Comprehensive Analysis of Physicochemical Properties and Volatile Compounds in Different Strawberry Wines under Various Pre-Treatments
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Compositions and Color Properties
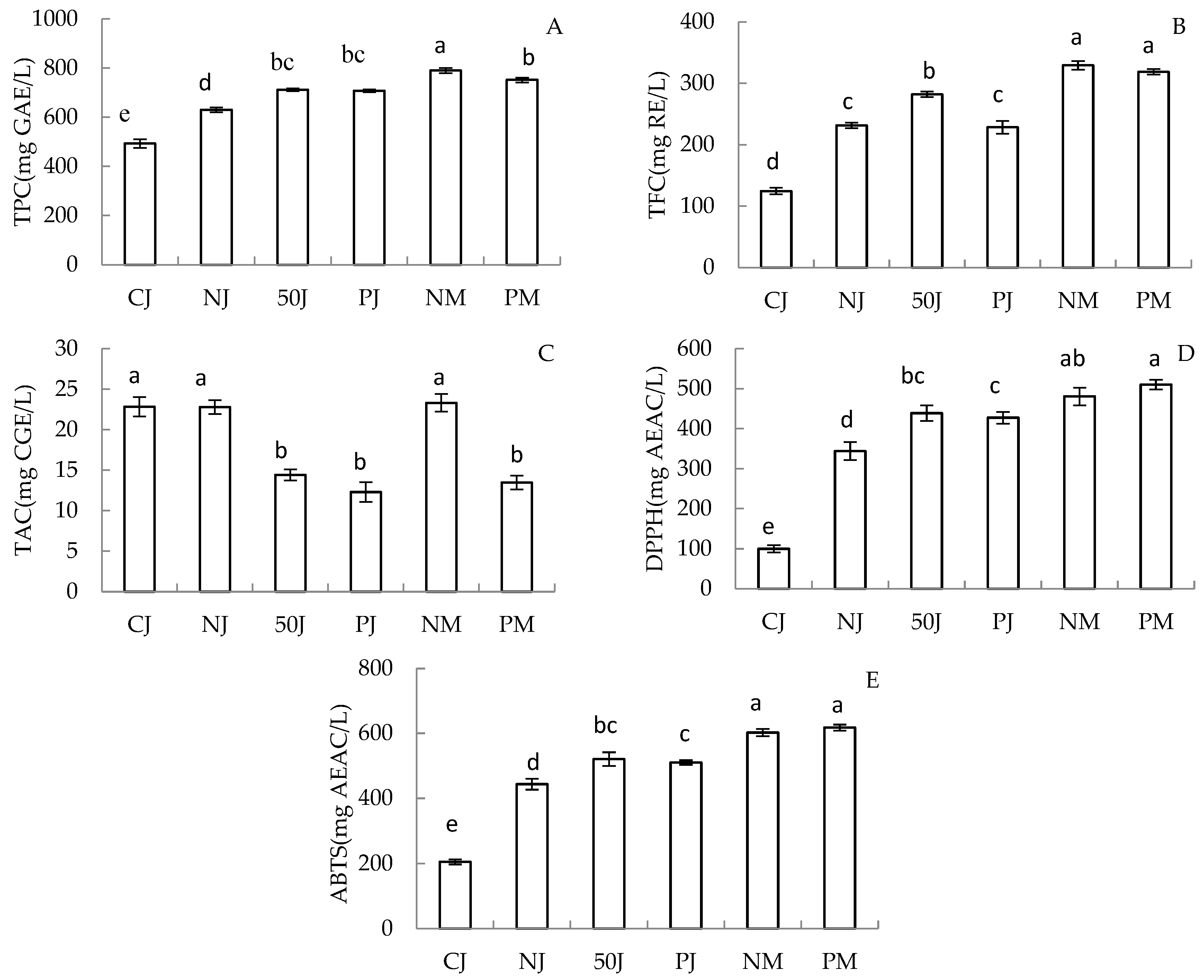
2.2. Total Phenolic Compounds and Antioxidant Capacity
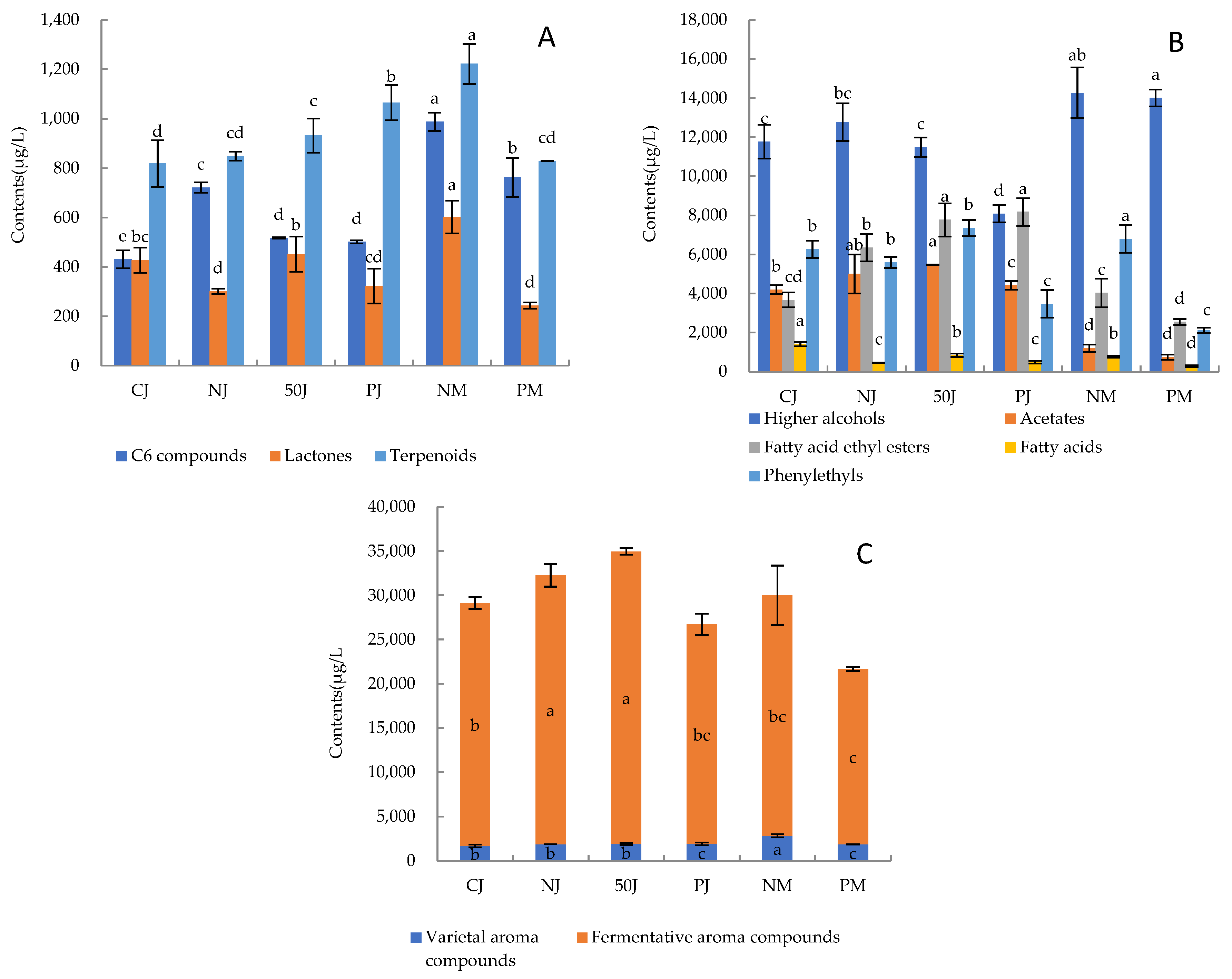
2.3. Volatile Organic Compounds (VOCs)
2.3.1. Varietal Aroma Compounds
2.3.2. Fermentative Aroma Compounds
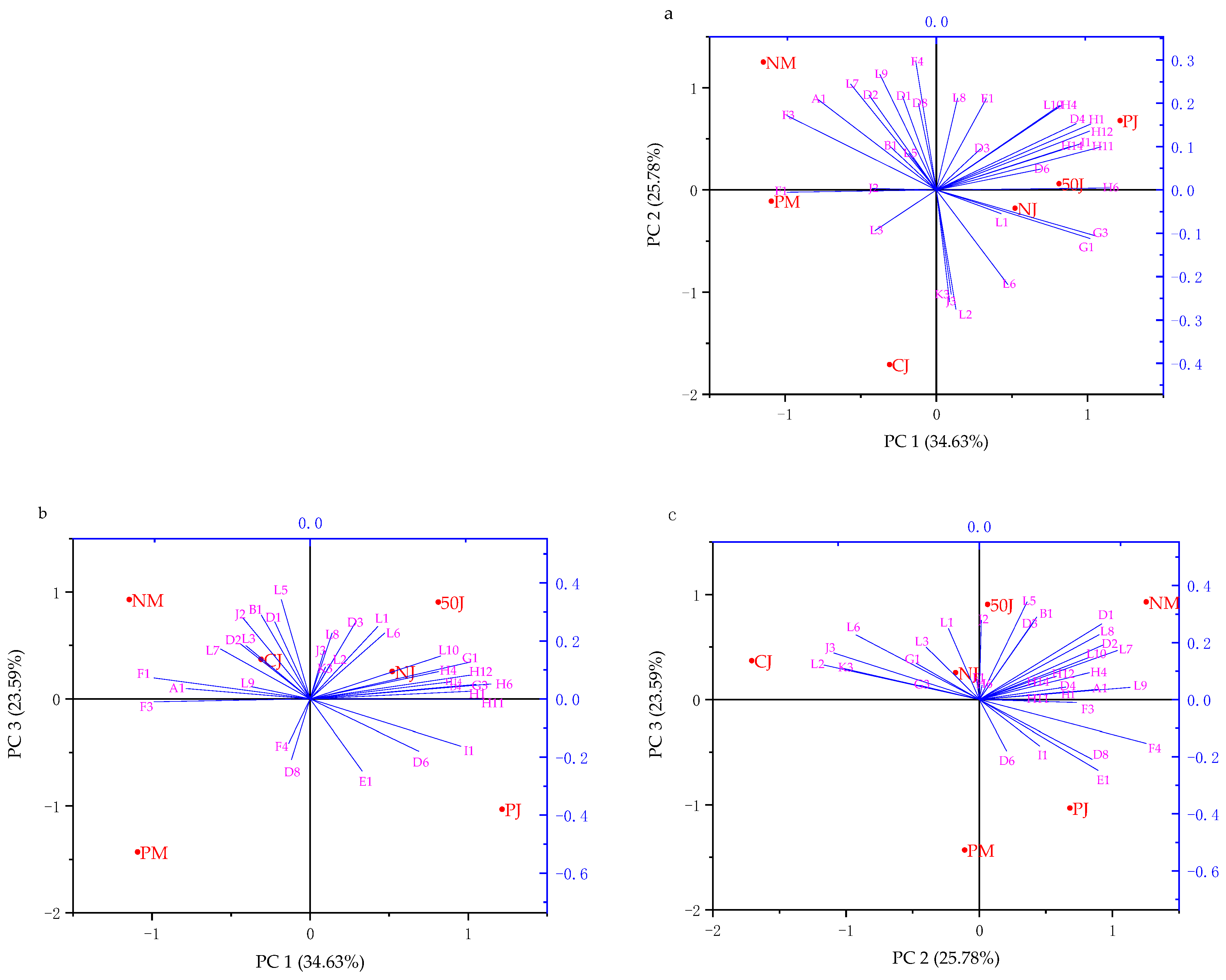
2.3.3. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Raw Material and Reagents
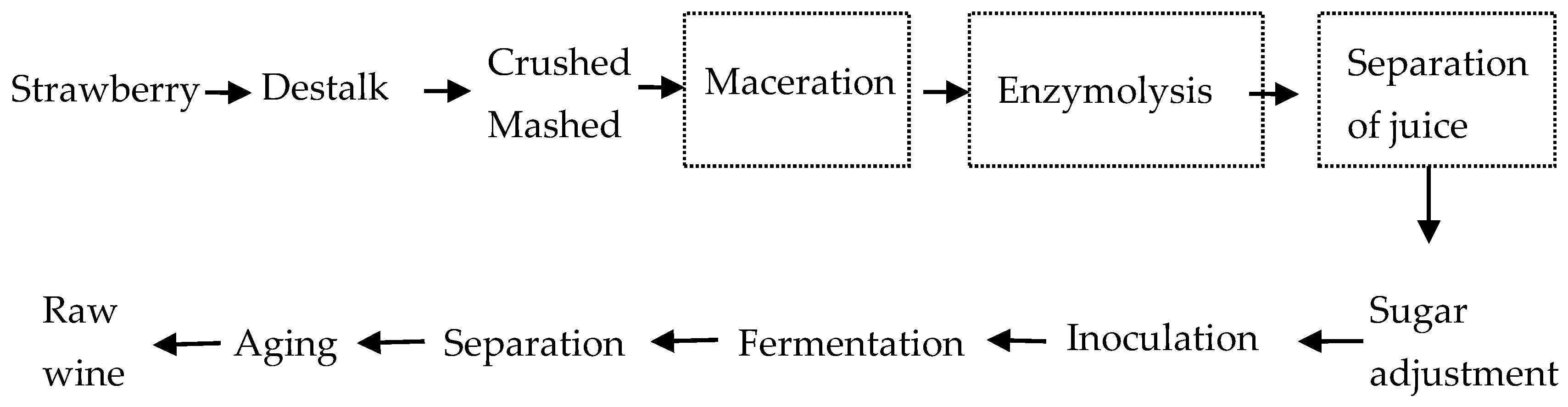
3.2. Winemaking and Maceration Techniques
3.3. Determination of Physicochemical Indicators and Color
3.4. High-Performance Liquid Chromatography (HPLC) Analysis of Organic Acids
3.5. Determination of Total Phenolics
3.6. Determination of Antioxidant Capacity
3.7. Determination of of Volatile Organic Compounds (VOCs)
3.8. Odor Activity Values (OAVs)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sturtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Alvarez-Fernandez, M.A.; Cerezo, A.B.; Garcia-Garcia, I.; Troncoso, A.M.; Garcia-Parrilla, M.C. Influence of Fermentation Process on the Anthocyanin Composition of Wine and Vinegar Elaborated from Strawberry. J. Food. Sci. 2017, 82, 364–372. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yoshimoto, K.; Okada, Y.; Nomura, M. Effect of Impregnation Using Sucrose Solution on Stability of Anthocyanin in Strawberry Jam. LWT Food Sci. Technol. 2011, 44, 891–895. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Szulc, K.; Jakubczyk, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. The Influence of a Chocolate Coating on the State Diagrams and Thermal Behaviour of Freeze-Dried Strawberries. Appl. Sci. 2022, 12, 1342. [Google Scholar] [CrossRef]
- Cakar, U.; Petrovic, A.; Colovic, M.; Krstic, D.; Dordevic, B. Strawberry Wine Antioxidant Properties in The Protection Against Free Radicals. Free Radic. Biol. Med. 2021, 177, s106. [Google Scholar] [CrossRef]
- Liu, J.; Yang, W.; Lv, Z.; Liu, H.; Zhang, C.; Jiao, Z. Effects of different pretreatments on physicochemical properties and phenolic compounds of hawthorn wine. CyTA J. Food 2020, 18, 518–526. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Longobardi, F.; Ventrella, A.; Agostiano, A.; Del Nobile, M.A. Effects of different vinification technologies on physical and chemical characteristics of Sauvignon blanc wines. Food Chem. 2012, 135, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdylo, A.; Chmielewska, J.; Oszmianski, J. The effects of flash release conditions on the phenolic compounds and antioxidant activity of Pinot noir red wine. Eur. Food Res. Technol. 2017, 243, 999–1007. [Google Scholar] [CrossRef]
- Lukic, I.; Budic-Leto, I.; Bubola, M.; Damijanic, K.; Staver, M. Pre-fermentative cold maceration, soignee, and various thermal treatments as options for modulating volatile aroma and phenol profiles of red wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- Fischer, U.; Strasser, M.; Gutzler, K. Impact of fermentation technology on the phenolic and volatile composition of German red wines. Int. J. Food Sci. Technol. 2000, 35, 81–94. [Google Scholar] [CrossRef]
- Sener, H. Effect of Temperature and Duration of Maceration on Colour and Sensory Properties of Red Wine—A Review. S. Afr. J. Enol. Vitic. 2018, 39, 227–234. [Google Scholar] [CrossRef]
- Piccardo, D.; Gonzalez-Neves, G.; Favre, G.; Pascual, O.; Canals, J.M.; Zamora, F. Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines. Fermentation 2019, 5, 80. [Google Scholar] [CrossRef]
- Jacob, N.; Sukumaran, R.K.; Prema, P. Optimization of Enzymatic Clarification of Sapodilla Juice: A Statistical Perspective. Appl. Biochem. Biotechnol. 2008, 151, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, S.S.; Ferruzzi, M.; Morgan, M.T. Effect of a flash vacuum expansion process on grape juice yield and quality. LWT Food Sci. Technol. 2012, 48, 147–155. [Google Scholar] [CrossRef]
- Sun, Y.M.; Zhang, T.; Lu, H.W.; Yu, Z.M.; Li, X.Z. Effect of added sulphur dioxide levels on the fermentation characteristics of strawberry wine. J. Inst. Brew. 2016, 122, 446–451. [Google Scholar] [CrossRef]
- Wei, X.; Francoise, U.; Qin, M.; Chen, Q.; Li, Y.; Sun, X.; Fang, Y.-L. Effects of different fermentation and storage conditions on methanol content in Chinese spine grape (Vitis davidii Foex) wine. CyTA J. Food 2020, 18, 367–374. [Google Scholar] [CrossRef]
- Miljic, U.; Puskas, V.; Vucurovic, V. Investigation of technological approaches for reduction of methanol formation in plum wines. J. Inst. Brew. 2016, 122, 635–643. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Characterization of Phenolic Compounds in Strawberry Fruits by RP-HPLC-DAD and Investigation of Their Antioxidant Capacity. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2495–2504. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.-J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties, sugar and organic acid profiles, and antioxidant compositions of strawberries. Food Sci. Biotechnol. 2019, 28, 1659–1667. [Google Scholar] [CrossRef]
- Liu, M.; Yang, K.; Qi, Y.; Fan, M.; Wei, X. Physicochemical characteristics and antioxidant activity of persimmon wine by technology of pectinase addition and different pre-macerations. J. Food Process. Pres. 2018, 42, e13452. [Google Scholar] [CrossRef]
- Vilela, A. Biological Demalication and Deacetification of Musts and Wines: Can Wine Yeasts Make the Wine Taste Better? Fermentation 2017, 3, 51. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Liu, N.; Ye, D.; Gong, X.; Qin, Y.; Liu, Y. Volatile compounds in wild strawberry and their odorants of wild strawberry wines: Effects of different stages of fermentation. Int. J. Food Prop. 2017, 20 (Suppl. S1), S399–S415. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, G.; Yu, Y.; Xu, Y.; Wu, J.; Zou, B.; Li, L. Low-oxygen pulping combined with high hydrostatic pressure improve the stability of blueberry pulp anthocyanins and color during storage. Food Control 2022, 138, 10891. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. Thermal stability of primary and secondary metabolites in highbush blueberry (Vaccinium corymbosum L.) purees. LWT Food Sci. Technol. 2017, 76, 79–86. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Lopez, R.; Feilhes, C.; Violleau, F.; Kleiber, D.; Favarel, J.-L.; Ferreira, V. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 2018, 257, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines.: Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition.: Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Synos, K.; Reynolds, A.G.; Bowen, A.J. Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Nuzzi, M.; Lo Scalzo, R.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Anal. Method. 2008, 1, 270–282. [Google Scholar] [CrossRef]
- López, R.; Ortín, N.; Pérez-Trujillo, J.P.; Cacho, J.; Ferreira, V. Impact odorants of different young white wines from the Canary Islands. J. Agric. Food Chem. 2003, 51, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tao, Y.-S.; Wang, H.; Zhang, L. Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 2007, 227, 287–292. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT Food Sci. Technol. 2019, 109, 233–240. [Google Scholar] [CrossRef]
- Peng, C.-T.; Wen, Y.; Tao, Y.-S.; Lan, Y.-Y. Modulating the Formation of Meili Wine Aroma by Prefermentative Freezing Process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y.; Jiang, W.; Li, J. Identification and Quantification of Impact Aroma Compounds in 4 Nonfloral Vids vinifera Varieties Grapes. J. Food Sci. 2010, 75, S81–S88. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas Chromatography−Olfactometry and Chemical Quantitative Study of the Aroma of Six Premium Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT Food Sci. Technol. 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef]
- Crandles, M.; Reynolds, A.G.; Khairallah, R.; Bowen, A. The effect of yeast strain on odor active compounds in Riesling and Vidal blanc icewines. LWT Food Sci. Technol. 2015, 64, 243–258. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y.Y. Seasonal variation of volatile composition and odor activity value of ‘Marion’ (Rubus spp. hyb) and ‘Thornless Evergreen’ (R. laciniatus L.) blackberries. J. Food Sci. 2005, 70, C13–C20. [Google Scholar] [CrossRef]
- Mihnea, M.; Gonzalez-Sanjose, M.L.; Ortega-Heras, M.; Perez-Magarino, S. A comparative study of the volatile content of Mencia wines obtained using different pre-fermentative maceration techniques. LWT-Food Sci. Technol. 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zeliou, K.; Papasotiropoulos, V.; Manoussopoulos, Y.; Lamari, F.N. Physical and chemical quality characteristics and antioxidant properties of strawberry cultivars (Fragaria × ananassa Duch.) in Greece: Assessment of their sensory impact. J. Sci. Food Agric. 2018, 98, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Cui, M.-Y.; Fu, Y.; Wang, X.-H.; Yang, Q.; Zhu, Y.; Yang, X.-H.; Bi, H.-J.; Gao, X.-L. Aroma enhancement of blueberry wine by postharvest partial dehydration of blueberries. Food Chem. 2023, 426, 136593. [Google Scholar] [CrossRef]
- Radeka, S.; Lukic, I.; Persuric, D. Influence of Different Maceration Treatments on the Aroma Profile of Rose and Red Wines from Croatian Aromatic cv. Muskat ruza porecki (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 442–453. [Google Scholar]
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Effect of different pre-fermentation treatments on polyphenols, color, and volatile compounds of three wine varieties. Food Sci. Biotechnol. 2016, 25, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Callejon, R.M.; Margulies, B.; Hirson, G.D.; Ebeler, S.E. Dynamic Changes in Volatile Compounds during Fermentation of Cabernet Sauvignon Grapes with and without Skins. Am. J. Enol. Viticult. 2012, 63, 301–312. [Google Scholar] [CrossRef]
- Hernandez Orte, P.; Guitart, A.; Ferreira, V.; Gracia, J.; Cacho, J. Effect of maceration time and the addition of enzymes on the amino acid composition of musts and wines and its influence on wine aroma. Food Sci. Technol. Int. 1998, 4, 407–418. [Google Scholar] [CrossRef]
- Alvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Albanese, D.; Attanasio, G.; Cinquanta, L.; Di Matteo, M. Volatile Compounds in Red Wines Processed on an Industrial Scale by Short Pre-fermentative Cold Maceration. Food Bioprocess Tech. 2013, 6, 3266–3272. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biot. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martinez, M.C.; Simal-Gandara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef]
- Toci, A.T.; Crupi, P.; Gambacorta, G.; Dipalmo, T.; Antonacci, D.; Coletta, A. Free and bound aroma compounds characterization by GC-MS of Negroamaro wine as affected by soil management. J. Mass Spectrom. 2012, 47, 1104–1112. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. Standardization Administration of China: Beijing, China, 2006.
- Li, J.X.; Zhang, C.L.; Liu, H.; Liu, J.C.; Jiao, Z.G. Profiles of Sugar and Organic Acid of Fruit Juices: A Comparative Study and Implication for Authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Zhang, Q.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Changes in nutritional composition, volatile organic compounds and antioxidant activity of peach pulp fermented by lactobacillus. Food Biosci. 2022, 49, 101894. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Lv, Z.; Yang, W.; Zhang, C.; Chen, D.; Jiao, Z. Effect of dehydration techniques on bioactive compounds in hawthorn slices and their correlations with antioxidant properties. J. Food Sci. Technol. 2019, 56, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, D.; Chen, X.; Kilmartin, P.; Quek, S.Y. The Influence of Vinification Methods and Cultivars on the Volatile and Phenolic Profiles of Fermented Alcoholic Beverages from Cranberry. Antioxidants 2019, 8, 144. [Google Scholar] [CrossRef]

| Number | Name and Abbreviation of Fermentation Method | Fermentation Raw Material | Maceration Time/h | Maceration Temperature/°C | Pectinase |
|---|---|---|---|---|---|
| A | control vinification of juice pressed from must without pretreatment (CJ) | juice | / | / | / |
| B | vinification of juice pressed from must macerated at normal temperature (NJ) | juice | 3 | 25 | / |
| C | vinification of juice pressed from must macerated at 50 °C (50J) | juice | 3 | 50 | / |
| D | vinification of juice pressed from must macerated at 50 °C with pectinase (PJ) | juice | 3 | 50 | added |
| E | vinification of must macerated at normal temperature (NM) | must | 3 | 25 | / |
| F | vinification of must macerated at 50 °C with pectinase (PM) | must | 3 | 50 | added |
| CJ | NJ | 50J | PJ | NM | PM | |
|---|---|---|---|---|---|---|
| Alcoholic content (% v/v) | 12.17 ± 0.23 b | 12.3 ± 0.00 b | 12.3 ± 0.00 b | 12.5 ± 0.17 a | 12.27 ± 0.1 b | 12.3 ± 0.00 b |
| Sugar-free extract (g/L) | 16.48 ± 0.20 c | 18.72 ± 0.3 b | 18.51 ± 0.26 b | 17.48 ± 1.37 b | 19.39 ± 0.23 a | 19.32 ± 0.39 a |
| Total sugar (g/L) | 1.83 ± 0.11 d | 2.51 ± 0.19 bc | 2.73 ± 0.21 b | 5.32 ± 0.42 a | 2.26 ± 0.19 c | 2.23 ± 0.15 c |
| Methanol (mg/L) | 8.87 ± 1.01 e | 67.12 ± 4.58 c | 72.43 ± 2.14 c | 136.72 ± 11.5 a | 88.16 ± 7.52 b | 134.08 ± 2.65 a |
| Titratable acidity (g/L) | 7.72 ± 0.05 e | 8.11 ± 0.05 bc | 8.04 ± 0.09 c | 7.87 ± 0.05 d | 8.54 ± 0.07 a | 7.90 ± 0.08 d |
| Malic acid(g/L) | 1.21 ± 0.05 a | 1.11 ± 0.04 b | 0.98 ± 0.01 de | 0.92 ± 0.07 e | 1.07 ± 0.05 bc | 1.01 ± 0.02 cd |
| Lactic acid(g/L) | 0.25 ± 0.05 b | 0.17 ± 0.04 b | 0.22 ± 0.01 b | 0.24 ± 0.00 b | 0.38 ± 0.07 a | 0.18 ± 0.02 b |
| Acetic acid(g/L) | 0.12 ± 0.02 c | 0.10 ± 0.03 c | 0.11 ± 0.01 c | 0.57 ± 0.09 b | 0.97 ± 0.34 a | 0.35 ± 0.03 bc |
| Citric acid(g/L) | 4.47 ± 0.02 bc | 4.53 ± 0.03 bc | 4.71 ± 0.04 ab | 4.34 ± 0.12 c | 4.93 ± 0.43 a | 4.88 ± 0.02 a |
| Succinic acid(g/L) | 0.20 ± 0.01 f | 0.49 ± 0.01 cd | 0.52 ± 0.03 b | 0.42 ± 0.03 e | 0.61 ± 0.02 a | 0.50 ± 0.00 bc |
| L* | 16.86 ± 0.06 e | 17.25 ± 0.01 d | 17.58 ± 0.04 c | 16.53 ± 0.03 f | 18.09 ± 0.04 b | 18.44 ± 0.02 a |
| a* | 28.05 ± 0.14 a | 18.00 ± 0.05 c | 15.60 ± 0.02 e | 21.90 ± 0.03 b | 16.35 ± 0.02 cd | 15.60 ± 0.07 e |
| b* | 26.70 ± 0.15 b | 25.05 ± 0.02 bc | 26.85 ± 0.05 b | 29.70 ± 0.03 a | 28.95 ± 0.04 a | 29.10 ± 0.09 a |
| c* | 38.72 ± 0.21 a | 30.85 ± 0.02 d | 31.05 ± 0.03 d | 36.90 ± 0.02 b | 33.25 ± 0.02 c | 33.02 ± 0.11 c |
| h | 43.56 ± 0.71 c | 54.24 ± 1.59 b | 59.85 ± 1.11 a | 53.60 ± 0.81 b | 60.54 ± 0.99 a | 61.80 ± 0.77 a |
| No. | Compounds | RT/min | CJ | NJ | 50J | PJ | NM | PM | Descriptor | Thresholds (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 1-Hexanol | 10.85 | 0.05 | 0.09 | 0.06 | 0.06 | 0.12 | 0.09 | Resin, green | 8000 [31,32] |
| B1 | γ-Decanolide | 46.30 | 42.72 | 30.12 | 45.16 | 32.27 | 60.19 | 24.35 | Peach | 11 [33] |
| D1 | Linalool | 21.18 | 37.02 | 39.84 | 44.33 | 38.25 | 52.78 | 34.45 | Floral, fruity, musky | 15 [32,34] |
| D2 | Geranyl acetone | 24.18 | 0.09 | 0.32 | 0.13 | 0.16 | 0.81 | 0.05 | Tropical fruits | 60 [32] |
| D3 | α-Terpinol | 27.00 | 0.43 | 0.46 | 0.43 | 0.43 | 0.52 | 0.26 | Pleasant, sweet, anise | 250 [31,35] |
| D4 | Myrtenol | 28.76 | 0.00 | 2.95 | 2.27 | 2.63 | 1.13 | 0.28 | Floral, mint | 7 [36] |
| D6 | (E,E)-Farnesol | 34.66 | 0.39 | 0.33 | 0.35 | 0.61 | 0.33 | 0.35 | Lemon, anise, floral, honey, pollen, raspberry | 100 [35] |
| D8 | Trans-nerolidol | 38.99 | 0.15 | 0.09 | 0.14 | 0.40 | 0.30 | 0.29 | Floral, fruity, orange, light flavor | 700 [37] |
| E1 | Methyl salicylate | 28.33 | 0.16 | 0.29 | 0.32 | 0.80 | 0.40 | 0.52 | Holly | 40 [38] |
| F1 | 1-Butanol, 3-methyl- | 2.34 | 0.39 | 0.42 | 0.38 | 0.27 | 0.46 | 0.46 | Whiskey, harsh, bitter | 30,000 [34,35] |
| F3 | 1-Octanol | 20.85 | 0.47 | 0.56 | 0.68 | 0.42 | 1.97 | 1.70 | Roses, citrus | 120 [37] |
| F4 | 2-Nonanol | 20.68 | 0.00 | 0.07 | 0.07 | 0.10 | 0.10 | 0.11 | Fat wax, rose, citrus | 50 [37] |
| G1 | 1-Butanol, 3-methyl-, acetate | 5.05 | 2.45 | 2.84 | 3.27 | 2.50 | 0.75 | 0.47 | Banana | 1500 [39] |
| G3 | Acetic acid, hexyl ester | 13.43 | 51.50 | 71.67 | 55.06 | 65.14 | 6.21 | 3.79 | Apple, banana, pear | 10 [37] |
| H1 | Hexanoic acid, ethyl ester | 12.09 | 112.47 | 210.67 | 241.02 | 246.77 | 152.57 | 125.71 | Apple, strawberry | 14 [31,32] |
| H4 | Heptanoic acid, ethyl ester | 17.11 | 0.04 | 0.11 | 0.12 | 0.11 | 0.09 | 0.06 | Pineapple, fruity | 220 [37] |
| H6 | Octanoic acid, ethyl ester | 21.66 | 759.46 | 1007.75 | 1180.29 | 1333.33 | 440.28 | 234.82 | Pineapple, apple, brandy | 2 [37] |
| H11 | Decanoic acid, ethyl ester | 29.77 | 1.78 | 3.16 | 4.18 | 5.70 | 1.76 | 0.72 | Pear, brandy | 200 [32,37] |
| H12 | Ethyl 9-decenoate | 30.98 | 0.75 | 4.73 | 7.59 | 6.72 | 2.56 | 0.22 | Fruity | 100 [40] |
| H14 | Dodecanoic acid, ethyl ester | 36.97 | 0.04 | 0.06 | 0.33 | 0.25 | 0.07 | 0.02 | Oil, floral | 400 [40] |
| L1 | Styrene | 8.08 | 5.26 | 6.85 | 7.12 | 6.34 | 5.09 | 4.36 | Flowery | 65 [41] |
| L2 | Benzaldehyde | 19.19 | 0.42 | 0.10 | 0.23 | 0.13 | 0.09 | 0.09 | Almond | 2000 [41] |
| L3 | Acetophenone | 25.17 | 1.21 | 0.33 | 1.05 | 0.57 | 1.03 | 0.69 | Almond | 65 [42] |
| L5 | Phenylethyl Alcohol | 30.40 | 0.20 | 0.23 | 0.28 | 0.10 | 0.35 | 0.08 | Honey, rose | 14,000 [31,32] |
| L6 | Acetic acid, 2-phenylethyl ester | 31.95 | 7.51 | 4.61 | 6.96 | 2.82 | 2.45 | 0.94 | Floral | 250 [37] |
| L7 | Benzoic acid, ethyl ester | 26.44 | 0.05 | 0.11 | 0.14 | 0.08 | 0.32 | 0.10 | Holly oil and fruit | 575 [32] |
| L8 | Benzeneacetic acid, ethyl ester | 31.25 | 0.03 | 0.10 | 0.10 | 0.06 | 0.10 | 0.05 | Floral, honey | 73 [37] |
| L9 | Benzenepropanoic acid, ethyl ester | 34.97 | 0.45 | 0.24 | 0.86 | 1.00 | 1.53 | 0.79 | Floral | 18.5 [43] |
| L10 | 2-Propenoic acid, 3-phenyl-, ethyl ester | 41.09 | 228.80 | 424.48 | 516.99 | 508.17 | 423.87 | 155.73 | Honey, cinnamon | 1.1 [34] |
| I1 | Octanoic acid, methyl ester | 18.83 | 0.01 | 0.06 | 0.06 | 0.14 | 0.01 | 0.03 | Orange | 200 [44] |
| J2 | Hexanoic acid | 23.60 | 1.16 | 0.56 | 0.91 | 0.57 | 1.36 | 0.44 | Cheese | 420 [39] |
| J3 | Octanoic acid | 31.63 | 1.65 | 0.37 | 0.77 | 0.38 | 0.41 | 0.09 | Fatty acid | 500 [39] |
| K3 | Decanal | 24.87 | 1.07 | 1.32 | 0.81 | 0.30 | 0.42 | 0.67 | Orange peel | 10 [39] |
| Total | 1177.33 | 1741.68 | 2029.59 | 2182.76 | 1044.24 | 532.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Liu, H.; Yang, W.; Zhang, Q.; Chen, D.; Jiao, Z.; Liu, J. Comprehensive Analysis of Physicochemical Properties and Volatile Compounds in Different Strawberry Wines under Various Pre-Treatments. Molecules 2024, 29, 2045. https://doi.org/10.3390/molecules29092045
Lv Z, Liu H, Yang W, Zhang Q, Chen D, Jiao Z, Liu J. Comprehensive Analysis of Physicochemical Properties and Volatile Compounds in Different Strawberry Wines under Various Pre-Treatments. Molecules. 2024; 29(9):2045. https://doi.org/10.3390/molecules29092045
Chicago/Turabian StyleLv, Zhenzhen, Hui Liu, Wenbo Yang, Qiang Zhang, Dalei Chen, Zhonggao Jiao, and Jiechao Liu. 2024. "Comprehensive Analysis of Physicochemical Properties and Volatile Compounds in Different Strawberry Wines under Various Pre-Treatments" Molecules 29, no. 9: 2045. https://doi.org/10.3390/molecules29092045
APA StyleLv, Z., Liu, H., Yang, W., Zhang, Q., Chen, D., Jiao, Z., & Liu, J. (2024). Comprehensive Analysis of Physicochemical Properties and Volatile Compounds in Different Strawberry Wines under Various Pre-Treatments. Molecules, 29(9), 2045. https://doi.org/10.3390/molecules29092045








