Insights on Stability Constants and Structures of Complexes between Coumarin Derivatives and Pb(II) in Aqueous Media
Abstract
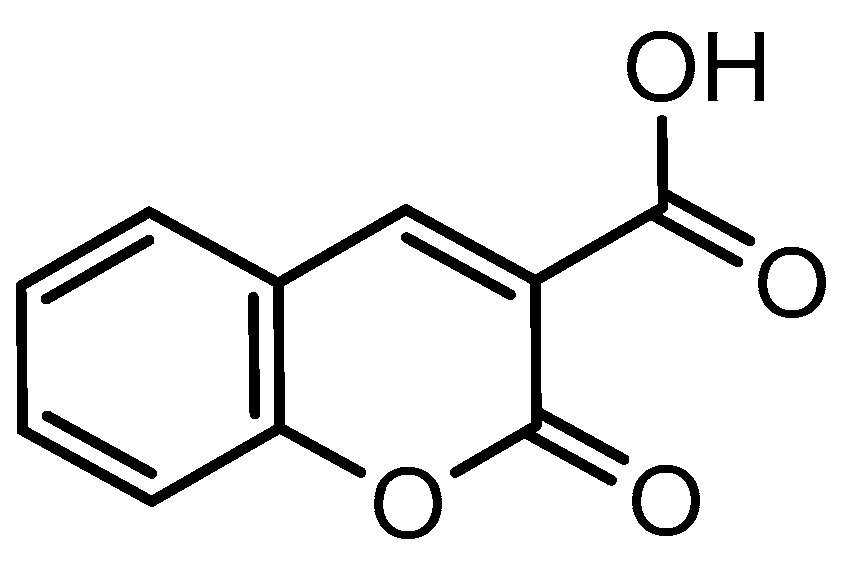
1. Introduction
2. Results and Discussion
2.1. Potentiometric Measurements
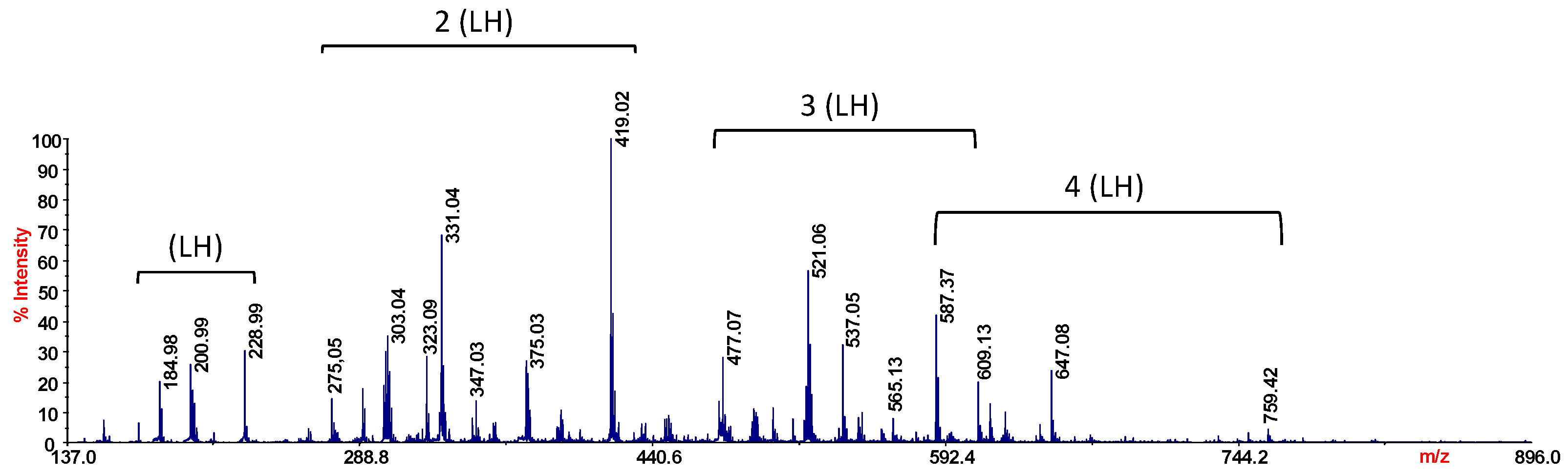
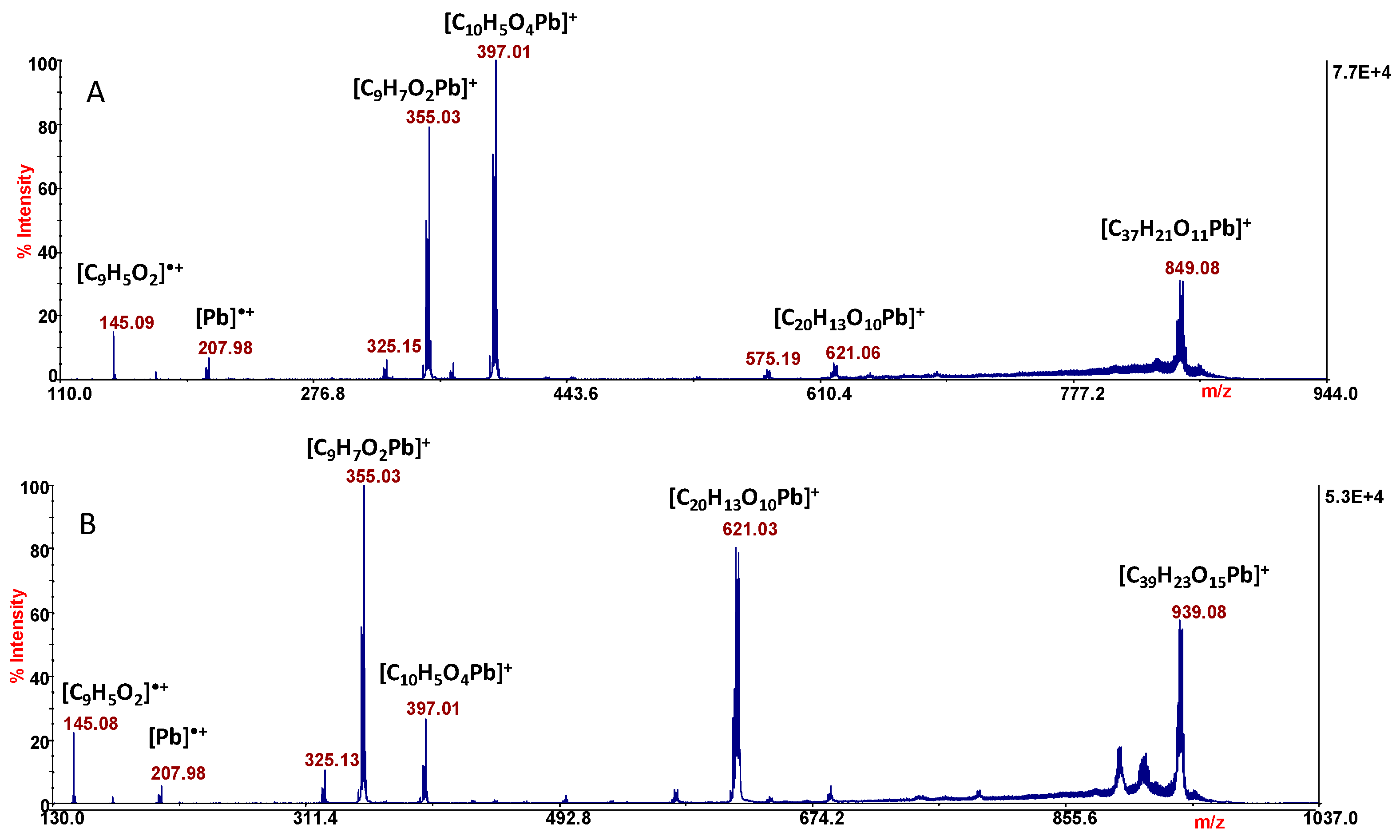
2.2. Mass Spectrometry Analysis
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Potentiometric Measurements
3.3. Mass Spectrometry Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, K.; Zeng, Z.; Tian, Q.; Huang, J.; Zhong, Q.; Huo, X. Epidemiological evidence for the effect of environmental heavy metal exposure on the immune system in children. Sci. Total Environ. 2023, 868, 161691. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Tan, S.H.; Lee, H.K. Extraction of lead ions by electromembrane isolation. J. Chrom. A 2008, 1213, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Koller, K.; Brown, T.; Spurgeon, A.; Levy, L. Recent Developments in Low-Level Lead Exposure and Intellectual Impairment in Children. Environ. Health Perspect. 2004, 112, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Shrivas, K.; Patel, D.K. Separation and preconcentration of trace level of lead in one drop of blood sample by using graphite furnace atomic absorption spectrometry. J. Hazard. Mater. 2010, 176, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Huang, W.-P. A Highly Selective Fluorescent Chemosensor for Lead Ions. J. Am. Chem. Soc. 2002, 124, 6246–6247. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Borsari, M.; Menabue, L.; Saladini, M.; Sola, M. Amide Group Coordination to the Pb2+ Ion. Inorg. Chem. 1996, 35, 4239–4247. [Google Scholar] [CrossRef]
- Kourgiantakis, M.; Matzapetakis, M.; Raptopoulou, C.P.; Terzis, A.; Salifoglou, A. Lead–citrate chemistry. Synthesis, spectroscopic and structural studies of a novel lead(II)–citrate aqueous complex. Inorg. Chim. Acta 2000, 297, 134–138. [Google Scholar] [CrossRef]
- Bryant, R.G.; Chacko, V.P.; Etter, M.C. 13C P/MAS NMR and crystallographic investigations of the structure and solid-state transformations of lead(II) acetate trihydrate. Inorg. Chem. 1984, 23, 3580–3584. [Google Scholar] [CrossRef]
- Harrison, P.G.; Steel, A.T. Lead(II) carboxylate structures. J. Organomet. Chem. 1982, 239, 105–113. [Google Scholar] [CrossRef]
- Matveev, E.Y.; Dontsova, O.S.; Avdeeva, V.V.; Kubasov, A.S.; Zhdanov, A.P.; Nikiforova, S.E.; Goeva, L.V.; Zhizhin, K.Y.; Malinina, E.A.; Kuznetsov, N.T. Synthesis and Structures of Lead(II) Complexes with Substituted Derivatives of the Closo-Decaborate Anion with a Pendant N3 Group. Molecules 2023, 28, 8073. [Google Scholar] [CrossRef]
- Karaliota, A.; Kretsi, O.; Tzougraki, C. Synthesis and characterization of a binuclear coumarin-3-carboxylate copper(II) complex. J. Inorg. Biochem. 2001, 84, 33–37. [Google Scholar] [CrossRef]
- Laskova, Y.N.; Serdyukov, A.A.; Sivaev, I.B. Boron-Containing Coumarins (Review). Russ. J. Inorg. Chem. 2023, 68, 621–643. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Georgieva, I.; Karcz, D.; McCann, M.; Trendafilova, N.; Walsh, M. Molecular structure and spectroscopic studies on novel complexes of coumarin-3-carboxylic acid with Ni(II), Co(II), Zn(II) and Mn(II) ions based on density functional theory. Spectrochim. Acta Part A 2011, 84, 275–285. [Google Scholar] [CrossRef]
- Al-Hazmy, S.M.; Zouaghi, M.O.; Al-Johani, J.N.; Arfaoui, Y.; Al-Ashwal, R.; Hammami, B.; Alhagri, I.A.; Alhemiary, N.A.; Hamdi, N. Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation. Molecules 2022, 27, 5921. [Google Scholar] [CrossRef]
- Wang, Y.H.; Avula, B.; Nanayakkara, N.P.D.; Zhao, J.; Khan, I.A. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef]
- Song, P.P.; Zhao, J.; Liu, Z.L.; Duan, Y.B.; Hou, Y.P.; Zhao, C.Q.; Wu, M.; Wei, M.; Wang, N.H.; Lv, Y.; et al. Evaluation of antifungal activities and structure–activity relationships of coumarin derivatives. Pest. Man. Sci. 2017, 73, 94–101. [Google Scholar] [CrossRef]
- Wang, T.; Peng, T.; We, X.; Wang, G.; Sun, Y.; Liu, S.; Zhang, S.; Wang, L. Design, synthesis and preliminary biological evaluation of benzylsulfone coumarin derivatives as anti-cancer agents. Molecules 2019, 24, 4034. [Google Scholar] [CrossRef]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini-Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylato silver(I) complexes. Inorg. Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef]
- de Alcantara, F.C.; Lozano, V.F.; Vale Velosa, A.S.; dos Santos, M.R.M.; Pereira, R.M.S. New coumarin complexes of Zn, Cu, Ni and Fe with antiparasitic activity. Polyhedron 2015, 101, 165–170. [Google Scholar] [CrossRef]
- de Souza, D.A.G.; Tranquilin, R.L.; dos Santos, M.L.; Pereira, R.M.S. Synthesis of Ni(II), Cu(II) and Zn(II) coumarin-3-carboxilic acid derivates and their physical-chemical properties. Res. Soc. Dev. 2021, 10, e47910313430. [Google Scholar] [CrossRef]
- Malacaria, L.; Bruno, R.; Corrente, G.A.; Armentano, D.; Furia, E.; Beneduci, A. Experimental insights on the coordination modes of coumarin-3-carboxilic acid towards Cr(III)-, Co(II)-, Ni(II)-, Cu(II)- and Zn(II): A detailed potentiometric and spectroscopic investigation in aqueous media. J. Mol. Liq. 2022, 346, 118302. [Google Scholar] [CrossRef]
- Klepka, M.T.; Drzewiecka-Antonik, A.; Wolska, A.; Rejmak, P.; Struga, M. Structural studies of Cu(II) complexes with obtained using direct and electrochemical synthesis coumarin acid derivatives. Chem. Phys. Lett. 2018, 691, 190–195. [Google Scholar] [CrossRef]
- Malacaria, L.; Corrente, G.A.; Furia, E. Thermodynamic Study on the Dissociation and Complexation of Coumarinic Acid with Neodymium(III) and Dioxouranium(VI) in Aqueous Media. Appl. Sci. 2021, 11, 4475. [Google Scholar] [CrossRef]
- Furia, E.; Beneduci, A.; Russo, N.; Marino, T. Structural characterization of aluminium(III) and iron(III) complexes of coumarinic acid in aqueous solution from combined experimental and theoretical investigations. New J. Chem. 2018, 42, 11006–11012. [Google Scholar] [CrossRef]
- Martin, R.B. Practical hardness scales for metal ion complexes. Inorg. Chim. Acta 2002, 339, 27–33. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Furia, E.; Aiello, D.; Di Donna, L.; Mazzotti, F.; Tagarelli, A.; Thangavel, H.; Napoli, A.; Sindona, G. Mass spectrometry and potentiometry studies of Pb(II)-, Cd(II)- and Zn(II)-cystine complexes. Dalton Trans. 2014, 43, 1055–1062. [Google Scholar] [CrossRef]
- Sillén, L.G. Some Graphical Methods for Determining Equilibrium Constants. II. On “Curve-fitting” Methods for Two-variable Data. Acta Chem. Scand. 1956, 10, 186–202. [Google Scholar] [CrossRef]
- Carnamucio, F.; Aiello, D.; Foti, C.; Napoli, A.; Giuffrè, O. Aqueous chemistry of nalidixic acid and its complexes with biological relevant cations: A combination of potentiometric, UV spectrophotometric, MS and MS/MS study. J. Inorg. Biochem. 2023, 249, 112366. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Aiello, D.; Cordaro, M.; Giuffrè, O.; Napoli, A.; Foti, C. Binding ability of L-carnosine towards Cu2+, Mn2+ and Zn2+ in aqueous solution. J. Mol. Liq. 2022, 368, 120772. [Google Scholar] [CrossRef]
- Zenobi, R.; Knochenmuss, R. Ion formation in MALDI mass spectrometry. Mass Spectrom. Rev. 1998, 17, 337–366. [Google Scholar] [CrossRef]
- Wyatt, M.F. MALDI-TOF MS analysis of coordination and organometallic complexes: A nic(h)e area to work in. J. Mass Spectrom. 2011, 46, 712–719. [Google Scholar] [CrossRef]
- Karas, M.; Krüger, R. Ion formation in MALDI: The cluster ionization mechanism. Chem. Rev. 2003, 103, 427–440. [Google Scholar]
- Lehmann, E.; Knochenmuss, R.; Zenobi, R. Ionization mechanisms in matrix-assisted laser desorption/ionization mass spectrometry: Contribution of pre-formed ions. Rapid Commun. Mass Spectrom. 1997, 11, 1483–1492. [Google Scholar] [CrossRef]
- Kruger, R.; Karas, M. Formation and Fate of Ion Pairs during MALDI Analysis: Anion Adduct Generation as an Indicative Tool to Determine Ionization Processes. J. Am. Soc. Mass Spectrom. 2002, 13, 1218–1226. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhou, L.H.; Zhao, S.K.; Deng, H.M. 3-Hydroxycoumarin as a New Matrix for Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry of DNA. J. Am. Soc. Mass Spectrom. 2006, 17, 1665–1668. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Dai, B.; Liu, B.; Lu, H. Coumarins as new matrices for matrix-assisted laser-desorption/ionization Fourier transform ion cyclotron resonance mass spectrometric analysis of hydrophobic compounds. Anal. Chim. Acta. 2015, 882, 49–57. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Funakoshi, N.; Takeyama, K.; Hioki, Y.; Nishikaze, T.; Kaneshiro, K.; Kawabata, S.; Iwamoto, S.; Tanaka, K. 3-Aminoquinoline/p-Coumaric Acid as a MALDI Matrix for Glycopeptides, Carbohydrates, and Phosphopeptides. Anal. Chem. 2014, 86, 1937–1942. [Google Scholar] [CrossRef]
- Saeed, A.; Ashraf, S.; Florke, U.; Delgado Espinoza, Z.Y.; Erben, M.F.; Perez, H. Supramolecular self-assembly of a coumarine-based acylthiourea synthon directed by p-stacking interactions: Crystal structure and Hirshfeld surface analysis. J. Mol. Struct. 2016, 1111, 76–83. [Google Scholar] [CrossRef]
- Deblonde, G.J.-P.; Lohrey, T.D.; An, D.D.; Abergel, R.J. Toxic heavy metal—Pb, Cd, Sn—Complexation by the octadentate hydroxypyridinonate ligand archetype 3,4,3-LI(1,2-HOPO). New J. Chem. 2018, 42, 7649. [Google Scholar] [CrossRef]
- O’Hair, R.A.J.; Khairallah, G.N. Gas Phase Ion Chemistry of Transition Metal Clusters: Production, Reactivity, and Catalysis. J. Clust. Sci. 2004, 15, 331–363. [Google Scholar] [CrossRef]
- Armentrout, P.B. The thermochemistry of adsorbates on transition metal cluster ions: Relationship to bulk-phase properties. Eur. J. Mass Spectrom. 2003, 9, 531. [Google Scholar] [CrossRef]
- Dietz, T.G.; Duncan, M.A.; Powers, D.E.; Smalley, R.E.J. Laser production of supersonic metal cluster beams. Chem. Phys. 1981, 74, 6511. [Google Scholar] [CrossRef]
- Bondybey, V.E.; English, J.H. Laser excitation spectra and lifetimes of Pb2 and Sn2 produced by YAG laser vaporization. J. Chem. Phys. 1982, 76, 2165. [Google Scholar] [CrossRef]
- McIndoe, J.S. Laser synthesis of transition metal clusters. Trans. Metal Chem. 2003, 28, 122. [Google Scholar] [CrossRef]
- Barman, S.; Rajesh, C.; Das, G.P.; Majumder, C. Structural and electronic properties of Snn−1Pb and Pbn−1Sn clusters. Eur. Phys. J. D 2009, 55, 613–625. [Google Scholar] [CrossRef]
- Waldschmidt, B.; Barman, S.; Rajesh, C.; Majumder, C.; Das, G.P.; Schafer, R. Energetics and fragmentation of single-doped tin and lead clusters. Phys. Rev. B 2009, 79, 045422. [Google Scholar] [CrossRef]
- Schafer, S.; Heiles, S.; Becker, J.A.; Shafer, R. Electric deflection studies on lead clusters. J. Chem. Phys. 2008, 129, 044304. [Google Scholar] [CrossRef]
- Giuffrè, O.; Aiello, D.; Chillè, D.; Napoli, A.; Foti, C. Binding ability of arsenate towards Cu2+ and Zn2+: Thermodynamic behavior and simulation under natural water conditions. Environ. Sci. Process. Impacts 2020, 22, 1731–1742. [Google Scholar] [CrossRef]

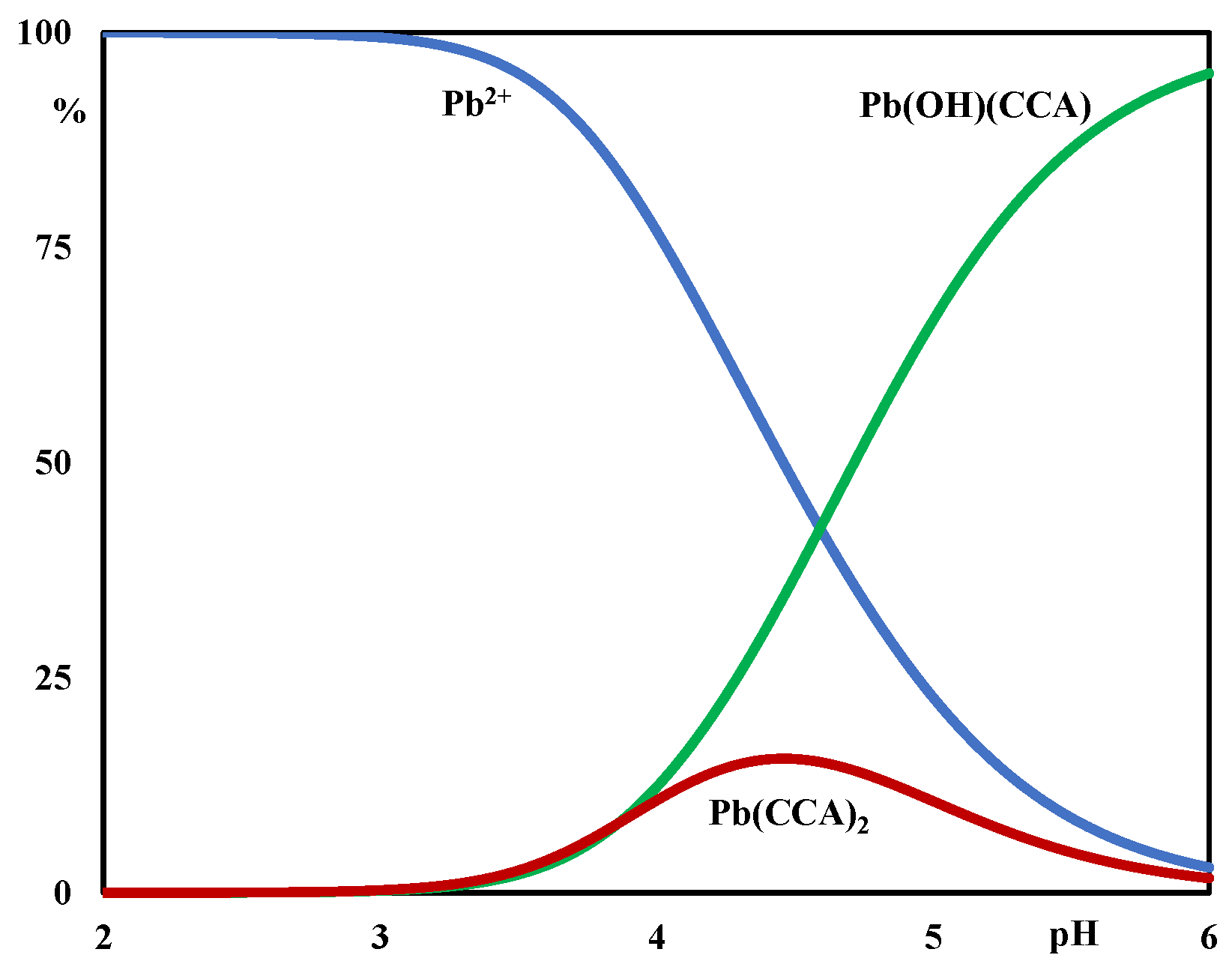


| (p,q,r) | Complexes | log βpqr ± 3σ |
|---|---|---|
| (1,1,1) | Pb(OH)(CCA) | 10.77 ± 0.01 |
| (1,0,2) | Pb(CCA)2 | 3.6 ± 0.2 |
| Species | m/z | Error (ppm) | Chemical Formula |
|---|---|---|---|
| [LH+K]+ | 228.99 | 3.5 | [C10H6O4K]+ |
| 200.99 | 3.6 | [C9H6O3K]+ | |
| [LH-CO2+K]+ | 184.98 | 3.1 | [C9H6O2K]+ |
| [2LH+K]+ | 419.02 | 3.1 | [C20H12O8K]+ |
| [2LH-CO2+K]+ | 375.03 | 3.0 | [C19H12O6K]+ |
| 347.03 | 3.5 | [C18H12O5K]+ | |
| [2LH-2CO2+K]+ | 331.04 | 3.9 | [C18H12O4K]+ |
| 323.09 | 4.0 | [C19H15O5]+ | |
| 303.04 | 3.8 | [C17H12O3K]+ | |
| 275.05 | 3.5 | [C16H12O2K]+ | |
| [3LH+K]+ | 609.05 | 5.0 | [C30H18O12K]+ |
| [3LH-CO2+K]+ | 565.05 | 3.1 | [C29H18O10K]+ |
| 537.05 | 3.4 | [C28H18O9K]+ | |
| [3LH-2CO2+K]+ | 521.06 | 3.5 | [C28H18O8K]+ |
| [3LH-3CO2+K]+ | 477.07 | 3.5 | [C27H18O6K]+ |
| [4LH-CO2+K]+ | 755.08 | 3.9 | [C39H24O14K]+ |
| 647.12 | 3.6 | [C36H23O12]+ | |
| 587.13 | 3.1 | [C35H23O9]+ |
| m/z | Error (ppm) | Chemical Formula |
|---|---|---|
| 466.92 | 3.0 | [OClPb2]+ |
| 530.90 | 3.1 | [O5ClPb2]+ |
| 690.89 | 3.4 | [O2ClPb3]+ |
| 754.91 | 3.0 | [H3O8Pb3]+ |
| 914.91 | 3.5 | [H3O5Pb4]+ |
| m/z | Error (ppm) | Chemical Formula |
|---|---|---|
| 983.08 | 3.5 | [C40H23O17Pb]+ |
| 981.06 | 3.7 | [C40H21O17Pb]+ |
| 893.07 | 3.7 | [C38H21O13Pb]+ |
| 891.06 | 3.8 | [C38H19O13Pb]+ |
| MS/MS Fragments | ||
| m/z | Error (ppm) | Chemical Formula |
| 939.08 | 4.1 | [C39H23O15Pb]+ |
| 849.08 | 3.9 | [C37H21O11Pb]+ |
| 621.03 | 3.8 | [C20H13O10Pb]+ |
| 397.01 | 4.0 | [C10H5O4Pb]+ |
| 355.03 | 4.2 | [C9H7O2Pb]+ |
| 207.98 | 3.7 | [Pb]•+ |
| 145.03 | 4.0 | [C9H5O2]•+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furia, E.; Lettera, V.; Napoli, A.; Aiello, D. Insights on Stability Constants and Structures of Complexes between Coumarin Derivatives and Pb(II) in Aqueous Media. Molecules 2024, 29, 1911. https://doi.org/10.3390/molecules29091911
Furia E, Lettera V, Napoli A, Aiello D. Insights on Stability Constants and Structures of Complexes between Coumarin Derivatives and Pb(II) in Aqueous Media. Molecules. 2024; 29(9):1911. https://doi.org/10.3390/molecules29091911
Chicago/Turabian StyleFuria, Emilia, Vincenzo Lettera, Anna Napoli, and Donatella Aiello. 2024. "Insights on Stability Constants and Structures of Complexes between Coumarin Derivatives and Pb(II) in Aqueous Media" Molecules 29, no. 9: 1911. https://doi.org/10.3390/molecules29091911
APA StyleFuria, E., Lettera, V., Napoli, A., & Aiello, D. (2024). Insights on Stability Constants and Structures of Complexes between Coumarin Derivatives and Pb(II) in Aqueous Media. Molecules, 29(9), 1911. https://doi.org/10.3390/molecules29091911








