hubei Univ. Technol. 2000, 04, 42–44. [Google Scholar]
- Lee, I.K.; Kim, D.H.; Lee, S.Y.; Kim, K.R.; Kang, R.L. Triterpenoic acids of Prunella vulgarisvar. lilacina and their cytotoxic activities in vitro. Arch. Pharm. Res. 2008, 31, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Iuchi, M.; Fujita, Y.; Minami, H.; Fukuyama, Y. Coumaroyl triterpenes from Casuarina equisetifolia. Phytochemistry 1999, 51, 543–550. [Google Scholar] [CrossRef]
- Zhu, L.L.; Zhong, L.J.; Xu, M.J.; Chen, J.K.; Liang, L.F. Chemical Constituents of Triterpenes from Sabia discolor Dunn. Chem. Ind. Forest Prod. 2022, 42, 95–100. [Google Scholar]
- Siddiqui, S.; Hafeez, F.; Begum, S.; Siddiqui, B.S. Kaneric Acid, a new triterpene from the leaves of Nerium oleander. J. Nat. Prod. 1986, 49, 1086–1090. [Google Scholar] [CrossRef]
- Shiojima, K.; Suzuki, H.; Kodera, N.; Ageta, H.; Chang, H.C.; Chen, Y.P. Composite constituents: Thirty-nine triterpenoids including two novel compounds from Ixeris chinensis. Chem. Pharm. Bull. 1996, 44, 509–514. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.M.; Chen, J.J.; Zhang, Y.; Lin, X.K.; Zhou, L. Chemical constituents from root of Actinidia chinensis. China J. Chin. Mater. Med. 2007, 32, 1663–1665. [Google Scholar]
- Xu, J.; Gao, H.Y.; Ma, S.L.; Li, H.H.; Bai, L.M. Chemical constituents and bioactivity of Kalimeris indica. Chin. Trad. Herb. Drugs 2014, 45, 3246–3250. [Google Scholar]
- Lee, S.R.; Lee, S.; Moon, E.; Park, H.J.; Park, H.B.; Kim, K.H. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated RAW 264. 7 cells. Bioorg. Chem. 2017, 70, 94–99. [Google Scholar] [CrossRef]
- Kim, A.T.; Kim, D.O. Anti-inflammatory effects of vanadium-binding protein from Halocynthia roretzi in LPS-stimulated RAW 264. 7 macrophages through NF-κB and MAPK pathways. Int. J. Biol. Macromol. 2019, 133, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Park, G.; Jeong, S.J. The anti-neuroinflammatory activity of tectorigenin pretreatment via downregulated NF-κB and ERK/JNK pathways in BV-2 microglial and microglia inactivation in mice with lipopolysaccharide. Front. Pharmacol. 2018, 9, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Stefani, H.A.; Gueogjan, K.; Manarin, F.; Farsky Sandra, H.P.; Zukerman-Schpector, J.; Caracelli, I.; Pizano Rodrigues, S.R.; Muscará, M.N.; Teixeira, S.A.; Santin, J.R.; et al. Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: Effect on inducible nitric oxide synthase. Eur. J. Med. Chem. 2012, 58, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Rupa, E.J.; Zheng, S.; Nahar, J.; Yang, D.C.; Kang, S.C.; Wang, Y. Panos-fermented extract-mediated nanoemulsion: Preparation, characterization, and in vitro anti-inflammatory effects on RAW 264.7 cells. Molecules 2022, 27, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Huang, X.S.; Chen, H.C.; Zhou, D.X.; Yang, Z.M.; Wang, K.; Liu, W.; Deng, S.P.; Yang, R.Y.; Li, J.; et al. Discovery of anti-inflammatory terpenoids from Mallotus conspurcatus Croizat. J. Ethnopharmacol. 2019, 231, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, X.; Ma, J.; Yang, Y.; Xie, C.; Tuerhong, M.; Jin, D.Q.; Xu, J.; Lee, D.; Ohizumi, Y.; et al. Nitric oxide inhibitory daphnane diterpenoids as potential anti-neuroinflammatory agents for AD from the twigs of Trigonostemon thyrsoideus. Bioorg. Chem. 2017, 75, 149–156. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Nicholls, P.R.; Mallinder, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).