Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of GC-MS/MS Conditions
2.2. Optimization of Extraction and Purification
2.3. Method Validation
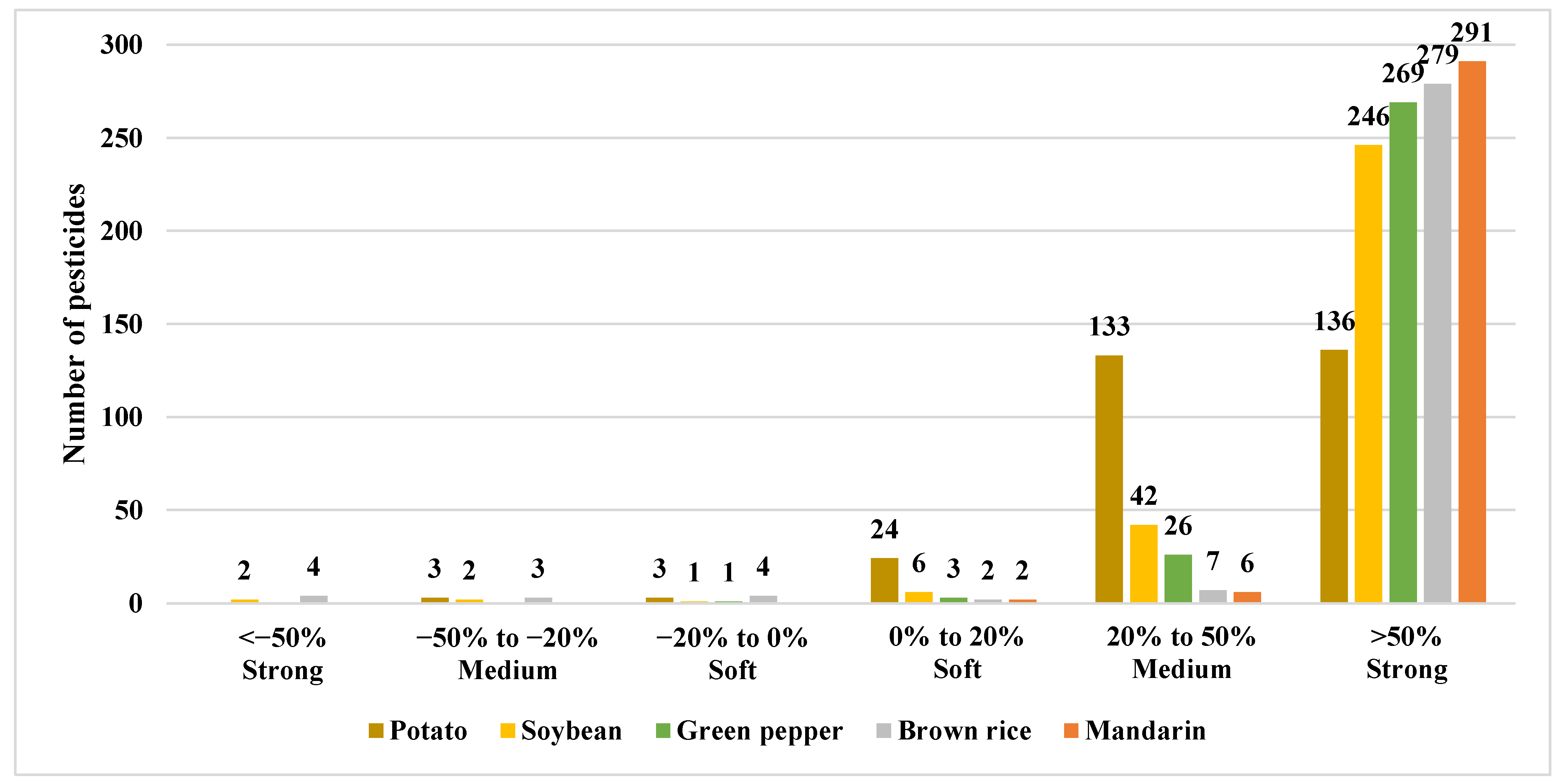
2.4. Matrix Effects
2.5. Application of the Multi-Residue Analysis Method in Real Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Analytical Standard
3.3. Sample Preparation
3.4. Optimization of Analytical GC-MS/MS Conditions
3.5. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. Current status and regulatory aspects of pesticides considered to be persistent organic pollutants (POPs) in Taiwan. Int. J. Environ. Res. Public Health 2010, 7, 3615–3627. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, K.; Reynolds, C.; Mateo, R.; Amar, A. A global review of the temporal and spatial patterns of DDT and dieldrin monitoring in raptors. Sci. Total Environ. 2023, 858, 159734. [Google Scholar] [CrossRef] [PubMed]
- Codex Allimentarius Commission. Pesticide Database-Maximum Residue Limits. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ (accessed on 4 October 2023).
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef]
- Seok, J.H.; Kim, G. Impact of the positive list system (PLS) on the banana market in Korea. J. Asia Pac. Econ. 2020, 25, 718–732. [Google Scholar] [CrossRef]
- Zhang, M.; Zeiss, M.R.; Geng, S. Agricultural pesticide use and food safety: California’s model. J. Integr. Agric. 2015, 14, 2340–2357. [Google Scholar] [CrossRef]
- Diop, A.; Diop, Y.M.; Thiare, D.D.; Cazier, F.; Sarr, S.O.; Kasprowiak, A.; Landy, D.; Delattre, F. Monitoring survey of the use patterns and pesticide residues on vegetables in the Niayes zone, Senegal. Chemosphere 2016, 144, 1715–1721. [Google Scholar] [CrossRef]
- Li, Z.; Nie, J.; Yan, Z.; Cheng, Y.; Lan, F.; Huang, Y.; Chen, Q.; Zhao, X.; Li, A. A monitoring survey and dietary risk assessment for pesticide residues on peaches in China. Regul. Toxicol. Pharmacol. 2018, 97, 152–162. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. Food Code (No.2020-18, 2020.3.20.). Available online: https://www.mfds.go.kr/brd/m_211/view.do?seq=14464&srchFr=&srchTo=&srchWord=%EC%8B%9D%ED%92%88%EC%9D%98+%EA%B8%B0%EC%A4%80+%EB%B0%8F+%EA%B7%9C%EA%B2%A9&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=4 (accessed on 4 October 2023).
- Li, P.; Duan, Y.; Ge, H.; Zhang, Y.; Wu, X. Multiresidue analysis of 113 pesticides in different maturity levels of mangoes using an optimized QuEChERS method with GC-MS/MS and UHPLC-MS/MS. Food Anal. Methods 2018, 11, 2742–2757. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B.J.; Kim, J.-H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere 2018, 207, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Balkan, T.; Karaağaçlı, H. Determination of 301 pesticide residues in tropical fruits imported to Turkey using LC–MS/MS and GC-MS. Food Control 2023, 147, 109576. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 000010151520150109. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, L.; Shin, Y.; Lee, J.; Lee, J.; Kim, E.; Moon, J.-K.; Kim, J.-H. Rapid and simultaneous analysis of 360 pesticides in brown rice, spinach, orange, and potato using microbore GC-MS/MS. J. Agric. Food Chem. 2017, 65, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission (CAC). Guidelines on Good Laboratory Practice in Pesticide Residue Analysis, CAC/GL 40-1993; Rev.1-2003; CAC: Rome, Italy, 2003; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B40-1993%252Fcxg_040e.pdf (accessed on 6 October 2023).
- Bicchi, C.; Brunelli, C.; Galli, M.; Sironi, A. Conventional inner diameter short capillary columns: An approach to speeding up gas chromatographic analysis of medium complexity samples. J. Chromatogr. A 2001, 931, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Shin, Y.; Choi, H. Simultaneous analytical method for 296 pesticide multiresidues in root and rhizome based herbal medicines with GC-MS/MS. PLoS ONE 2023, 18, e0288198. [Google Scholar] [CrossRef]
- Peris, A.; Eljarrat, E. Multi-residue methodologies for the analysis of non-polar pesticides in water and sediment matrices by GC–MS/MS. Chromatographia 2021, 84, 425–439. [Google Scholar] [CrossRef]
- Yudthavorasit, S.; Meecharoen, W.; Leepipatpiboon, N. New practical approach for using an analyte protectant for priming in routine gas chromatographic analysis. Food Control 2015, 48, 25–32. [Google Scholar] [CrossRef]
- Schenck, F.J.; Lehotay, S.J. Does further clean-up reduce the matrix enhancement effect in gas chromatographic analysis of pesticide residues in food? J. Chromatogr. A 2000, 868, 51–61. [Google Scholar] [CrossRef]
- Cho, S.-M.; Lee, H.-S.; Park, J.-S.; Lee, S.-J.; Shin, H.-S.; Chung, Y.-M.; Choi, H.-N.; Jung, Y.-H.; Oh, J.-H.; Yun, S.-S. Determination of residual triflumezopyrim insecticide in agricultural products through a modified QuEChERS method. Foods 2021, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Maštovská, K.; Lehotay, S.J. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J. Chromatogr. A 2004, 1040, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.-Y.; Bae, J.-Y.; Park, C.-W.; Jang, G.-H.; Choe, W.-J. Determination of Modified QuEChERS Method for Chlorothalonil Analysis in Agricultural Products Using Gas Chromatography–Mass Spectrometry (GC-MS/MS). Foods 2023, 12, 3793. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.D.C.; Caldas, S.S.; Prestes, O.D.; Primel, E.G.; Zanella, R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-L.; Zhao, Z.-D.; Zhang, X.-L.; Tang, L.-L.; Zhang, Y.; Zhang, C.-H. Simultaneous analysis of herbicide metribuzin and its transformation products in tomato using QuEChERS-based gas chromatography coupled to a triple quadrupole mass analyzer. Microchem. J. 2017, 133, 468–473. [Google Scholar] [CrossRef]
- Hajšlová, J.; Holadova, K.; Kocourek, V.; Poustka, J.; Godula, M.; Cuhra, P.; Kempný, M. Matrix-induced effects: A critical point in the gas chromatographic analysis of pesticide residues. J. Chromatogr. A 1998, 800, 283–295. [Google Scholar] [CrossRef]
- Frenich, A.G.; Vidal, J.L.M.; Moreno, J.L.F.; Romero-González, R. Compensation for matrix effects in gas chromatography–tandem mass spectrometry using a single point standard addition. J. Chromatogr. A 2009, 1216, 4798–4808. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Wang, Z.; Wang, X.; Shu, J.; Yang, J.; Zhang, G.; Zeng, Z. Selection of a representative matrix for the multiresidue analysis of nine β-agonists in animal tissues and urine with LC-MS/MS. Analyst 2013, 138, 4579–4584. [Google Scholar] [CrossRef]
- Ferrer, C.; Martínez-Bueno, M.J.; Lozano, A.; Fernández-Alba, A.R. Pesticide residue analysis of fruit juices by LC–MS/MS direct injection. One year pilot survey. Talanta 2011, 83, 1552–1561. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Park, E.; Lee, J.; Lee, H.S.; Kim, J.-H. A quantitative tandem mass spectrometry and scaled-down QuEChERS approach for simultaneous analysis of pesticide multiresidues in human urine. Molecules 2019, 24, 1330. [Google Scholar] [CrossRef] [PubMed]
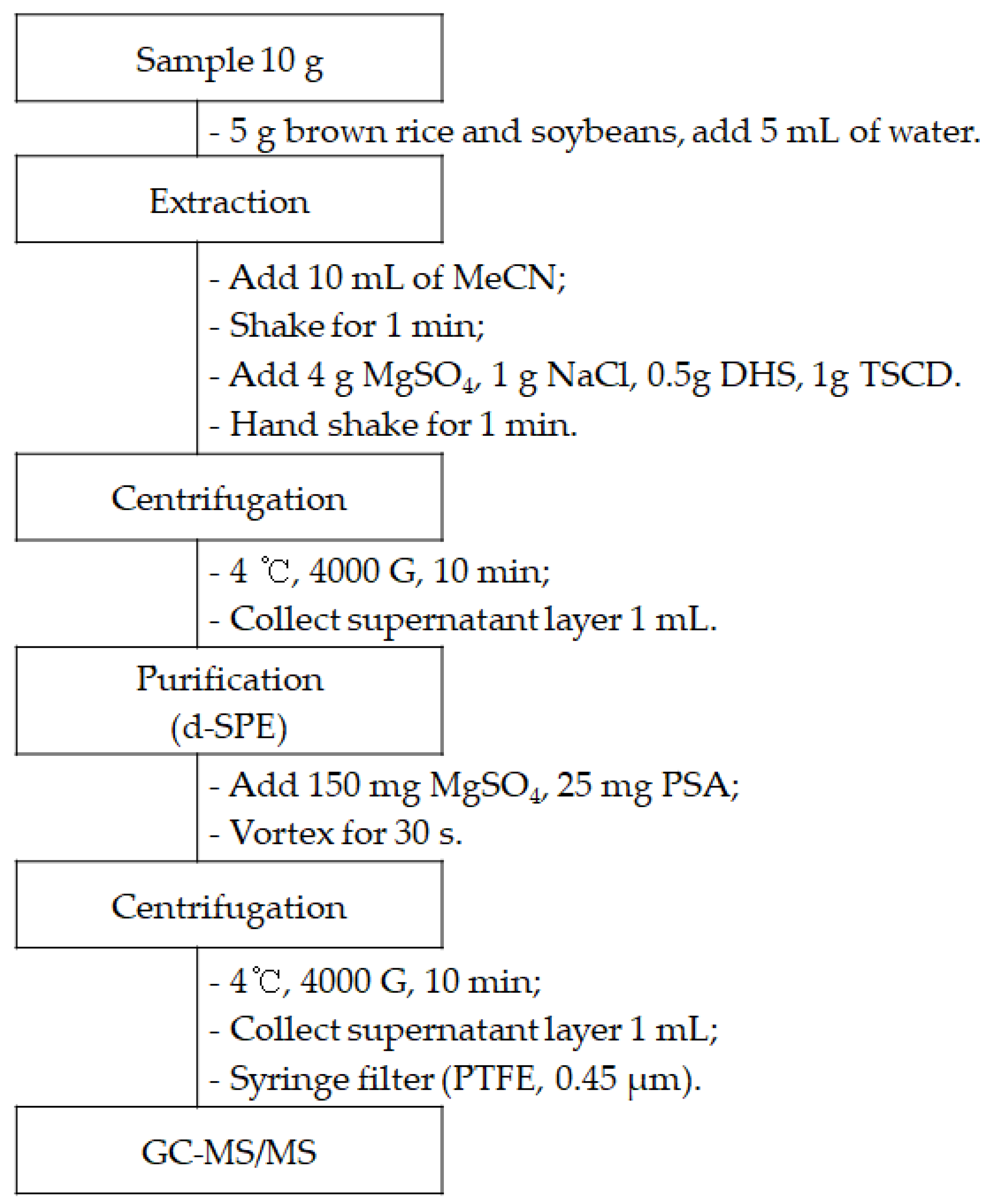
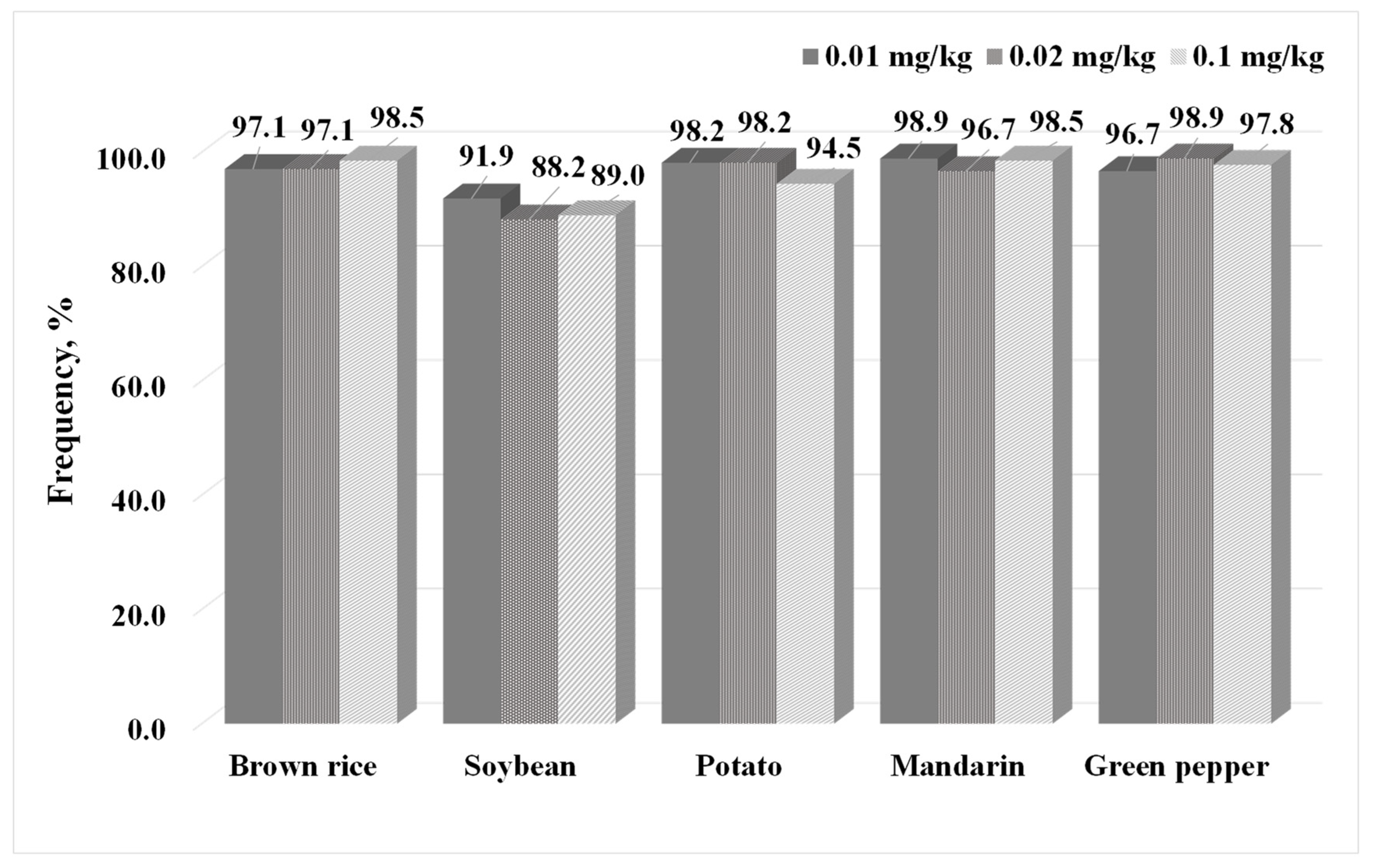
| d-SPE Sorbents | Brown Rice | Green Pepper | ||
|---|---|---|---|---|
| No. of Analytes | % of Analytes | No. of Analytes | % of Analytes | |
| 150 mg of MgSO4, 25 mg of PSA | 264 | 97.1 | 267 | 98.2 |
| 150 mg of MgSO4, 25 mg of PSA, 25 mg of C18 | 261 | 96.0 | 264 | 97.1 |
| 150 mg of MgSO4, 25 mg of PSA, 2.5 mg of GCB | 262 | 96.3 | 257 | 94.5 |
| No. | Analyte | Soybean (n = 11) | |||||
|---|---|---|---|---|---|---|---|
| 0.01 mg/kg | 0.02 mg/kg | 0.1 mg/kg | |||||
| Rec. (%) | CV (%) | Rec. (%) | CV (%) | Rec. (%) | CV (%) | ||
| 1 | Aldrin | 85.9 | 7.9 | 79.9 | 3.9 | 90.0 | 4.9 |
| Dieldrin | 69.6 | 2.6 | 114.2 | 2.7 | 119.0 | 4.5 | |
| 2 | Anilofos | 88.9 | 8.3 | 106.4 | 4.3 | 101.9 | 4.8 |
| 3 | BHC, γ- | 76.9 | 9.2 | 89.6 | 4.9 | 88.6 | 12.3 |
| 4 | Butylate | 104.0 | 6.0 | 71.7 | 5.8 | 74.8 | 13.7 |
| 5 | Chlorbenside | 92.9 | 7.8 | 112.7 | 4.2 | 86.2 | 2.3 |
| 6 | Dichlofenthion | 62.3 | 9.3 | 72.9 | 3.0 | 82.3 | 18.6 |
| 7 | Dicloran | 112.2 | 4.6 | 114.8 | 9.8 | 110.7 | 8.3 |
| 8 | Fenclorim | 87.3 | 15.2 | 77.7 | 8.1 | 76.1 | 12.0 |
| 9 | Fenoxanil | 84.2 | 6.8 | 107.3 | 5.6 | 102.0 | 2.6 |
| 10 | Flucythrinate-1 | 90.8 | 7.3 | 105.7 | 4.1 | 100.8 | 2.6 |
| Flucythrinate-2 | 90.7 | 7.7 | 108.3 | 3.7 | 101.3 | 2.2 | |
| 11 | Indoxacarb | 75.2 | 9.9 | 95.0 | 5.8 | 97.9 | 1.6 |
| 12 | Pyraclofos | 86.8 | 9.8 | 114.9 | 3.8 | 109.6 | 11.3 |
| 13 | Tridiphane | 70.4 | 9.7 | 80.7 | 6.2 | 85.6 | 18.7 |
| Commodity | Sample Number | Detected Number | Pesticides | Concentration | MRLs (Korea) |
|---|---|---|---|---|---|
| (mg/kg) | |||||
| Persimmon | 20 | 9 | Buprofezin | 0.015 | 0.5 |
| Difenoconazole | 0.012 | 1 | |||
| Difenoconazole | 0.017 | 1 | |||
| Difenoconazole | 0.048 | 1 | |||
| Tebuconazole | 0.017 | 2 | |||
| Cyprodinil | 0.02 | 1 | |||
| Tebuconazole | 0.031 | 2 | |||
| Buprofezin | 0.018 | 0.5 | |||
| Tebuconazole | 0.078 | 2 | |||
| Trifloxystrobin | 0.046 | 0.7 | |||
| Buprofezin | 0.034 | 0.5 | |||
| Tebuconazole | 0.021 | 2 | |||
| Buprofezin | 0.01 | 0.5 | |||
| Mandarin | 19 | 7 | Chlorfenapyr | 0.016 | 1 |
| Indoxacarb | 0.034 | 0.5 | |||
| Chlorfenapyr | 0.07 | 1 | |||
| Deltamethrin | 0.011 | 0.5 | |||
| Etoxazole | 0.025 | 1 | |||
| Deltamethrin | 0.017 | 0.5 | |||
| Chlorfenapyr | 0.01 | 1 | |||
| Boscalid | 0.015 | 0.5 | |||
| Chlorfenapyr | 0.017 | 1 | |||
| Pepper | 18 | 6 | Chlorfenapyr | 0.016 | 1 |
| Deltamethrin | 0.03 | 0.2 | |||
| Bifenthrin | 0.021 | 1 | |||
| Boscalid | 0.023 | 3 | |||
| Chlorfenapyr | 0.222 | 1 | |||
| Chlorfenapyr | 0.034 | 1 | |||
| Procymidone | 0.015 | 5 | |||
| Boscalid | 0.011 | 3 | |||
| Indoxacarb | 0.014 | 1 | |||
| Chlorfenapyr | 0.062 | 1 | |||
| Indoxacarb | 0.024 | 1 | |||
| Spiromesifen | 0.028 | 3 | |||
| Tebufenpyrad | 0.026 | 0.5 | |||
| Radish | 18 | 2 | Metalaxyl | 0.019 | 0.05 |
| Tebuconazole | 0.03 | 0.2 | |||
| Korean cabbage | 18 | 3 | Diniconazole | 0.018 | 0.1 |
| Metalaxyl | 0.014 | 0.2 | |||
| Diniconazole | 0.038 | 0.1 | |||
| Peach | 19 | 7 | Indoxacarb | 0.014 | 1 |
| Fenitrothion | 0.045 | 0.1 | |||
| Boscalid | 0.019 | 1 | |||
| Deltamethrin | 0.039 | 0.5 | |||
| Difenoconazole | 0.026 | 2 | |||
| Indoxacarb | 0.678 | 1 | |||
| Difenoconazole | 0.028 | 2 | |||
| Indoxacarb | 0.011 | 1 | |||
| Trifloxystrobin | 0.061 | 2 | |||
| Kresoxim- methyl | 0.024 | 1 | |||
| Indoxacarb | 0.03 | 1 | |||
| Apple | 18 | 12 | Tebuconazole | 0.023 | 1 |
| Bifenthrin | 0.013 | 0.5 | |||
| Tebuconazole | 0.032 | 0.5 | |||
| Tebuconazole | 0.048 | 1 | |||
| Bifenthrin | 0.015 | 0.5 | |||
| Deltamethrin | 0.019 | 0.5 | |||
| Propiconazole | 0.069 | 1 | |||
| Spiromesifen | 0.101 | 1 | |||
| Tebuconazole | 0.125 | 1 | |||
| Trifloxystrobin | 0.011 | 0.7 | |||
| Chlorpyrifos | 0.287 | 1 | |||
| Difenoconazole | 0.049 | 1 | |||
| Indoxacarb | 0.023 | 0.3 | |||
| Propiconazole | 0.033 | 1 | |||
| Tebuconazole | 0.029 | 1 | |||
| Tebuconazole | 0.016 | 1 | |||
| Trifloxystrobin | 0.014 | 0.7 | |||
| Bifenthrin | 0.043 | 0.5 | |||
| Difenoconazole | 0.011 | 1 | |||
| Tebuconazole | 0.091 | 1 | |||
| Trifloxystrobin | 0.043 | 0.7 | |||
| Tebuconazole | 0.069 | 1 | |||
| Tebuconazole | 0.017 | 1 | |||
| Brown rice | 18 | 2 | Fenoxanil | 0.013 | 1 |
| Fenoxanil | 0.02 | 1 | |||
| Tomato | 18 | 5 | Fenpyrazamine | 0.012 | 3 |
| Spiromesifen | 0.016 | 1 | |||
| Buprofezin | 0.035 | 3 | |||
| Spiromesifen | 0.015 | 1 | |||
| Spiromesifen | 0.014 | 1 | |||
| Spiromesifen | 0.019 | 1 |
| Instrument | |||
| GC | 7890B GC system (Agilent Technologies, Santa Clara, CA, USA) | ||
| MS/MS | GC/MS Triple Quad (Agilent Technologies, Santa Clara, CA, USA) | ||
| GC conditions | |||
| Column | DB-5MS (30 m × 0.25 mm, 0.25 μm) | ||
| Flow rate | 1.2 mL/min (He 99%) | ||
| Injection volume | 1 μL | ||
| Injection mode | splitless | ||
| Oven temp. | Rate (°C/min) | Temperature (°C) | Hold (min) |
| Initial | 60 | - | |
| 20 | 180 | - | |
| 5 | 300 | 5 | |
| MS/MS condition | |||
| Ionization mode | Electron ionization (EI) | ||
| Transfer line temp. | 280 °C | ||
| Ion source temp. | 280 °C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, D.-Y.; Bae, J.-Y.; Kang, Y.-J.; Lim, C.-U.; Jang, G.-H.; Eom, M.-O.; Choe, W.-J. Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry. Molecules 2024, 29, 2114. https://doi.org/10.3390/molecules29092114
Yun D-Y, Bae J-Y, Kang Y-J, Lim C-U, Jang G-H, Eom M-O, Choe W-J. Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry. Molecules. 2024; 29(9):2114. https://doi.org/10.3390/molecules29092114
Chicago/Turabian StyleYun, Da-Young, Ji-Yeon Bae, Yoon-Jung Kang, Chae-Uk Lim, Gui-Hyun Jang, Mi-Ok Eom, and Won-Jo Choe. 2024. "Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry" Molecules 29, no. 9: 2114. https://doi.org/10.3390/molecules29092114
APA StyleYun, D.-Y., Bae, J.-Y., Kang, Y.-J., Lim, C.-U., Jang, G.-H., Eom, M.-O., & Choe, W.-J. (2024). Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry. Molecules, 29(9), 2114. https://doi.org/10.3390/molecules29092114







