Effect of Particle Heterogeneity in Catalytic Copper-Containing Single-Chain Polymeric Nanoparticles Revealed by Single-Particle Kinetics
Abstract
1. Introduction
2. Results
2.1. Design, Synthesis, and Characterization of Cu(I) SCPNs
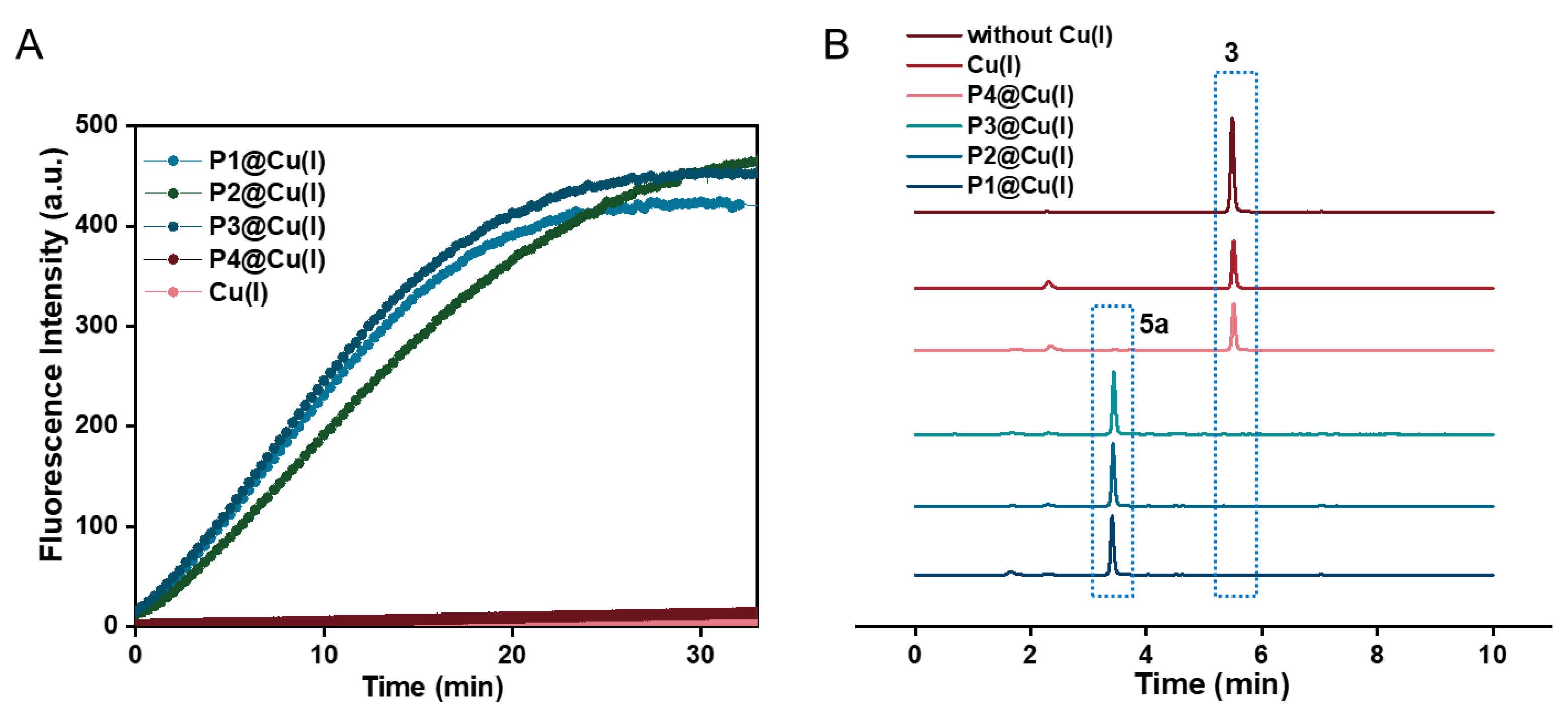
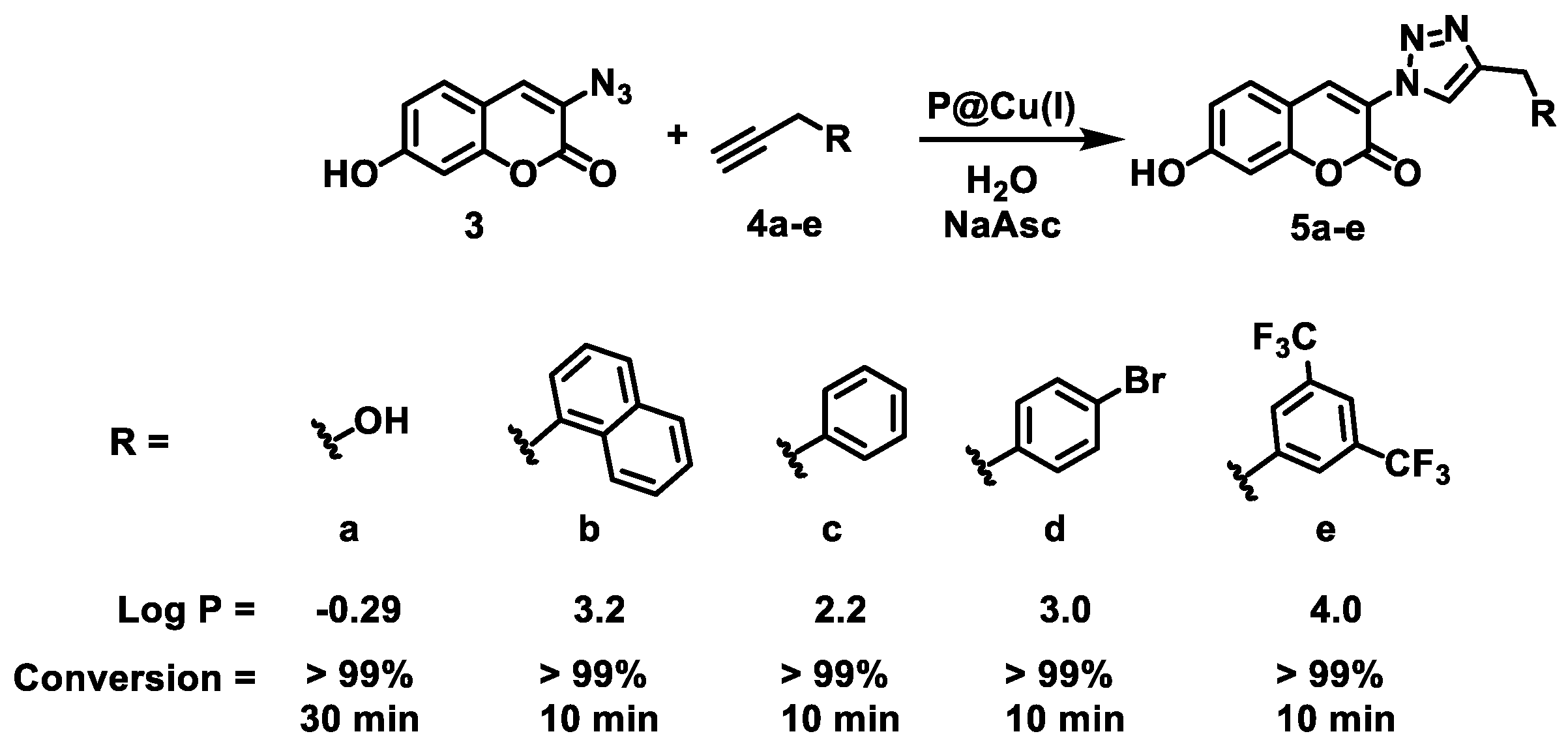
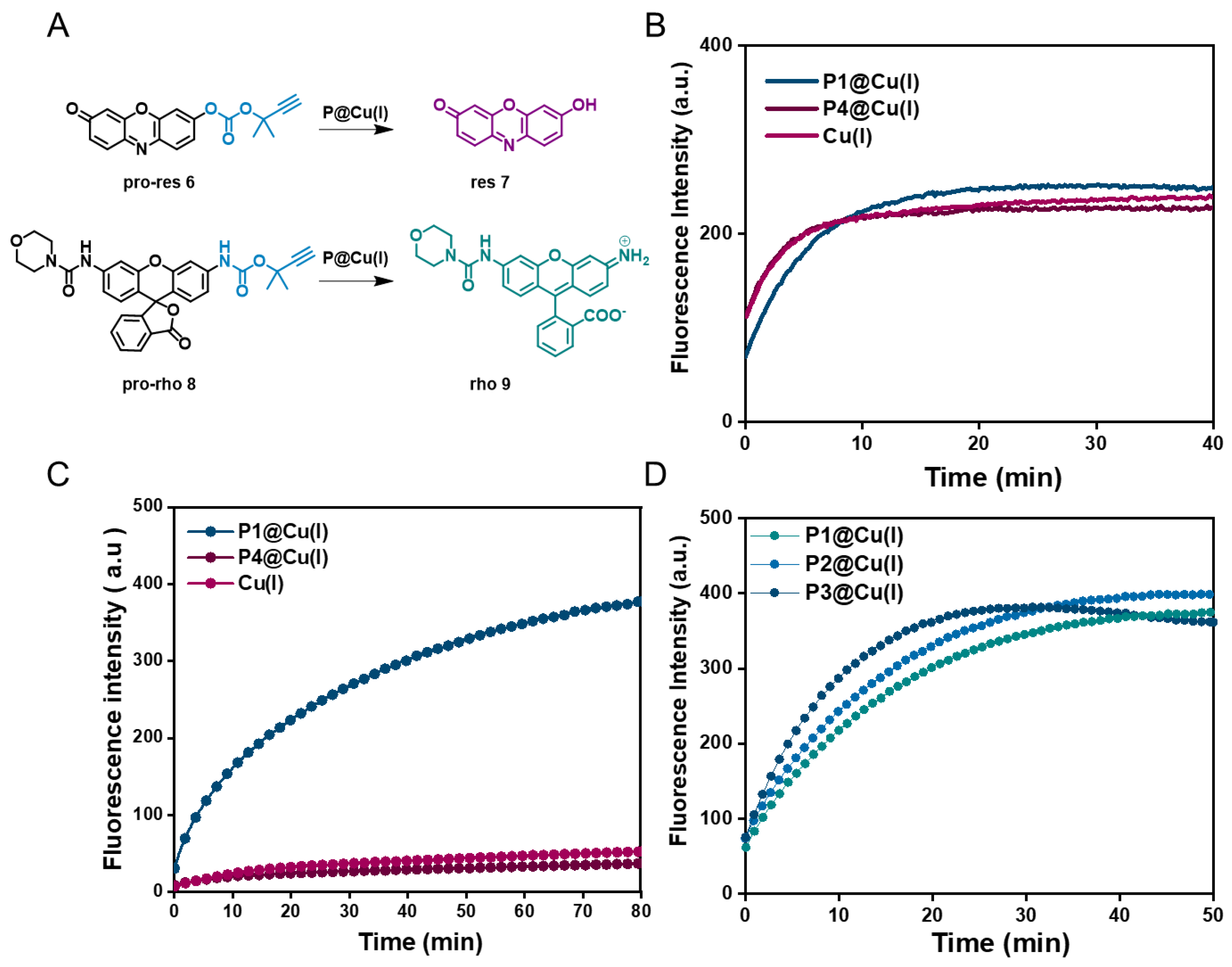
2.2. Catalytic Efficiency of Cu(I) SCPNs—Depropargylation and CuAAC Reactions
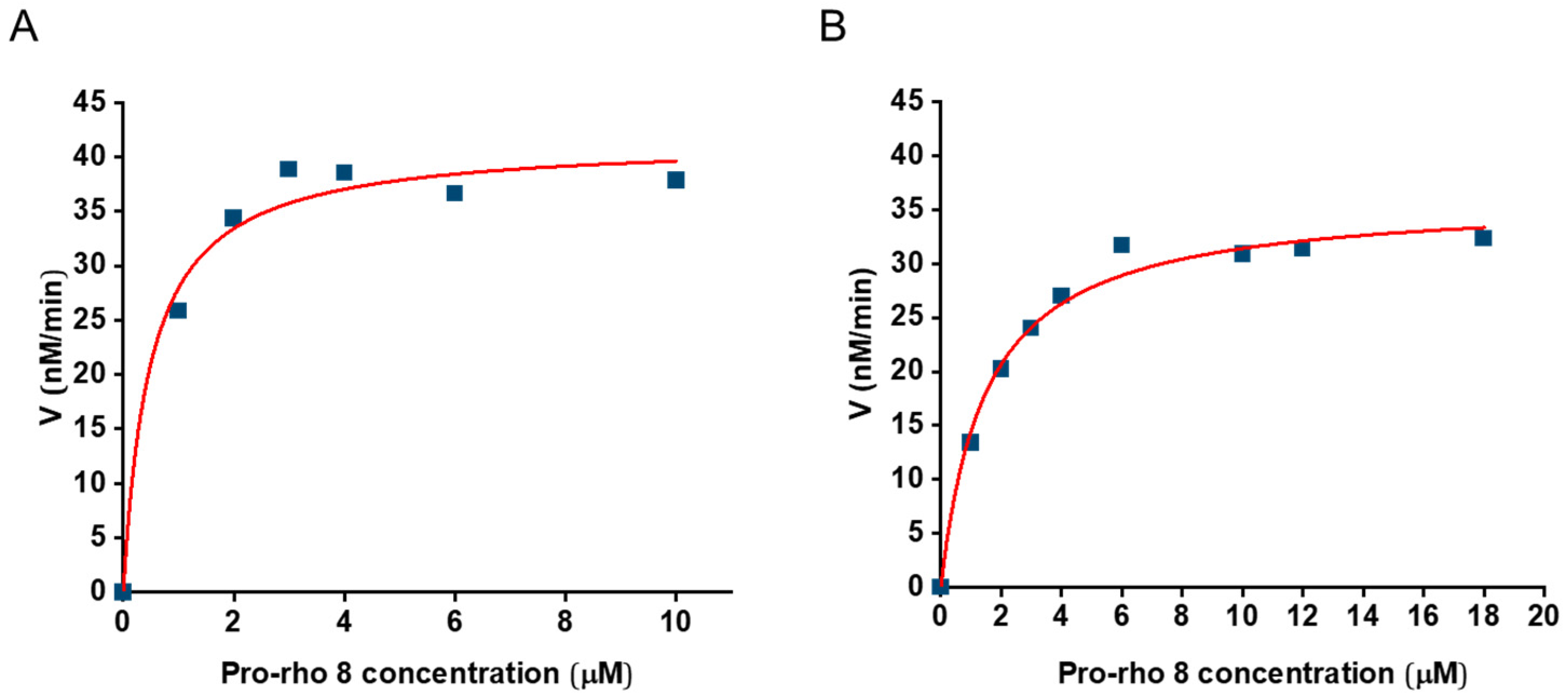
2.3. Cu(I) SCPNs Enzyme Kinetics—Michaelis–Menten Model
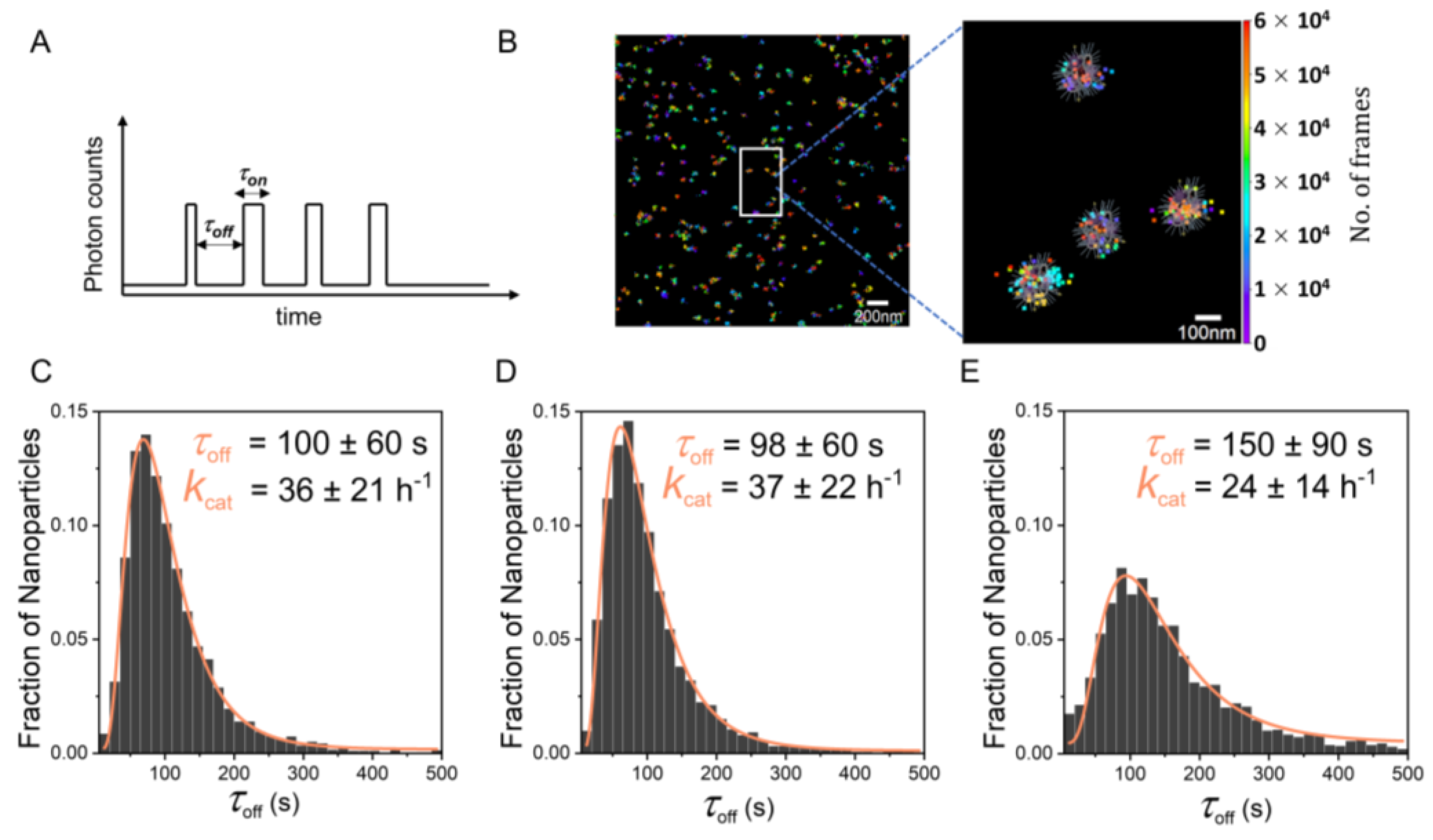
2.4. Single-Particle Kinetics Using Single-Molecule Fluorescence Microscopy
3. Discussion and Conclusions
4. Materials and Methods
4.1. Materials and Instruments
4.2. Pro-Dye and Polymer Synthesis
4.2.1. Pro-Res 6 (2-Methylbut-3-yn-2-yl (3-oxo-3H-Phenoxazin-7-yl) Carbonate)
- Yield = 15 mg, 6%. 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.7 Hz, 1H), 7.43 (d, J = 9.8 Hz, 1H), 7.29 (s, 1H), 7.23 (d, J = 2.5 Hz, 1H), 6.87 (dd, J = 9.8, 2.0 Hz, 1H), 6.33 (d, J = 2.0 Hz, 1H), 2.65 (s, 1H), 1.82 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 144.34, 135.22, 134.80, 131.15, 118.64, 109.13, 107.30, 83.07, 77.21, 76.05, 73.92, 28.65. FT-IR (ATR): v (cm−1) 3290, 3048, 2994, 1772, 1623, 1608, 1572, 1515, 1246, 1220, 1196, 1129, 885, 848, 772, 667. MALDI-TOF-MS: m/z calc: 323.0; found: 323 [M]−, deprotected product resorufin 212 [M − H]−.
4.2.2. Post-Functionalization of p-PFPA180 to P1a–P3a
- Yield: 380 mg, Mtheoretical = 161 kD, Mn, SEC-DMF = 59.1 kD, Ð = 1.21. 1H NMR (400 MHz, CDCl3) δ 6.92–6.01 (br), 4.15–3.90 (br), 3.84–3.14 (m), 1.90–1.82 (br), 1.72–1.52 (s), 1.41–0.93 (m). FT-IR (ATR): v(cm−1) 3520.56, 2865.86, 2096.31, 1650.15, 1544.45, 1454.07, 1348.63, 1325.4, 1296.42, 1249.42, 1096.62, 947.46, 848.32, 522.91. Mtheoretical = 161 kD, Mn, SEC-DMF = 59.1 kD, Ð = 1.21.
- P2a: p-PFPA180 (180 mg, 1 eq, 0.0042 mmol), 12-azidododecan-1-amine (34 mg, 36 eq, 0,15 mmol), BTA amine (25 mg, 9 eq, 0.037 mmol), biotinylated PEG amine (57 mg, 6 eq, 0.025 mmol), and Jeffamine@1000 (890 mg, 212 eq, 0.8 mmol). The polymer was dried under vacuum at 50 °C to yield a colorless solid and was stored at −19 °C. 1H NMR (400 MHz, CDCl3) δ 8.51–8.39 (br), 6.86–6.32 (br), 4.18–3.13 (m), 1.91–1.51 (m), 1.41–1.04 (m). FT-IR (ATR): v (cm−1) 3519.56, 2866.02, 2096.51, 1650.73, 1543.94, 1454.53, 1348.76, 1325.35, 1294.99, 1249.98, 1094.69, 947.35, 848.13, 523.42. Mtheoretical = 158 kD, Mn, SEC-DMF = 44.3 kD, Ð = 1.20.
- P3a: p-PFPA180 (150 mg, 1 eq, 0.0031 mmol), 12-azidododecan-1-amine (25 mg, 36 eq, 0,11 mmol), dodecyl amine (15.4 mg, 27 eq, 0.083 mmol), biotinylated PEG amine (47 mg, 6 eq, 0.018 mmol), and Jeffamine@1000 (657 mg, 212 eq, 0.65 mmol). The polymer was dried under vacuum at 50 °C to yield a colorless solid and was stored at −19 °C. 1H NMR (400 MHz, CDCl3) δ 6.86–6.13 (br), 4.27–3.95 (br), 3.87–3.13 (m), 1.94–0.82 (m). FT-IR (ATR): v (cm−1): 3301.87, 2863.67, 2096.15, 1649.47, 1540.03, 1454.47, 1346.79, 1324.91, 1295.06, 1249.38, 1199.43, 1098.22, 1039.84, 947.47, 845.32, 522.96. Mtheoretical = 165 kD, Mn,SEC-DMF = 47.5 kD, Ð = 1.25.
4.2.3. Incorporation of the diyne 2 to P1–P3
- P1: 1H NMR (400 MHz, D2O) δ 8.45–8.40 (br), 8.22–8.16 (m), 4.85–4.81 (d), 3.91–3.27 (m), 2.12–1.76 (s), 1.27–0.95 (br). FT-IR (ATR): v (cm−1): 3504.07, 2866.26, 1648.32, 1542.17, 1453.42, 1348.53, 1297.19, 1249.3, 1094.89, 946.76, 845.98. Mtheoretical = 164 kD, Mn, SEC-PBS = 15.5 kD, Ð = 1.38.
- P2: 1H NMR (400 MHz, D2O) δ 8.47–8.43 (br), 8.23–8.14 (m), 4.85–4.81 (s), 3.81–3.29 (m), 1.31–0.99 (br). FT-IR (ATR): v (cm−1): 3437.19, 2867.07, 1647.18, 1544.54, 1452.73, 1348.65, 1294.81, 1249.96, 1094.27, 947.04, 846.59, 521.88. Mtheoretical = 161 kD, Mn, SEC-PBS = 23.4 kD, Ð = 1.35.
- P3: 1H NMR (400 MHz, D2O) δ 8.41–8.37 (br), 8.22–8.16 (m), 4.85–4.81 (s), 4.53–4.35 (s), 3.92–3.27 (m), 1.36–1.12 (br). FT-IR (ATR): v (cm−1): 3436.86, 2869.53, 1646.34, 1548.24, 1454.48, 1348.8, 1296.07, 1250.18, 1091.99, 947.74, 844.89, 805.17, 523.09. Mtheoretical = 168 kD, Mn, SEC-PBS = 16.3 kD, Ð = 1.35.
4.3. Ensemble Catalysis Measurements
4.4. Depropargylation Reactions
4.5. Michaelis–Menten Kinetics
4.6. Single-Particle Kinetic Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssen, K.P.F.; De Cremer, G.; Neely, R.K.; Kubarev, A.V.; Van Loon, J.; Martens, J.A.; De Vos, D.E.; Roeffaers, M.B.J.; Hofkens, J. Single Molecule Methods for the Study of Catalysis: From Enzymes to Heterogeneous Catalysts. Chem. Soc. Rev. 2014, 43, 990–1006. [Google Scholar] [CrossRef]
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme Mimics: Advances and Applications. Chem. Eur. J. 2016, 22, 8404–8430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, R.; Gopalakrishnan, S.; Cao-Milán, R.; Rotello, V.M. Bioorthogonal Nanozymes: Progress towards Therapeutic Applications. Trends Chem. 2019, 1, 90–98. [Google Scholar] [CrossRef]
- Fedeli, S.; Im, J.; Gopalakrishnan, S.; Elia, J.L.; Gupta, A.; Kim, D.; Rotello, V.M. Nanomaterial-Based Bioorthogonal Nanozymes for Biological Applications. Chem. Soc. Rev. 2021, 50, 13467–13480. [Google Scholar] [CrossRef]
- Garcia, E.S.; Xiong, T.M.; Lifschitz, A.; Zimmerman, S.C. Tandem Catalysis Using an Enzyme and a Polymeric Ruthenium-Based Artificial Metalloenzyme. Polym. Chem. 2021, 12, 6755–6760. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, K.; Wang, Y.; Gruebele, M.; Ferguson, A.L.; Zimmerman, S.C. Polymeric “Clickase” Accelerates the Copper Click Reaction of Small Molecules, Proteins, and Cells. J. Am. Chem. Soc. 2019, 141, 9693–9700. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Bai, Y.; Li, K.; Garcia, E.S.; Ferguson, A.L.; Zimmerman, S.C. Enzyme-like Click Catalysis by a Copper-Containing Single-Chain Nanoparticle. J. Am. Chem. Soc. 2018, 140, 13695–13702. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Feng, X.; Xing, H.; Xu, Y.; Kim, B.K.; Baig, N.; Zhou, T.; Gewirth, A.A.; Lu, Y.; Oldfield, E.; et al. A Highly Efficient Single-Chain Metal-Organic Nanoparticle Catalyst for Alkyne-Azide “Click” Reactions in Water and in Cells. J. Am. Chem. Soc. 2016, 138, 11077–11080. [Google Scholar] [CrossRef]
- Liu, Y.; Pujals, S.; Stals, P.J.M.; Paulöhrl, T.; Presolski, S.I.; Meijer, E.W.; Albertazzi, L.; Palmans, A.R.A. Catalytically Active Single-Chain Polymeric Nanoparticles: Exploring Their Functions in Complex Biological Media. J. Am. Chem. Soc. 2018, 140, 3423–3433. [Google Scholar] [CrossRef]
- Cole, J.P.; Hanlon, A.M.; Rodriguez, K.J.; Berda, E.B. Protein-like Structure and Activity in Synthetic Polymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 191–206. [Google Scholar] [CrossRef]
- Huerta, E.; Stals, P.J.M.; Meijer, E.W.; Palmans, A.R.A. Consequences of Folding a Water-Soluble Polymer Around an Organocatalyst. Angew. Chem. 2013, 125, 2978–2982. [Google Scholar] [CrossRef]
- Liu, Y.; Turunen, P.; De Waal, B.F.M.; Blank, K.G.; Rowan, A.E.; Palmans, A.R.A.; Meijer, E.W. Catalytic Single-Chain Polymeric Nanoparticles at Work: From Ensemble towards Single-Particle Kinetics. Mol. Syst. Des. Eng. 2018, 3, 609–618. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule Plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef]
- Elenko, M.P.; Szostak, J.W.; Van Oijen, A.M. Single-Molecule Imaging of an in Vitro-Evolved RNA Aptamer Reveals Homogeneous Ligand Binding Kinetics. J. Am. Chem. Soc. 2009, 131, 9866–9867. [Google Scholar] [CrossRef]
- Leake, M.C. The Physics of Life: One Molecule at a Time. Philos. Trans. R. Soc. B 2013, 368, 20120248. [Google Scholar] [CrossRef][Green Version]
- Leake, M.C.; Greene, N.P.; Godun, R.M.; Granjon, T.; Buchanan, G.; Chen, S.; Berry, R.M.; Palmer, T.; Berks, B.C. Variable Stoichiometry of the TatA Component of the Twin-Arginine Protein Transport System Observed by in Vivo Single-Molecule Imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 15376–15381. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.P.; Xun, L.; Xie, X.S. Single-Molecule Enzymatic Dynamics. Science 1998, 282, 1877–1882. [Google Scholar] [CrossRef]
- Wang, Y. A Plasmonic Nanotorch: Pushing Plasmon-Enhanced Fluorescence for Applications in Single-Molecule Enzymology. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2020. [Google Scholar]
- Turunen, P.; Rowan, A.E.; Blank, K. Single-Enzyme Kinetics with Fluorogenic Substrates: Lessons Learnt and Future Directions. FEBS Lett. 2014, 588, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kong, J.S.; Yeh, Y.T.E.; Chen, P. Single-Molecule Nanocatalysis Reveals Heterogeneous Reaction Pathways and Catalytic Dynamics. Nat. Mater. 2008, 7, 992–996. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-Dependent Catalytic Activity and Dynamics of Gold Nanoparticles at the Single-Molecule Level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Zhou, X.; Andoy, N.M.; Liu, G.; Choudhary, E.; Han, K.S.; Shen, H.; Chen, P. Quantitative Super-Resolution Imaging Uncovers Reactivity Patterns on Single Nanocatalysts. Nat. Nanotechnol. 2012, 7, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dong, B.; Chen, K.; Zhao, F.; Cheng, X.; Ma, C.; Lee, S.; Zhang, P.; Kang, S.H.; Ha, J.W.; et al. Optical Super-Resolution Imaging of Surface Reactions. Chem. Rev. 2017, 117, 7510–7537. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Xu, W. Single-Molecule Nanocatalysis Reveals Catalytic Activation Energy of Single Nanocatalysts. J. Am. Chem. Soc. 2016, 138, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Archontakis, E.; Deng, L.; Zijlstra, P.; Palmans, A.R.A.; Albertazzi, L. Spectrally PAINTing a Single Chain Polymeric Nanoparticle at Super-Resolution. J. Am. Chem. Soc. 2022, 144, 23698–23707. [Google Scholar] [CrossRef] [PubMed]
- Sathyan, A.; Croke, S.; Pérez-López, A.M.; de Waal, B.F.M.; Unciti-Broceta, A.; Palmans, A.R.A. Developing Pd(II) Based Amphiphilic Polymeric Nanoparticles for pro-Drug Activation in Complex Media. Mol. Syst. Des. Eng. 2022, 7, 1736–1748. [Google Scholar] [CrossRef]
- Deng, L.; Sathyan, A.; Adam, C.; Unciti-Broceta, A.; Sebastian, V.; Palmans, A.R.A. Enhanced Efficiency of Pd(0)-Based Single Chain Polymeric Nanoparticles for in Vitro Prodrug Activation by Modulating the Polymer’s Microstructure. Nano. Lett. 2024, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Lozhkin, B.; Ward, T.R. Bioorthogonal Strategies for the in Vivo Synthesis or Release of Drugs. Bioorg. Med. Chem. 2021, 45, 116310. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Zhang, B.; Wang, L.; Huang, S.; Wang, S.; You, Y.; Zhu, G.; Zhu, A.; Geng, M.; Li, L. An Efficient and Easily-Accessible Ligand for Cu(I)-Catalyzed Azide–Alkyne Cycloaddition Bioconjugation. Chem. Commun. 2020, 56, 14401–14403. [Google Scholar] [CrossRef] [PubMed]
- Huerta, E.; Van Genabeek, B.; Stals, P.J.M.; Meijer, E.W.; Palmans, A.R.A. A Modular Approach to Introduce Function into Single-Chain Polymeric Nanoparticles. Macromol. Rapid Commun. 2014, 35, 1320–1325. [Google Scholar] [CrossRef]
- ter Huurne, G.M.; de Windt, L.N.J.; Liu, Y.; Meijer, E.W.; Voets, I.K.; Palmans, A.R.A. Improving the Folding of Supramolecular Copolymers by Controlling the Assembly Pathway Complexity. Macromolecules 2017, 50, 8562–8569. [Google Scholar] [CrossRef]
- Wijker, S.; Deng, L.; Eisenreich, F.; Voets, I.K.; Palmans, A.R.A. En Route to Stabilized Compact Conformations of Single-Chain Polymeric Nanoparticles in Complex Media. Macromolecules 2022, 55, 6220–6230. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, V.O.; Presolski, S.I.; Díaz, D.D.; Fokin, V.V.; Finn, M.G. Ligand-Accelerated Cu-Catalyzed Azide-Alkyne Cycloaddition: A Mechanistic Report. J. Am. Chem. Soc. 2007, 129, 12705–12712. [Google Scholar] [CrossRef] [PubMed]
- English, B.P.; Min, W.; Van Oijen, A.M.; Kang, T.L.; Luo, G.; Sun, H.; Cherayil, B.J.; Kou, S.C.; Xie, X.S. Ever-Fluctuating Single Enzyme Molecules: Michaelis-Menten Equation Revisited. Nat. Chem. Biol. 2005, 2, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kong, J.S.; Chen, P. Single-Molecule Kinetic Theory of Heterogeneous and Enzyme Catalysis. J. Phys. Chem. C 2009, 113, 2393–2404. [Google Scholar] [CrossRef]
- Ye, R.; Mao, X.; Sun, X.; Chen, P. Analogy between Enzyme and Nanoparticle Catalysis: A Single-Molecule Perspective. ACS Catal. 2019, 9, 1985–1992. [Google Scholar] [CrossRef]
- Latocheski, E.; Dal Forno, G.M.; Ferreira, T.M.; Oliveira, B.L.; Bernardes, G.J.L.; Domingos, J.B.; Forno, G.M.D.; Ferreira, T.M.; Oliveira, B.L.; Bernardes, G.J.L.; et al. Mechanistic Insights into Transition Metal-Mediated Bioorthogonal Uncaging Reactions. Chem. Soc. Rev. 2020, 49, 7710–7729. [Google Scholar] [CrossRef]
- Shieh, P.; Dien, V.T.; Beahm, B.J.; Castellano, J.M.; Wyss-Coray, T.; Bertozzi, C.R. CalFluors: A Universal Motif for Fluorogenic Azide Probes across the Visible Spectrum. J. Am. Chem. Soc. 2015, 137, 7145–7151. [Google Scholar] [CrossRef]
- Sathyan, A.; Loman, T.; Deng, L.; Palmans, A.R.A. Amphiphilic Polymeric Nanoparticles Enable Homogenous Rhodium-Catalysed NH Insertion Reactions in Living Cells. Nanoscale 2023, 15, 12710–12717. [Google Scholar] [CrossRef]
- Leenders, C.M.A.; Jansen, G.; Frissen, M.M.M.; Lafleur, R.P.M.; Voets, I.K.; Palmans, A.R.A.; Meijer, E.W. Monosaccharides as Versatile Units for Water-Soluble Supramolecular Polymers. Chem. Eur. J. 2016, 22, 4608–4615. [Google Scholar] [CrossRef]

| Polymer | a | b | c | d | e | Ð | Mn, SEC (kDa) | Mn, theoretical (kDa) | RH (nm) |
|---|---|---|---|---|---|---|---|---|---|
| pPFPA180 | 1.19 x | 30.2 x | 42 | - | |||||
| P1a | 3 | 18 | - | - | 71 | 1.21 y | 59.1 y | 161 | 4.3 |
| P2a | 3 | 18 | - | 5 | 69 | 1.20 y | 44.3 y | 158 | 5.0 |
| P3a | 3 | 18 | 12 | - | 67 | 1.25 y | 47.5 y | 165 | 5.0 |
| P1 | 3 | 18 | - | - | 71 | 1.38 z | 15.5 z | 164 | 3.4 |
| P2 | 3 | 18 | - | 5 | 69 | 1.35 z | 23.4 z | 161 | 4.9 |
| P3 | 3 | 18 | 12 | - | 67 | 1.35 z | 16.3 z | 168 | 4.8 |
| P4 | - | - | 20 | - | 80 | 1.16 y | 24.4 y | 181.0 | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathyan, A.; Archontakis, E.; Spiering, A.J.H.; Albertazzi, L.; Palmans, A.R.A. Effect of Particle Heterogeneity in Catalytic Copper-Containing Single-Chain Polymeric Nanoparticles Revealed by Single-Particle Kinetics. Molecules 2024, 29, 1850. https://doi.org/10.3390/molecules29081850
Sathyan A, Archontakis E, Spiering AJH, Albertazzi L, Palmans ARA. Effect of Particle Heterogeneity in Catalytic Copper-Containing Single-Chain Polymeric Nanoparticles Revealed by Single-Particle Kinetics. Molecules. 2024; 29(8):1850. https://doi.org/10.3390/molecules29081850
Chicago/Turabian StyleSathyan, Anjana, Emmanouil Archontakis, A. J. H. Spiering, Lorenzo Albertazzi, and Anja R. A. Palmans. 2024. "Effect of Particle Heterogeneity in Catalytic Copper-Containing Single-Chain Polymeric Nanoparticles Revealed by Single-Particle Kinetics" Molecules 29, no. 8: 1850. https://doi.org/10.3390/molecules29081850
APA StyleSathyan, A., Archontakis, E., Spiering, A. J. H., Albertazzi, L., & Palmans, A. R. A. (2024). Effect of Particle Heterogeneity in Catalytic Copper-Containing Single-Chain Polymeric Nanoparticles Revealed by Single-Particle Kinetics. Molecules, 29(8), 1850. https://doi.org/10.3390/molecules29081850






