Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations
Abstract
1. Introduction
2. Results
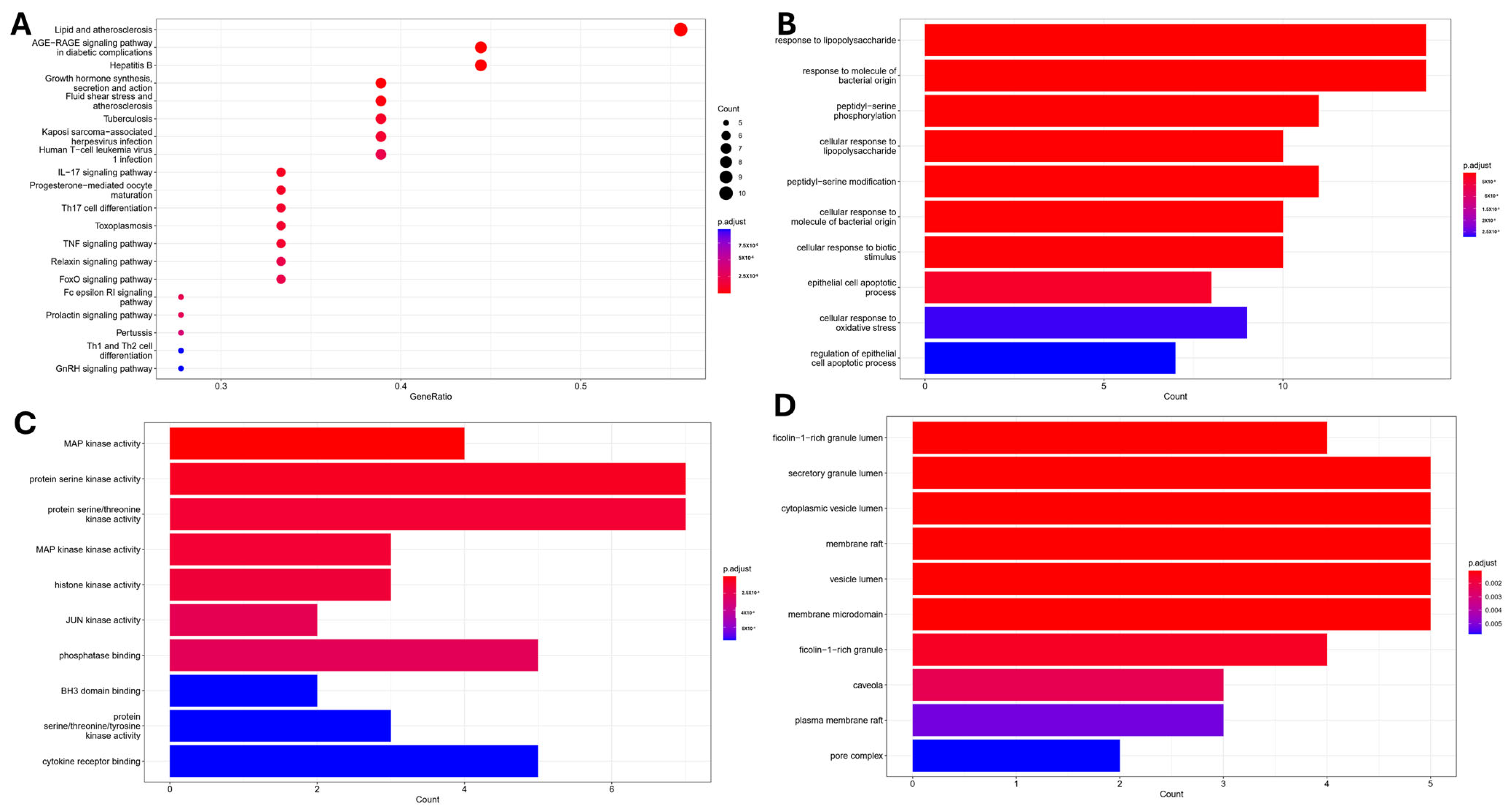
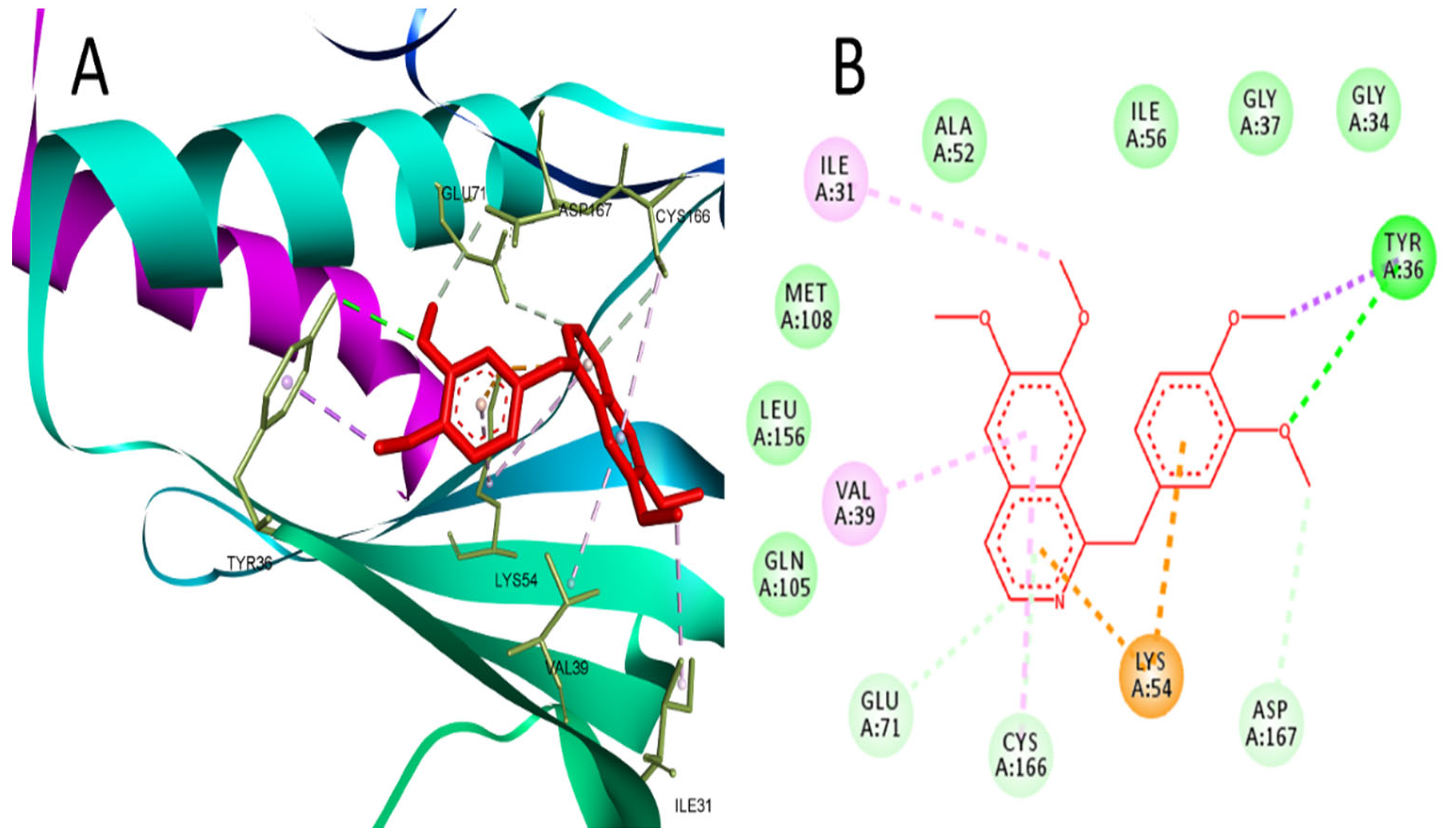
2.1. Network Pharmacology and Molecular Docking Analysis
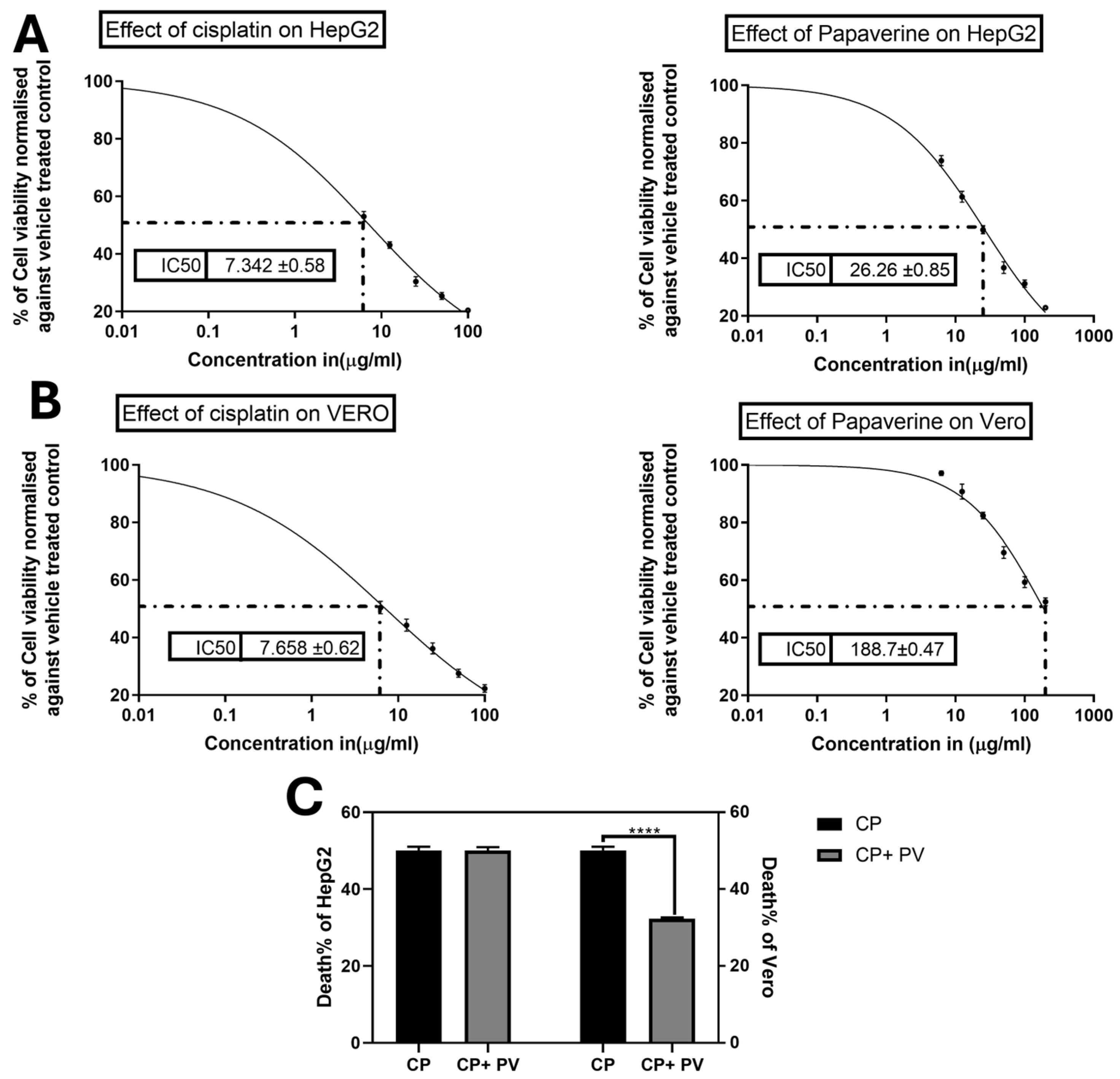
2.2. Effect of PV on CP-Induced Cytotoxicity on Cancer and Normal Cells
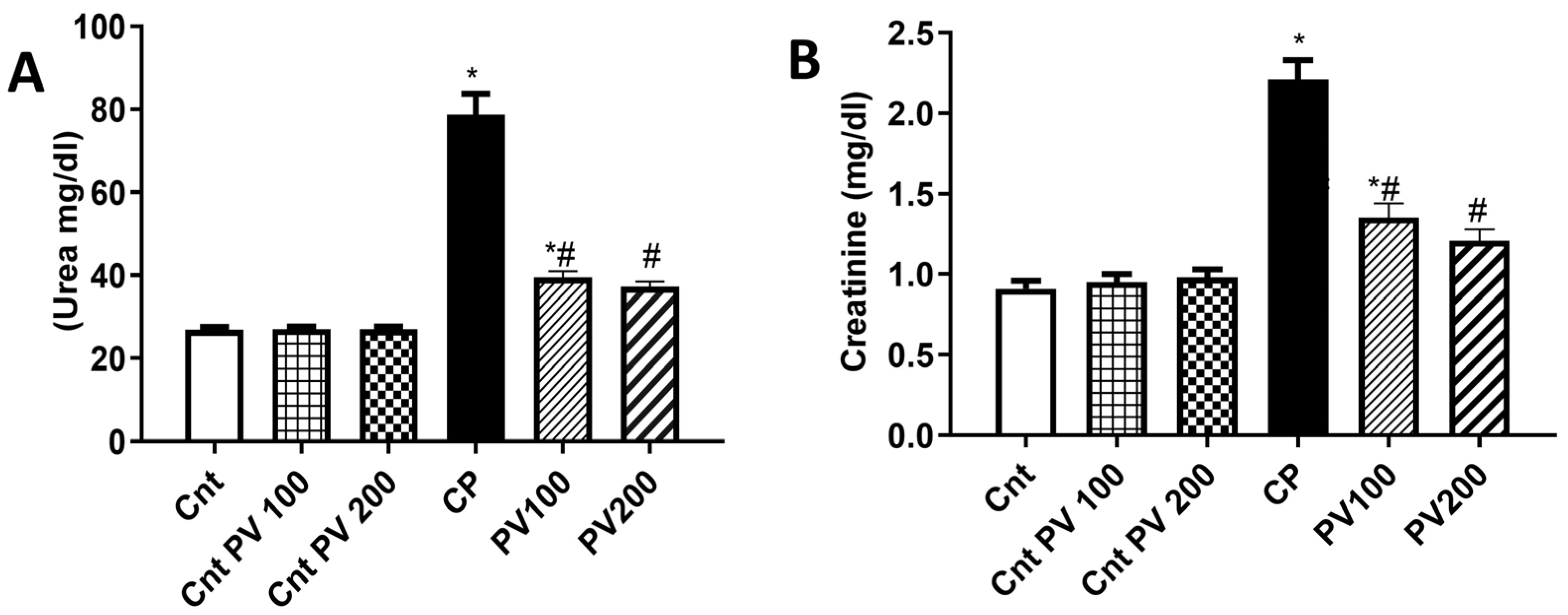
2.3. PV Decreased Serum Levels of CP-Triggered Renal Damage Parameters
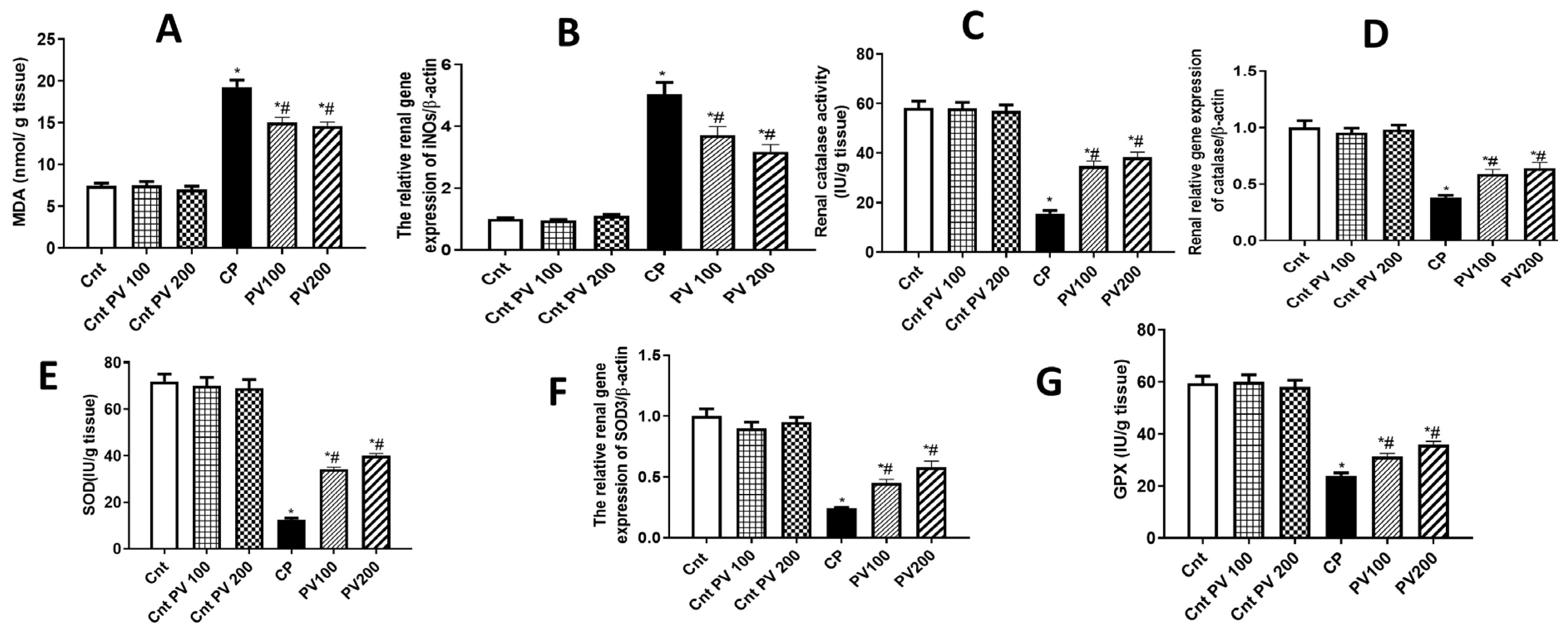
2.4. PV Diminished CP-Promoted Renal Oxidative Stress
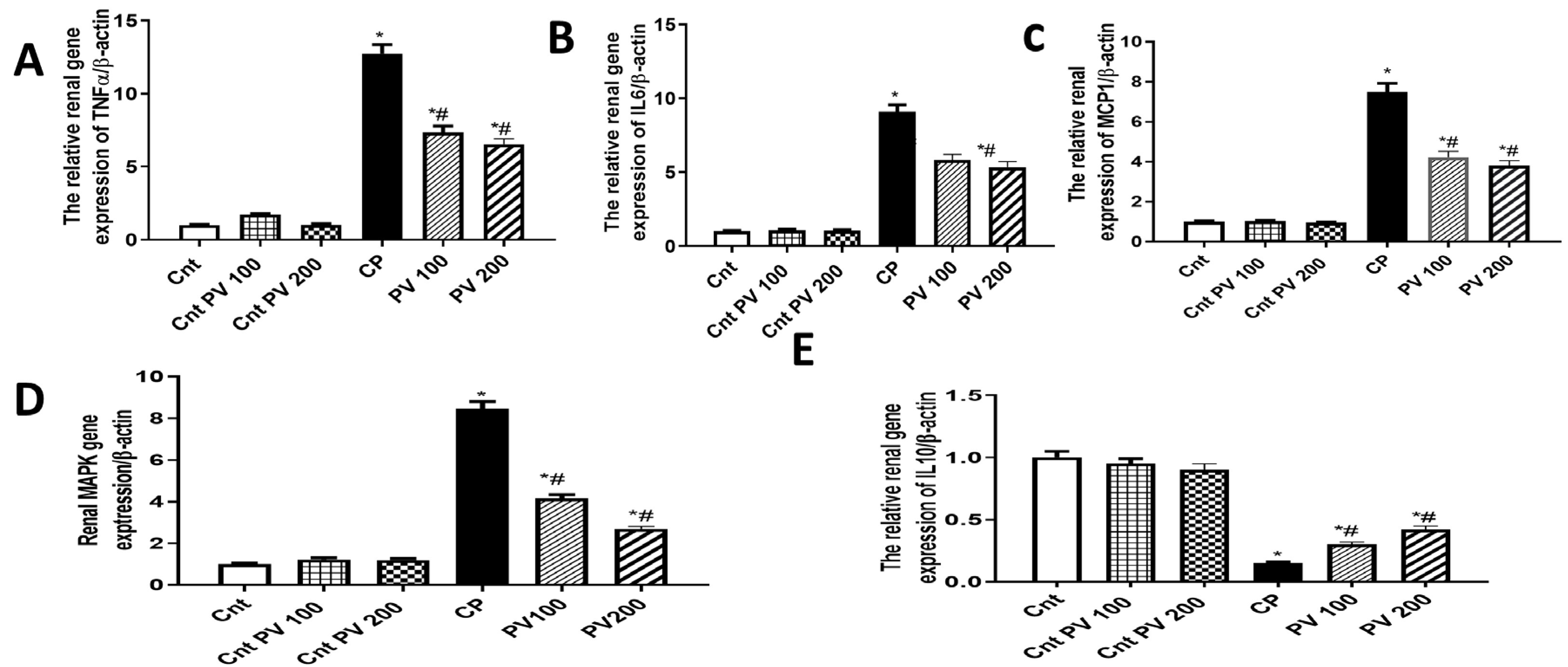
2.5. PV Inhibited Renal Inflammation Caused by CP
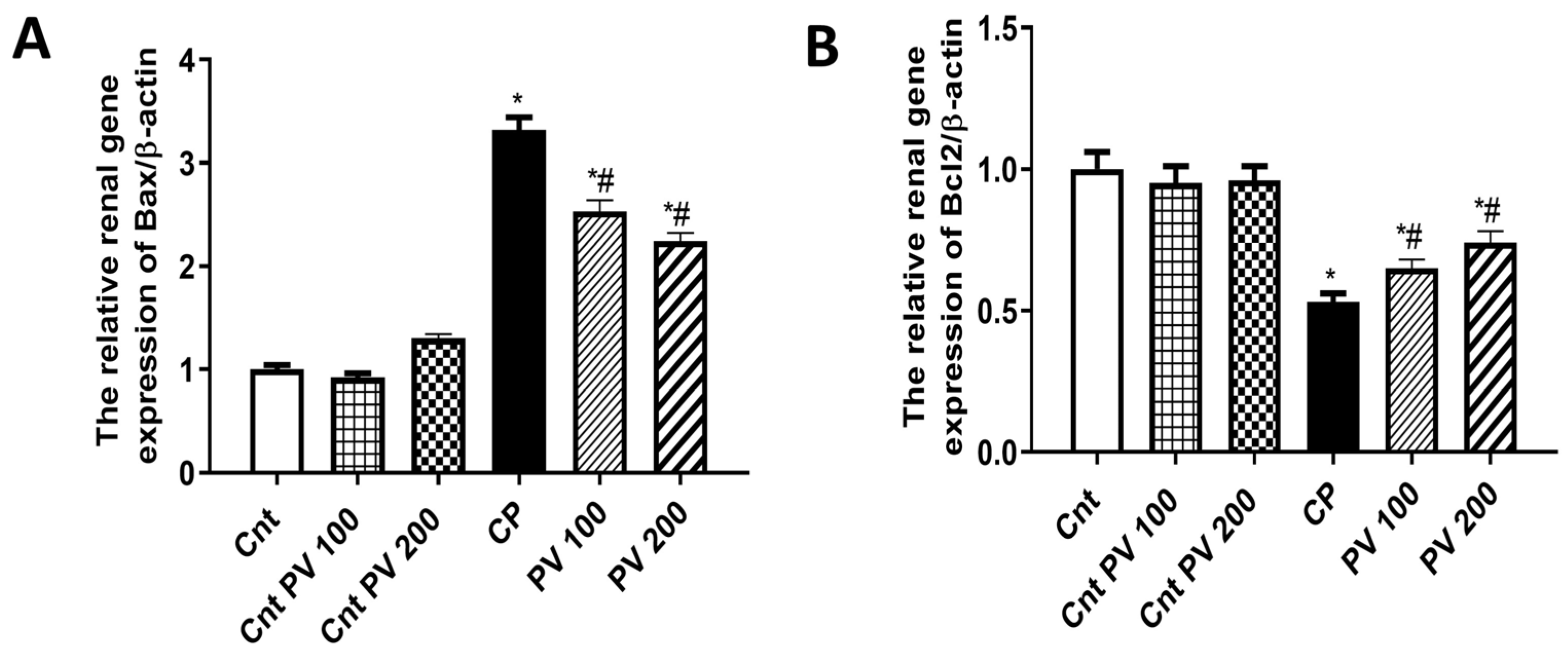
2.6. PV Alleviated CP-Induced Renal Apoptosis
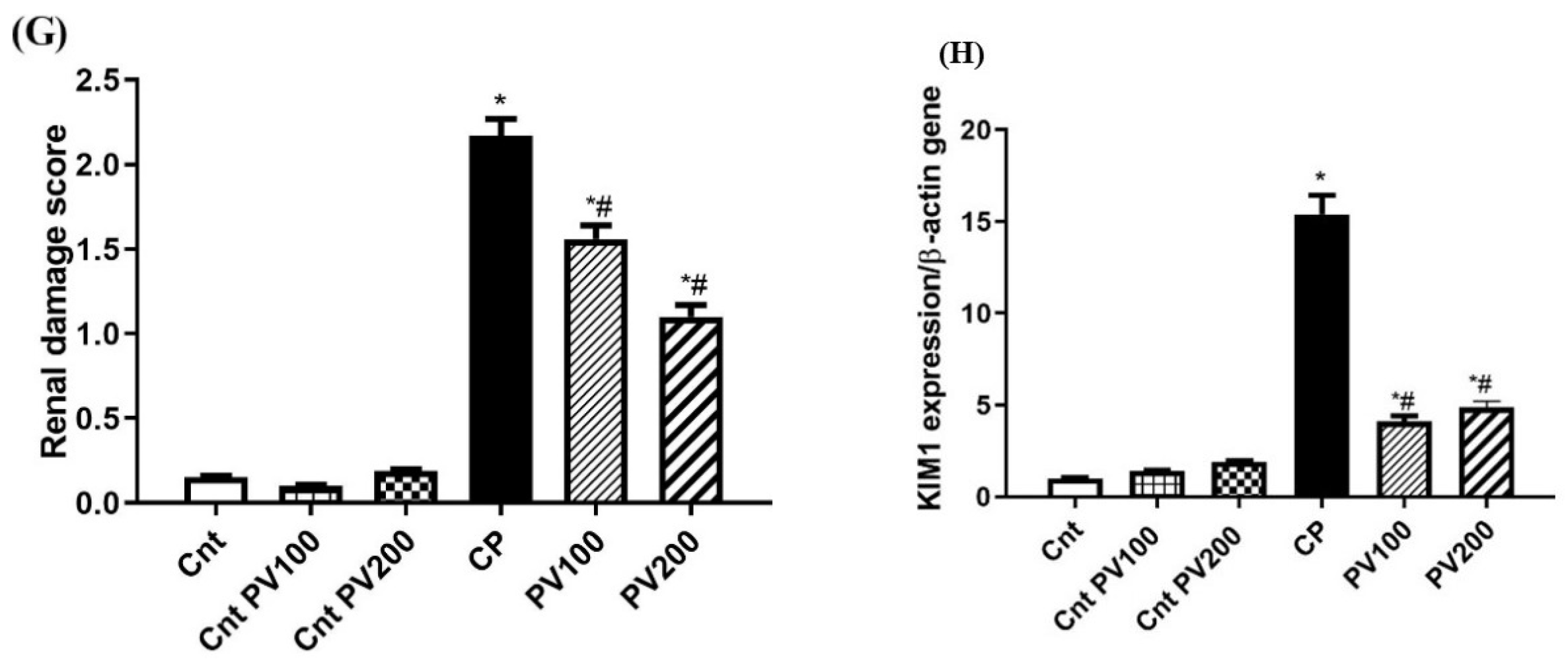
2.7. PV Restored Renal Damage Cause by CP
2.8. PV Repressed CP-Induced Renal Damage
3. Discussion
4. Materials and Methods
4.1. Network Pharmacology and In Silico Prediction of PV Targets
4.2. Drugs, Chemicals, and Kits
4.3. Cytotoxicity by the MTT Assay
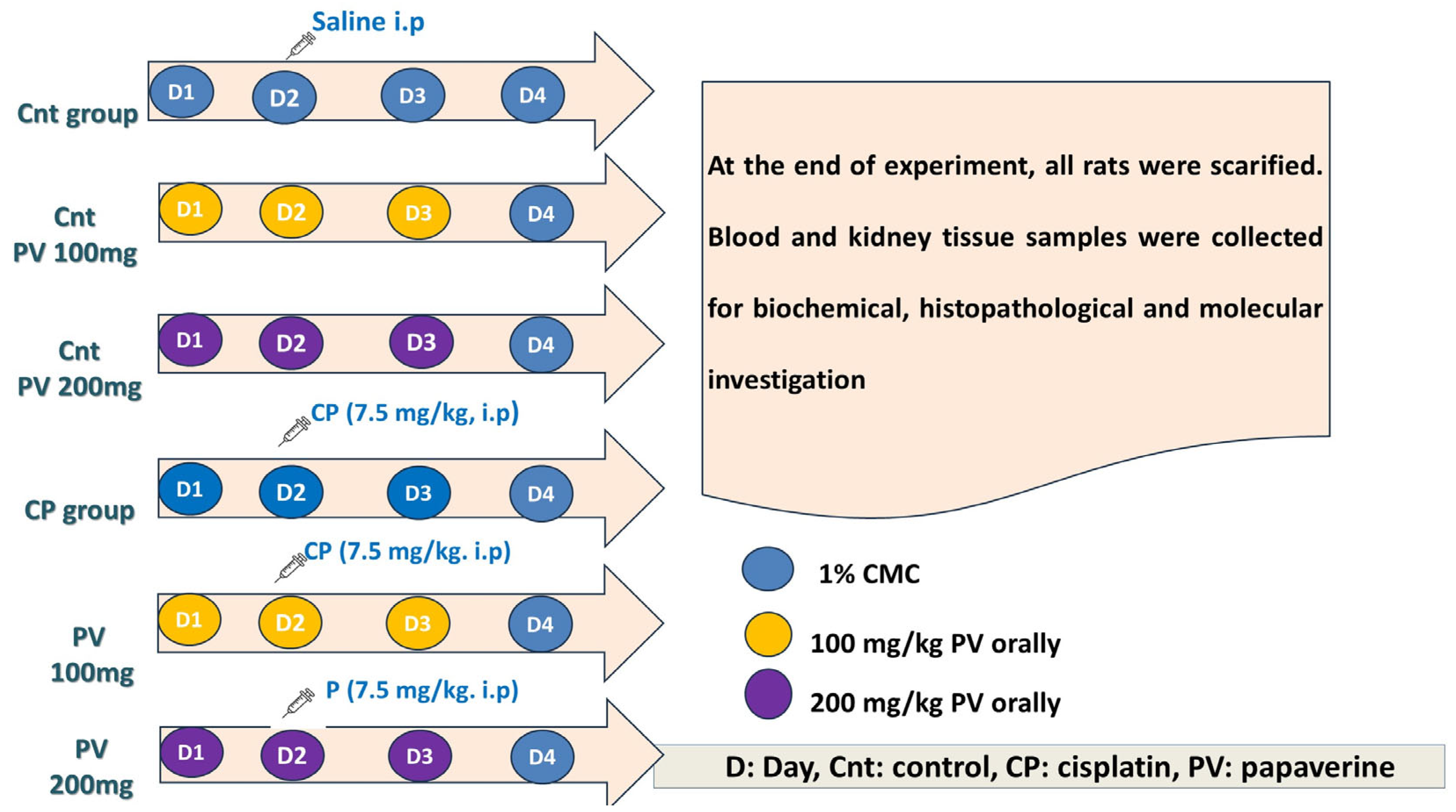
4.4. Experimental Design
4.5. Sampling
4.6. Kidney Function Tests
4.7. Biochemical Assays of Oxidative Stress
4.8. Real-Time PCR
4.9. Histopathological Examination of Kidney Tissues
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klumpers, M.J.; Witte, W.D.; Gattuso, G.; Schiavello, E.; Terenziani, M.; Massimino, M.; Gidding, C.E.; Vermeulen, S.H.; Driessen, C.M.; Van Herpen, C.M. Genome-wide analyses of nephrotoxicity in platinum-treated cancer patients identify association with genetic variant in RBMS3 and acute kidney injury. J. Pers. Med. 2022, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic implications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Lou, D.-Y.; Zhou, L.-Q.; Wang, J.-C.; Yang, B.; He, Q.-J.; Wang, J.-J.; Weng, Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hou, J.; Yan, X.; Leng, J.; Li, R.; Zhang, J.; Xing, J.; Chen, C.; Wang, Z.; Li, W. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways. Nutrients 2018, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; Radford, R.; Slyne, J.; O’Connell, S.; Slattery, C.; Ryan, M.P.; McMorrow, T. The role of MAPK in drug-induced kidney injury. J. Signal Transduct. 2012, 2012, 463617. [Google Scholar] [CrossRef] [PubMed]
- Prša, P.; Karademir, B.; Biçim, G.; Mahmoud, H.; Dahan, I.; Yalçın, A.S.; Mahajna, J.; Milisav, I. The potential use of natural products to negate hepatic, renal and neuronal toxicity induced by cancer therapeutics. Biochem. Pharmacol. 2020, 173, 113551. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for Cancer Prevention With Natural Compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Nie, R.-C.; Yin, Y.-X.; Cai, X.-X.; Xie, D.; Cai, M.-Y. Protective Effect of Natural Antioxidants on Reducing Cisplatin-Induced Nephrotoxicity. Dis. Markers 2022, 2022, 1612348. [Google Scholar] [CrossRef]
- Elgazar, A.A.; El-Domany, R.A.; Eldehna, W.M.; Badria, F.A. 3-Acetyl-11-keto-β-boswellic Acid-Based Hybrids Alleviate Acetaminophen-Induced Hepatotoxicity in HepG2 by the Regulation of Inflammatory and Oxidative Stress Pathways: An Integrated Approach. ACS Omega 2023, 8, 39490–39510. [Google Scholar] [CrossRef]
- Ridzuan, N.R.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Radovits, T.; Szabó, G.; Mózes, M.M.; Rosivall, L.; Kökény, G. Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3′,5′ guanosine monophosphate (cGMP) level in podocytes. Nephrol. Dial. Transplant. 2012, 28, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Hotta, Y.; Naiki-Ito, A.; Hirano, K.; Kataoka, T.; Maeda, Y.; Takahashi, S.; Kimura, K. The phosphodiesterase 5 inhibitor tadalafil has renoprotective effects in a rat model of chronic kidney disease. Physiol. Rep. 2020, 8, e14556. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. Phosphodiesterase inhibition in the treatment of autoimmune and inflammatory diseases: Current status and potential. J. Recept. Ligand Channel Res. 2015, 8, 19–30. [Google Scholar] [CrossRef]
- Gomes, D.A.; Joubert, A.M.; Visagie, M.H. The Biological Relevance of Papaverine in Cancer Cells. Cells 2022, 11, 3385. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, V.; Kaya, M.; Uslu, F.B.; Atasoy, O.; Erbaş, O. Papaverine Has Therapeutic Potential for Sepsis-Induced Neuropathy in Rats, Possibly via the Modulation of HMGB1-RAGE Axis and Its Antioxidant Prosperities. J. Investig. Surg. 2022, 35, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lu, W.; Wu, D.; Luo, A.; Bian, H.; Li, J.; Li, W.; Liu, G.; Huang, J.; Cheng, F.; et al. In silico prediction of chemical mechanism of action via an improved network-based inference method. Br. J. Pharmacol. 2016, 173, 3372–3385. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Huang, J.; Wang, N.; Tan, H.-Y.; Cheung, F.; Chen, F.; Feng, Y. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2019, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Li, B.; Ding, P.; Jin, D.; Hou, S.; Cai, X.; Sheng, X. The effect of monotropein on alleviating cisplatin-induced acute kidney injury by inhibiting oxidative damage, inflammation and apoptosis. Biomed. Pharmacother. 2020, 129, 110408. [Google Scholar] [CrossRef]
- Assar, D.H.; Asa, S.A.; El-Abasy, M.A.; Elbialy, Z.I.; Shukry, M.; Latif, A.A.E.; BinMowyna, M.N.; Althobaiti, N.A.; El-Magd, M.A. Aspergillus awamori attenuates ochratoxin A-induced renal and cardiac injuries in rabbits by activating the Nrf2/HO-1 signaling pathway and downregulating IL1β, TNFα, and iNOS gene expressions. Environ. Sci. Pollut. Res. 2022, 29, 69798–69817. [Google Scholar] [CrossRef]
- Zahran, R.; Ghozy, A.; Elkholy, S.S.; El-Taweel, F.; El-Magd, M.A. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia–reperfusion injury in a rat model. Int. J. Urol. 2020, 27, 1039–1049. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin ameliorates cisplatin induced acute kidney injury by upregulating Nrf-2 via the activation of PI3K and exhibits synergistic anticancer activity with cisplatin. Front. Pharmacol. 2018, 9, 638. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for Bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Elgazar, A.A.; Alwasify, H.A.; Abed, A.; Foda, M.; Abouzeid, S.; Lewerenz, L.; Selmar, D.; Badria, F. Bio-evaluation of Untapped Alkaloids from Vinca minor Enriched by Methyl-jasmonate-induced Stress: An Integrated Approach. Planta Med. 2023, 89, 964–978. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, H.J.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, J.M. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Appl. Toxicol. Int. J. 2005, 25, 374–382. [Google Scholar] [CrossRef]
- Hamdi, A.; Yaseen, M.; Ewes, W.A.; Bhat, M.A.; Ziedan, N.I.; El-Shafey, H.W.; Mohamed, A.A.B.; Elnagar, M.R.; Haikal, A.; Othman, D.I.A.; et al. Development of new thiazolidine-2,4-dione hybrids as aldose reductase inhibitors endowed with antihyperglycaemic activity: Design, synthesis, biological investigations, and in silico insights. J. Enzym. Inhib. Med. Chem. 2023, 38, 2231170. [Google Scholar] [CrossRef]
- Wang, Q.; Xi, Y.; Chen, B.; Zhao, H.; Yu, W.; Xie, D.; Liu, W.; He, F.; Xu, C.; Cheng, J. Receptor of Advanced Glycation End Products Deficiency Attenuates Cisplatin-Induced Acute Nephrotoxicity by Inhibiting Apoptosis, Inflammation and Restoring Fatty Acid Oxidation. Front. Pharmacol. 2022, 13, 907133. [Google Scholar] [CrossRef]
- El-Far, A.H.A.M.; Munesue, S.; Harashima, A.; Sato, A.; Shindo, M.; Nakajima, S.; Inada, M.; Tanaka, M.; Takeuchi, A.; Tsuchiya, H. In vitro anticancer effects of a RAGE inhibitor discovered using a structure-based drug design system. Oncol. Lett. 2018, 15, 4627–4634. [Google Scholar] [CrossRef]
- Tamada, K.; Nakajima, S.; Ogawa, N.; Inada, M.; Shibasaki, H.; Sato, A.; Takasawa, R.; Yoshimori, A.; Suzuki, Y.; Watanabe, N.; et al. Papaverine identified as an inhibitor of high mobility group box 1/receptor for advanced glycation end-products interaction suppresses high mobility group box 1-mediated inflammatory responses. Biochem. Biophys. Res. Commun. 2019, 511, 665–670. [Google Scholar] [CrossRef]
- Li, M.; Li, C.-M.; Ye, Z.-C.; Huang, J.; Li, Y.; Lai, W.; Peng, H.; Lou, T.-Q. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell. Mol. Med. 2020, 24, 5109–5121. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Tsai, M.-S.; Hsieh, P.-C.; Shih, J.-H.; Wang, T.-S.; Wang, Y.-C.; Lin, T.-H.; Wang, S.-H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 2017, 329, 128–139. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Reif, G.A.; Calvet, J.P.; Wallace, D.P. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am. J. Physiol.-Ren. Physiol. 2010, 299, F944–F951. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y. Cascade Signals of Papaverine Inhibiting LPS-Induced Retinal Microglial Activation. J. Mol. Neurosci. 2019, 68, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Leser, G.P.; Lamb, R.A. Repurposing papaverine as an antiviral agent against influenza viruses and paramyxoviruses. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Gao, L.; Liu, M.-M.; Zang, H.-M.; Ma, Q.-Y.; Yang, Q.; Jiang, L.; Ren, G.-L.; Li, H.-D.; Wu, W.-F.; Wang, J.-N. Restoration of E-cadherin by PPBICA protects against cisplatin-induced acute kidney injury by attenuating inflammation and programmed cell death. Lab. Investig. 2018, 98, 911–923. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Du, W.; Mickelsen, D.M.; Shi, H.; Yu, H.; Kumar, S.; Yan, C. PDE10A Inactivation Prevents Doxorubicin-Induced Cardiotoxicity and Tumor Growth. Circ. Res. 2023, 133, 138–157. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.S.; Leem, Y.H.; Park, J.E.; Kim, D.Y.; Choi, Y.H.; Park, E.M.; Kang, J.L.; Kim, H.S. The phosphodiesterase 10 inhibitor papaverine exerts anti-inflammatory and neuroprotective effects via the PKA signaling pathway in neuroinflammation and Parkinson’s disease mouse models. J. Neuroinflamm. 2019, 16, 246. [Google Scholar] [CrossRef]
- Bhat, A.; Tan, V.; Heng, B.; Chow, S.; Basappa, S.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J. Papaverine, a Phosphodiesterase 10A Inhibitor, Ameliorates Quinolinic Acid-Induced Synaptotoxicity in Human Cortical Neurons. Neurotox. Res. 2021, 39, 1238–1250. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Tian, C.; Ren, Z.; Song, X.; Wang, X.; Xu, N.; Jing, H.; Li, S.; Liu, W. Characterization, antioxidation, anti-inflammation and renoprotection effects of selenized mycelia polysaccharides from Oudemansiella radicata. Carbohydr. Polym. 2018, 181, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.E.; Ma, J.Y.; Sablad, M.; Sonee, M.; Varacallo, L.; Louden, C.; Guy, A.; Vegas, J.; Liu, X.; La, D. Time course of renal proximal tubule injury, reversal, and related biomarker changes in rats following cisplatin administration. Int. J. Toxicol. 2013, 32, 251–260. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef]
- Abu Gazia, M.; El-Magd, M.A. Effect of pristine and functionalized multiwalled carbon nanotubes on rat renal cortex. Acta Histochem. 2018, 121, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Elmoslemany, A.M.; El-Magd, M.A.; Ghamry, H.I.; Alshahrani, M.Y.; Zidan, N.S.; Zedan, A.M.G. Avocado Seeds Relieve Oxidative Stress-Dependent Nephrotoxicity but Enhance Immunosuppression Induced by Cyclosporine in Rats. Antioxidants 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodríguez-Muñoz, R.; Reyes, J.L.; Loredo, M.L.; Barrera-Oviedo, D.; Pinzón, E.; Rodríguez-Rangel, D.S.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: Relation to oxidative stress. Food Funct. 2016, 7, 279–293. [Google Scholar] [CrossRef]
- Sallam, A.A.; Ahmed, M.M.; El-Magd, M.A.; Magdy, A.; Ghamry, H.I.; Alshahrani, M.Y.; Abou El-Fotoh, M.F. Quercetin Ameliorated Multi-Walled Carbon Nanotubes-Induced Immunotoxic, Inflammatory, and Oxidative Effects in Mice. Molecules 2022, 27, 2117. [Google Scholar] [CrossRef]
- Peres, L.A.B.; Cunha Júnior, A.D.d. Acute nephrotoxicity of cisplatin: Molecular mechanisms. Braz. J. Nephrol. 2013, 35, 332–340. [Google Scholar] [CrossRef]
- Fawzy, M.H.; Khodeer, D.M.; El-Sayed, N.M.; Saeed, N.M.; Ahmed, Y.M. Molecular mechanisms of cisplatin induced nephrotoxicity. Rec. Pharm. Biomed. Sci. 2022, 6, 128–135. [Google Scholar] [CrossRef]
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-κB signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Sangomla, S.; Khurana, A.; Godugu, C. Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res. 2019, 189, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kohda, Y.; Chiao, H.; Wang, Y.; Hu, X.; Hewitt, S.M.; Miyaji, T.; Mcleroy, P.; Nibhanupudy, B.; Li, S. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001, 60, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Nisansala, T.; Weerasekera, M.; Ranasinghe, N.; Marasinghe, C.; Gamage, C.; Fernando, N.; Gunasekara, C. Importance of KIM-1 and MCP-1 in Determining the Leptospirosis-Associated AKI: A Sri Lankan Study. BioMed Res. Int. 2021, 2021, 1752904. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Xue, D.-F.; Pan, S.-T.; Huang, G.; Qiu, J.-X. ROS enhances the cytotoxicity of cisplatin by inducing apoptosis and autophagy in tongue squamous cell carcinoma cells. Int. J. Biochem. Cell Biol. 2020, 122, 105732. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Yoshikawa, H.; Shiiki, K.; Hamada, Y.; Akamatsu, N.; Tasaka, K. Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8,-3 and-6 in osteosarcoma. Cancer Chemother. Pharmacol. 2000, 45, 199–206. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Elgazar, A.A.; El-Domany, R.A.; Eldehna, W.M.; Badria, F.A. Theophylline-based hybrids as acetylcholinesterase inhibitors endowed with anti-inflammatory activity: Synthesis, bioevaluation, in silico and preliminary kinetic studies. RSC Adv. 2023, 13, 25616–25634. [Google Scholar] [CrossRef]
- Elgazar, A.A.; Selim, N.M.; Abdel-Hamid, N.M.; El-Magd, M.A.; El Hefnawy, H.M. Isolates from Alpinia officinarum Hance attenuate LPS-induced inflammation in HepG2: Evidence from in silico and in vitro studies. Phytother. Res. 2018, 32, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Othman, D.I.A.; Hamdi, A.; Tawfik, S.S.; Elgazar, A.A.; Mostafa, A.S. Identification of new benzimidazole-triazole hybrids as anticancer agents: Multi-target recognition, in vitro and in silico studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 2166037. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; Hamdi, A.; Mohamed, A.A.B.; El-Shafey, H.W.; Moustafa, M.; Elgazar, A.A.; Eldehna, W.M.; Ur Rahman, H.; Parambi, D.G.T.; Elbargisy, R.M.; et al. New benzothiazole hybrids as potential VEGFR-2 inhibitors: Design, synthesis, anticancer evaluation, and in silico study. J. Enzym. Inhib. Med. Chem. 2023, 38, 2166036. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Aneja, R.; Rewal, C.; Konduri, R.; Dass, S.K.; Agarwal, S. An opium alkaloid-papaverine ameliorates ethanol-induced hepatotoxicity: Diminution of oxidative stress. Indian J. Clin. Biochem. 2000, 15, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kelada, M.N.; Elagawany, A.; El Sekily, N.M.; El Mallah, M.; Abou Nazel, M.W. Protective Effect of Platelet-Rich Plasma on Cisplatin-Induced Nephrotoxicity in Adult Male Albino Rats: Histological and Immunohistochemical Study. Biol. Trace Elem. Res. 2023, 202, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Patton, C.J.; Crouch, S. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967, 118, 29–32. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Kahilo, K.A.; Nasr, N.E.; Kamal, T.; Shukry, M.; Saleh, A.A. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia 2016, 49, e12750. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.M.G.; Sakran, M.I.; Bahattab, O.; Hawsawi, Y.M.; Al-Amer, O.; Oyouni, A.A.A.; Nasr Eldeen, S.K.; El-Magd, M.A. Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells. Molecules 2021, 26, 3303. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Sung, P.H.; Chen, K.H.; Shao, P.L.; Yang, C.C.; Cheng, B.C.; Lin, K.C.; Chen, C.H.; Chai, H.T.; Chang, H.W.; et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am. J. Transl. Res. 2018, 10, 1053–1070. [Google Scholar] [PubMed]


| ID | Gene Symbol | Numbers Related KEGG | Numbers Related BP | Numbers Related MF |
|---|---|---|---|---|
| 1. | MAPK1 | 100/144 | 133/1331 | 7/92 |
| 2. | MAPK8 | 70/144 | 90/1331 | 9/92 |
| 3. | MAPK10 | 70/144 | 13/1331 | 6/92 |
| 4. | TNF | 63/144 | 536/1331 | 5/92 |
| 5. | MAPK14 | 60/144 | 151/1331 | 9/92 |
| 6. | PRKCA | 53/144 | 98/1331 | 5/92 |
| 7. | IL6 | 47/144 | 340/1331 | 6/92 |
| 8. | BAX | 43/144 | 243/1331 | 5/92 |
| 9. | BCL2 | 40/144 | 288/1331 | 6/92 |
| 10. | CREBBP | 23/144 | 40/1331 | 1/92 |
| 11. | JAK2 | 22/144 | 278/1331 | 11/92 |
| 12. | IL10 | 20/144 | 258/1331 | 5/92 |
| 13. | CDK2 | 17/144 | 38/1331 | 3/92 |
| 14. | CCL2 | 16/144 | 129/1331 | 5/92 |
| 15. | ICAM1 | 15/144 | 59/1331 | 1/92 |
| 16. | HSP90AA1 | 14/144 | 107/1331 | 14/92 |
| 17. | NOS2 | 12/144 | 84/1331 | 11/92 |
| 18. | ATR | 6/144 | 56/1331 | 5/92 |
| 19. | SELE | 6/144 | 25/1331 | 2/92 |
| 20. | NQO1 | 4/144 | 59/1331 | 6/92 |
| 21. | CYP1A1 | 3/144 | 75/1331 | 16/92 |
| 22. | G6PD | 2/144 | 80/1331 | 2/92 |
| 23. | S100A9 | 1/144 | 65/1331 | 10/92 |
| 24. | ANXA5 | 0/144 | 5/1331 | 1/92 |
| 25. | DPEP1 | 0/144 | 31/1331 | 4/92 |
| 26. | ALAD | 0/144 | 45/1331 | 2/92 |
| Gene | Forward Primer (5′-----3′) | Reverse Primer (5′-----3′) |
|---|---|---|
| MAPK1 | AGGGCGATGTGACGTTT | CTGGCAGGGTGAAGTTGG |
| iNOs | CACCACCCTCCTTGTTCAAC | CAATCCACAACTCGCTCCAA |
| SOD3 | AAGGAGCAAGGTCGCTTACA | ACACATCAATCCCCAGCAGT |
| CAT | GAATGGCTATGGCTCACACA | CAAGTTTTTGATGCCCTGGT |
| TNFα | GCATGATCCGCGACGTGGAA | AGATCCATGCCGTTGGCCAG |
| MCP1 | TCGCTTCTGACACCATGCA | TGCTACAGGCAGCAAATGTGA |
| IL6 | TCCTACCCCAACTTCCAATGCTC | TTGGATGGTCTTGGTCCTTAGCC |
| IL10 | GTTGCCAAGCCTTGTCAGAAA | TTTCTGGGCCATGGTTCTCT |
| Bax | ACACCTGAGCTGACCTTG | AGCCCATGATGGTTCTGATC |
| Bcl2 | ATCGCTCTGTGGATGACTGAGTAC | AGAGACAGCCAGGAGAAATCAAAC |
| KIM1 | TGGCACTGTGACATCCTCAGA | GCAACGGACATGCCAACATA |
| β-actin | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abass, S.A.; Elgazar, A.A.; El-kholy, S.S.; El-Refaiy, A.I.; Nawaya, R.A.; Bhat, M.A.; Farrag, F.A.; Hamdi, A.; Balaha, M.; El-Magd, M.A. Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations. Molecules 2024, 29, 1927. https://doi.org/10.3390/molecules29091927
Abass SA, Elgazar AA, El-kholy SS, El-Refaiy AI, Nawaya RA, Bhat MA, Farrag FA, Hamdi A, Balaha M, El-Magd MA. Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations. Molecules. 2024; 29(9):1927. https://doi.org/10.3390/molecules29091927
Chicago/Turabian StyleAbass, Shimaa A., Abdullah A. Elgazar, Sanad S. El-kholy, Amal I. El-Refaiy, Reem A. Nawaya, Mashooq Ahmad Bhat, Foad A. Farrag, Abdelrahman Hamdi, Marwa Balaha, and Mohammed A. El-Magd. 2024. "Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations" Molecules 29, no. 9: 1927. https://doi.org/10.3390/molecules29091927
APA StyleAbass, S. A., Elgazar, A. A., El-kholy, S. S., El-Refaiy, A. I., Nawaya, R. A., Bhat, M. A., Farrag, F. A., Hamdi, A., Balaha, M., & El-Magd, M. A. (2024). Unraveling the Nephroprotective Potential of Papaverine against Cisplatin Toxicity through Mitigating Oxidative Stress and Inflammation: Insights from In Silico, In Vitro, and In Vivo Investigations. Molecules, 29(9), 1927. https://doi.org/10.3390/molecules29091927








