Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of C3-Symmetric N-Centered BTA (N-BTA)
2.2. Gelation Studies
2.3. Thermal Stability
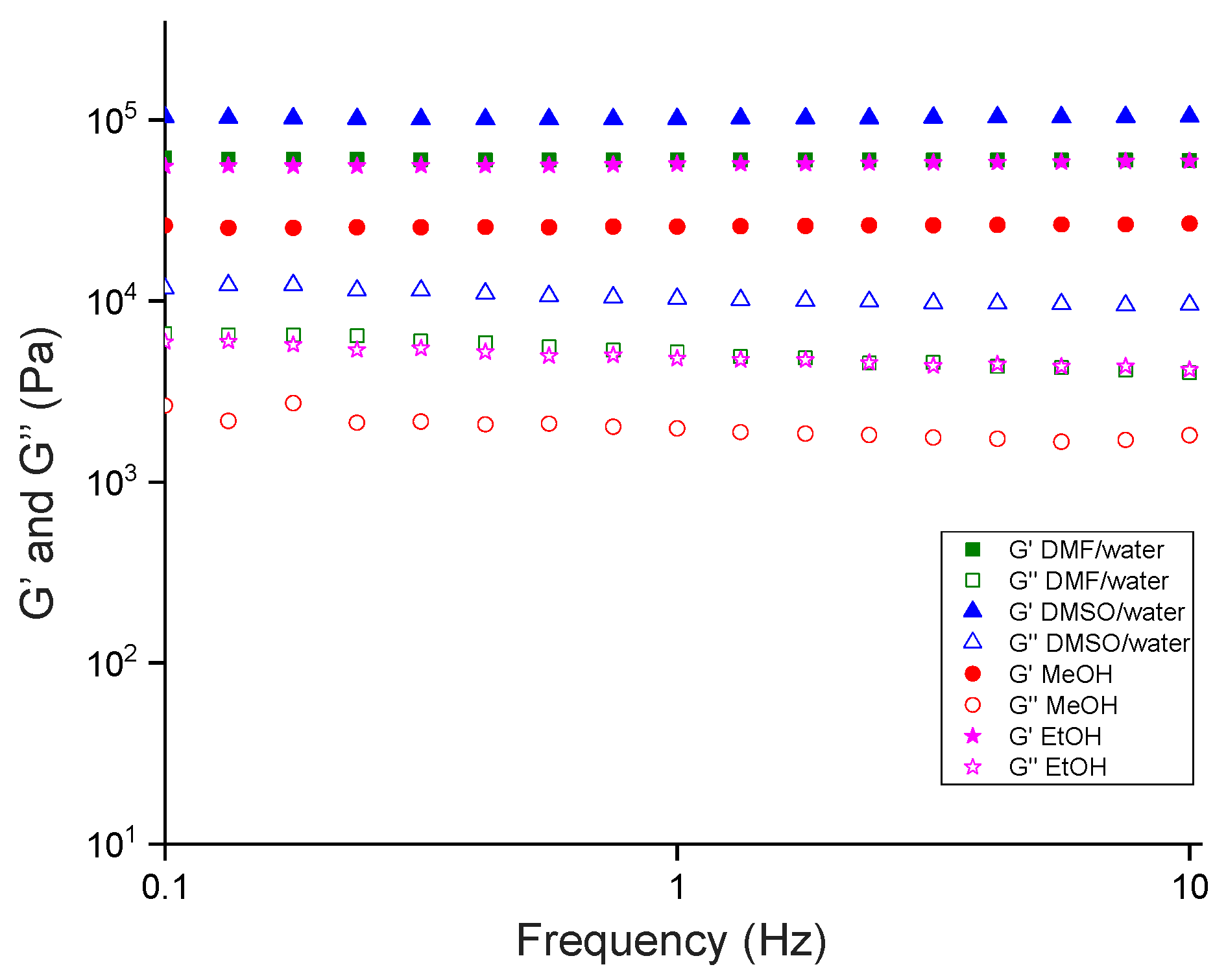
2.4. Rheology
2.5. Gel Morphology
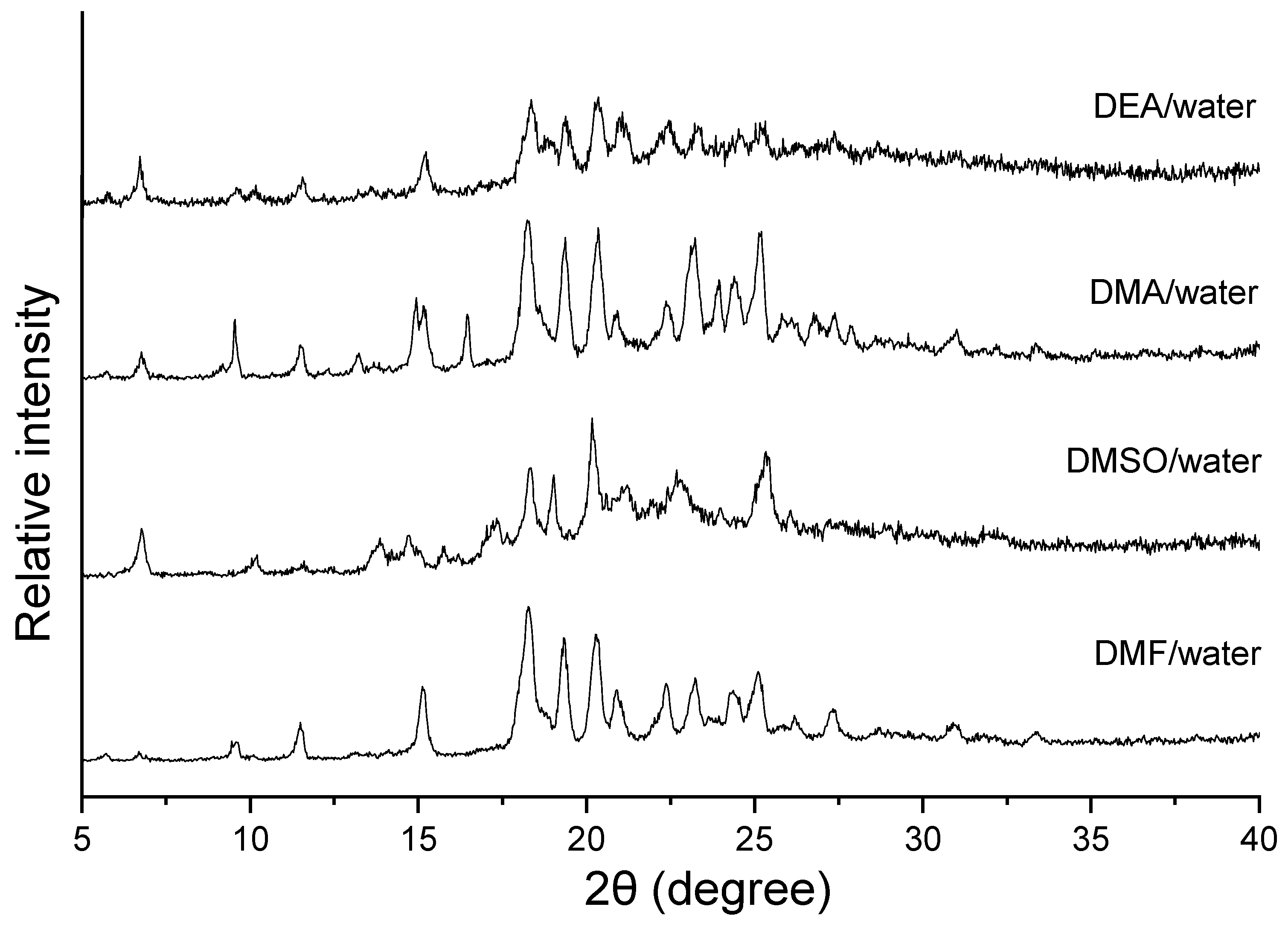
2.6. Powder X-ray Powder Diffraction (PXRD)
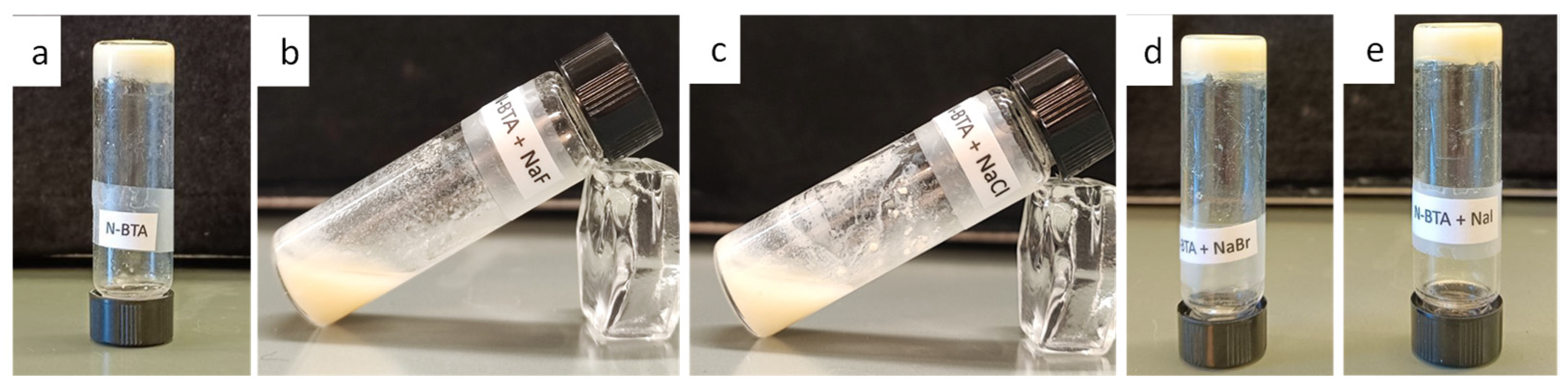
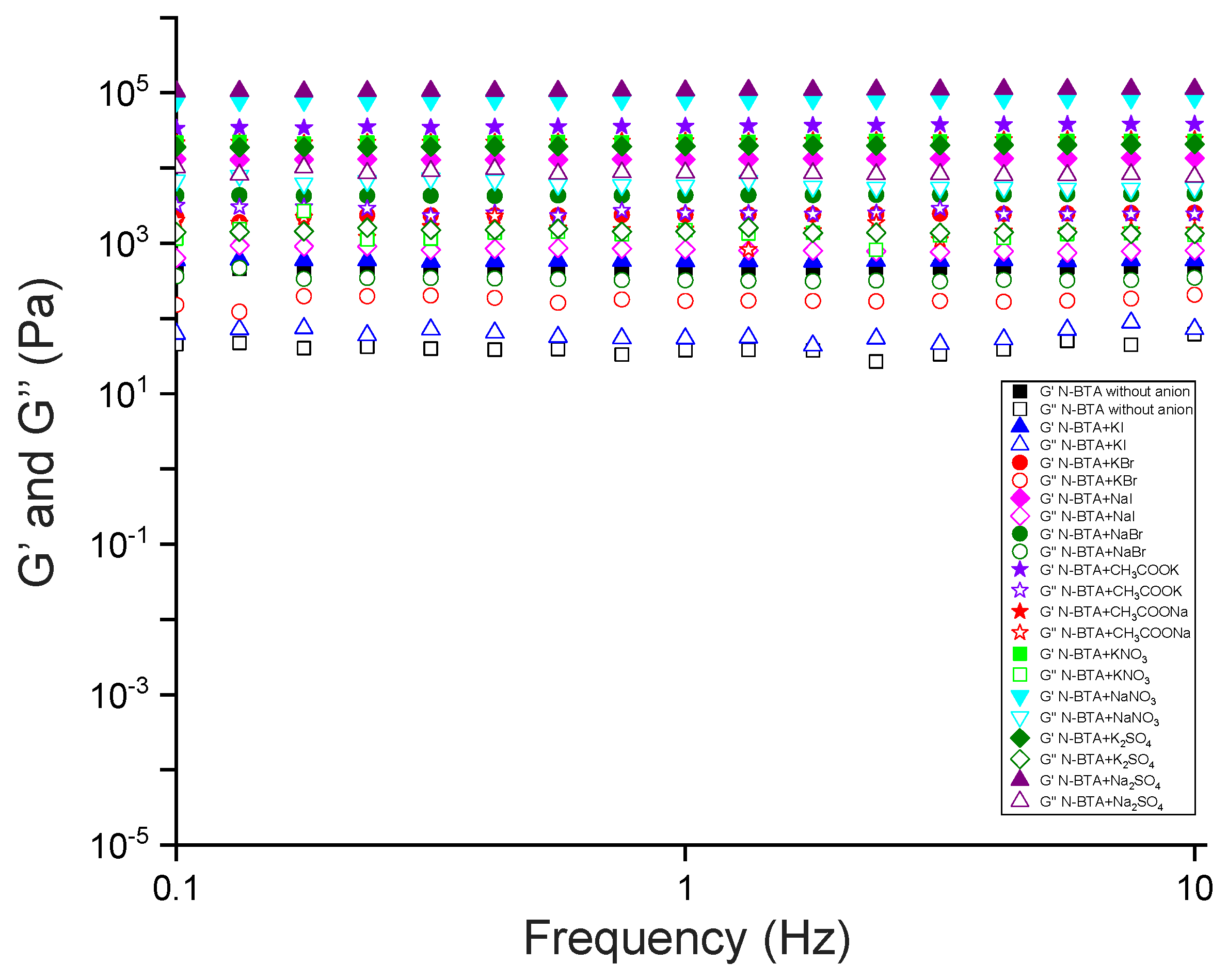
2.7. Stimuli-Responsive Properties
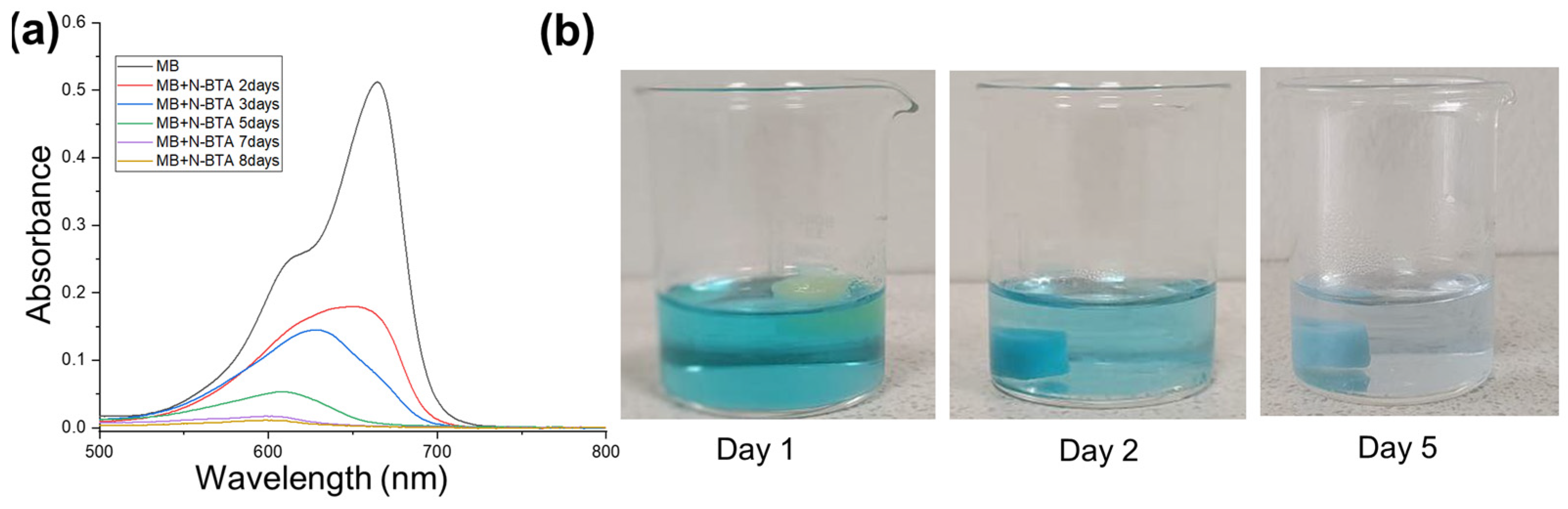
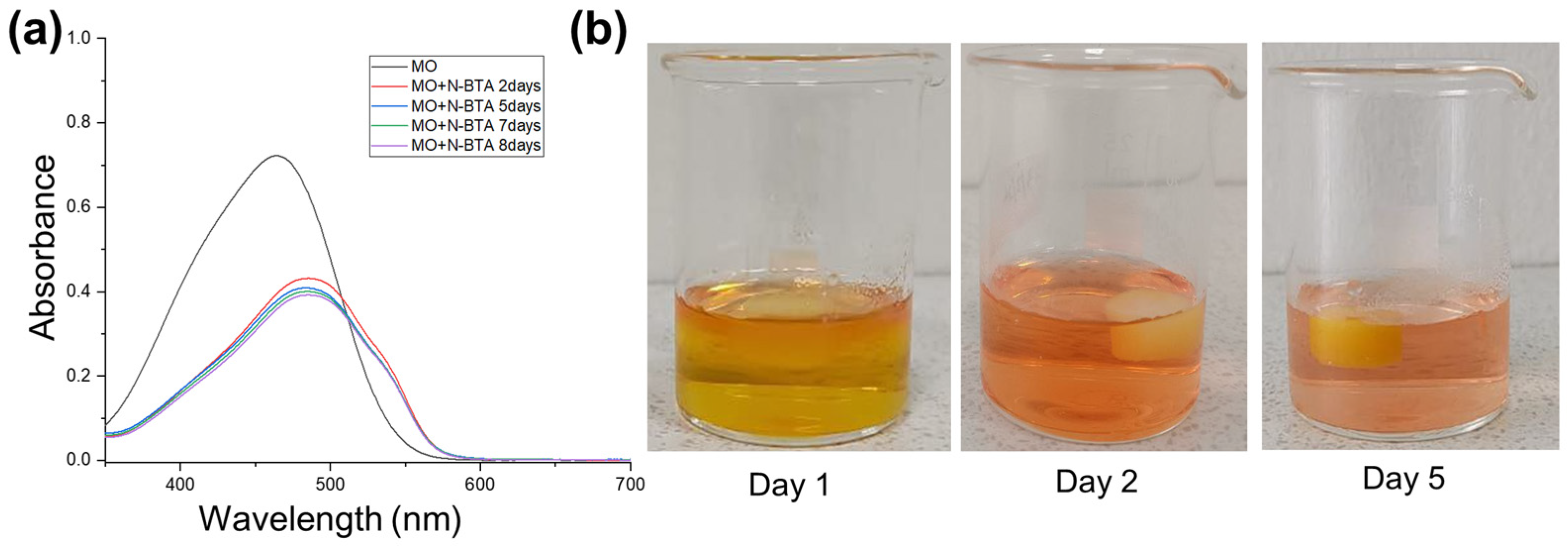
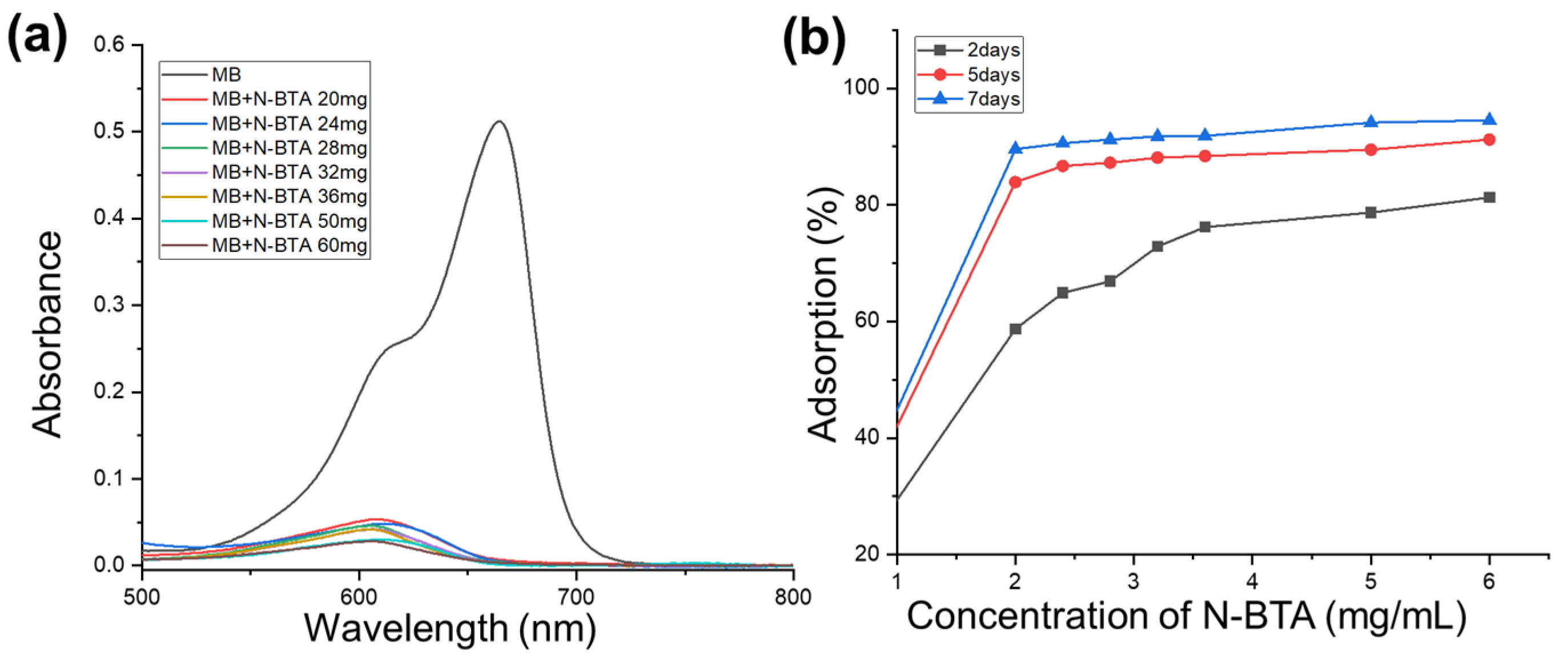
2.8. Dye Adsorption Studies
3. Materials and Methods
3.1. Synthesis of Ligands
3.1.1. Synthesis of Benzene-1,3,5-Triyltrimethanamine
3.1.2. Synthesis of Triethyl 4,4′,4″-(((benzene-1,3,5-triyltris(methylene))tris(azanediyl))tris(carbonyl))tribenzoate (N-BTA)
3.2. Gelation Studies
3.2.1. Minimum Gelator Concentration (MGC)
3.2.2. Tgel Experiments
3.3. Rheology
3.4. Scanning Electron Microscopy (SEM)
3.5. Powder X-ray Diffraction
3.6. Stimuli-Responsive Properties
3.7. UV–Visible Spectroscopy
3.7.1. Dye Adsorption Studies with Varying Concentrations of N-BTA
3.7.2. Dye Adsorption Studies of MO with N-BTA Gel from Other Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, M.N.V.; Elchuri, S.V. Environmental Contaminants of Emerging Concern: Occurrence and Remediation. Chem. Didact. Ecol. Metrol. 2023, 28, 57–77. [Google Scholar] [CrossRef]
- Feng, W.; Deng, Y.; Yang, F.; Miao, Q.; Ngien, S.K. Systematic Review of Contaminants of Emerging Concern (CECs): Distribution, Risks, and Implications for Water Quality and Health. Water 2023, 15, 3922. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mudhoo, A.; Ramasamy, D.L.; Bhatnagar, A.; Usman, M.; Sillanpää, M. An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol. Environ. Saf. 2020, 197, 110587. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.C.; Jadhav, N.C. Chapter Ten—Treatment of textile wastewater using adsorption and adsorbents. In Sustainable Technologies for Textile Wastewater Treatments; Muthu, S.S., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 235–273. [Google Scholar]
- de Loos, M.; Feringa, B.L.; van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- Kumar, D.K.; Steed, J.W. Supramolecular gel phase crystallization: Orthogonal self-assembly under non-equilibrium conditions. Chem. Soc. Rev. 2014, 43, 2080–2088. [Google Scholar] [CrossRef]
- Steed, J.W. Anion-tuned supramolecular gels: A natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 2010, 39, 3686–3699. [Google Scholar] [CrossRef]
- Smith, D.K. Supramolecular gels—A panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 2024, 20, 10–70. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—Self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, R.; Zheng, R.; Huang, Y. Anions-responsive supramolecular gels: A review. Mater. Des. 2021, 205, 109759. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-W.; Schalley, C.A. Recent Advances on Supramolecular Gels: From Stimuli-Responsive Gels to Co-Assembled and Self-Sorted Systems. Org. Mater. 2021, 3, 025–040. [Google Scholar] [CrossRef]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45, 6546–6596. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2012, 22, 38–50. [Google Scholar] [CrossRef]
- Patel, A.M.; Bhardwaj, V.; Ray, D.; Aswal, V.K.; Ballabh, A. A library of benzimidazole based amide and urea derivatives as supramolecular gelators—A comparative study. J. Mol. Liq. 2024, 395, 123858. [Google Scholar] [CrossRef]
- Cheng, N.; Hu, Q.; Guo, Y.; Wang, Y.; Yu, L. Efficient and Selective Removal of Dyes Using Imidazolium-Based Supramolecular Gels. ACS Appl. Mater. Interfaces 2015, 7, 10258–10265. [Google Scholar] [CrossRef]
- Roy, R.; Adalder, T.K.; Dastidar, P. Supramolecular Gels Derived from the Salts of Variously Substituted Phenylacetic Acid and Dicyclohexylamine: Design, Synthesis, Structures, and Dye Adsorption. Chem. Asian J. 2018, 13, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Wang, Z.; Xie, L.; Feng, C.; He, G.; Hu, H.; Sun, R.; Zhu, H. A supramolecular gel made from an azobenzene-based phenylalanine derivative: Synthesis, self-assembly, and dye adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127289. [Google Scholar] [CrossRef]
- Ray, S.; Das, A.K.; Banerjee, A. pH-Responsive, Bolaamphiphile-Based Smart Metallo-Hydrogels as Potential Dye-Adsorbing Agents, Water Purifier, and Vitamin B12 Carrier. Chem. Mater. 2007, 19, 1633–1639. [Google Scholar] [CrossRef]
- Samai, S.; Biradha, K. Chemical and Mechano Responsive Metal–Organic Gels of Bis(benzimidazole)-Based Ligands with Cd(II) and Cu(II) Halide Salts: Self Sustainability and Gas and Dye Sorptions. Chem. Mater. 2012, 24, 1165–1173. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Song, S.; Feng, L.; Song, A.; Hao, J. Hydrogels Facilitated by Monovalent Cations and Their Use as Efficient Dye Adsorbents. J. Phys. Chem. B 2014, 118, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Bhavya, P.V.; Soundarajan, K.; Malecki, J.G.; Mohan Das, T. Sugar-Based Phase-Selective Supramolecular Self-Assembly System for Dye Removal and Selective Detection of Cu2+ Ions. ACS Omega 2022, 7, 39310–39324. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Versatile supramolecular pH-tolerant hydrogels which demonstrate pH-dependent selective adsorption of dyes from aqueous solution. Chem. Commun. 2013, 49, 11164–11166. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Weiss, R.G.; Terech, P. (Eds.) Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006; p. 978. [Google Scholar]
- Fages, F.; Voegtle, F.; Zinic, M. Systematic design of amide- and urea-type gelators with tailored properties. Top. Curr. Chem. 2005, 256, 77–131. [Google Scholar]
- Moulin, E.; Armao, J.J.; Giuseppone, N. Triarylamine-Based Supramolecular Polymers: Structures, Dynamics, and Functions. Acc. Chem. Res. 2019, 52, 975–983. [Google Scholar] [CrossRef]
- Wang, Y.; de Kruijff, R.M.; Lovrak, M.; Guo, X.; Eelkema, R.; van Esch, J.H. Access to Metastable Gel States Using Seeded Self-Assembly of Low-Molecular-Weight Gelators. Angew. Chem. Int. Ed. 2019, 58, 3800–3803. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Chaudhary, P.; Pradeep, A.; Singh, S.; Rangasamy, J.; Damodaran, K.K. Structural modification induced hydrogelation and antibacterial properties in supramolecular gels. J. Mol. Liq. 2023, 382, 122023. [Google Scholar] [CrossRef]
- Ghosh, D.; Farahani, A.D.; Martin, A.D.; Thordarson, P.; Damodaran, K.K. Unraveling the Self-Assembly Modes in Multicomponent Supramolecular Gels Using Single-Crystal X-ray Diffraction. Chem. Mater. 2020, 32, 3517–3527. [Google Scholar] [CrossRef]
- Dastidar, P. Supramolecular gelling agents: Can they be designed? Chem. Soc. Rev. 2008, 37, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bera, S.; Nandi, S.K.; Haldar, D. The effect of amide bond orientation and symmetry on the self-assembly and gelation of discotic tripeptides. Soft Matter 2021, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, G.; Gan, C.; Cai, G.; Zhang, J.; Ji, H. Effective adsorption of arsenate, dyes and eugenol from aqueous solutions by cationic supramolecular gel materials. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126238. [Google Scholar] [CrossRef]
- Fang, H.; Qu, W.-J.; Yang, H.-H.; He, J.-X.; Yao, H.; Lin, Q.; Wei, T.-B.; Zhang, Y.-M. A self-assembled supramolecular gel constructed by phenazine derivative and its application in ultrasensitive detection of cyanide. Dyes Pigm. 2020, 174, 108066. [Google Scholar] [CrossRef]
- Hao, C.; Gao, J.; Wu, Y.; Wang, X.; Zhao, R.; Mei, S.; Yang, J.; Zhai, X.; Qiu, H. Design of folic acid based supramolecular hybrid gel with improved mechanical properties in NMP/H2O for dye adsorption. React. Funct. Polym. 2018, 122, 140–147. [Google Scholar] [CrossRef]
- Lou, X.; Lafleur, R.P.M.; Leenders, C.M.A.; Schoenmakers, S.M.C.; Matsumoto, N.M.; Baker, M.B.; van Dongen, J.L.J.; Palmans, A.R.A.; Meijer, E.W. Dynamic diversity of synthetic supramolecular polymers in water as revealed by hydrogen/deuterium exchange. Nat. Commun. 2017, 8, 15420. [Google Scholar] [CrossRef]
- Lynes, A.D.; Hawes, C.S.; Ward, E.N.; Haffner, B.; Möbius, M.E.; Byrne, K.; Schmitt, W.; Pal, R.; Gunnlaugsson, T. Benzene-1,3,5-tricarboxamide n-alkyl ester and carboxylic acid derivatives: Tuneable structural, morphological and thermal properties. CrystEngComm 2017, 19, 1427–1438. [Google Scholar] [CrossRef]
- Daly, R.; Kotova, O.; Boese, M.; Gunnlaugsson, T.; Boland, J.J. Chemical Nano-Gardens: Growth of Salt Nanowires from Supramolecular Self-Assembly Gels. ACS Nano 2013, 7, 4838–4845. [Google Scholar] [CrossRef]
- Kumar, D.K.; Jose, D.A.; Dastidar, P.; Das, A. Nonpolymeric Hydrogelators Derived from Trimesic Amides. Chem. Mater. 2004, 16, 2332–2335. [Google Scholar] [CrossRef]
- Goodwin, J.W.; Hughes, R.W. Rheology for Chemists: An Introduction; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Guenet, J.-M. Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Does Drying Affect Gel Networks? Gels 2018, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Lebedytė, I.; Yufit, D.S.; Damodaran, K.K.; Steed, J.W. Selective gelation of N-(4-pyridyl)nicotinamide by copper(ii) salts. CrystEngComm 2015, 17, 8130–8138. [Google Scholar] [CrossRef]
- Ghosh, D.; Mulvee, M.T.; Damodaran, K.K. Tuning Gel State Properties of Supramolecular Gels by Functional Group Modification. Molecules 2019, 24, 3472. [Google Scholar] [CrossRef]
- Ghosh, D.; Deepa; Damodaran, K.K. Metal complexation induced supramolecular gels for the detection of cyanide in water. Supramol. Chem. 2020, 32, 276–286. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, P.; Kaushik, R.; Damodaran, K.K.; Jose, D.A. Anion responsive and morphology tunable tripodal gelators. RSC Adv. 2016, 6, 83303–83311. [Google Scholar] [CrossRef]
- Sudhakaran Jayabhavan, S.; Kuppadakkath, G.; Damodaran, K.K. The Role of Functional Groups in Tuning the Self-Assembly Modes and Physical Properties of Multicomponent Gels. ChemPlusChem 2023, 88, e202300302. [Google Scholar] [CrossRef]
- Jayabhavan, S.S.; Kristinsson, B.; Ghosh, D.; Breton, C.; Damodaran, K.K. Stimuli-Responsive Properties of Supramolecular Gels Based on Pyridyl-N-oxide Amides. Gels 2023, 9, 89. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Gao, Y.; Hao, J.; Hu, J.; Ju, Y. Multi-stimuli-responsive hydrogels of gluconamide-tailored anthracene. Soft Matter 2019, 15, 4662–4668. [Google Scholar] [CrossRef] [PubMed]
- Panja, A.; Ghosh, S.; Ghosh, K. A sulfonyl hydrazone cholesterol conjugate: Gelation, anion interaction and its application in dye adsorption. New J. Chem. 2019, 43, 10270–10277. [Google Scholar] [CrossRef]
- Pati, C.; Ghosh, K. A 1,8-naphthalimide–pyridoxal conjugate as a supramolecular gelator for colorimetric read out of F− ions in solution, gel and solid states. New J. Chem. 2019, 43, 2718–2725. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Peng, X.; Sun, J.; Li, C.; Zhang, X.; Zhang, H.; Chen, J.; Zhou, X.; Zeng, H.; et al. Hydrogels for the removal of the methylene blue dye from wastewater: A review. Environ. Chem. Lett. 2022, 20, 2665–2685. [Google Scholar] [CrossRef]
- Dutta, S.K.; Amin, M.K.; Ahmed, J.; Elias, M.; Mahiuddin, M. Removal of toxic methyl orange by a cost-free and eco-friendly adsorbent: Mechanism, phytotoxicity, thermodynamics, and kinetics. S. Afr. J. Chem. Eng. 2022, 40, 195–208. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. [Google Scholar] [CrossRef]
- Mancuso, L.; Knobloch, T.; Buchholz, J.; Hartwig, J.; Möller, L.; Seidel, K.; Collisi, W.; Sasse, F.; Kirschning, A. Preparation of Thermocleavable Conjugates Based on Ansamitocin and Superparamagnetic Nanostructured Particles by a Chemobiosynthetic Approach. Chem. Eur. J. 2014, 20, 17541–17551. [Google Scholar] [CrossRef]


| Solvent | MGC (wt/v%) | Tgel (°C) |
|---|---|---|
| Methanol | 1.9 | 79.9 |
| Ethanol | 1.9 | 82.9 |
| Isopropanol | 2.0 | 94.3 |
| n-butanol | 2.0 | 96.1 |
| DMF/water (1:1, v/v) | 2.8 | 92.8 |
| DMSO/water (1:1, v/v) | 3.6 | 99.1 |
| DMA/water (1:1, v/v) | 2.5 | 58.3 |
| DEA/water (1:1, v/v) | 2.7 | 83.9 |
| Day | Adsorption Capacity (mg/g) | Adsorption Efficiency (%) | ||
|---|---|---|---|---|
| MB | MO | MB | MO | |
| 2 | 0.78 | 4.92 | 64.94 | 60.11 |
| 5 | 1.07 | 5.09 | 89.58 | 62.19 |
| 7 | 1.15 | 5.14 | 96.64 | 62.81 |
| 8 | 1.17 | 5.21 | 97.87 | 63.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuppadakkath, G.; Jayabhavan, S.S.; Damodaran, K.K. Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water. Molecules 2024, 29, 2149. https://doi.org/10.3390/molecules29092149
Kuppadakkath G, Jayabhavan SS, Damodaran KK. Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water. Molecules. 2024; 29(9):2149. https://doi.org/10.3390/molecules29092149
Chicago/Turabian StyleKuppadakkath, Geethanjali, Sreejith Sudhakaran Jayabhavan, and Krishna K. Damodaran. 2024. "Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water" Molecules 29, no. 9: 2149. https://doi.org/10.3390/molecules29092149
APA StyleKuppadakkath, G., Jayabhavan, S. S., & Damodaran, K. K. (2024). Supramolecular Gels Based on C3-Symmetric Amides: Application in Anion-Sensing and Removal of Dyes from Water. Molecules, 29(9), 2149. https://doi.org/10.3390/molecules29092149






