Novel Withanolides from Tubocapsicum anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and p53-ASCT2-SLC7A11-Mediated Ferroptosis
Abstract
1. Introduction
2. Results and Discussion
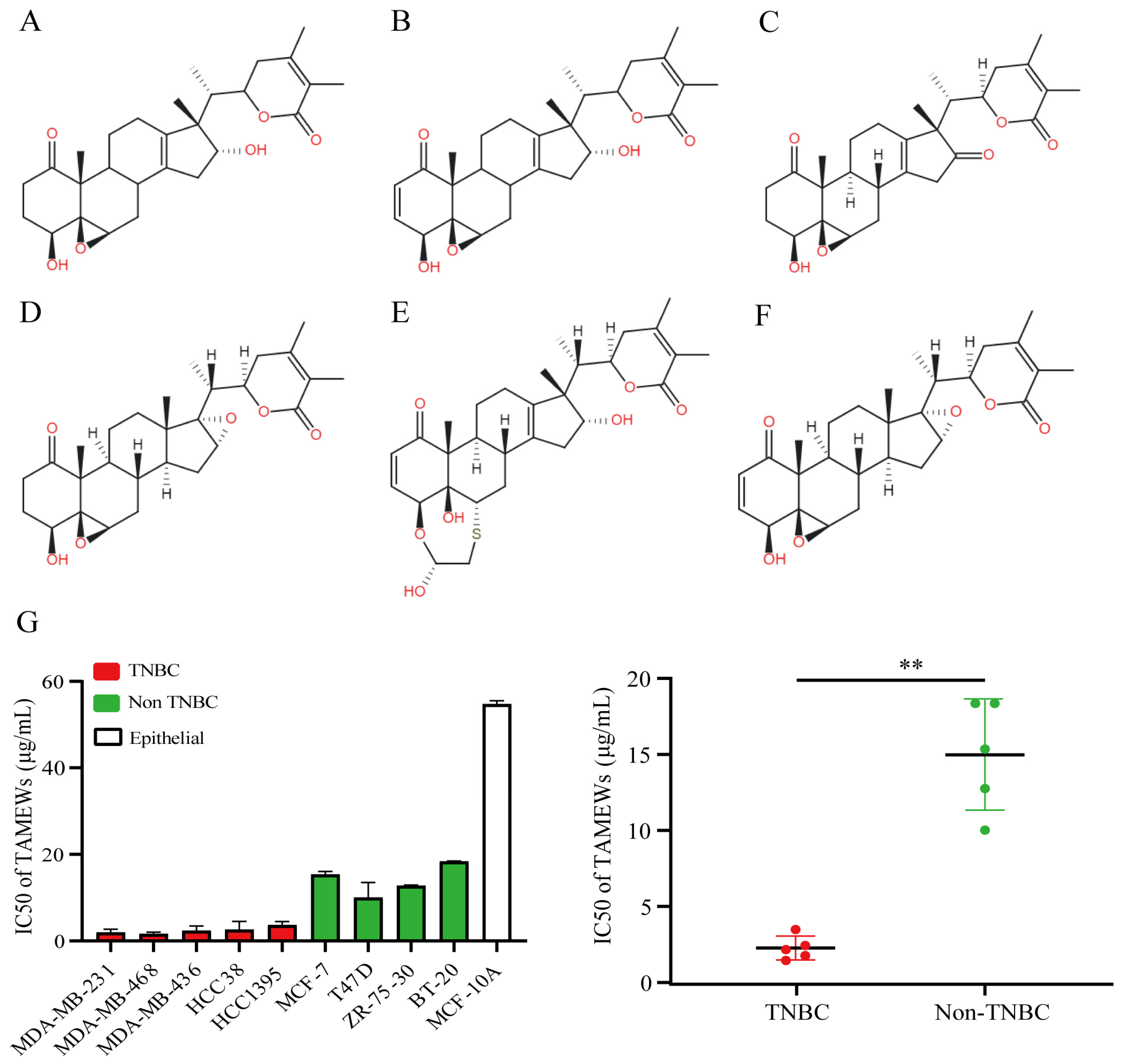
2.1. TAMEWs Suppresses the Viability of TNBC Cells In Vitro
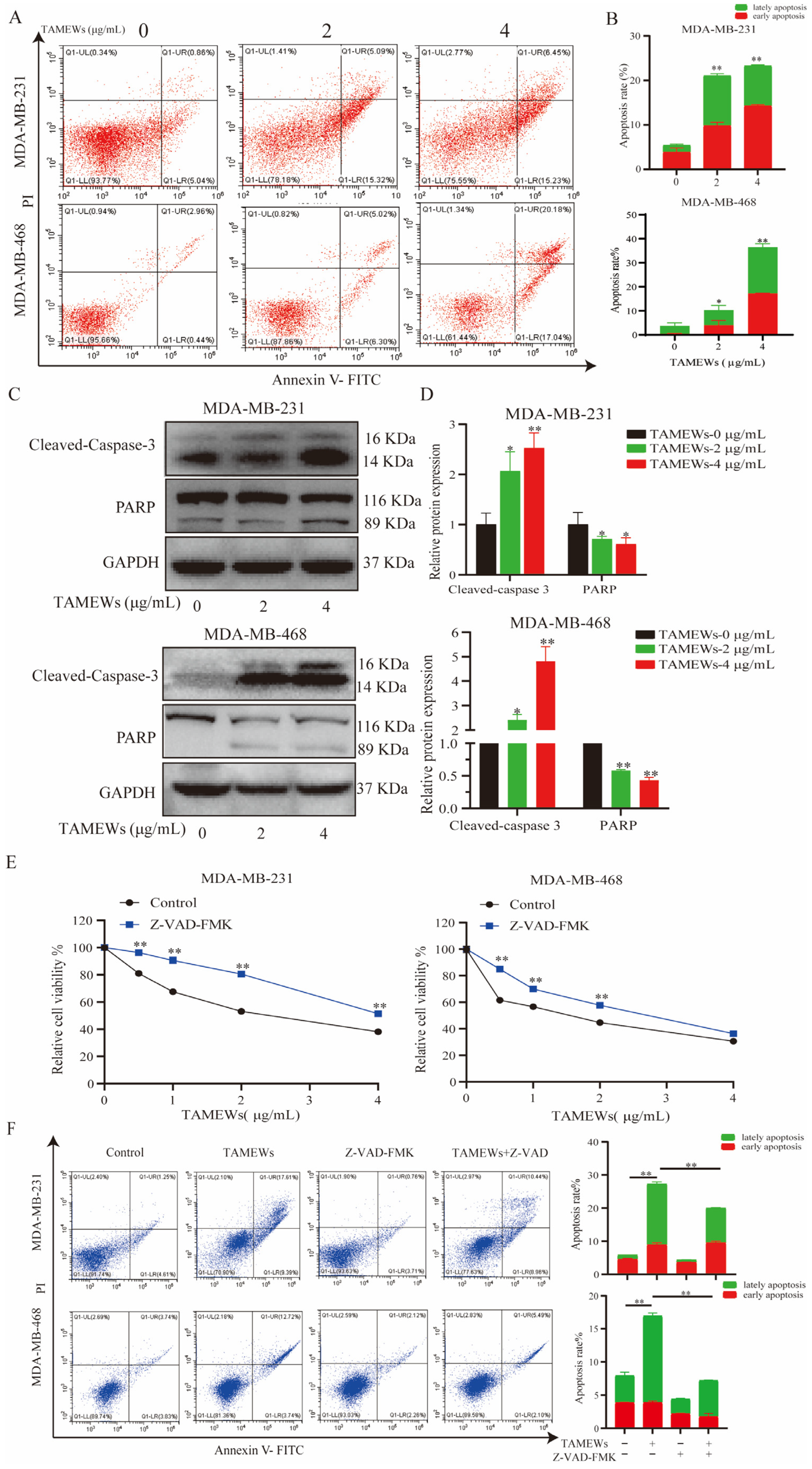
2.2. TNBC Cell Inhibition by TAMEWs Might Be via Apoptosis and the Ferroptosis Pathway
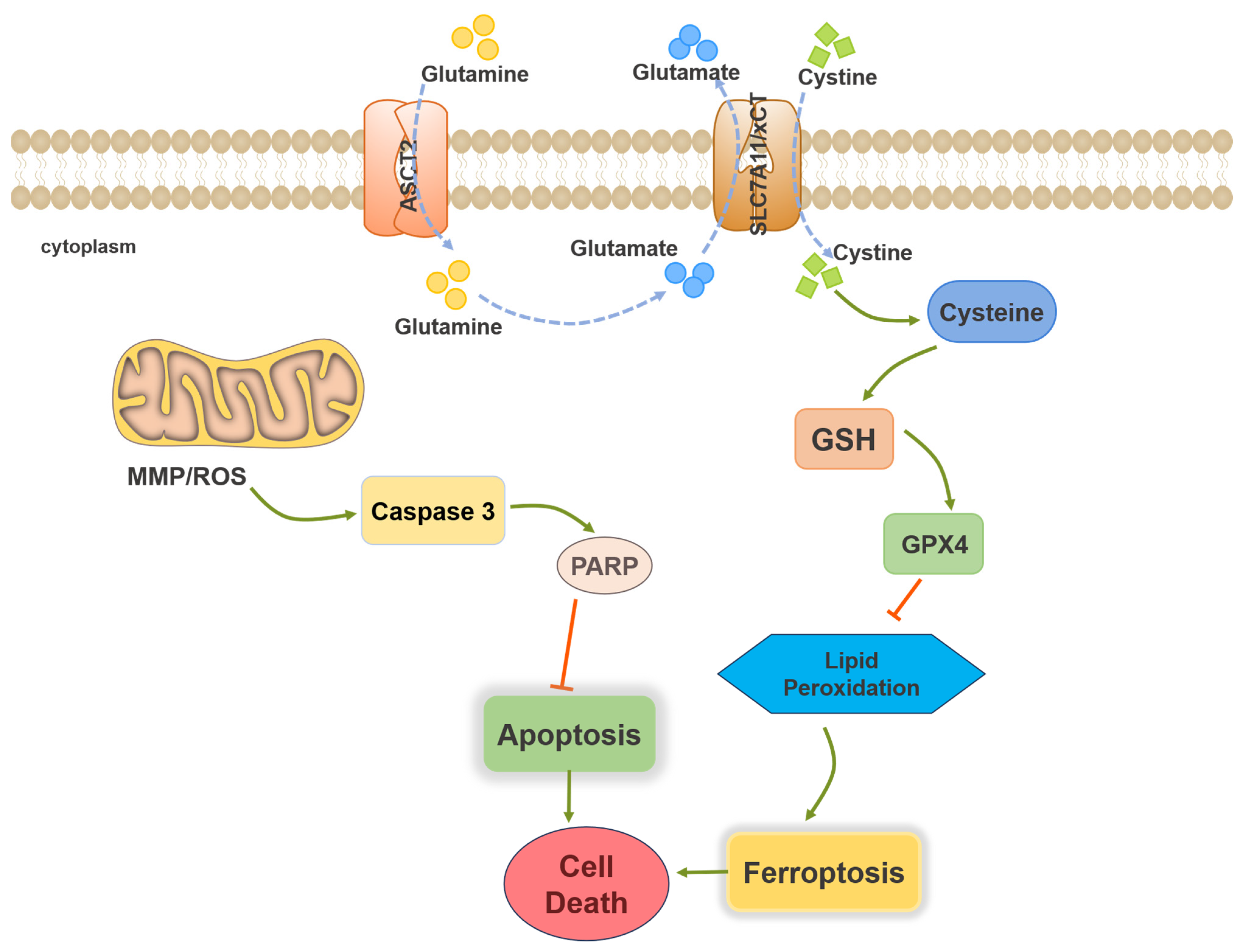
2.3. TAMEWs Induce Apoptosis via Mitochondrial Dysfunction in TNBC Cells
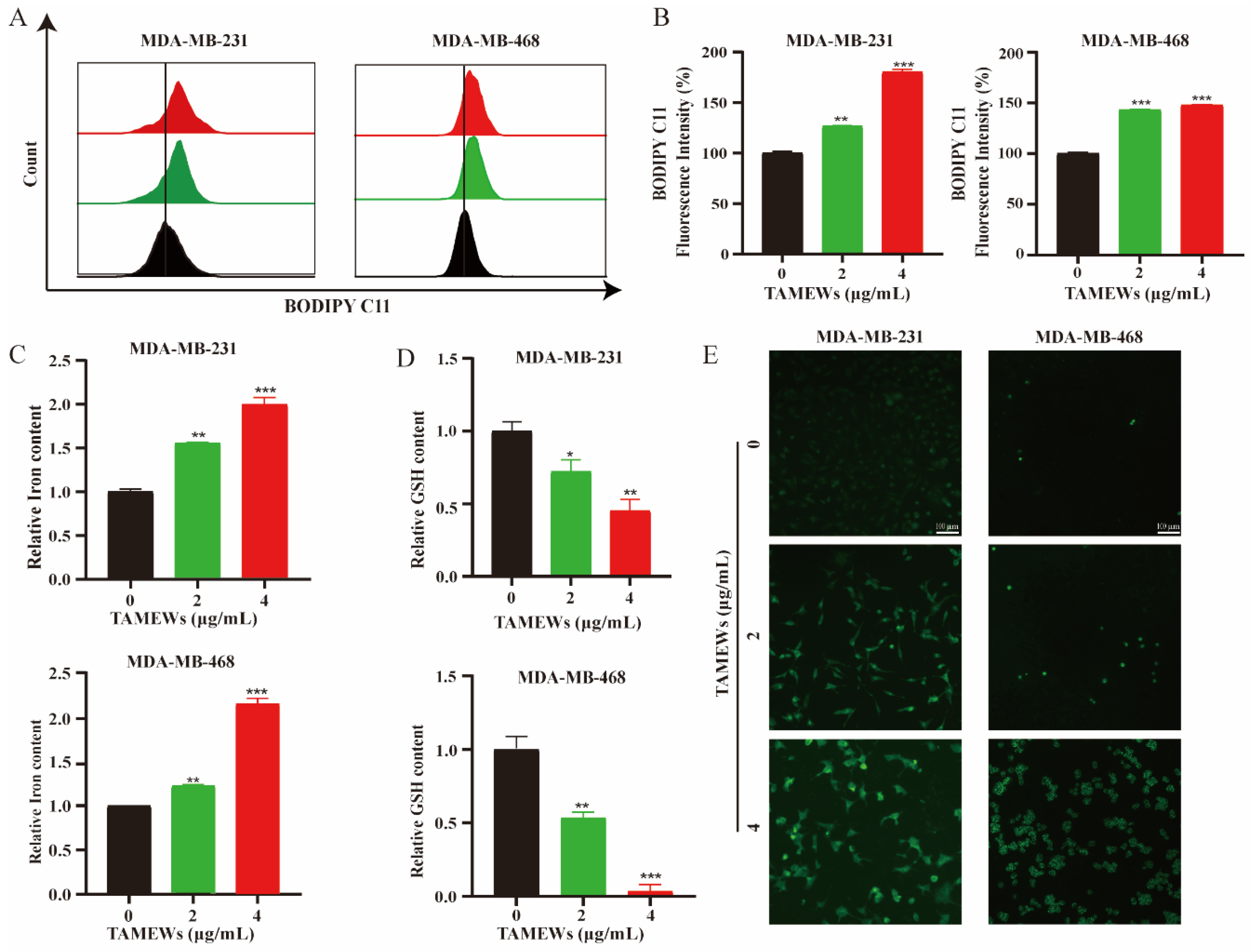
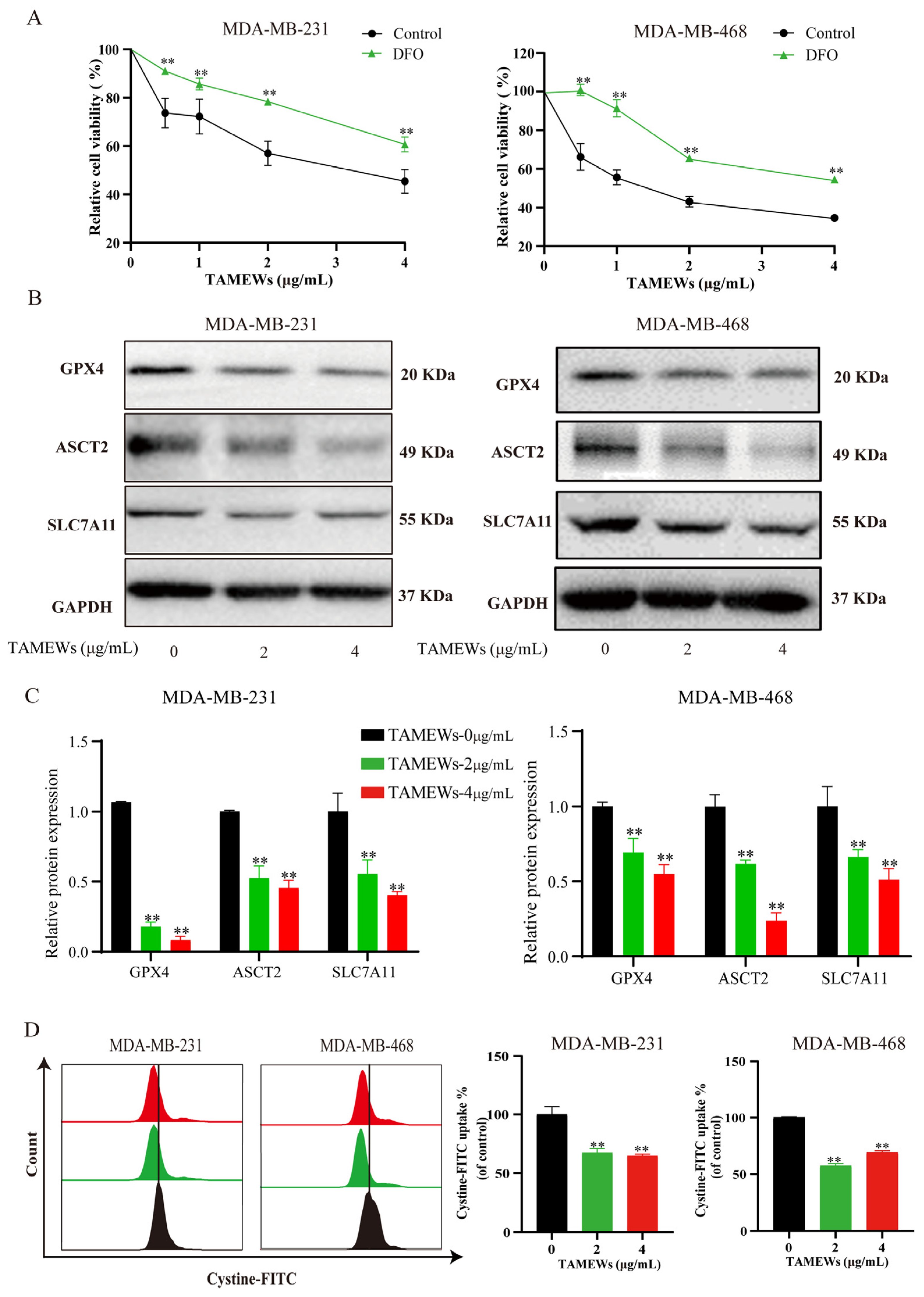
2.4. TAMEWs Induce Ferroptosis by Regulating Amino Acid Metabolism in TNBC Cells
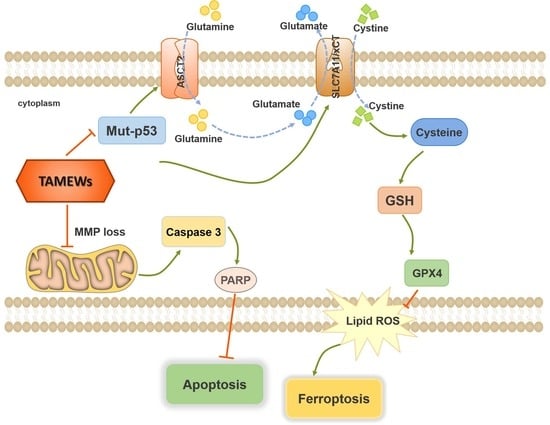
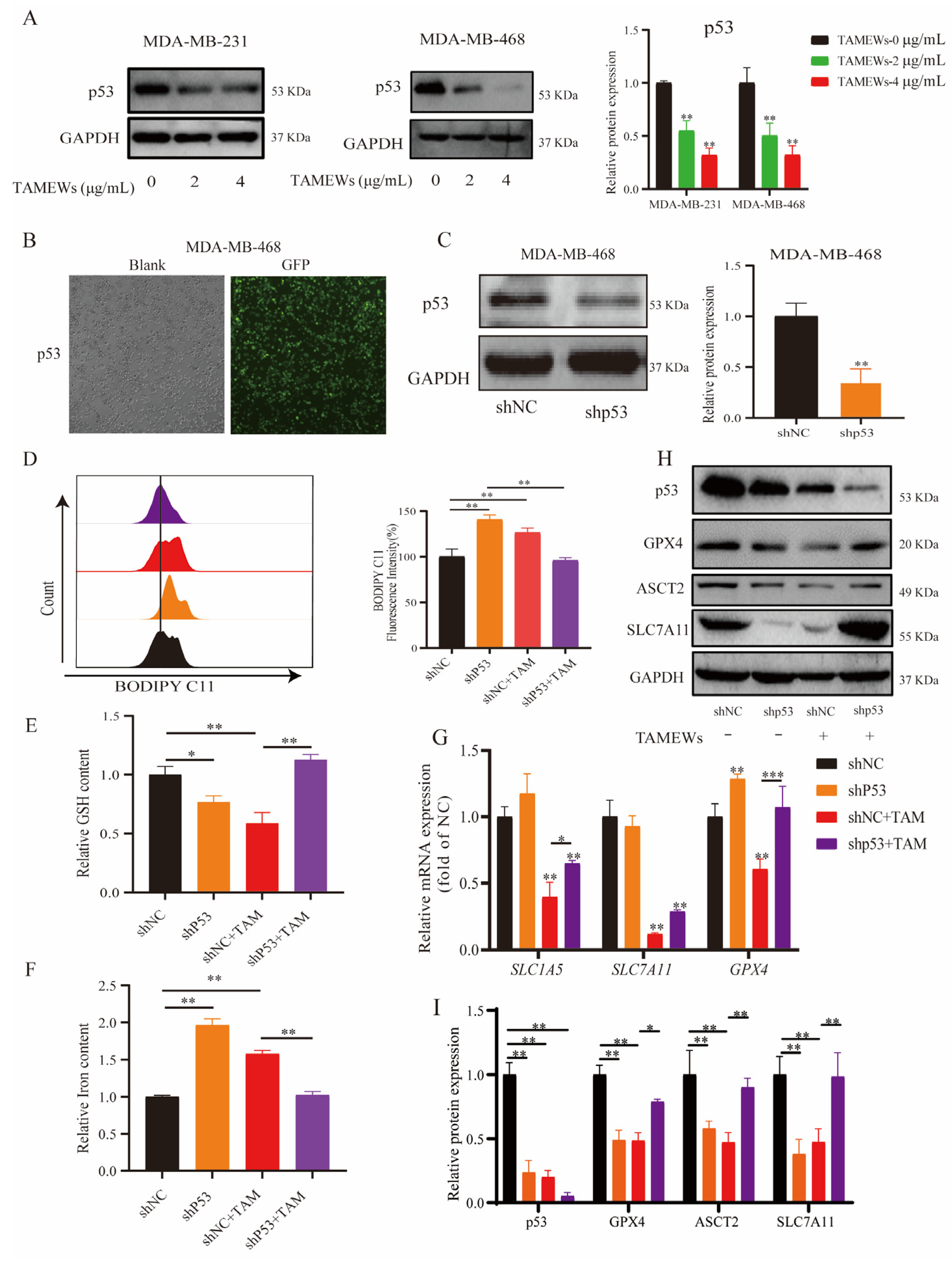
2.5. TAMEWs Induce Ferroptosis via Regulation of p53 in the ASCT2-SLC7A11-GPX4 Axis
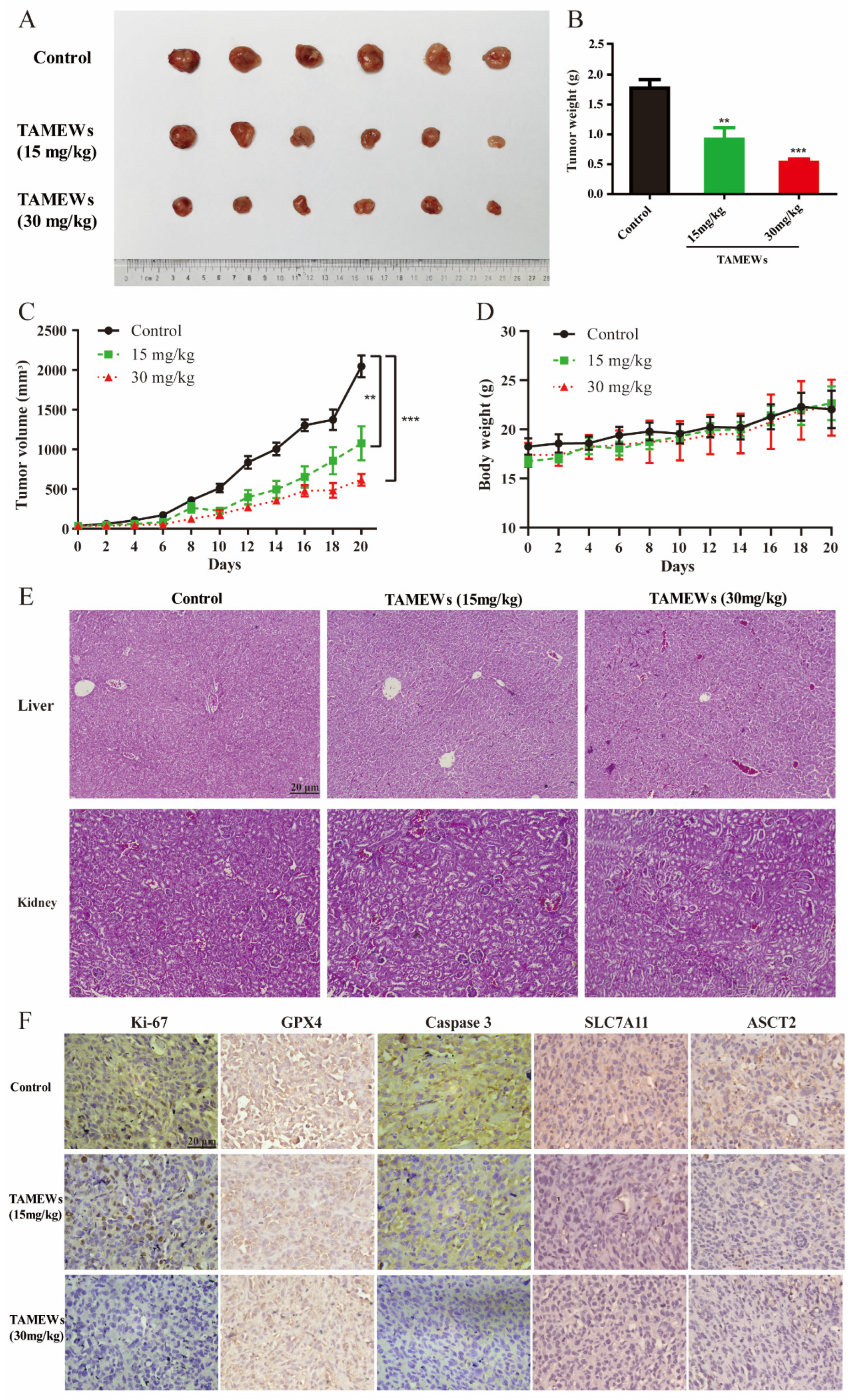
2.6. TAMEWs Suppress Tumor Growth of TNBC In Vivo
2.7. TAMEWs Inhibit TNBC In Vivo through Apoptosis and Ferroptosis
2.8. Discussion
3. Methods and Materials
3.1. Reagents
3.2. Preparation of TAMEWs
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. RNA-Sequencing (RNA-Seq) and Proteomic Analysis
3.6. Mitochondrial Membrane Potential (MMP) Assay
3.7. Apoptosis Assessment
3.8. Detection of Mitochondrial ROS (Mito-ROS)
3.9. Lipid Peroxidation Detection
3.10. Intracellular Glutathione (GSH) Assay
3.11. Iron Assay
3.12. Cystine Uptake Assay
3.13. Plasmid and Cell Line Generation
3.14. Quantitative Real-Time PCR Analysis
3.15. Western Blotting
3.16. Tumor Xenograft
3.17. HE Staining and IHC
3.18. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trapani, D.; Ginsburg, O.; Fadelu, T.; Lin, N.U.; Hassett, M.; Ilbawi, A.M.; Anderson, B.O.; Curigliano, G. Global challenges and policy solutions in breast cancer control. Cancer Treat. Rev. 2022, 104, 102339. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tian, W.; Zheng, S.; Zou, Y.; Xie, J.; Zhang, J.; Li, X.; Sun, Y.; Lan, J.; Li, N.; et al. Dissection of FOXO1-Induced LYPLAL1-DT Impeding Triple-Negative Breast Cancer Progression via Mediating hnRNPK/β-Catenin Complex. Research 2023, 6, 0289. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, T.; Li, R.; Du, Y.; Lv, H.; Zhang, L.; Chen, H.; Shi, X.; Li, Q.; Shen, J. The discovery of a novel series of potential ERRα inverse agonists based on p-nitrobenzenesulfonamide template for triple-negative breast cancer in vivo. J. Enzyme Inhib. Med. Chem. 2022, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Shingu, K.; Yamaguchi, K.; Yoshitake, Y.; Harano, K.; Yoshimitsu, H.; Miyashita, H.; Ikeda, T.; Tagawa, C.; Nohara, T. New C(28) steroidal glycosides from Tubocapsicum anomalum. Chem. Pharm. Bull. 2008, 56, 1038–1040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKenna, M.K.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.N.; Rangnekar, V.M.; Gupta, R.C.; Bondada, S. Anti-cancer activity of withaferin A in B-cell lymphoma. Cancer Biol. Ther. 2015, 16, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Li, C.; Li, M.X.; Song, Z.R.; Ma, X.X.; Sun, D.J.; Li, H.; Chen, L.X. Withanolides isolated from Tubocapsicum anomalum and their antiproliferative activity. Bioorg. Chem. 2021, 110, 104809. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Tsai, Y.L.; Wu, Y.C.; Chang, F.R.; Liu, M.H.; Chen, W.Y.; Wu, C.C. Withanolides-Induced Breast Cancer Cell Death Is Correlated with Their Ability to Inhibit Heat Protein 90. PLoS ONE 2012, 7, e37764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Xu, W.; Liu, Y.; Zhang, J.H.; Yang, Y.Y.; Wang, Z.W.; Sun, D.J.; Li, H.; Liu, B.; Chen, L.X. Anomanolide C suppresses tumor progression and metastasis by ubiquitinating GPX4-driven autophagy-dependent ferroptosis in triple negative breast cancer. Int. J. Biol. Sci. 2023, 19, 2531–2550. [Google Scholar] [CrossRef]
- Xiang, K.; Liu, Y.; Zhu, R.; Xu, Y.; Sun, D.J.; Yang, Y.Y.; Yan, Y.S.; Yang, B.Y.; Li, H.; Chen, L.X. Cytotoxic withanolides from the stems and leaves of Physalis ixocarpa. Food Chem. 2024, 439, 138136. [Google Scholar] [CrossRef] [PubMed]
- Shou, P.; Li, J.; Zhang, P.; Wei, Y.; Yan, M.; Zhang, M.; Feng, K.; Lin, N.; Zhao, H.; Yang, B. Pharmacophore-probe reaction guided purification to precisely identify electrophilic withanolides from Tubocapsicum anomalum Makino and their anti-TNBC activity. Fitoterapia 2022, 158, 105169. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Rehm, M.; Huber, H.J.; Hellwig, C.T.; Anguissola, S.; Dussmann, H.; Prehn, J.H.M. Dynamics of outer mitochondrial membrane permeabilization during apoptosis. Cell Death Differ. 2009, 16, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Ye, Z.; Zhuo, Q.F.; Hu, Q.S.; Xu, X.W.; Liu, M.Q.; Zhang, Z.; Xu, W.Y.; Liu, W.S.; Fan, G.X.; Qin, Y.; et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021, 38, 101807. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yuan, H.S.; Pratte, J.; Giardina, C. Ferroptosis and its potential as a therapeutic target. Biochem. Pharmacol. 2021, 186, 114486. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Xu, X.T.; Shen, C.J.; Yuan, J.T.; Lou, S.Y.; Ma, X.L.; Chen, X.; Yang, B.; Zhao, H.J. A novel sesquiterpene lactone fraction from Eupatorium chinense L. suppresses hepatocellular carcinoma growth by triggering ferritinophagy and mitochondrial damage. Phytomedicine 2023, 112, 154671. [Google Scholar] [CrossRef]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, Z.; Hu, H.; Guan, J.; Yang, B.; Zhao, H. Eupaformosanin induces apoptosis and ferroptosis through ubiquitination of mutant p53 in triple-negative breast cancer. Eur. J. Pharmacol. 2022, 924, 174970. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, L.J.; Chen, G.; Chao, T.I.; Wang, C.Y. SHP-1/STAT3-Signaling-Axis-Regulated Coupling between BECN1 and SLC7A11 Contributes to Sorafenib-Induced Ferroptosis in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 11092. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, J.; You, J.H.; Kim, D.; Roh, J.L. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020, 30, 101418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Z.; Tu, M.; Meng, W.; Gao, H.; Li, M.D.; Li, L. Correlation Between Prognostic Biomarker SLC1A5 and Immune Infiltrates in Various Types of Cancers Including Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 608641. [Google Scholar] [CrossRef]
- Hassanein, M.; Hoeksema, M.D.; Shiota, M.; Qian, J.; Harris, B.K.; Chen, H.; Clark, J.E.; Alborn, W.E.; Eisenberg, R.; Massion, P.P. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 2013, 19, 560–570. [Google Scholar] [CrossRef]
- van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 1675613. [Google Scholar] [CrossRef]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef]
- Tian, X.; Gu, L.; Zeng, F.; Liu, X.; Zhou, Y.; Dou, Y.; Han, J.; Zhao, Y.; Zhang, Y.; Luo, Q.; et al. Strophanthidin Induces Apoptosis of Human Lung Adenocarcinoma Cells by Promoting TRAIL-DR5 Signaling. Molecules 2024, 29, 877. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, D. The Preparation of Ginsenoside Rg5, Its Antitumor Activity against Breast Cancer Cells and Its Targeting of PI3K. Nutrients 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Davids, M.S. BCL-2 Antagonism to Target the Intrinsic Mitochondrial Pathway of Apoptosis. Clin. Cancer Res. 2015, 21, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Favaron, C.; Gabano, E.; Zanellato, I.; Gaiaschi, L.; Casali, C.; Bottone, M.G.; Ravera, M. Effects of Ferrocene and Ferrocenium on MCF-7 Breast Cancer Cells and Interconnection with Regulated Cell Death Pathways. Molecules 2023, 28, 6469. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van ‘t Veer, L.J.; et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, C.K.; Wu, J.; Sjol, J.; Wardell, S.; Spasojevic, I.; George, D.; McDonnell, D.P.; Hsu, D.S.; Chang, J.T.; et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene 2017, 36, 4235–4242. [Google Scholar] [CrossRef]
- Briggs, K.J.; Koivunen, P.; Cao, S.; Backus, K.M.; Olenchock, B.A.; Patel, H.; Zhang, Q.; Signoretti, S.; Gerfen, G.J.; Richardson, A.L.; et al. Paracrine Induction of HIF by Glutamate in Breast Cancer: EglN1 Senses Cysteine. Cell 2016, 166, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting p53 for the treatment of cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef]
- He, W.; Tao, W.; Zhang, F.; Jie, Q.; He, Y.; Zhu, W.; Tan, J.; Shen, W.; Li, L.; Yang, Y.; et al. Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J. Cell. Mol. Med. 2020, 24, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Chen, J.; Li, X.; Dong, N. Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation. Biosci. Rep. 2020, 40, BSR20201807. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Wei, Y.; Ni, M.; Hu, H.; Xi, L.; Wang, C.; Zhu, Z.; Yang, B.; Zhao, H. Novel Withanolides from Tubocapsicum anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and p53-ASCT2-SLC7A11-Mediated Ferroptosis. Molecules 2024, 29, 1838. https://doi.org/10.3390/molecules29081838
Huang L, Wei Y, Ni M, Hu H, Xi L, Wang C, Zhu Z, Yang B, Zhao H. Novel Withanolides from Tubocapsicum anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and p53-ASCT2-SLC7A11-Mediated Ferroptosis. Molecules. 2024; 29(8):1838. https://doi.org/10.3390/molecules29081838
Chicago/Turabian StyleHuang, Lili, Yingying Wei, Maowei Ni, Hongtao Hu, Luyi Xi, Chen Wang, Zhihui Zhu, Bo Yang, and Huajun Zhao. 2024. "Novel Withanolides from Tubocapsicum anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and p53-ASCT2-SLC7A11-Mediated Ferroptosis" Molecules 29, no. 8: 1838. https://doi.org/10.3390/molecules29081838
APA StyleHuang, L., Wei, Y., Ni, M., Hu, H., Xi, L., Wang, C., Zhu, Z., Yang, B., & Zhao, H. (2024). Novel Withanolides from Tubocapsicum anomalum Suppress Triple-Negative Breast Cancer by Triggering Apoptosis and p53-ASCT2-SLC7A11-Mediated Ferroptosis. Molecules, 29(8), 1838. https://doi.org/10.3390/molecules29081838