Poria cocos Attenuated DSS-Induced Ulcerative Colitis via NF-κB Signaling Pathway and Regulating Gut Microbiota
Abstract
1. Introduction
2. Results
2.1. Chemical Profile Analysis and Determination of Polysaccharide of PC
2.2. PCE Alleviates the Symptoms of DSS-Induced Ulcerative Colitis in Mice
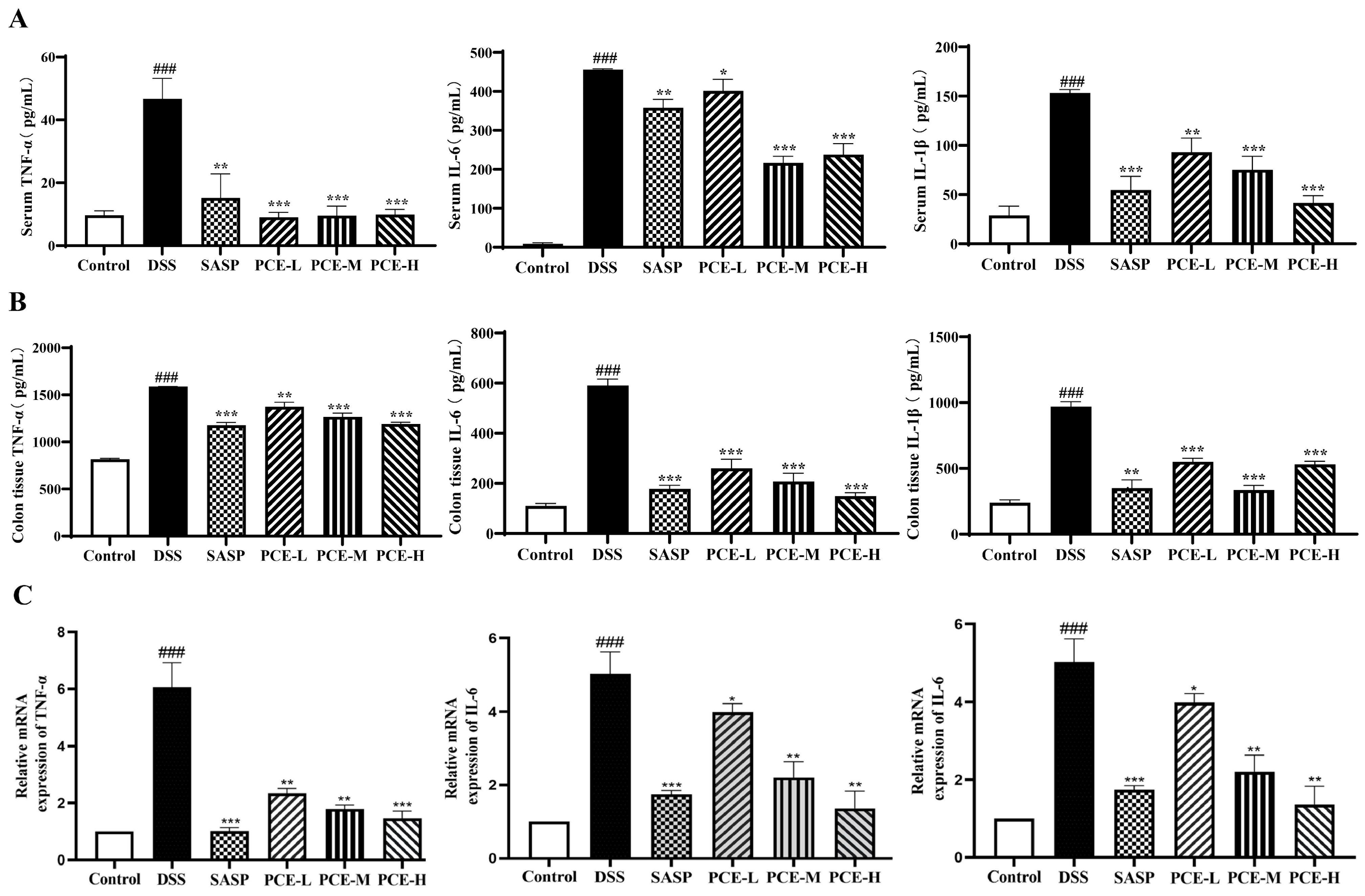
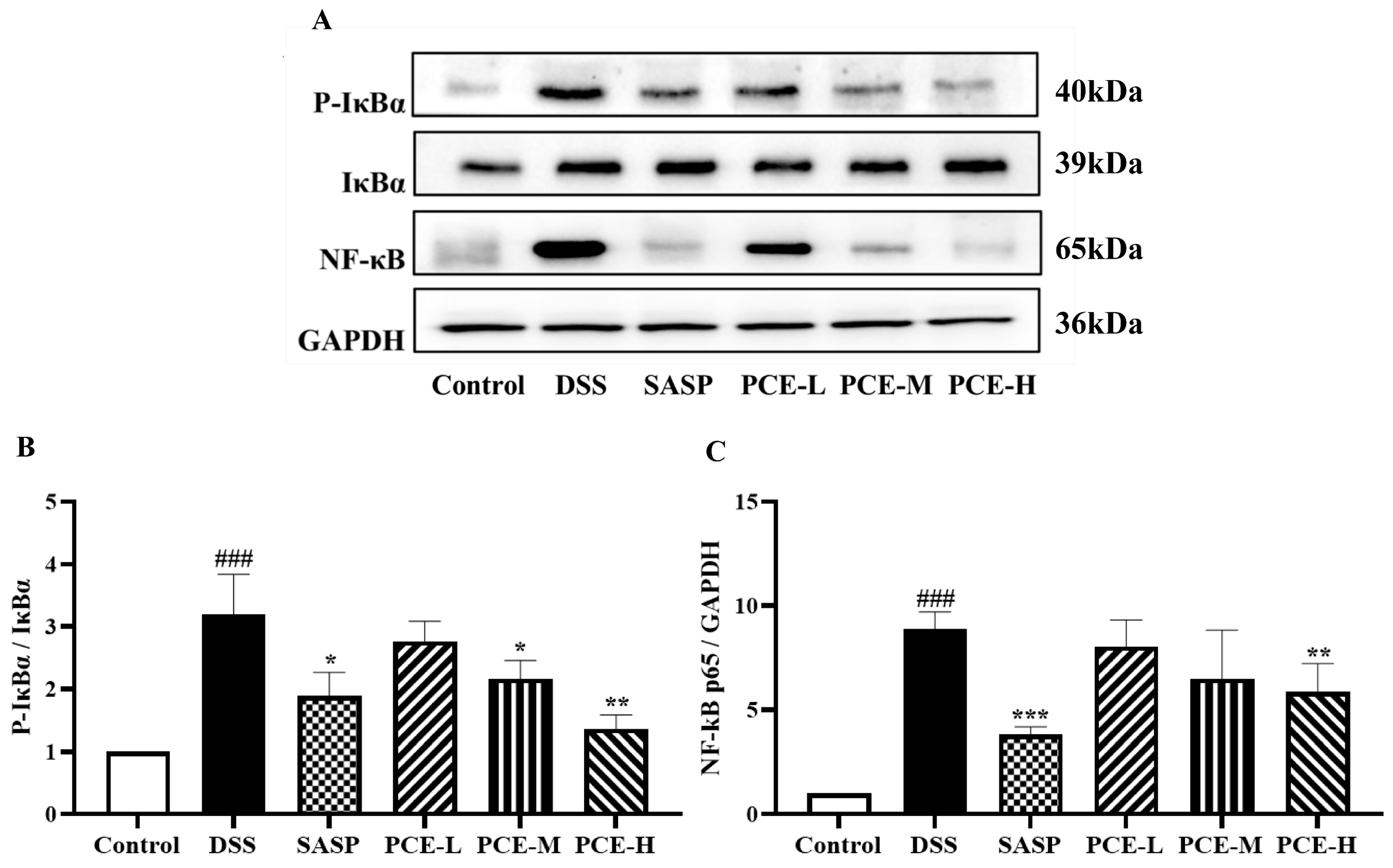
2.3. PCE Alleviates Colonic Inflammation in DSS-Induced UC Mice
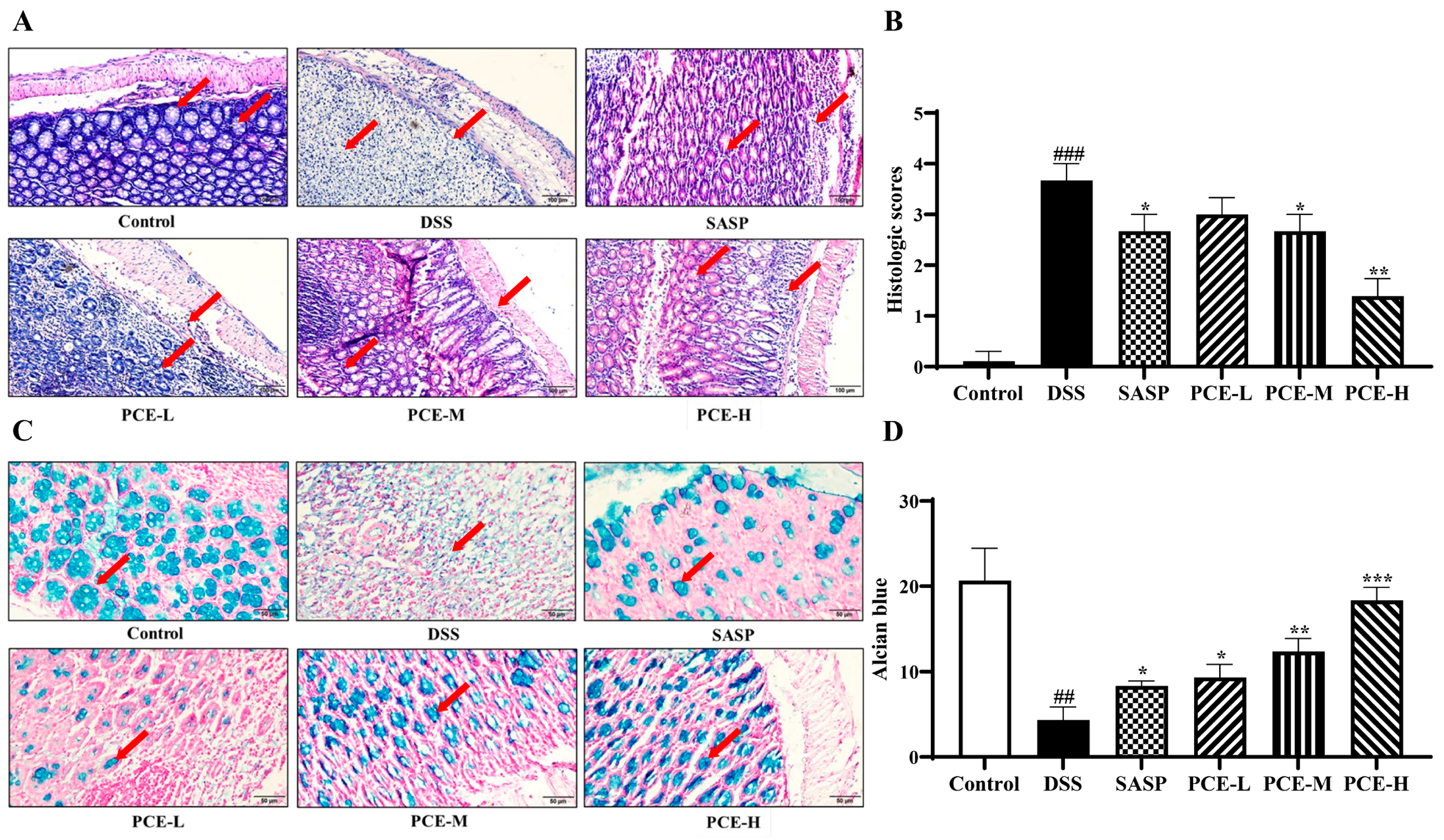
2.4. PCE Ameliorates Intestinal Barrier Function in DSS-Induced UC Mice
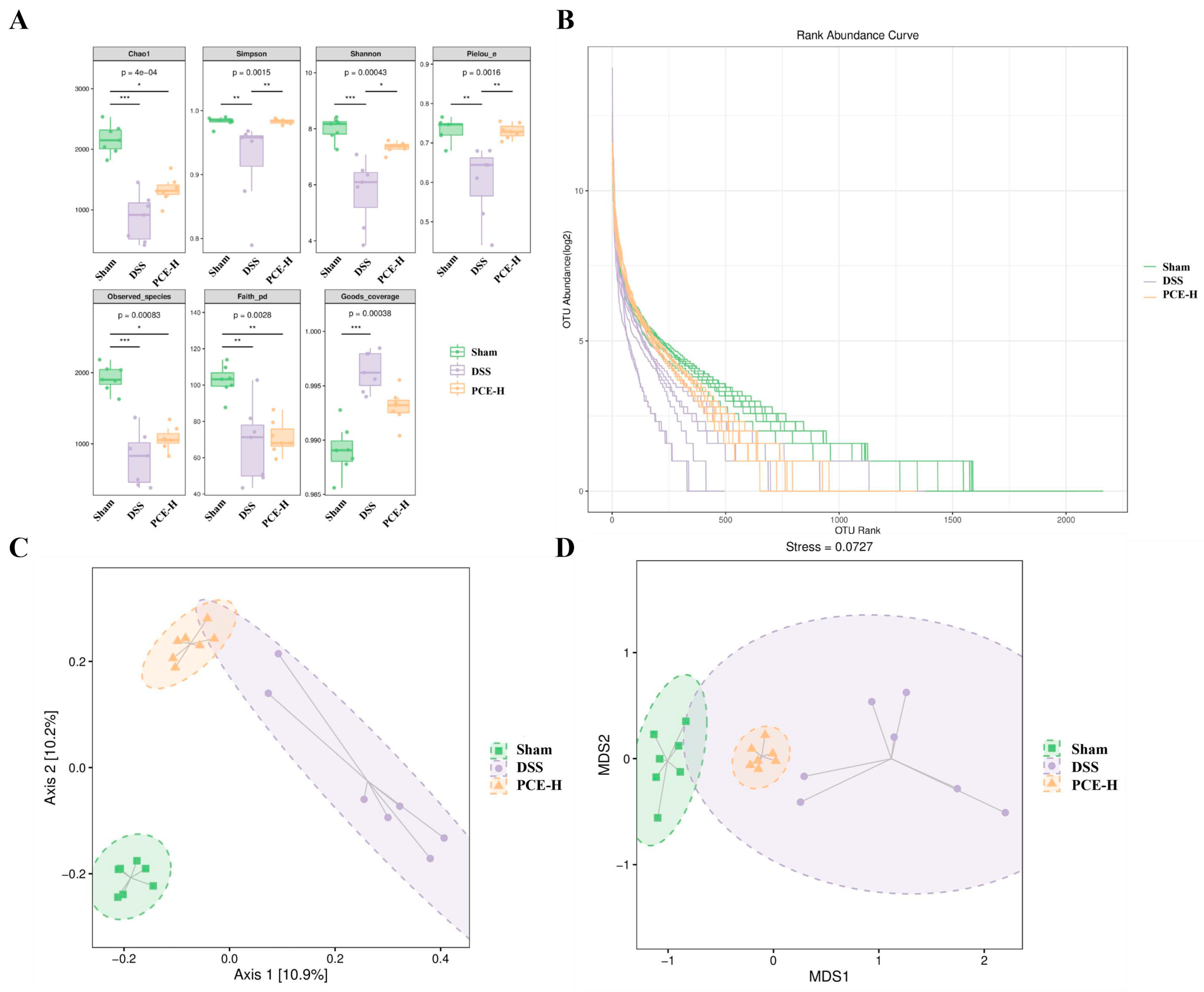
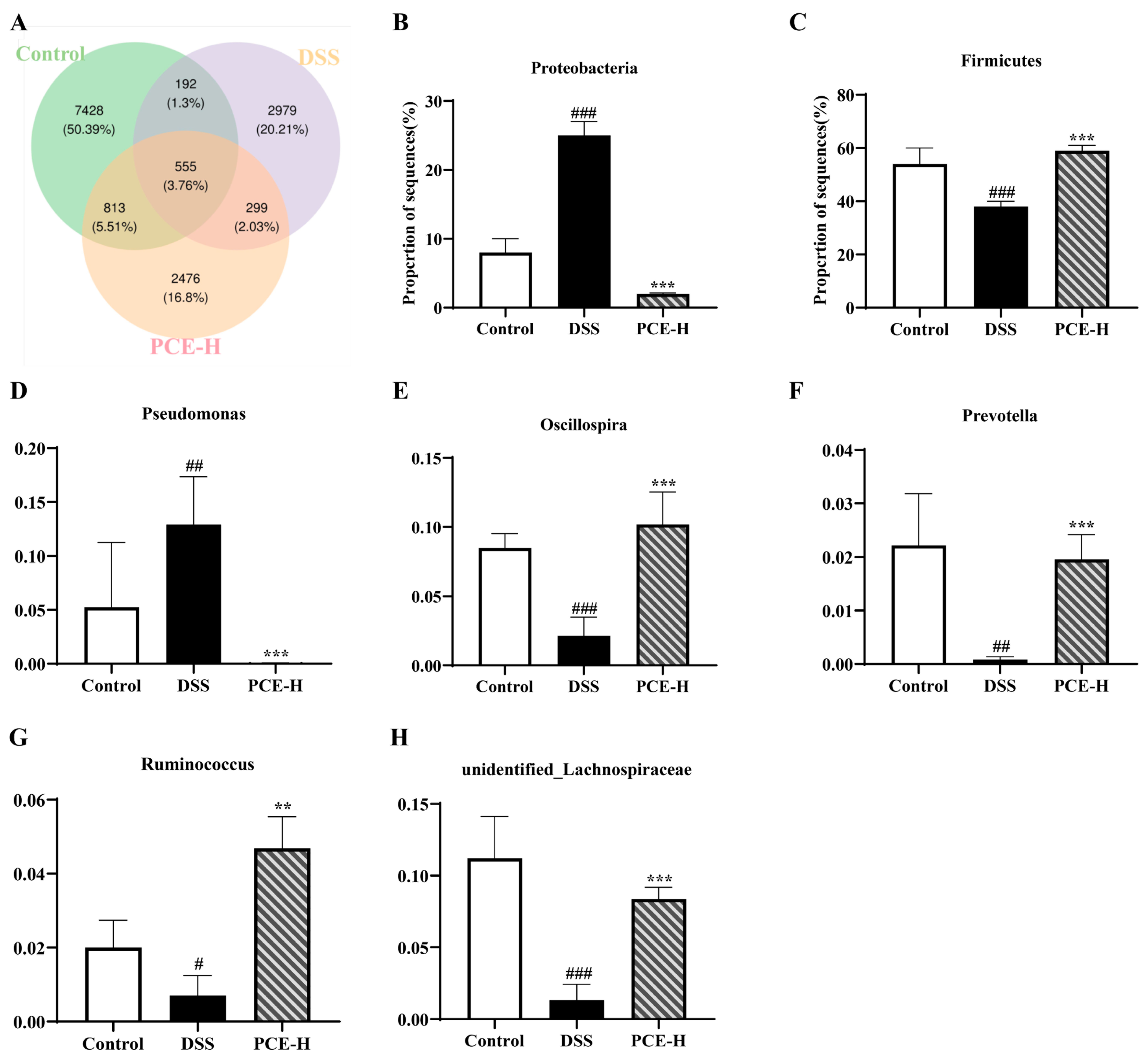
2.5. PCE Regulates Gut Microbiota Disorders in DSS-Induced UC Mice
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Preparation of Poria cocos Extraction
4.3. UPLC-Q-Exactive Orbitrap MS/MS Analysis and Determination of Polysaccharides from Poria cocos Extraction
4.3.1. Instrumentation and UPLC-Q-Exactive Orbitrap MS Conditions
4.3.2. MS Data Preprocessing and Statistical Analysis
4.4. Animals
4.5. Establishment and Treatment of UC
4.6. Hematoxylin & Eosin Staining
4.7. Alician Staining
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Quantitative PCR (qPCR) Analysis
4.10. Immunohistochemistry Staining
4.11. Western Blotting
4.12. Gut Microbiota Profiling by 16S rRNA Sequencing
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pabla, B.S.; Schwartz, D.A. Assessing Severity of Disease in Patients with Ulcerative Colitis. Clin. Gastroenterol. 2020, 49, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.Y.; Shao, B.L.; Tian, F.; Ye, M.; Li, Y.Q.; Wang, X.L.; Wang, L.; Yang, S.Q.; Lv, X.P.; Jia, Y.; et al. Trends in medication use and treatment patterns in Chinese patients with inflammatory bowel disease. World J. Gastroenterol. 2022, 28, 4102–4119. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Berre, C.L.; Wei, S.C.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Roda, G.; Nedeljkovic Protic, M.; Danese, S.; Peyrin-Biroulet, L. Modern use of 5-aminosalicylic acid compounds for ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Shahabi, A.; Seabury, S.A.; Lakdawalla, D.N.; Espinosa, O.D.; Green, S.; Baldassano, R.N. Lifetime economic burden of crohn’s disease and ulcerative colitis by age at diagnosis. Clin. Gastroenterol. Hepatol. 2020, 18, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Chacko, A. Influence of environmental factors on the onset and course of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1088–1100. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.G.; Coffey, J.C.; Sahebally, S.M.; Tibbitts, P.; Lyons, E.M.; O’Leary, E.; Owolabi, F.; Dunne, C.P. Systemic molecular mediators of inflammation differentiate between crohn’s disease and ulcerative colitis, implicating threshold levels of IL-10 and relative ratios of pro-inflammatory cytokines in therapy. J. Crohn’s Colitis 2020, 14, 118–129. [Google Scholar] [CrossRef]
- Wang, G.; Ma, D.; Wang, R. Effect of butylphthalide on serum CRP, PARK7, NT-3 and neurological function in patients with acute cerebral infarction. Am. J. Transl. Res. 2021, 13, 10388–10395. [Google Scholar]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef]
- Lu, P.D.; Zhao, Y.H. Targeting NF-κB pathway for treating ulcerative colitis: Comprehensive regulatory characteristics of Chinese medicines. Chin. Med. 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, C.; Wang, X.; Tang, J.; Wu, Z.; Huang, Y.; Shao, W.; Geng, K.; Xie, H.; Pu, Z. Schisandrin protects against ulcerative colitis by inhibiting the SGK1/NLRP3 signaling pathway and reshaping gut microbiota in mice. Chin. Med. 2023, 18, 112. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, Y.; He, W.; Tian, Z.; Lin, J.; Liu, Z.; Li, Y.; Chen, M.; Han, S.; Liang, J.; et al. Gut microbiota and metabolite changes in patients with ulcerative colitis and clostridioides difficile infection. Front. Microbiol. 2022, 13, 802823. [Google Scholar] [CrossRef]
- Huang, S.W.; Fu, Y.J.; Xu, B.; Liu, C.; Wang, Q.; Luo, S.; Nong, F.F.; Wang, X.J.; Huang, S.Y.; Chen, J.Y. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine 2020, 68, 153179. [Google Scholar] [CrossRef]
- Zhou, H.; Li, H.; Wang, H. Potential protective effects of the water-soluble Chinese propolis on experimental ulcerative colitis. J. Tradit. Chin. Med. 2023, 43, 925–933. [Google Scholar]
- Ríos, J.L. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tan, X.; Shi, H.; Xia, D. Nutrition and traditional Chinese medicine (TCM): A system’s theoretical perspective. Eur. J. Clin. Nutr. 2021, 75, 267–273. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, N.H.; Bhattarai, G.; Kim, G.E.; Lee, I.K.; Yun, B.S.; Yi, H.K. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis. 2013, 19, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, Q.; Olatunji, O.J.; Li, Y.; Pan, S.; Wang, D.D.; Zuo, J. Activation of cholinergic anti-inflammatory pathway involved in therapeutic actions of α-mangostin on lipopolysaccharide-induced acute lung injury in rats. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420954941. [Google Scholar] [CrossRef]
- Zhang, D.D.; Li, H.J.; Zhang, H.R.; Ye, X.C. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct. 2022, 13, 6648–6664. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, L. Evaluation of anticancer activities of Poria cocos ethanol extract in breast cancer: In vivo and in vitro, identification and mechanism. J. Ethnopharmacol. 2020, 257, 112851. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Liu, Z.; Tian, H.; Bao, Y. The immunomodulatory effect of Poria cocos polysaccharides is mediated by the Ca2+/PKC/p38/NF-κB signaling pathway in macrophages. Int. Immunopharmacol. 2019, 72, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Claudia, F.; Beatriz, S.; Eugenia, L.M.; García, L.S.; González, S.R.; González, L.Y.; Yamile, Z.; Joaquín, H.; Barreiro, D.A.M.; Domingo, B.; et al. GADECCU 2022 guideline for the treatment of ulcerative colitis. adaptation and updating of the GETECCU 2020 guideline. Gastroenterol. Hepatol. 2023, 46, S1–S56. [Google Scholar]
- Nascimento, R.D.P.D.; Machado, A.P.D.F.; Galvez, J.; Betim Cazarin, C.B.; Junior, M.R.M. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.K.; Cheng, J.J.; Lin, C.Y.; Chang, C.C. Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1,6-branched 1,3-α-d-galactan from cultured mycelia of Poria cocos. Food Chem. 2010, 118, 349–356. [Google Scholar] [CrossRef]
- Chao, C.L.; Huang, H.W.; Su, M.H.; Lin, H.C.; Wu, W.M. The lanostane triterpenoids in Poria cocos play beneficial roles in immunoregulatory activity. Life 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, H.; Wang, S.; Xiang, Z.; Kong, H.; Xue, Q.; He, M.; Yu, X.; Li, Y.; Sun, D.; et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 2022, 217, 536–551. [Google Scholar] [CrossRef]
- Hyun, C.K. Molecular and pathophysiological links between metabolic disorders and inflammatory bowel diseases. Int. J. Mol. Sci. 2021, 22, 9139. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Parvinroo, S.; Kopper, J.; Wannemuehler, M.J. Rules of engagement: Epithelial-microbe interactions and inflammatory bowel disease. Front. Med. 2021, 8, 669913. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Xie, X.; Su, Y.; Pan, Z.; Li, Y.; Liang, J.; Zhang, M.; Pan, S.; Xu, B.; et al. Dahuang Mudan decoction repairs intestinal barrier in chronic colitic mice by regulating the function of ILC3. J. Ethnopharmacol. 2022, 299, 115652. [Google Scholar] [CrossRef]
- Barreau, F.; Hugot, J.P. Intestinal barrier dysfunction triggered by invasive bacteria. Curr. Opin. Microbiol. 2014, 17, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, N.; Boerner, K.; Waschke, J. Targeting desmosomal adhesion and signalling for intestinal barrier stabilization in inflammatory bowel diseases—Lessons from experimental models and patients. Acta Physiol. 2021, 231, e13492. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Wang, Z.H.; Xu, Q.C.; Chen, Q.; Wang, Z.; Lu, W.H. Sepsis induces variation of intestinal barrier function in different phase through nuclear factor kappa B signaling. Korean J. Physiol. Pharmacol. 2021, 25, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Zhang, Y.M.; Zhang, S.P.; Xu, Q.X.; Tian, Y.Q.; Li, P.; Cao, D.; Zheng, Y.Q. Suppression of PTRF alleviates post-infectious irritable bowel syndrome via downregulation of the TLR4 pathway in rats. Front. Pharmacol. 2021, 12, 724410. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tsugami, Y.; Suzuki, N.; Suzuki, T.; Nishimura, T. Suppressive effects of curcumin on milk production without inflammatory responses in lactating mammary epithelial cells. Phytomedicine 2020, 80, 153360. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, W.D.; Jiang, J.; Zhao, J.; Liu, Y.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juvenile Jian carp. Fish Shellfish Immunol. 2016, 58, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Weersma, R.K. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Butterworth, A.D.; Akobeng, A.K. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Wang, M.X.; Lin, L.; Chen, Y.D.; Zhong, Y.P.; Liao, Q.F. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 2020, 159, 104978. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Geng, W.; Chen, S.; Wang, L.; Lu, Y. Ginger Alleviates DSS-Induced Ulcerative Colitis Severity by Improving the Diversity and Function of Gut Microbiota. Front. Pharmacol. 2021, 12, 632569. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; You, S.; Beiyuan, J.; Luo, G.; Gupta, J.; Kumar, S.; Singh, L.; Zhang, S.; Tsang, D.C.W. Lignin valorization by bacterial genus Pseudomonas: State-of-the-art review and prospects. Bioresour. Technol. 2021, 320, 124412. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Lin, X.; Guo, X.; Qu, L.; Tu, J.; Li, S.; Cao, G.; Liu, Y. Preventive effect of Atractylodis Rhizoma extract on DSS-induced acute ulcerative colitis through the regulation of the MAPK/NF-κB signals in vivo and in vitro. J. Ethnopharmacol. 2022, 292, 115211. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Wang, W.; Liu, L.; Zhao, Z.; Shen, X.; Zhou, L.; Zhang, Y.; Peng, D.; Nian, S. Poria cocos Attenuated DSS-Induced Ulcerative Colitis via NF-κB Signaling Pathway and Regulating Gut Microbiota. Molecules 2024, 29, 2154. https://doi.org/10.3390/molecules29092154
Song X, Wang W, Liu L, Zhao Z, Shen X, Zhou L, Zhang Y, Peng D, Nian S. Poria cocos Attenuated DSS-Induced Ulcerative Colitis via NF-κB Signaling Pathway and Regulating Gut Microbiota. Molecules. 2024; 29(9):2154. https://doi.org/10.3390/molecules29092154
Chicago/Turabian StyleSong, Xiaojun, Wei Wang, Li Liu, Zitong Zhao, Xuebin Shen, Lingyun Zhou, Yuanxiang Zhang, Daiyin Peng, and Sihui Nian. 2024. "Poria cocos Attenuated DSS-Induced Ulcerative Colitis via NF-κB Signaling Pathway and Regulating Gut Microbiota" Molecules 29, no. 9: 2154. https://doi.org/10.3390/molecules29092154
APA StyleSong, X., Wang, W., Liu, L., Zhao, Z., Shen, X., Zhou, L., Zhang, Y., Peng, D., & Nian, S. (2024). Poria cocos Attenuated DSS-Induced Ulcerative Colitis via NF-κB Signaling Pathway and Regulating Gut Microbiota. Molecules, 29(9), 2154. https://doi.org/10.3390/molecules29092154






