Hierarchically Structured Graphene Aerogel Supported Nickel–Cobalt Oxide Nanowires as an Efficient Electrocatalyst for Oxygen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
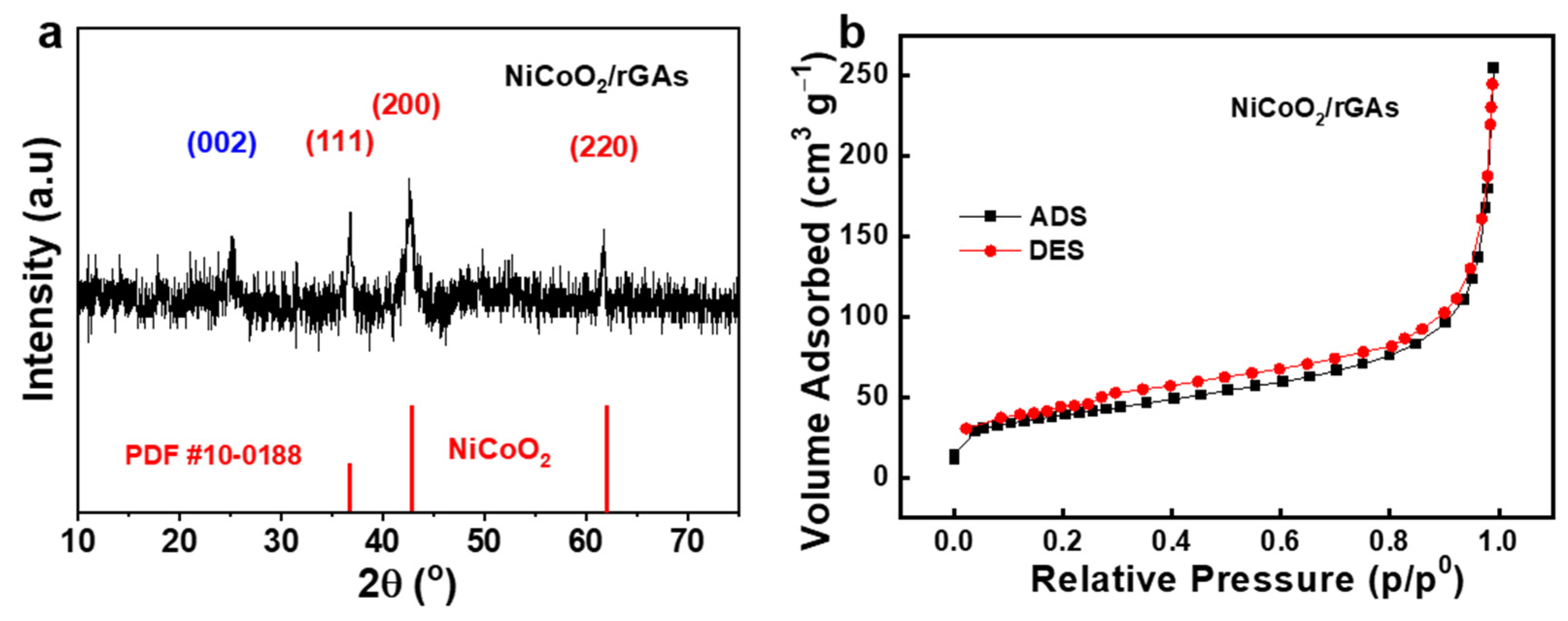
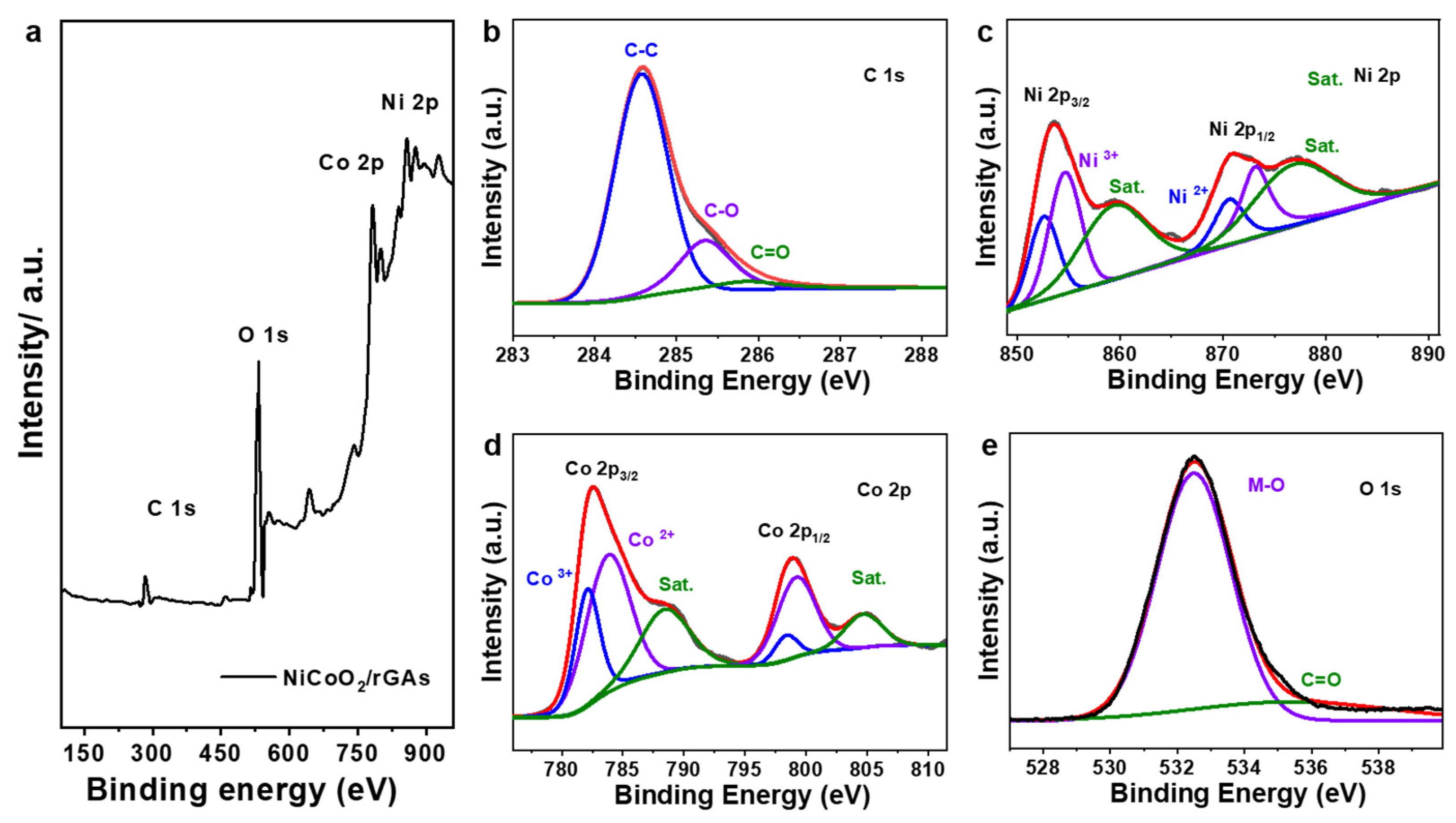
2.1. Morphological Structure of NiCoO2/rGAs
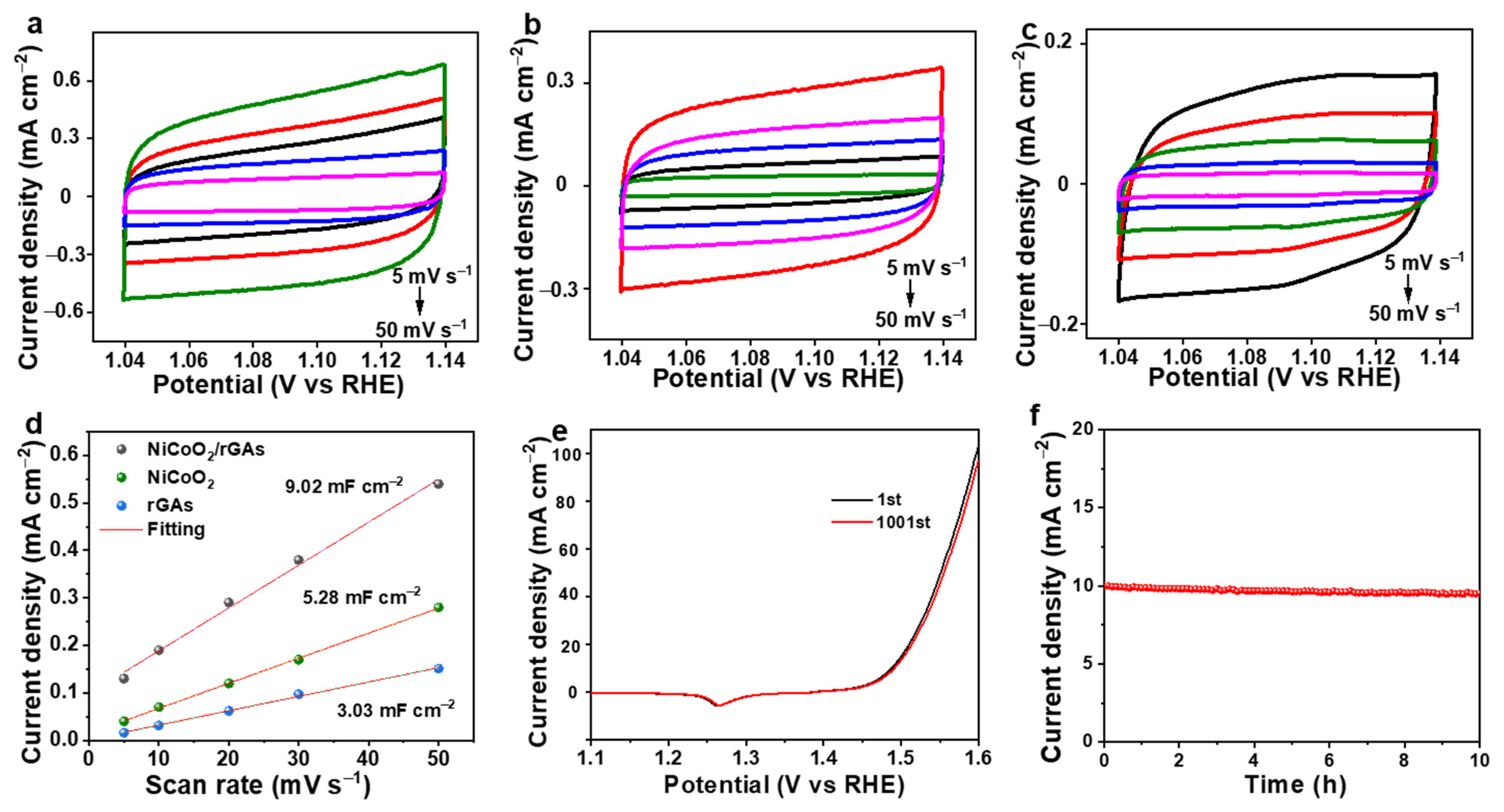
2.2. Electrocatalytic OER Performance
3. Materials and Methods
3.1. Preparation of NiCoO2
3.2. Preparation of rGAs
3.3. Preparation of NiCoO2/rGAs
3.4. Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Z.; Li, Y.; Gao, Y.; Meng, Z.; Sun, Y.; Bai, Y.; Suen, N.-T.; Chen, H.-C.; Pi, Y.; Pang, H. 2D MOF-assisted Pyrolysis-displacement-alloying Synthesis of High-entropy Alloy Nanoparticles Library for Efficient Electrocatalytic Hydrogen Oxidation. Angew. Chem. Int. Ed. 2023, 62, e202306881. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Qiu, Z.; Sun, Y.; Ishii, H.; Liao, Y.-F.; Zhang, X.; Chen, H.-Y.; Pang, H. Synergistic Mechanism of Sub-Nanometric Ru Clusters Anchored on Tungsten Oxide Nanowires for High-Efficient Bifunctional Hydrogen Electrocatalysis. Adv. Sci. 2023, 10, 2206096. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Jiao, Y.; Yan, H.; Fu, H. Subtle 2D/2D MXene-Based Heterostructures for High-Performance Electrocatalytic Water Splitting. Small Methods 2024, 2301602. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, S.; Li, F.; Zhang, L.; Jiang, Y.; Ma, H.; Cheng, K.; Qing, L. Defect engineering induces Mo-regulated Co9Se8/FeNiSe heterostructures with selenium vacancy for enhanced electrocatalytic overall water splitting in alkaline. J. Colloid Interface Sci. 2024, 655, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yu, L.; Wang, X.; Feng, L. Insights into Fe-doping effect-induced heterostructure formation for the oxygen evolution reaction. Chem. Commun. 2023, 59, 12294–12297. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, P.; Chen, S. Quantitative Understanding of the Sluggish Kinetics of Hydrogen Reactions in Alkaline Media Based on a Microscopic Hamiltonian Model for the Volmer Step. J. Phys. Chem. C 2019, 123, 17325–17334. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yu, T.; Deng, S.; Zhou, X.-Y.; Lin, D.; Zhang, Q.; Jin, Z.; Zhang, D.; He, Y.-B.; Qiu, H.-J.; et al. RuO2 electronic structure and lattice strain dual engineering for enhanced acidic oxygen evolution reaction performance. Nat. Commun. 2022, 13, 3784. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Dong, B.; Yang, F.; Feng, L. Ruthenium-nickel oxide derived from Ru-coupled Ni metal–organic framework for effective oxygen evolution reaction. J. Colloid Interface Sci. 2024, 654, 1080–1088. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Zhao, D.; Han, Z.; Wu, X.; Zhai, J.; Liu, Z.; Tang, Y.; Fu, G. l-Lysine-induced green synthesis of CoS/Co3O4 nanoframes for efficient electrocatalytic oxygen evolution. Green Chem. 2023, 25, 7309–7317. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, K.; Dai, F.; Liu, Y.; Zhang, C.; Su, J.; Wang, K. Hierarchical porous tri-metallic NiCoFe-Se/CFP derived from Ni-Co-Fe Prussian blue analogues as efficient electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2023, 642, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Xiong, D.; Zhang, B.; Liu, Y.; Wu, K.-H.; Amorim, I.; Li, W.; Liu, L. Trends in activity for the oxygen evolution reaction on transition metal (M = Fe, Co, Ni) phosphide pre-catalysts. Chem. Sci. 2018, 9, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, M.; Zhu, W.; Zhang, Y.; Hu, W.; Pang, H. Preparation of hierarchical hollow CoFe Prussian blue analogues and its heat-treatment derivatives for the electrocatalyst of oxygen evolution reaction. J. Colloid Interface Sci. 2023, 631, 8–16. [Google Scholar] [CrossRef]
- Lu, Y.; Pei, C.; Han, X.; Li, Y.; Park, H.S.; Kim, J.K.; Yu, X. Tri-metallic fluoride nanoplates immobilized on reduced graphene architectures as efficient oxygen evolution reaction catalyst. J. Electroanal. Chem. 2024, 957, 118108. [Google Scholar] [CrossRef]
- Pei, C.; Gu, Y.; Liu, Z.; Yu, X.; Feng, L. Fluoridated Iron–Nickel Layered Double Hydroxide for Enhanced Performance in the Oxygen Evolution Reaction. ChemSusChem 2019, 12, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Piao, S.; Choi, Y.; Lyu, L.; Hong, H.; Kim, D.; Lee, J.; Zhang, W.; Piao, Y. Nanostructured Transition Metal Nitrides as Emerging Electrocatalysts for Water Electrolysis: Status and Challenges. EnergyChem 2022, 4, 100072. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.; Feng, R.; Wang, S.; Hsu, C.-S.; Ni, Y.; Ahmad, A.; Zhang, C.; Wu, H.; Chen, H.-M.; et al. Oxygen Vacancies Unfold the Catalytic Potential of NiFe-Layered Double Hydroxides by Promoting Their Electronic Transport for Oxygen Evolution Reaction. ACS Catal. 2023, 13, 6000–6012. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.-J.; Li, Z.-Y.; Bu, X.-H. Recent Progress on NiFe-Based Electrocatalysts for the Oxygen Evolution Reaction. Small 2020, 16, 2003916. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Tu, J.; Zhang, Y.; Wang, X.; Gu, C.; Zhao, X.-B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Q.; Wang, A.; Duan, J.; Zhou, W.; Sang, Y.; Liu, H. Iron oxide embedded titania nanowires—An active and stable electrocatalyst for oxygen evolution in acidic media. Nano Energy 2018, 45, 118–126. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Mao, X.; Hu, X.; Gao, F.; Gao, M.; Wu, Q.-L.; Lyu, X.; Du, A.; Xu, X.; et al. Dual Integrating Oxygen and Sulphur on Surface of CoTe Nanorods Triggers Enhanced Oxygen Evolution Reaction. Adv. Sci. 2023, 10, 2206204. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Yang, D.; Liu, Z.; Wang, S.; Feng, L. Iron oxide promoted nickel/nickel oxide rough nanorods for efficient urea assisted water splitting. Electrochim. Acta 2020, 353, 136516. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Silva, M.M.; Raimundo, R.A.; Silva, T.R.; Araújo, A.J.; Macedo, D.A.; Morales, M.A.; Souza, C.P.; Santos, A.G.; Lopes-Moriyama, A.L. Morphology-controlled NiFe2O4 nanostructures: Influence of calcination temperature on structural, magnetic and catalytic properties towards OER. J. Electroanal. Chem. 2023, 933, 117277. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and Metal Oxide Supported on Ordered Mesoporous Carbon as Heterogeneous Catalysts. ACS Catal. 2023, 13, 4060–4090. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Yan, Z.; Liu, S.; Wang, E. CuO/Co3O4 heterostructures with carbon nanotubes composites as ORR/OER electrocatalysts for Zn-air batteries. J. Energy Storage 2023, 66, 107485. [Google Scholar] [CrossRef]
- He, H.; Lei, Y.; Liu, S.; Thummavichai, K.; Zhu, Y.; Wang, N. Tunable active-sites of Co– nanoparticles encapsulated in carbon nanofiber as high performance bifunctional OER/ORR electrocatalyst. J. Colloid Interface Sci. 2023, 630, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, G.; Kim, M.; Han, M.; Jang, J.; Yamauchi, Y.; Yuliarto, B.; Krüger, P.; Kim, J.; Park, N.; et al. Ni-single atom decorated mesoporous carbon electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2023, 468, 143733. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Pei, C. Surface oxidized iron-nickel nanorods anchoring on graphene architectures for oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 3579–3583. [Google Scholar] [CrossRef]
- Chen, W.; Yu, X.; Zhao, Z.; Ji, S.; Feng, L. Hierarchical architecture of coupling graphene and 2D WS2 for high-performance supercapacitor. Electrochim. Acta 2019, 298, 313–320. [Google Scholar] [CrossRef]
- Chang, J.; Hu, Z.; Wu, D.; Xu, F.; Chen, C.; Jiang, K.; Gao, Z. Prussian blue analog-derived nickel iron phosphide-reduced graphene oxide hybrid as an efficient catalyst for overall water electrolysis. J. Colloid Interface Sci. 2023, 638, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, K.; Ingavale, S.B.; Kakade, B. Three dimensional NiS2–Ni(OH)2/CNT nanostructured assembly for supercapacitor and oxygen evolution reaction. J. Alloys Compd. 2020, 812, 152126. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Wang, R.; Wang, S.; Lv, Y.-R.; Xu, H.; Zang, S.-Q. Metal–organic framework-derived Co9S8 embedded in N, O and S-tridoped carbon nanomaterials as an efficient oxygen bifunctional electrocatalyst. J. Mater. Chem. A 2019, 7, 7389–7395. [Google Scholar] [CrossRef]
- Wang, Z.; Denis, D.K.; Zhao, Z.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Unusual formation of hollow NiCoO2 sub-microspheres by oxygen functional group dominated thermally induced mass relocation towards efficient lithium storage. J. Mater. Chem. A 2019, 7, 18109–18117. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C. 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Majumdar, A.; Dutta, P.; Sikdar, A.; Lee, H.; Ghosh, D.; Jha, S.N.; Tripathi, S.; Oh, Y.; Maiti, U.N. Impact of Atomic Rearrangement and Single Atom Stabilization on MoSe2@NiCo2Se4 Heterostructure Catalyst for Efficient Overall Water Splitting. Small 2022, 18, 2200622. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Y.; Gu, C.; Yang, J.; Pang, H.; Li, J.; Xu, L.; Tang, Y. Fe incorporation-induced electronic modification of Co-tannic acid complex nanoflowers for high-performance water oxidation. Inorg. Chem. Front. 2022, 9, 1091–1099. [Google Scholar] [CrossRef]
- Chen, P.; Wang, T.; He, D.; Shi, T.; Chen, M.; Fang, K.; Lin, H.; Wang, J.; Wang, C.; Pang, H. Delocalized Isoelectronic Heterostructured FeCoOxSy Catalysts with Tunable Electron Density for Accelerated Sulfur Redox Kinetics in Li-S batteries. Angew. Chem. Int. Ed. 2023, 62, e202311693. [Google Scholar] [CrossRef] [PubMed]
- Kapałka, A.; Fóti, G.; Comninellis, C. Determination of the Tafel slope for oxygen evolution on boron-doped diamond electrodes. Electrochem. Commun. 2008, 10, 607–610. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The Pitfalls of Using Potentiodynamic Polarization Curves for Tafel Analysis in Electrocatalytic Water Splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning synergy between nickel and iron in Ruddlesden–Popper perovskites through controllable crystal dimensionalities towards enhanced oxygen-evolving activity and stability. Carbon Energy 2024, e465. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Xu, J.; Liu, G.; Yu, X. Hierarchically Structured Graphene Aerogel Supported Nickel–Cobalt Oxide Nanowires as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Molecules 2024, 29, 1805. https://doi.org/10.3390/molecules29081805
Guo D, Xu J, Liu G, Yu X. Hierarchically Structured Graphene Aerogel Supported Nickel–Cobalt Oxide Nanowires as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Molecules. 2024; 29(8):1805. https://doi.org/10.3390/molecules29081805
Chicago/Turabian StyleGuo, Donglei, Jiaqi Xu, Guilong Liu, and Xu Yu. 2024. "Hierarchically Structured Graphene Aerogel Supported Nickel–Cobalt Oxide Nanowires as an Efficient Electrocatalyst for Oxygen Evolution Reaction" Molecules 29, no. 8: 1805. https://doi.org/10.3390/molecules29081805
APA StyleGuo, D., Xu, J., Liu, G., & Yu, X. (2024). Hierarchically Structured Graphene Aerogel Supported Nickel–Cobalt Oxide Nanowires as an Efficient Electrocatalyst for Oxygen Evolution Reaction. Molecules, 29(8), 1805. https://doi.org/10.3390/molecules29081805





