Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study
Abstract
1. Introduction
2. Results and Discussion
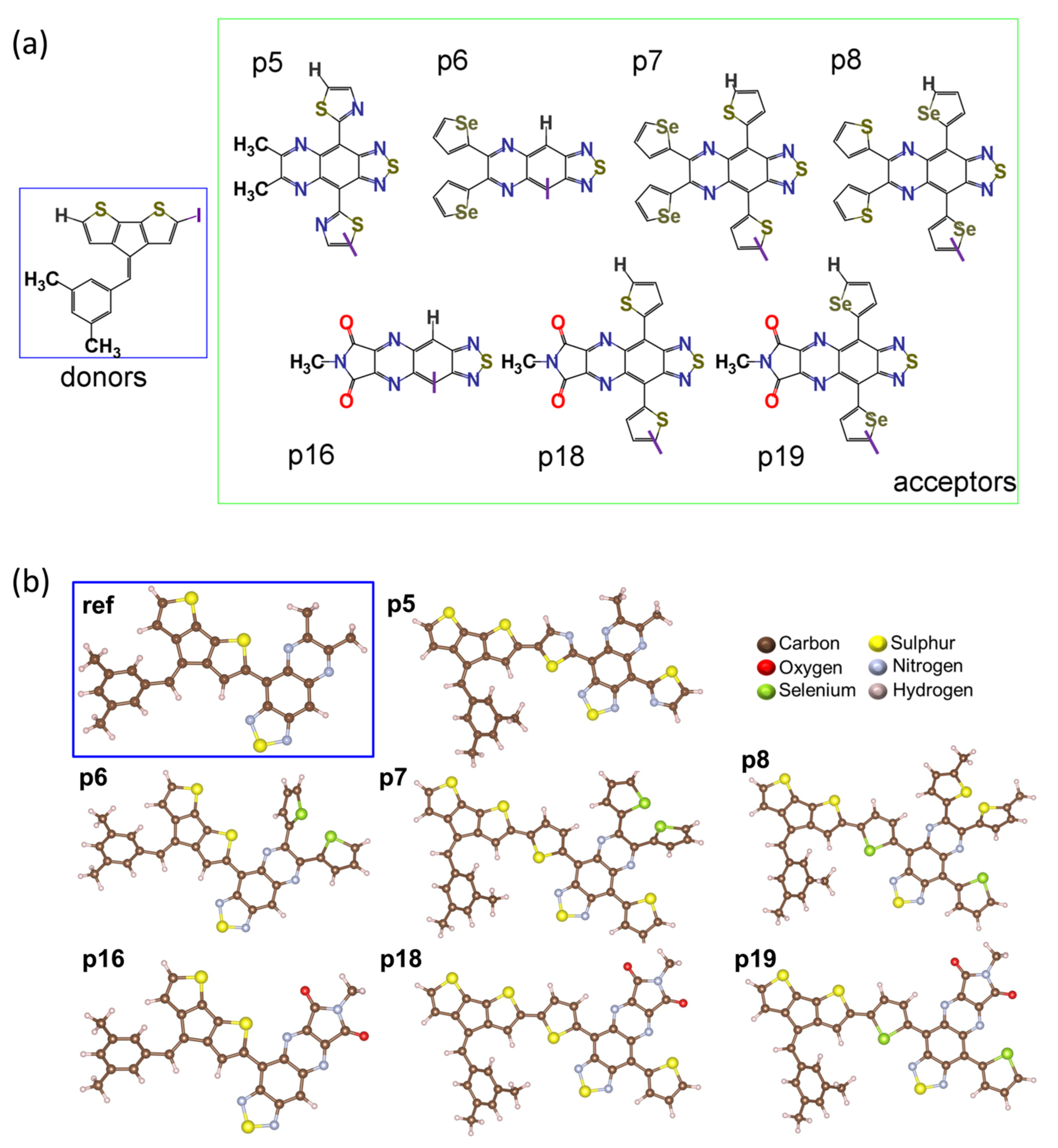
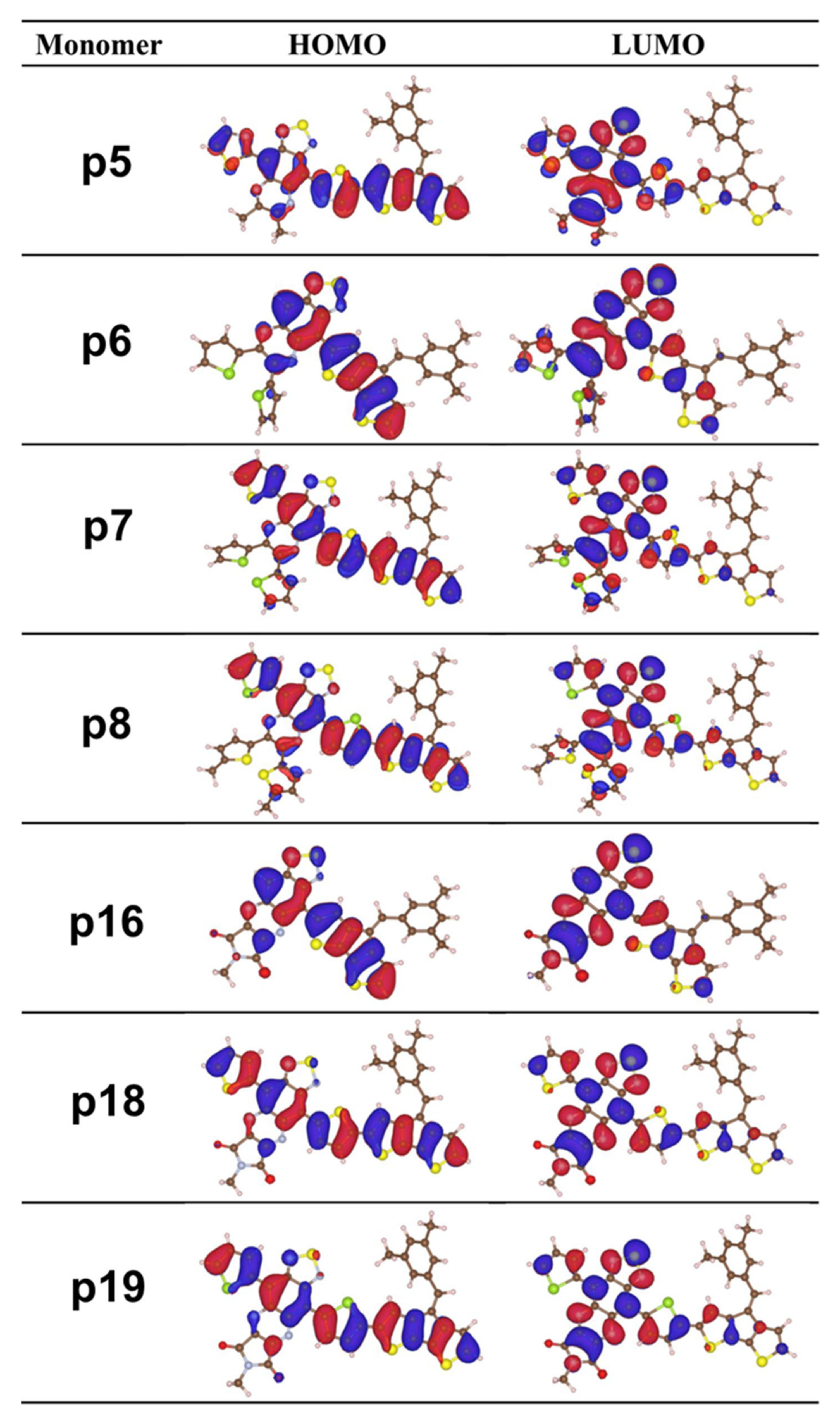
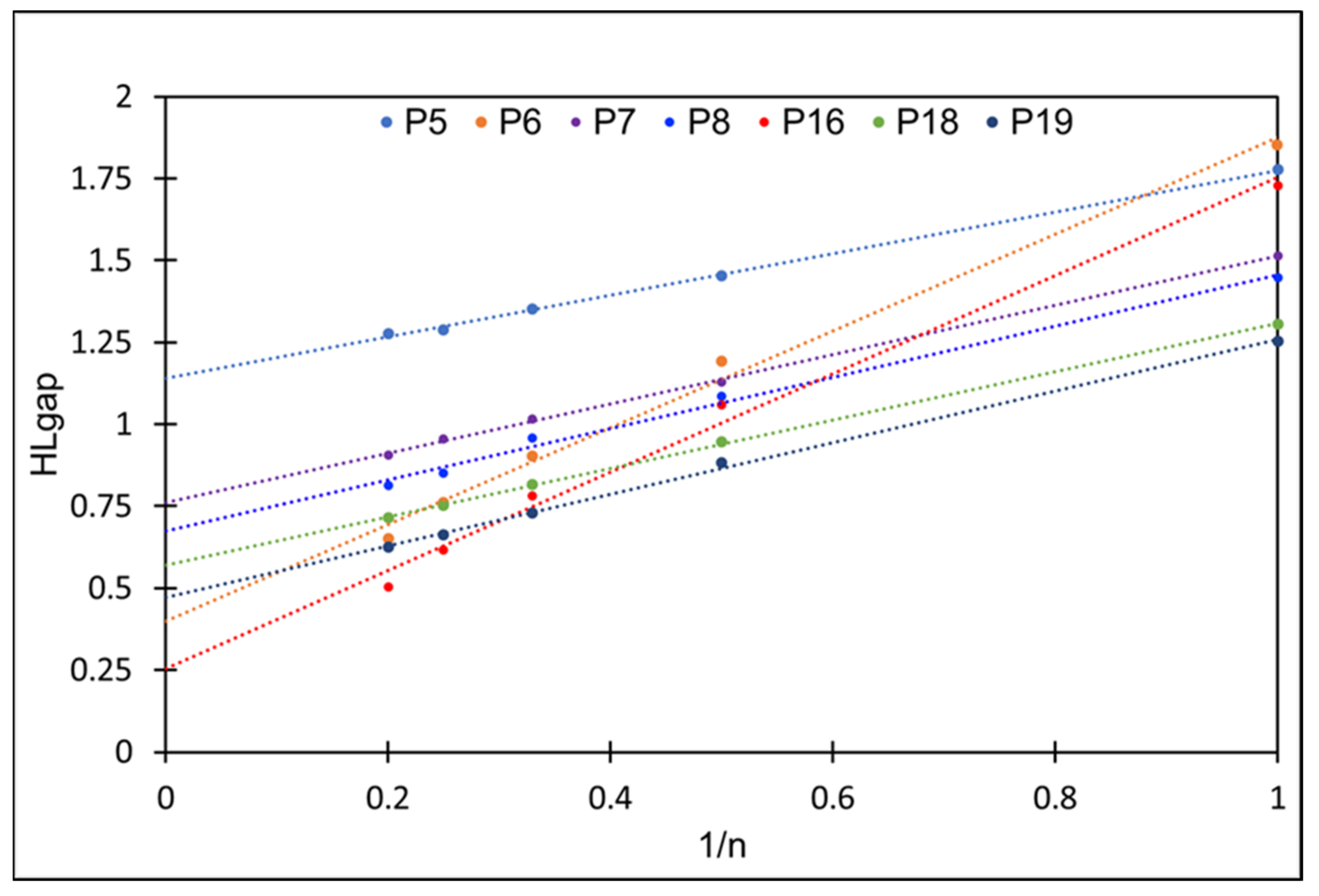
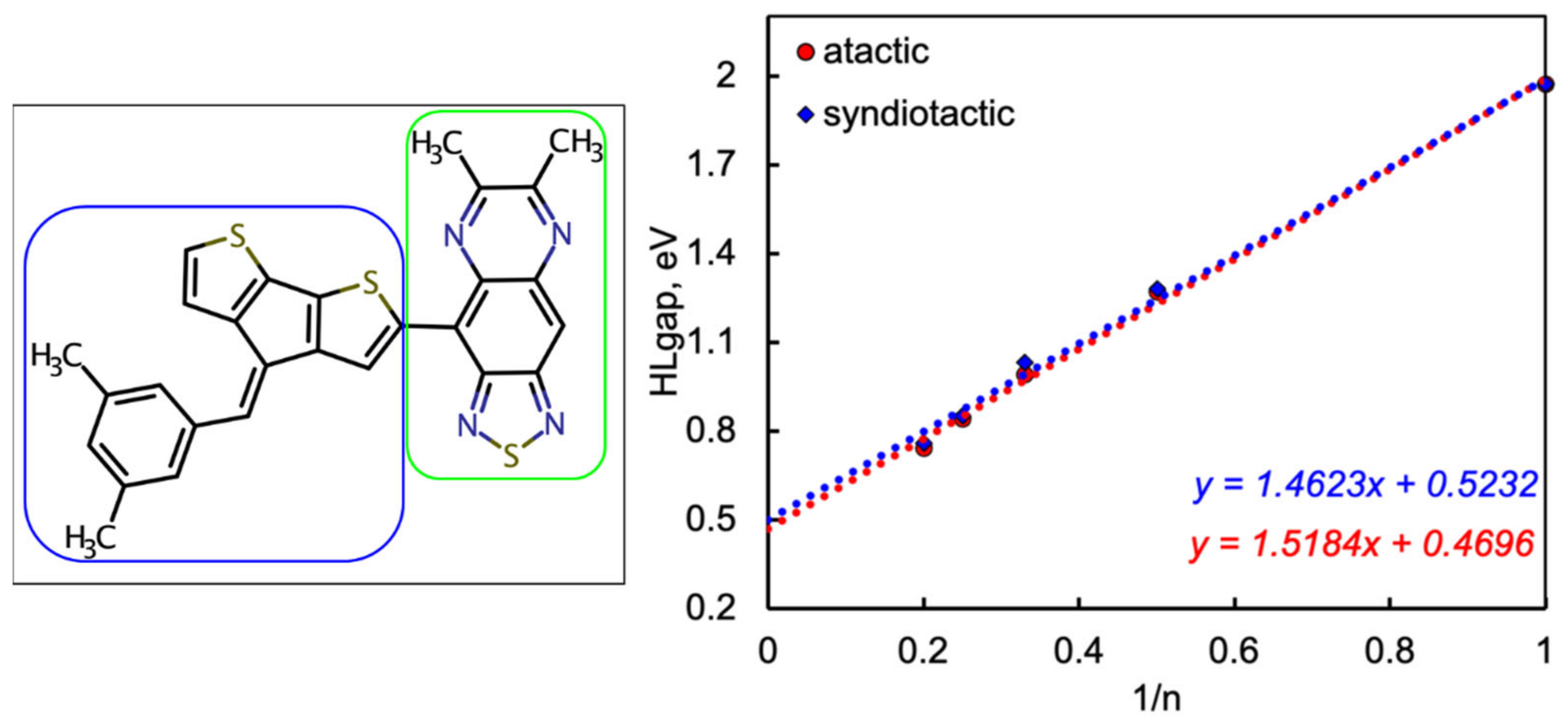
2.1. Frontier Molecular Orbitals and Energy Gap
2.2. Dipole Moment
2.3. UV-Vis Analysis
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- REN21. Global Status Report, Renewable 2017; REN21: Paris, France, 2017; Volume 72, ISBN 978-3-9818107-0-7. [Google Scholar]
- Roser, M. The World’s Energy Problem. Available online: https://ourworldindata.org/worlds-energy-problem (accessed on 14 July 2023).
- Santhoshi Kiran, K.S.; Preethi, V.; Kumar, S. A Brief Review of Organic Solar Cells and Materials Involved in Its Fabrication. Mater. Today Proc. 2022, 56, 3826–3829. [Google Scholar] [CrossRef]
- Kularatne, R.S.; Magurudeniya, H.D.; Sista, P.; Biewer, M.C.; Stefan, M.C. Donor-Acceptor Semiconducting Polymers for Organic Solar Cells. J. Polym. Sci. A Polym. Chem. 2013, 51, 743–768. [Google Scholar] [CrossRef]
- Chen, L.X. Organic Solar Cells: Recent Progress and Challenges. ACS Energy Lett. 2019, 4, 2537–2539. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Zhao, D.; Wang, L.; Jiao, Z.; Huang, Q.; Wang, P.; Sun, M.; Yuan, G. Recent Progress in Organic Solar Cells: A Review on Materials from Acceptor to Donor. Molecules 2022, 27, 1800. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhang, H.; Zhu, L.; Xu, L.; Zhang, F.; Tsang, C.-S.; Lee, L.Y.S.; Woo, H.Y.; He, Z.; et al. Metallated Terpolymer Donors with Strongly Absorbing Iridium Complex Enables Polymer Solar Cells with 16.71% Efficiency. Chem. Eng. J. 2022, 430, 132832. [Google Scholar] [CrossRef]
- Chamberlain, G.A. Organic Solar Cells: A Review. Sol. Cells 1983, 8, 47–83. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Gong, X.; Heeger, A.J. Low Bandgap Semiconducting Polymers for Polymeric Photovoltaics. Chem. Soc. Rev. 2016, 45, 4825–4846. [Google Scholar] [CrossRef]
- Roy, J.K.; Kar, S.; Leszczynski, J. Optoelectronic Properties of C60 and C70 Fullerene Derivatives: Designing and Evaluating Novel Candidates for Efficient P3HT Polymer Solar Cells. Materials 2019, 12, 2282. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Donor-Acceptor Block Copolymers: Synthesis and Solar Cell Applications. Materials 2014, 7, 3274–3290. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Sabuj, M.A.; Li, Y.; Zhu, W.; Zeng, M.; Sarap, C.S.; Huda, M.M.; Qiao, X.; Peng, X.; et al. Evolution of the Electronic Structure in Open-Shell Donor-Acceptor Organic Semiconductors. Nat. Commun. 2021, 12, 5889. [Google Scholar] [CrossRef]
- London, A.E.; Chen, H.; Sabuj, M.A.; Tropp, J.; Saghayezhian, M.; Eedugurala, N.; Zhang, B.A.; Liu, Y.; Gu, X.; Wong, B.M.; et al. A High-Spin Ground-State Donor-Acceptor Conjugated Polymer. Sci. Adv. 2019, 5, eaav2336. [Google Scholar] [CrossRef]
- Dallos, T.; Beckmann, D.; Brunklaus, G.; Baumgarten, M. Thiadiazoloquinoxaline–Acetylene Containing Polymers as Semiconductors in Ambipolar Field Effect Transistors. J. Am. Chem. Soc. 2011, 133, 13898–13901. [Google Scholar] [CrossRef]
- Steckler, T.T.; Henriksson, P.; Mollinger, S.; Lundin, A.; Salleo, A.; Andersson, M.R. Very Low Band Gap Thiadiazoloquinoxaline Donor–Acceptor Polymers as Multi-Tool Conjugated Polymers. J. Am. Chem. Soc. 2014, 136, 1190–1193. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Tse, S.C.; Zhou, J.; Du, X.; Tao, Y.; Ding, J. Synthesis and Applications of Difluorobenzothiadiazole Based Conjugated Polymers for Organic Photovoltaics. J. Mater. Chem. 2011, 21, 3226–3233. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational Design of High Performance Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Marsh, A.V.; Heeney, M. Conjugated Polymers Based on Selenophene Building Blocks. Polym. J. 2023, 55, 375–385. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J. Structures and Properties of Conjugated Donor–Acceptor Copolymers for Solar Cell Applications. J. Mater. Chem. 2012, 22, 4178. [Google Scholar] [CrossRef]
- Huang, L.; Eedugurala, N.; Benasco, A.; Zhang, S.; Mayer, K.S.; Adams, D.J.; Fowler, B.; Lockart, M.M.; Saghayezhian, M.; Tahir, H.; et al. Open-Shell Donor–Acceptor Conjugated Polymers with High Electrical Conductivity. Adv. Funct. Mater. 2020, 30, 1909805. [Google Scholar] [CrossRef]
- Foster, M.E.; Zhang, B.A.; Murtagh, D.; Liu, Y.; Sfeir, M.Y.; Wong, B.M.; Azoulay, J.D. Solution-Processable Donor-Acceptor Polymers with Modular Electronic Properties and Very Narrow Bandgaps. Macromol. Rapid Commun. 2014, 35, 1516–1521. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Najari, A.; Leclerc, M. Processable Low-Bandgap Polymers for Photovoltaic Applications. Chem. Mater. 2010, 23, 456–469. [Google Scholar] [CrossRef]
- Chang, S.-W.; Muto, T.; Kondo, T.; Liao, M.-J.; Horie, M. Double Acceptor Donor–Acceptor Alternating Conjugated Polymers Containing Cyclopentadithiophene, Benzothiadiazole and Thienopyrroledione: Toward Subtractive Color Organic Photovoltaics. Polym. J. 2017, 49, 113–122. [Google Scholar] [CrossRef]
- Sun, H.; Chen, S.; Zhong, A.; Sun, R.; Jin, J.; Yang, J.; Liu, D.; Niu, J.; Lu, S. Tuning Photophysical Properties via Positional Isomerization of the Pyridine Ring in Donor–Acceptor-Structured Aggregation-Induced Emission Luminogens Based on Phenylmethylene Pyridineacetonitrile Derivatives. Molecules 2023, 28, 3282. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef]
- Akkuratov, A.; Prudnov, F.; Mukhacheva, O.; Luchkin, S.; Sagdullina, D.; Obrezkov, F.; Kuznetsov, P.; Volyniuk, D.; Grazulevichus, J.V.; Troshin, P. New Cyclopentadithiophene-Based (X-DAD′AD)n Conjugated Polymers for Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2019, 193, 66–72. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Chen, C.-P.; Chan, S.-H.; Hwang, G.-W.; Ting, C. Thiophene/Phenylene/Thiophene-Based Low-Bandgap Conjugated Polymers for Efficient Near-Infrared Photovoltaic Applications. Chem. Mater. 2009, 21, 3262–3269. [Google Scholar] [CrossRef]
- Zhang, X.; Steckler, T.T.; Dasari, R.R.; Ohira, S.; Potscavage, W.J.; Tiwari, S.P.; Coppée, S.; Ellinger, S.; Barlow, S.; Brédas, J.-L.; et al. Dithienopyrrole-Based Donor–Acceptor Copolymers: Low Band-Gap Materials for Charge Transport, Photovoltaics and Electrochromism. J. Mater. Chem. 2010, 20, 123–134. [Google Scholar] [CrossRef]
- Zoombelt, A.P.; Fonrodona, M.; Wienk, M.M.; Sieval, A.B.; Hummelen, J.C.; Janssen, R.A.J. Photovoltaic Performance of an Ultrasmall Band Gap Polymer. Org. Lett. 2009, 11, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Abid, Z.; Ali, L.; Gulzar, S.; Wahad, F.; Ashraf, R.S.; Nielsen, C.B. Quinoxaline Derivatives as Attractive Electron-Transporting Materials. Beilstein J. Org. Chem. 2023, 19, 1694–1712. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.L.; McCormick, T.M.; Seferos, D.S. Effect of Group-14 and Group-16 Substitution on the Photophysics of Structurally Related Donor–Acceptor Polymers. J. Phys. Chem. C 2013, 117, 16606–16615. [Google Scholar] [CrossRef]
- Kim, H.S.; Song, E.; Lee, S.B.; Kang, I.-N.; Cho, K.; Hwang, D.-H. Effect of Methyl Substitution on the Diketopyrrolopyrrole-Based Semiconducting Polymers for Organic Thin Film Transistors. Org. Electron. 2018, 56, 129–138. [Google Scholar] [CrossRef]
- Sirin, P.S.; Civcir, P.U.; Unaleroglu, C. Theoretically Designated Synthesizable Donor-Acceptor Type Semiconducting Copolymers: Investigation into Molecular, Electronic, and Optical Properties. Mater. Chem. Phys. 2022, 275, 125238. [Google Scholar] [CrossRef]
- Kadam, V.S.; Machhi, H.K.; Soni, S.S.; Zade, S.S.; Patel, A.L. Donor–Acceptor π-Conjugated Polymers Based on Terthiophene-3,4-Dicarboxylate, Dithienopyrrolobenzothiadiazole and Thieno [3,4-c]Pyrrole-4,6-Dione Units and Their Hole Mobility. New J. Chem. 2022, 46, 8601–8610. [Google Scholar] [CrossRef]
- He, Z.; Chen, X.; Yu, H.; Du, Y.; Gao, M.; Wang, S.; Wang, C. Quinoxaline-Based Donor-Acceptor Conjugated Polymers for Nonvolatile Ternary Memory Devices. Chem. Eng. J. 2023, 457, 141365. [Google Scholar] [CrossRef]
- Civcir, P.Ü.; Özen, E.; Karadeniz, C. Narrow-Energy Gap Conjugated Polymers Based on Benzobisthiadiazole and Thiadiazoloquinoxaline: DFT and TDDFT Study. J. Mol. Model. 2020, 26, 289. [Google Scholar] [CrossRef] [PubMed]
- Usta, H.; Facchetti, A. Polymeric and Small-Molecule Semiconductors for Organic Field-Effect Transistors. In Large Area and Flexible Electronics; Wiley: Hoboken, NJ, USA, 2015; pp. 1–100. [Google Scholar]
- Chen, H.-Y.; Yeh, S.-C.; Chen, C.-T.; Chen, C.-T. Comparison of Thiophene- and Selenophene-Bridged Donor–Acceptor Low Band-Gap Copolymers Used in Bulk-Heterojunction Organic Photovoltaics. J. Mater. Chem. 2012, 22, 21549. [Google Scholar] [CrossRef]
- De Gier, H.D.; Broer, R.; Havenith, R.W.A. Non-Innocent Side-Chains with Dipole Moments in Organic Solar Cells Improve Charge Separation. Phys. Chem. Chem. Phys. 2014, 16, 12454–12461. [Google Scholar] [CrossRef] [PubMed]
- Bouzzine, S.M.; Salgado-Morán, G.; Hamidi, M.; Bouachrine, M.; Pacheco, A.G.; Glossman-Mitnik, D. DFT Study of Polythiophene Energy Band Gap and Substitution Effects. J. Chem. 2015, 2015, 296386. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, H.; Deng, Y.; Jiang, Y.; Wang, Z.; Shi, Y.; Han, Y.; Geng, Y. Low-Band Gap Conjugated Polymers with Strong Absorption in the Second Near-Infrared Region Based on Diketopyrrolopyrrole-Containing Quinoidal Units. Macromolecules 2021, 54, 3498–3506. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Momma, K.; Izumi, F. IUCr VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- McCormick, T.M.; Bridges, C.R.; Carrera, E.I.; Dicarmine, P.M.; Gibson, G.L.; Hollinger, J.; Kozycz, L.M.; Seferos, D.S. Conjugated Polymers: Evaluating DFT Methods for More Accurate Orbital Energy Modeling. Macromolecules 2013, 46, 3879–3886. [Google Scholar] [CrossRef]
- Roy, J.K.; Kar, S.; Leszczynski, J. Revealing the Photophysical Mechanism of N, N′-Diphenyl-Aniline Based Sensitizers with the D-D-π-A Framework: Theoretical Insights. ACS Sustain. Chem. Eng. 2020, 8, 13328–13341. [Google Scholar] [CrossRef]
- Sparks, N.E.; Vijayan, S.M.; Roy, J.K.; Dorris, A.; Lambert, E.; Karunathilaka, D.; Hammer, N.I.; Leszczynski, J.; Watkins, D.L. Synthesis and Characterization of Novel Thienothiadiazole-Based D−π–A−π–D Fluorophores as Potential NIR Imaging Agents. ACS Omega 2023, 8, 24513–24523. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.K.; Kar, S.; Leszczynski, J. Electronic Structure and Optical Properties of Designed Photo-Efficient Indoline-Based Dye-Sensitizers with D–A−π–A Framework. J. Phys. Chem. C 2019, 123, 3309–3320. [Google Scholar] [CrossRef]
- Roy, J.; Kaur, R.; Daniel, A.; Baumann, A.; Li, Q.; Delcamp, J.; Leszczynski, J. Photophysical Properties of Donor–Acceptor−π Bridge–Acceptor Sensitizers with a Naphthobisthiadiazole Auxiliary Acceptor: Toward Longer-Wavelength Access in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2022, 126, 11875–11888. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Fadili, D.; Bouzzine, S.M.; Hamidi, M. Study of the Structural and Optoelectronic Properties of Dye Solar Cells Based on Phosphonic Acid Anchoring by DFT Functionals. New J. Chem. 2021, 45, 2723–2733. [Google Scholar] [CrossRef]
- Halsey-Moore, C.; Jena, P.; McLeskey, J.T. Tuning Range-Separated DFT Functionals for Modeling the Peak Absorption of MEH-PPV Polymer in Various Solvents. Comput. Theor. Chem. 2019, 1162, 112506. [Google Scholar] [CrossRef]
- Tsuneda, T.; Hirao, K. Long-range Correction for Density Functional Theory. WIREs Comput. Mol. Sci. 2014, 4, 375–390. [Google Scholar] [CrossRef]
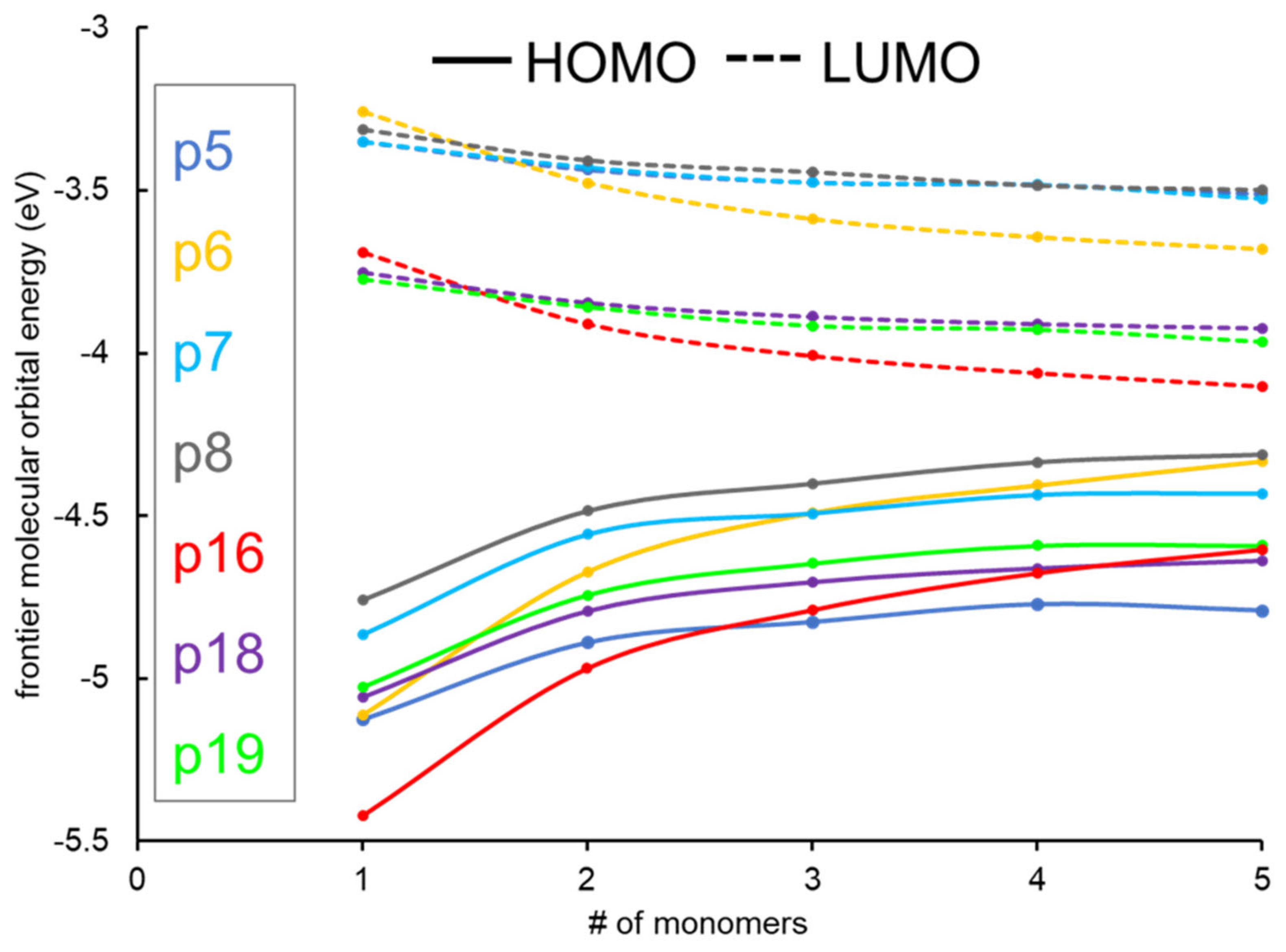

| Name | n | EHOMO (eV) | ELUMO (eV) | Egap (eV) | (eV/nm) | Eb (eV) | µD Debye | f | (Major Contributions) |
|---|---|---|---|---|---|---|---|---|---|
| P5 | 1 | −5.13 | −3.35 | 1.78 | 2.11/587.39 | 0.334 | 1.53 | 0.799 | (83.9%) |
| 2 | −4.89 | −3.44 | 1.45 | 1.79/691.15 | 0.341 | 2.30 | 2.293 | (71.6%) | |
| 3 | −4.82 | −3.48 | 1.35 | 1.70/728.54 | 0.352 | 1.26 | 3.730 | (59.8%) | |
| 4 | −4.77 | −3.48 | 1.29 | 1.64/755.73 | 0.353 | 3.80 | 5.063 | (48.2%) | |
| 5 | −4.80 | −3.51 | 1.28 | 1.63/759.91 | 0.354 | 3.28 | 7.089 | (40.4%) | |
| ∞ | 1.13 | ||||||||
| P6 | 1 | −5.11 | −3.26 | 1.85 | 1.98/726.97 | 0.126 | 3.41 | 0.417 | (95.9%) |
| 2 | −4.67 | −3.48 | 1.19 | 1.35/917.63 | 0.157 | 3.75 | 1.043 | (90.2%) | |
| 3 | −4.49 | −3.59 | 0.90 | 1.06/1142.61 | 0.183 | 6.37 | 1.887 | (84.1%) | |
| 4 | −4.41 | −3.64 | 0.76 | 0.96/1292.19 | 0.197 | 7.66 | 2.754 | (78.0%) | |
| 5 | −4.33 | −3.68 | 0.65 | 0.86/1439.09 | 0.209 | 10.52 | 3.590 | (71.5%) | |
| ∞ | 0.40 | ||||||||
| P7 | 1 | −4.87 | −3.35 | 1.52 | 1.70/729.69 | 0.184 | 0.56 | 0.577 | (91.8%) |
| 2 | −4.56 | −3.43 | 1.13 | 1.40/890.94 | 0.263 | 2.27 | 1.782 | (72.9%) | |
| 3 | −4.49 | −3.48 | 1.01 | 1.29/954.14 | 0.283 | 4.39 | 2.788 | (60.0%) | |
| 4 | −4.44 | −3.48 | 0.95 | 1.26/983.44 | 0.307 | 1.48 | 4.088 | (46.0%) | |
| 5 | −4.43 | −3.52 | 0.91 | 1.21/1027.44 | 0.300 | 5.10 | 5.102 | (41.3%) | |
| ∞ | 0.76 | ||||||||
| P8 | 1 | −4.76 | −3.32 | 1.45 | 1.61/768.01 | 0.17 | 2.28 | 0.614 | (93.0%) |
| 2 | −4.49 | −3.40 | 1.09 | 1.33/935.70 | 0.248 | 4.37 | 1.976 | (72.4%) | |
| 3 | −4.40 | −3.44 | 0.95 | 1.22/1014.08 | 0.265 | 5.70 | 3.424 | (64.5%) | |
| 4 | −4.34 | −3.48 | 0.85 | 1.16/1067.48 | 0.311 | 4.03 | 4.450 | (49.6%) | |
| 5 | −4.31 | −3.50 | 0.81 | 1.11/1118.29 | 0.295 | 3.91 | 5.361 | (41.3%) | |
| ∞ | 0.67 | ||||||||
| P16 | 1 | −5.42 | −3.69 | 1.73 | 1.83/678.57 | 0.010 | 7.13 | 0.446 | (96.1%) |
| 2 | −4.97 | −3.91 | 1.06 | 1.20/1036.29 | 0.140 | 12.52 | 1.118 | (89.5%) | |
| 3 | −4.79 | −4.01 | 0.78 | 0.95/1303.57 | 0.169 | 15.63 | 2.046 | (84.5%) | |
| 4 | −4.68 | −4.06 | 0.62 | 0.80/1559.70 | 0.179 | 21.60 | 2.955 | (78.5%) | |
| 5 | −4.60 | −4.10 | 0.50 | 0.69/1808.73 | 0.182 | 25.73 | 3.755 | (73.7%) | |
| ∞ | 0.25 | ||||||||
| P18 | 1 | −5.06 | −3.75 | 1.31 | 1.48/844.99 | 0.170 | 5.83 | 0.575 | (91.7%) |
| 2 | −4.79 | −3.84 | 0.95 | 1.18/1053.47 | 0.230 | 5.88 | 1.799 | (75.0%) | |
| 3 | −4.70 | −3.89 | 0.81 | 1.07/1163.67 | 0.250 | 8.07 | 3.157 | (65.2%) | |
| 4 | −4.66 | −3.91 | 0.75 | 1.01/1224.89 | 0.261 | 7.53 | 4.469 | (54.7%) | |
| 5 | −4.64 | −3.92 | 0.72 | 0.98/1260.96 | 0.269 | 8.66 | 5.788 | (46.2%) | |
| ∞ | 0.57 | ||||||||
| P19 | 1 | −5.03 | −3.77 | 1.26 | 1.40/885.14 | 0.151 | 6.18 | 0.590 | (92.1%) |
| 2 | −4.74 | −3.86 | 0.88 | 1.10/1123.60 | 0.082 | 11.19 | 1.868 | (74.4%) | |
| 3 | −4.65 | −3.92 | 0.73 | 0.97/1283.29 | 0.185 | 13.04 | 3.269 | (65.4%) | |
| 4 | −4.59 | −3.92 | 0.67 | 0.91/1357.46 | 0.218 | 19.16 | 4.404 | (54.4%) | |
| 5 | −4.60 | −3.97 | 0.63 | 0.88/1408.97 | 0.255 | 9.47 | 6.125 | (47.3%) | |
| ∞ | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorlus, T.A.; Roy, J.K.; Leszczynski, J. Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study. Molecules 2024, 29, 1580. https://doi.org/10.3390/molecules29071580
Dorlus TA, Roy JK, Leszczynski J. Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study. Molecules. 2024; 29(7):1580. https://doi.org/10.3390/molecules29071580
Chicago/Turabian StyleDorlus, Taylor A., Juganta K. Roy, and Jerzy Leszczynski. 2024. "Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study" Molecules 29, no. 7: 1580. https://doi.org/10.3390/molecules29071580
APA StyleDorlus, T. A., Roy, J. K., & Leszczynski, J. (2024). Designing Thiadiazoloquinoxaline-Based Conjugated Polymers for Efficient Organic Photovoltaics: A DFT/TDDFT Study. Molecules, 29(7), 1580. https://doi.org/10.3390/molecules29071580









