Inhibition of Proliferation and Induction of Apoptosis in Prostatic Carcinoma DU145 Cells by Polysaccharides from Yunnan Rosa roxburghii Tratt
Abstract
1. Introduction
2. Results
2.1. Impact of Single Factors on Polysaccharide Extraction Rate in Rosa roxburghii Tratt
2.1.1. Material–Liquid Ratio
2.1.2. Extraction Temperature
2.1.3. Extraction Time
2.1.4. Extracting Frequency
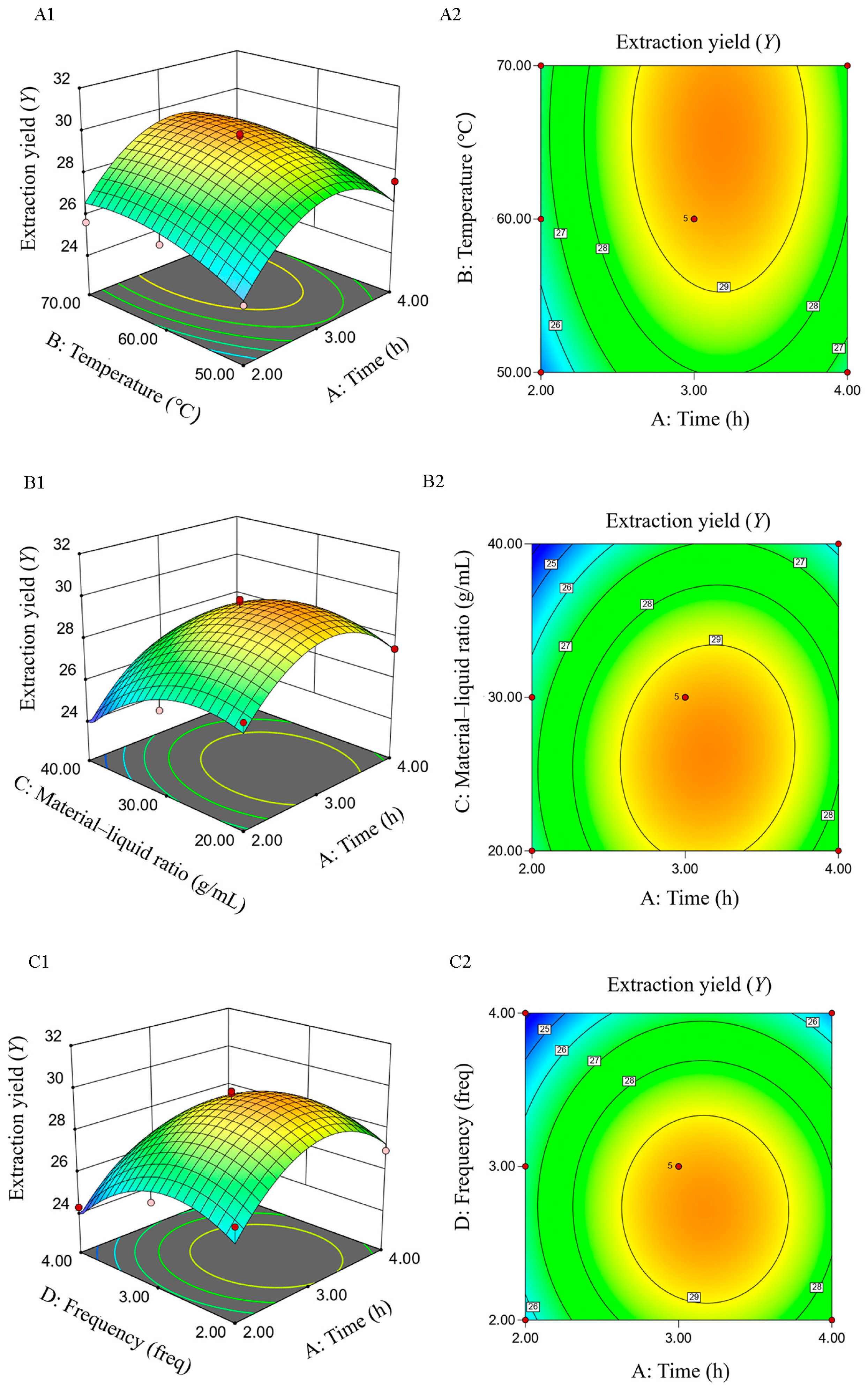
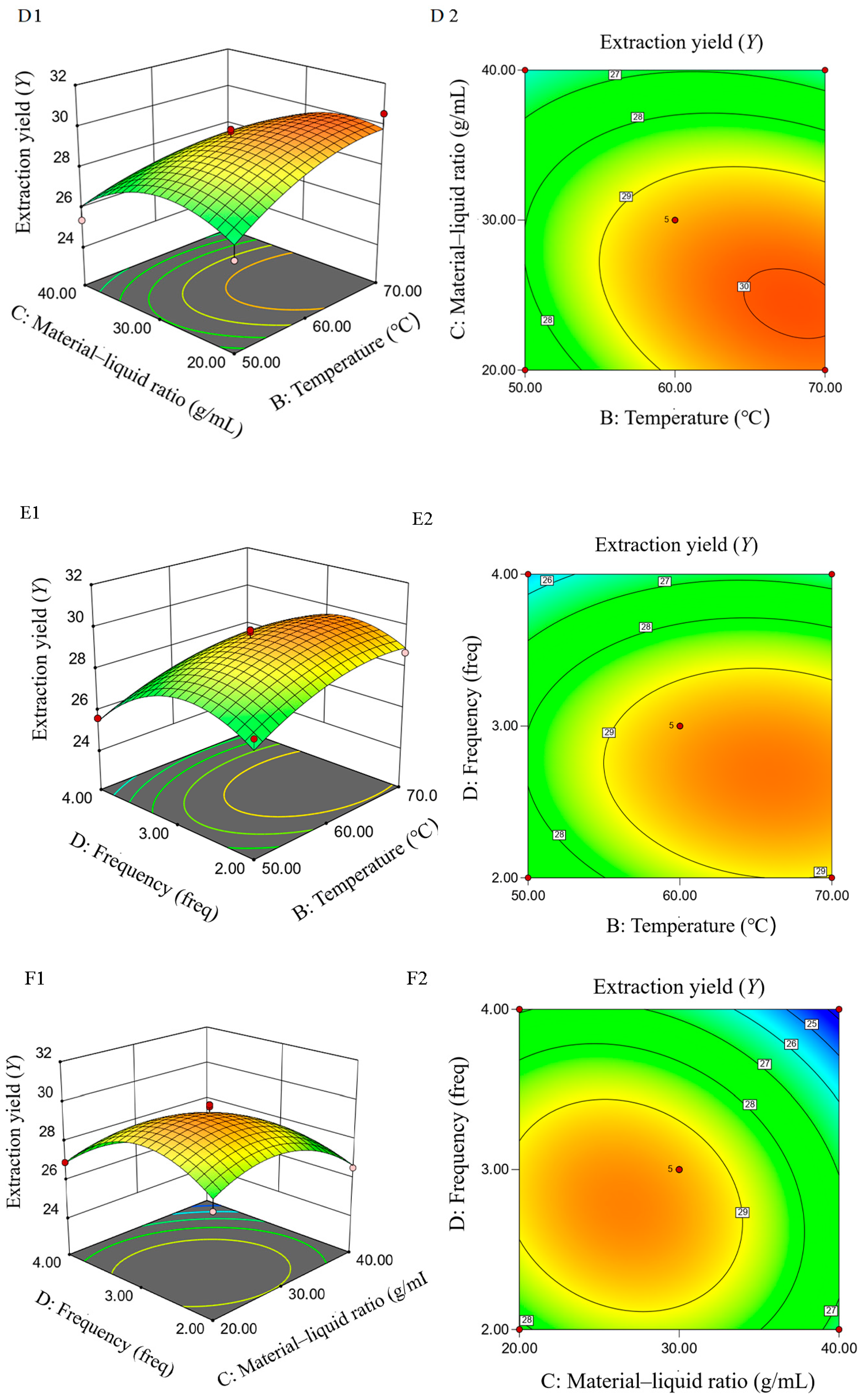
2.1.5. Response-Surface Test
2.1.6. Verification Test
2.2. Structural Characterization of RTDP
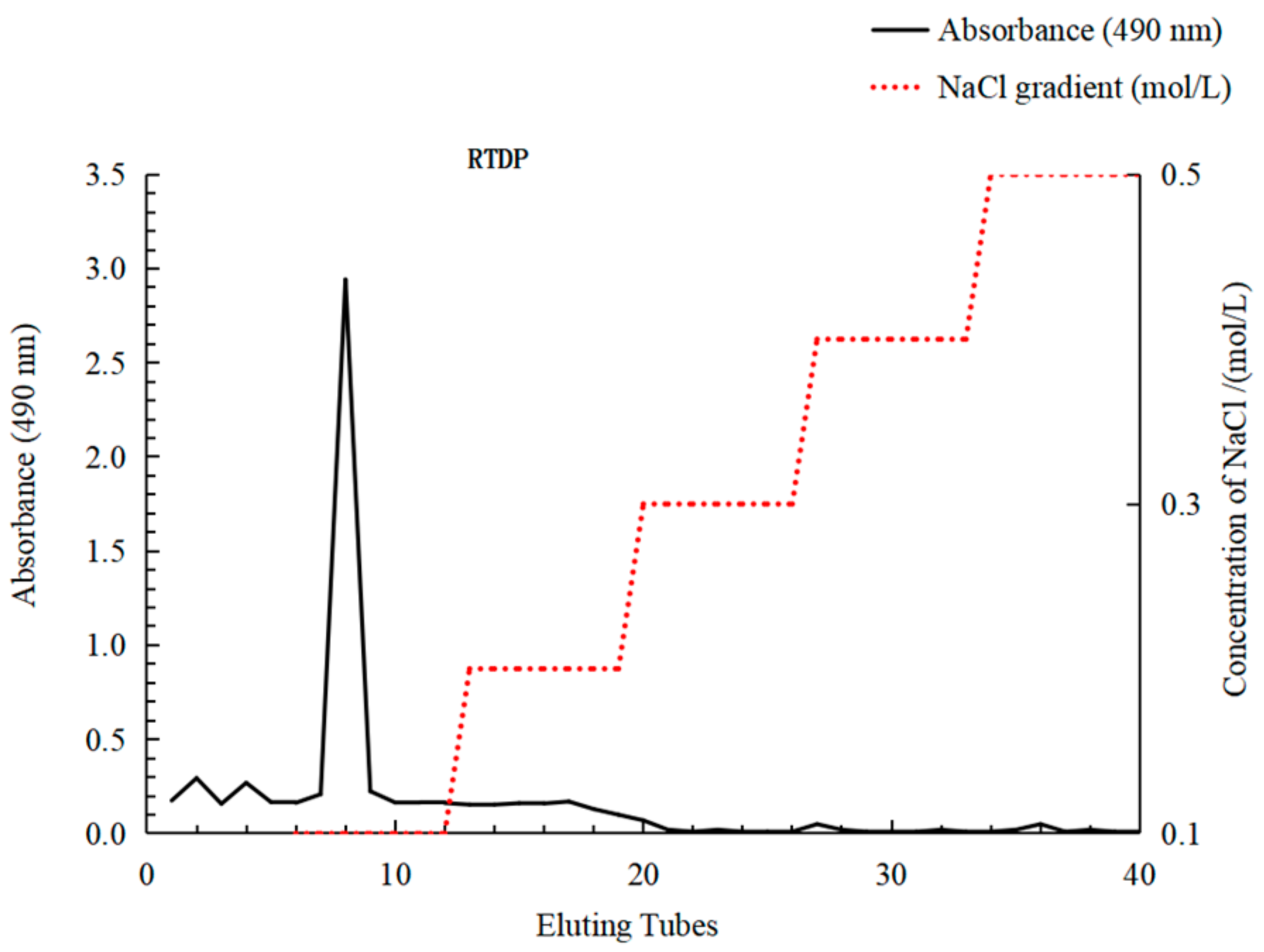
2.2.1. Isolation and Purification of RTDP
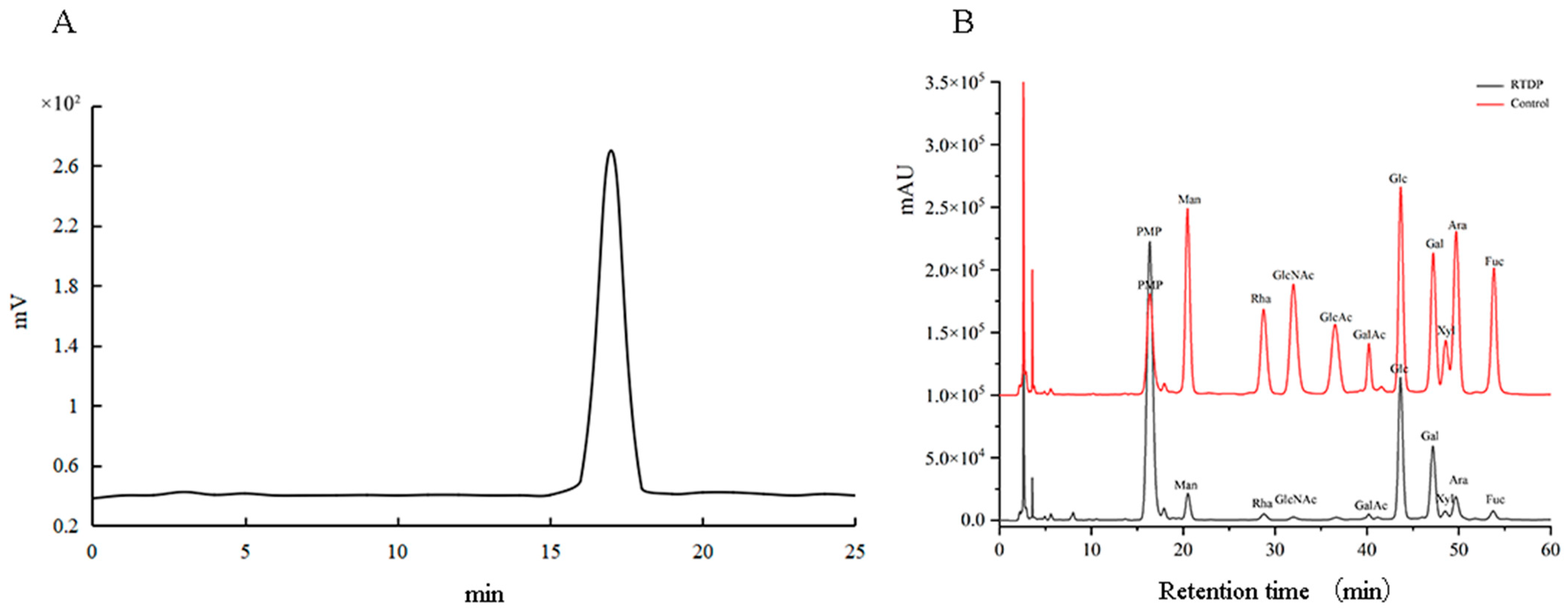
2.2.2. Analysis of Molecular Weight and Monosaccharide Composition
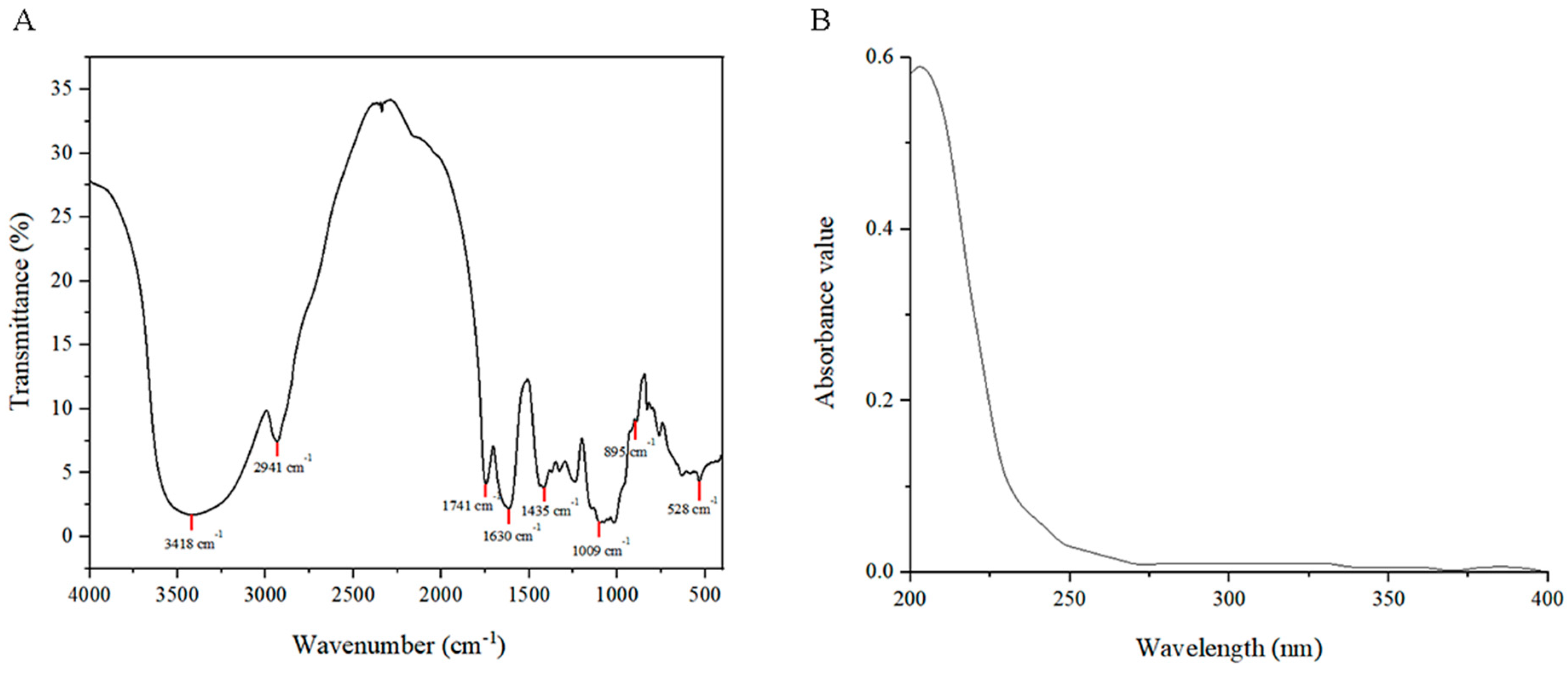
2.2.3. FT-IR and Ultraviolet–Visible Spectroscopy
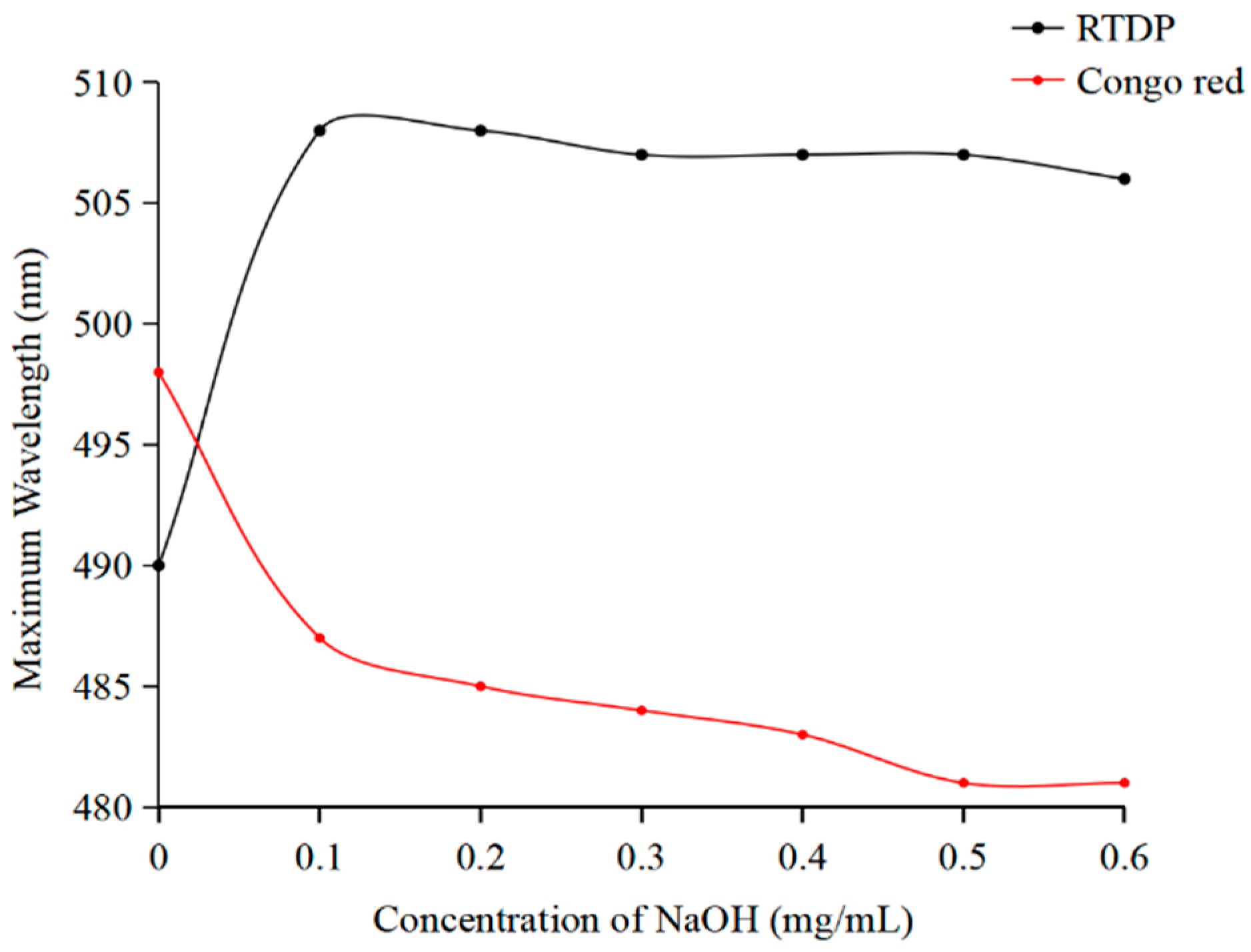
2.2.4. Congo Red Test
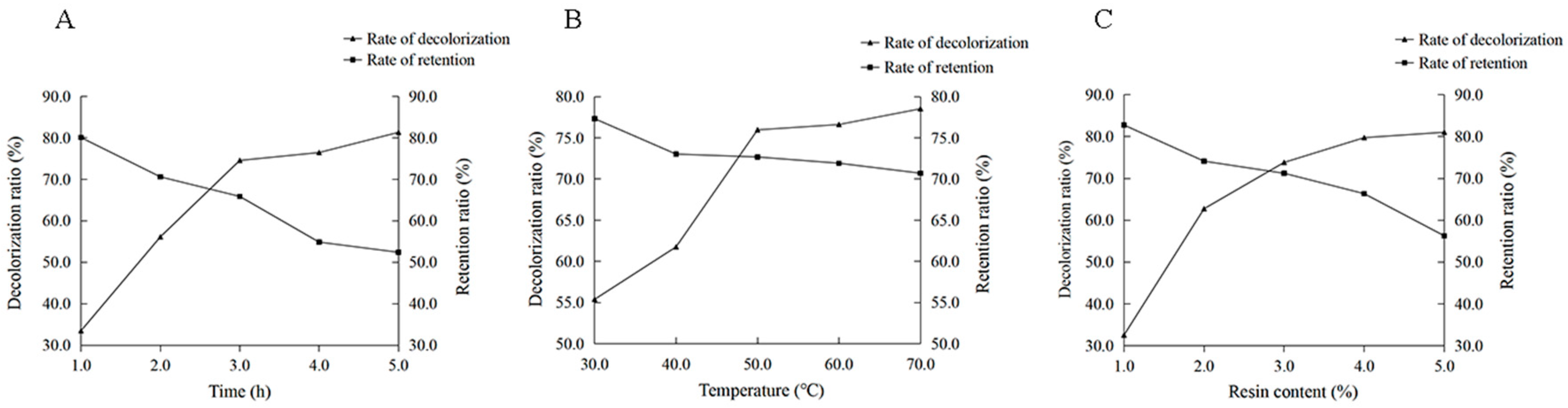
2.2.5. Effects of Decolorization Factors on the Decolorization Rate and Polysaccharide Retention Rate of Rosa roxburghii Tratt Polysaccharide
2.3. Results of the Response Surface Optimization Methodology
2.3.1. Response Surface Test Design and Results
2.3.2. Establishment of Fitting Model and Data Analysis
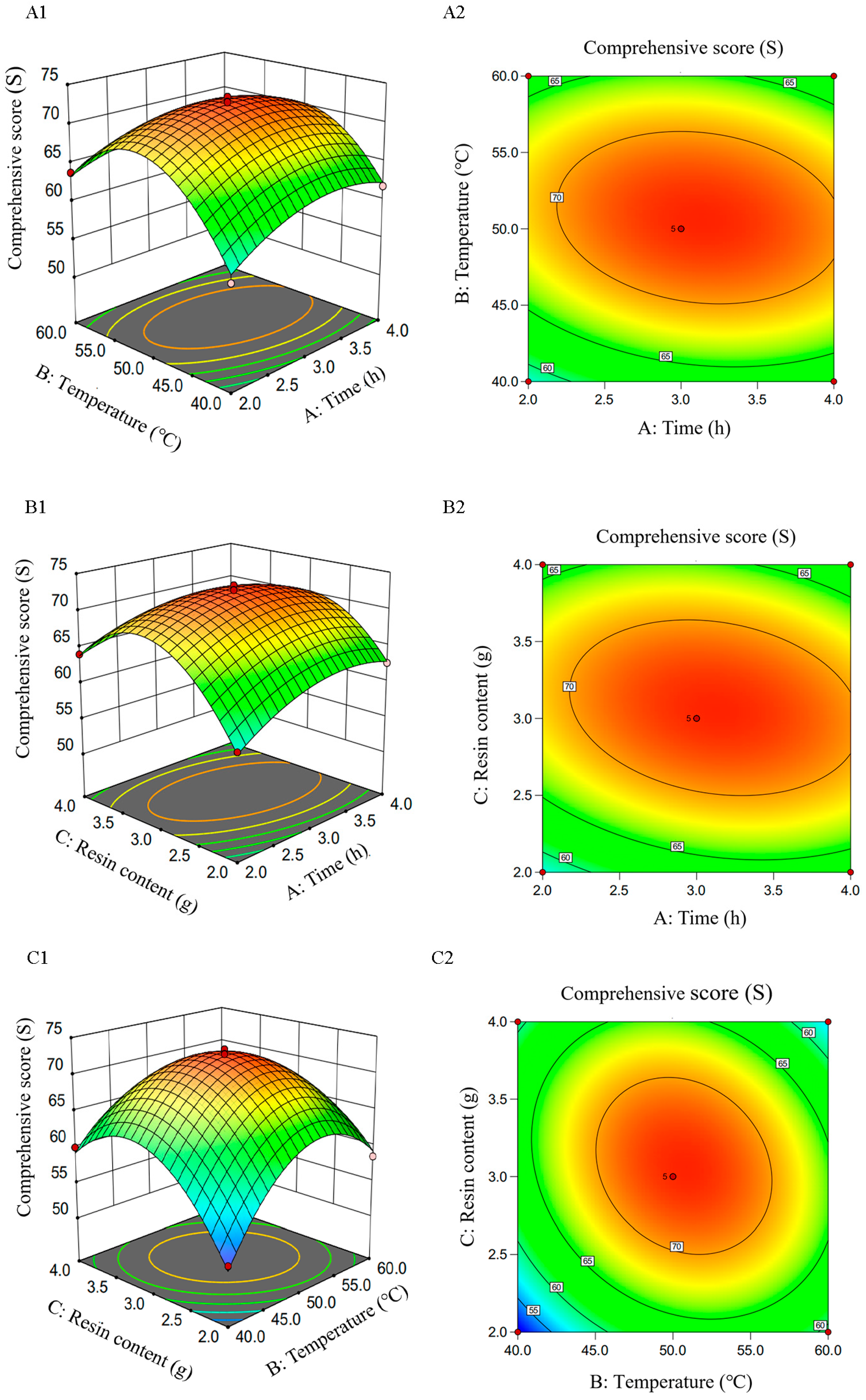
2.3.3. The Interaction Effect of Each Factor on the Comprehensive Score of Decolorizing Rate and Polysaccharide Retention Rate from RTDP
2.4. Verification Test
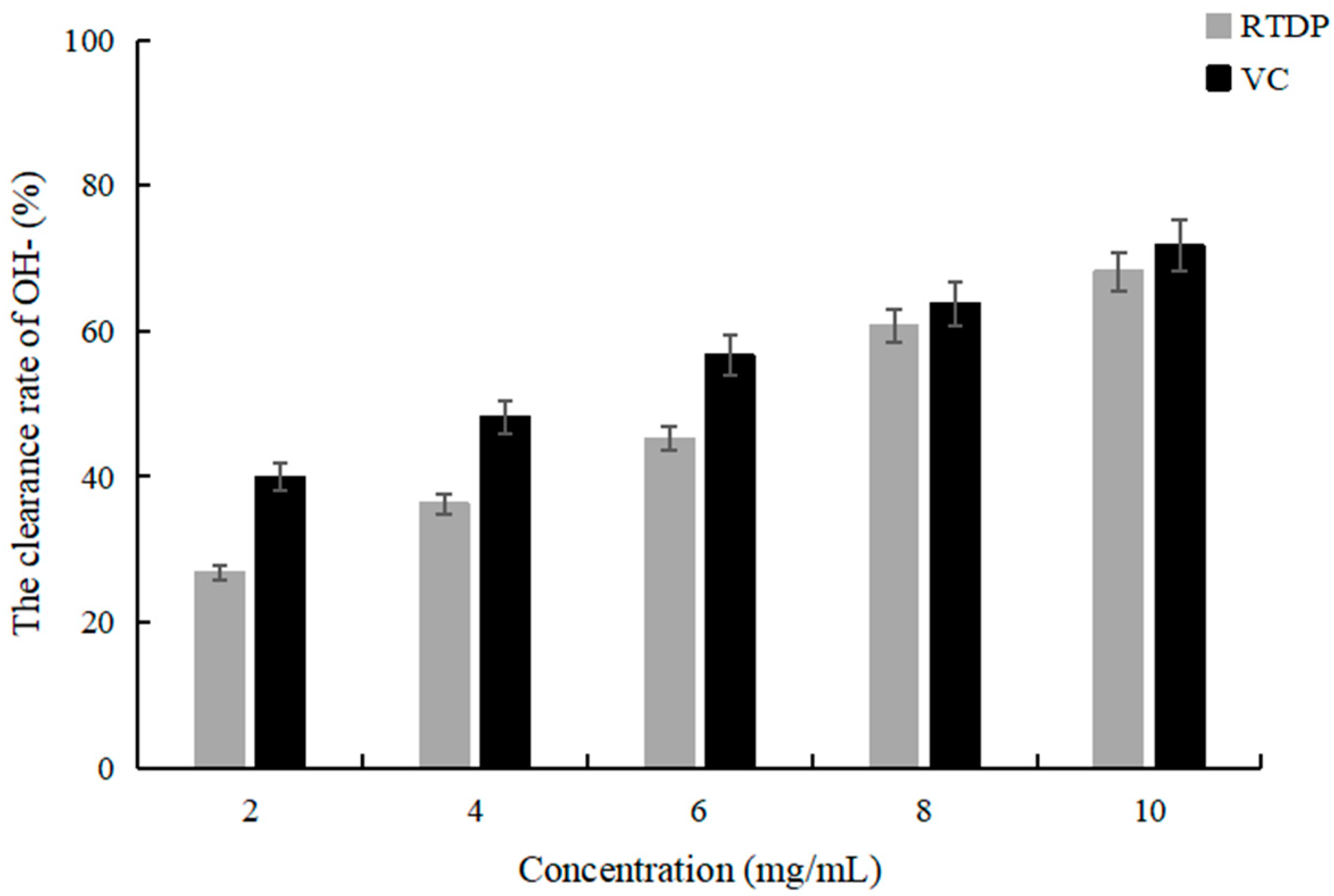
2.5. Detection of the OH− Scavenging Activity of Rosa roxburghii Tratt Polysaccharide
2.6. DU145 Cells Affected by RTDP
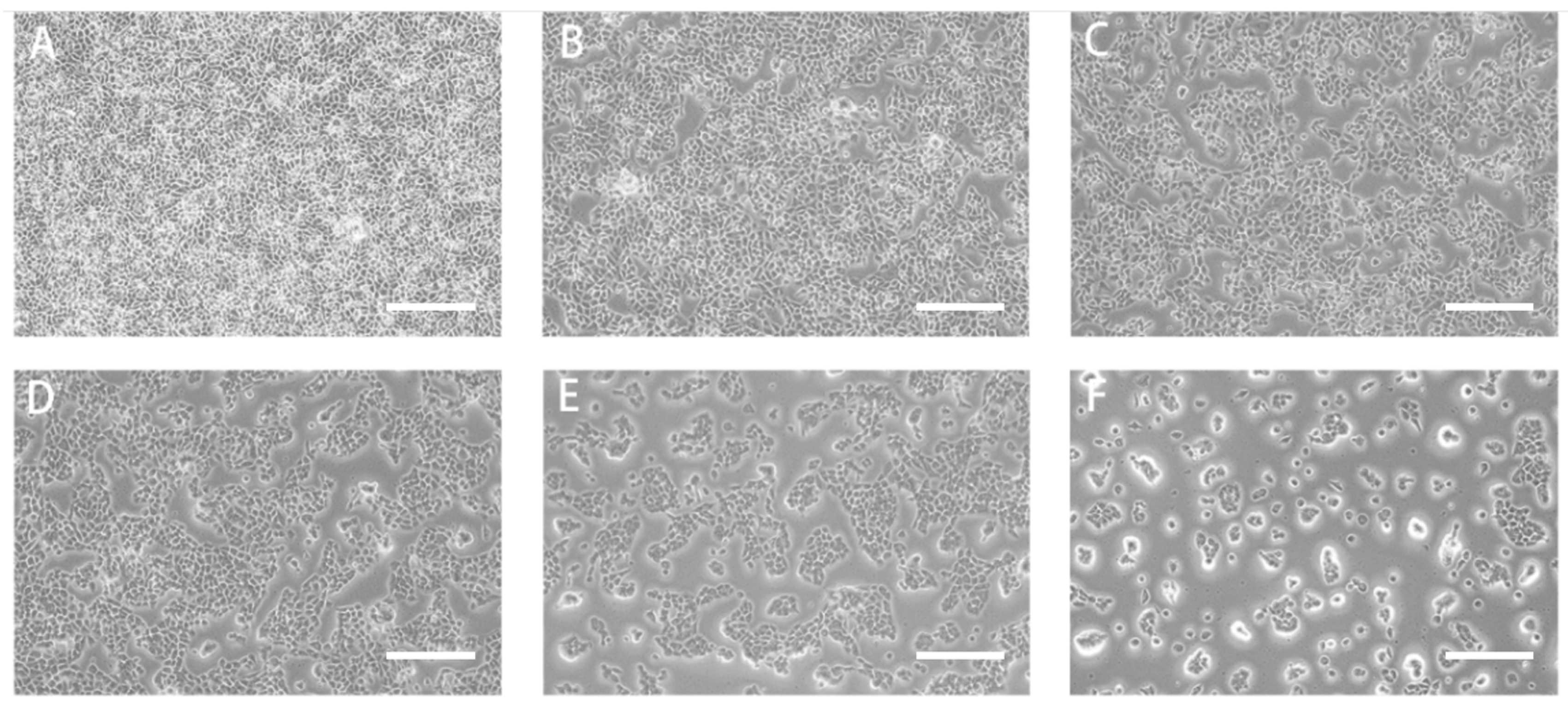
2.6.1. RTDP Decreased the Survival Rate of DU145 Cells
2.6.2. RTDP Inhibited the Migration of DU145 Cells
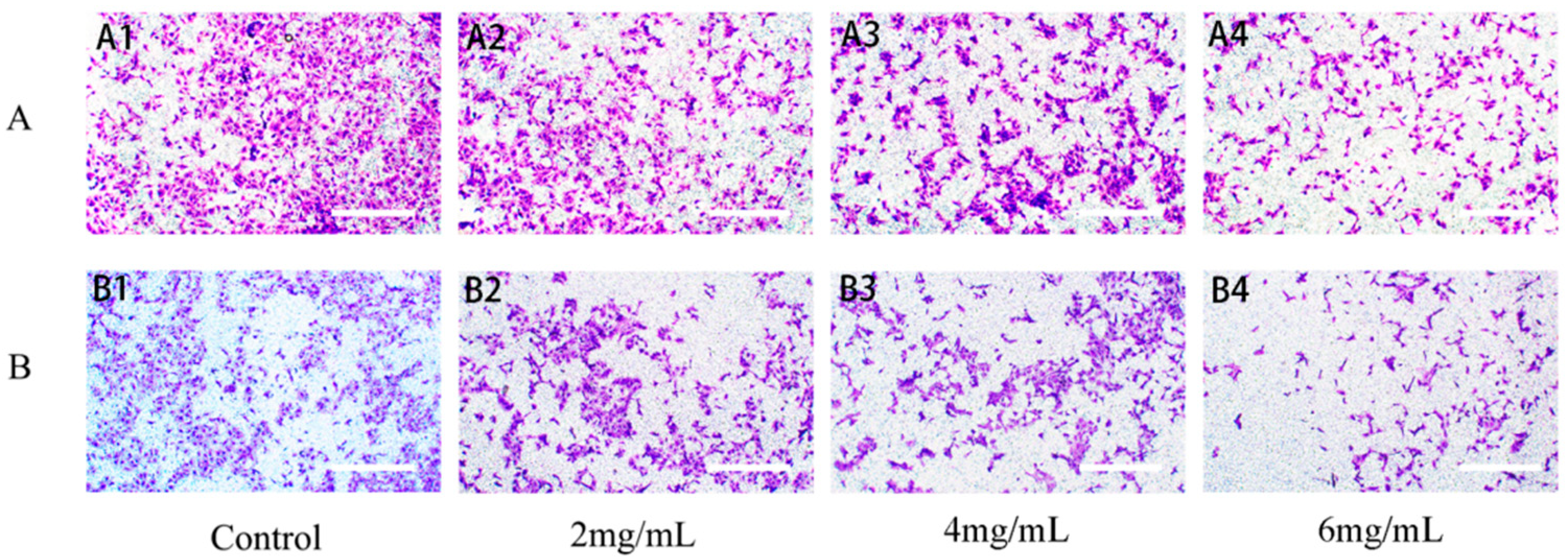
2.6.3. RTDP Inhibited the Invasion of DU145 Cells
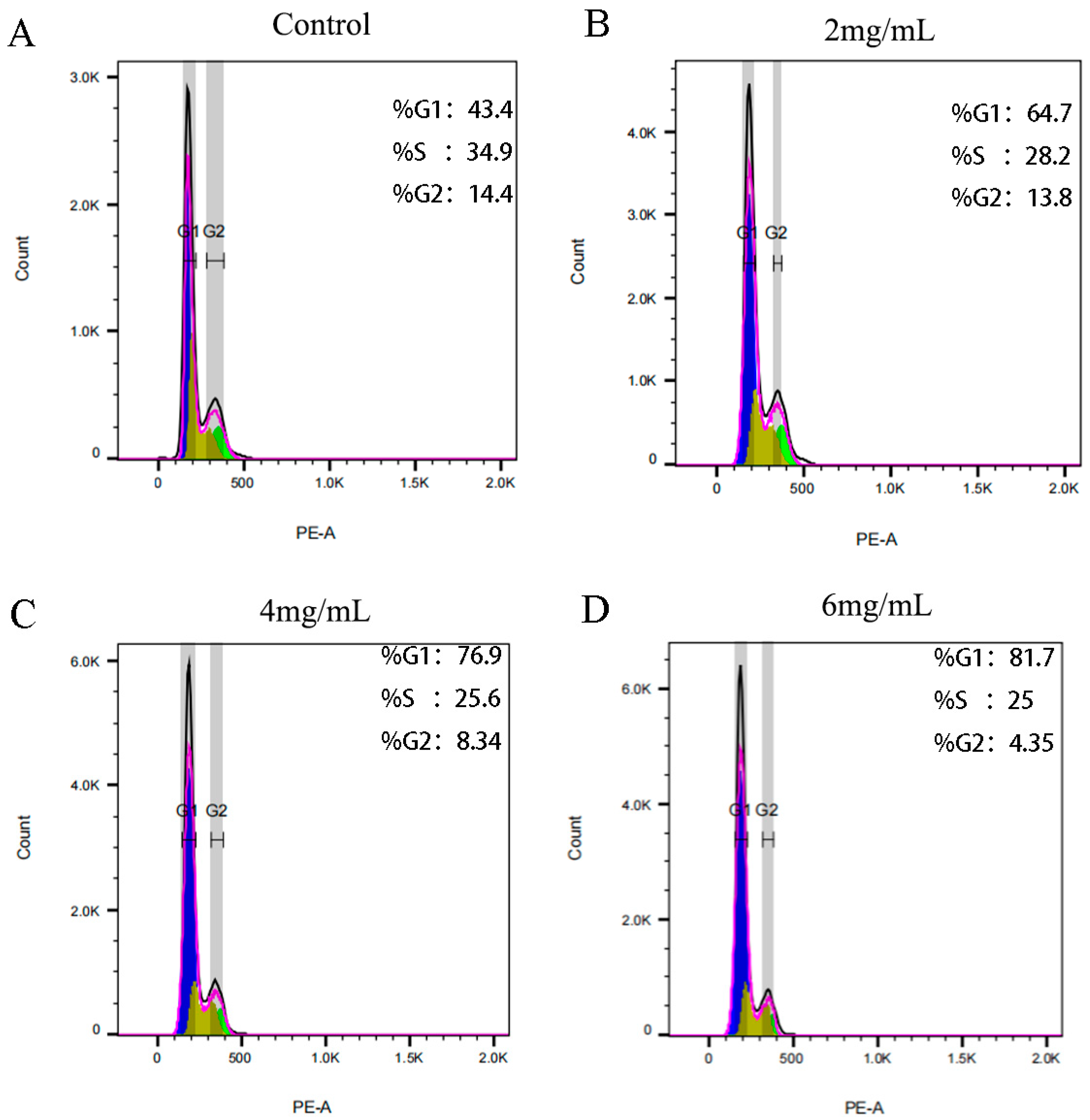
2.6.4. RTDP Inhibited the Cell Cycle of DU145 Cells
2.6.5. Induction of Apoptosis in DU145 Cells by RTDP
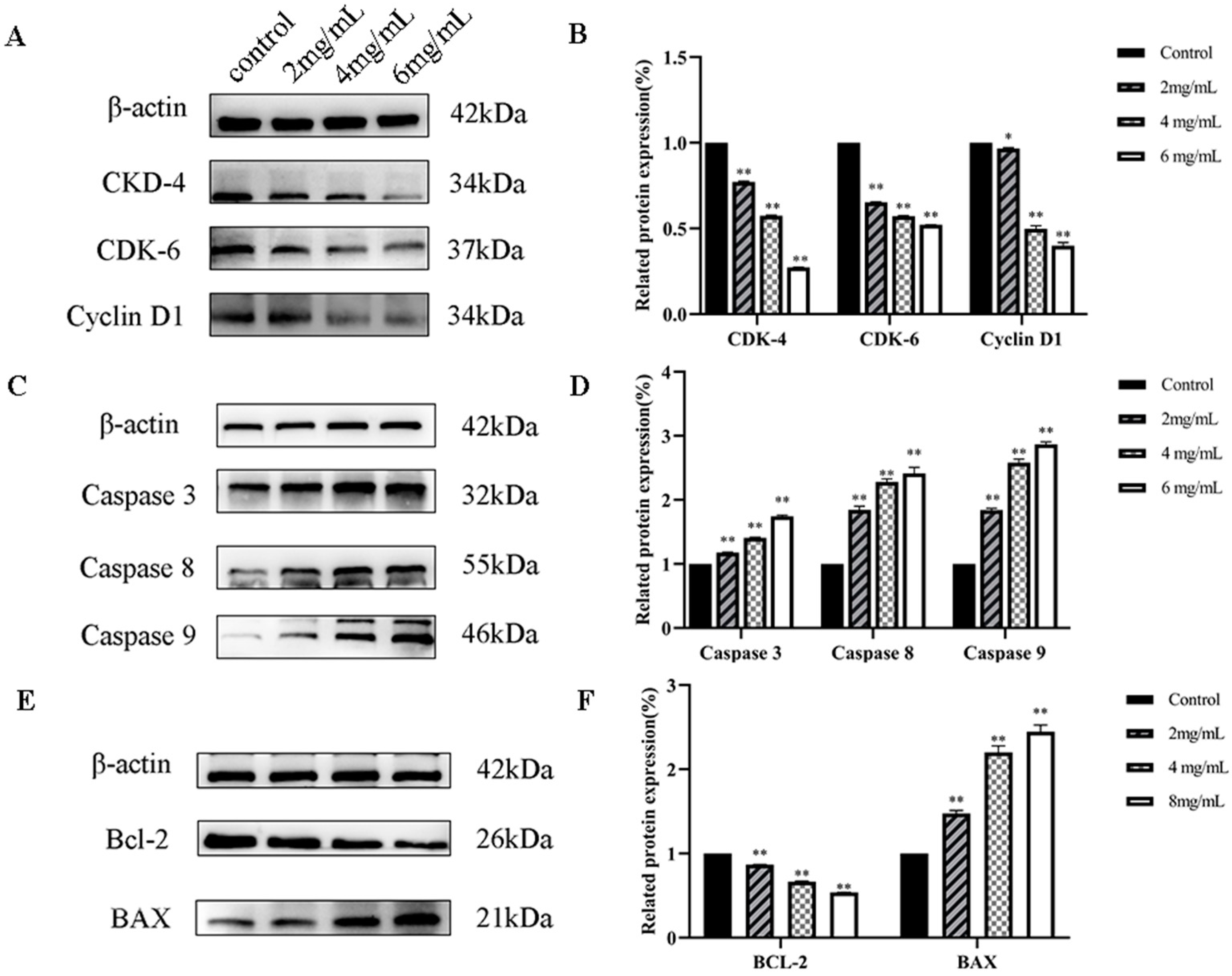
2.6.6. RTDP Effect on the Expression of Cell Cycle and Apoptosis-Related Proteins in Prostate Cancer DU145 Cell Assessed by Western Blotting
2.6.7. The Effects of RTDP on the Expression of DU145 Cell Cycle and Apoptosis-Related Genes Detected by Quantitative Real-Time PCR (RT-qPCR)
3. Materials and Methods
3.1. Instruments
3.2. Reagents
3.3. Methods
3.3.1. Extraction and Decoloration of Rosa roxburghii Tratt Polysaccharides
3.3.2. Isolation and Purification of the Polysaccharides
3.3.3. Determination of Molecular Weight and Monosaccharide Composition Analysis
3.3.4. FT-IR and Ultraviolet–Visible Spectroscopy
3.3.5. Congo Red Test
3.3.6. Preparation of the Glucose Standard Curve
3.3.7. Determination of the Extraction Yield of Polysaccharides from Rosa roxburghii Tratt
3.3.8. Single-Factor Experiment on Water Extraction and Alcohol Precipitation
3.4. Response-Surface Design for Polysaccharide Extraction
3.4.1. Resin Pretreatment
3.4.2. Pigment Removal from Rosa roxburghii Tratt Polysaccharides
3.4.3. Determination and Calculation of Decolorization Rate
3.4.4. Determination and Calculation of Polysaccharide Retention Rate
3.4.5. Comprehensive Score
3.4.6. Single-Factor Test
3.5. Response Surface Test Design for the Decolorization of Polysaccharide
3.6. The Antioxidant Activity of Rosa roxburghii Tratt Polysaccharide
3.7. Cell Culture
3.7.1. Cell Treatment
3.7.2. Cell Viability Assessment via CCK-8 Assay
3.7.3. Migration Rate Assessment via Scratch Assay
3.7.4. Invasiveness Rate of DU145 Cells Assessed by Transwell Assay
3.7.5. Cell Cycle Analysis of DU145 Cells by Flow Cytometry
3.7.6. Apoptosis Detection in DU145 Cells by Flow Cytometry
3.7.7. Detection of Cell Cycle and Apoptosis-Related Proteins in DU145 Cells by Western Blotting
3.7.8. Analysis of Cell Cycle and Apoptosis-Related Proteins in DU145 Cells by Quantitative Real-Time PCR
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, H.; Li, M.; Tian, F.; Yu, F.; Zhao, W. An Overview of Antitumour Activity of Polysaccharides. Molecules 2022, 27, 8083. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Half, E.E. Update on Screening for Urological Malignancies. Rambam Maimonides Med. J. 2017, 8, e0041. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, Z.; Liu, W.; Xiong, J.; Shi, Y.; Yang, L.; Gao, Q.; Le, K.; Zhang, B. RCC2 promotes prostate cancer cell proliferation and migration through Hh/GLI1 signaling pathway and cancer stem-like cells. Biol. Direct 2023, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Santer, F.R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014, 33, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Vyas, A.; Deshpande, K.; Vyas, D. Pharmacological cyclin dependent kinase inhibitors: Implications for colorectal cancer. World J. Gastroenterol. 2016, 22, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Golomb, L.; Meyerson, M. Functional Genomic Analysis of CDK4 and CDK6 Gene Dependency across Human Cancer Cell Lines. Cancer Res. 2022, 82, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Jiggens, E.; Mortoglou, M.; Grant, G.H.; Uysal-Onganer, P. The Role of CDK4 in the Pathogenesis of Pancreatic Cancer. Healthcare 2021, 9, 1478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wang, X.; Dong, H.; Min, W.; Hao, H.; Yang, P. Selective inhibition of CDK4/6: A safe and effective strategy for developing anticancer drugs. Acta Pharm. Sin. B 2021, 11, 30–54. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef]
- Romero-Pozuelo, J.; Figlia, G.; Kaya, O.; Martin-Villalba, A.; Teleman, A.A. Cdk4 and Cdk6 Couple the Cell-Cycle Machinery to Cell Growth via mTORC1. Cell Rep. 2020, 31, 107504. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Cui, Y.; Zhou, J.; Qin, X.; Zhang, L.; Li, X.; Li, Y.; Guo, E.; Yang, B.; et al. The Immunological Role of CDK4/6 and Potential Mechanism Exploration in Ovarian Cancer. Front. Immunol. 2021, 12, 799171. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, T.; Wang, F.; Hu, X.; Zhan, G.; Jin, X.; Zhang, L.; Li, Y. NUP37 silencing induces inhibition of cell proliferation, G1 phase cell cycle arrest and apoptosis in non-small cell lung cancer cells. Pathol. Res. Pract. 2020, 216, 152836. [Google Scholar] [CrossRef] [PubMed]
- Zu, M.L.; Duan, Y.; Xie, J.B.; Qi, Y.S.; Xie, P.; Borjigidai, A.; Piao, X.L. Gypenoside LI arrests the cell cycle of breast cancer in G0/G1 phase by down-regulating E2F1. J. Ethnopharmacol. 2021, 273, 114017. [Google Scholar] [CrossRef] [PubMed]
- Fann, L.Y.; Shih, J.H.; Tseng, J.H.; Huang, H.S.; Hsiao, S.H. CC12 Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Lines and Mouse Xenograft Model. Molecules 2020, 25, 1793. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, L.; Zhu, D.; Zhang, M.; Wang, K.; Chen, F. An effector caspase Sp-caspase first identified in mud crab Scylla paramamosain exhibiting immune response and cell apoptosis. Fish Shellfish. Immunol. 2020, 103, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Allani, M.; Akhilesh; Tiwari, V. Caspase-driven cancer therapies: Navigating the bridge between lab discoveries and clinical applications. Cell Biochem. Funct. 2024, 42, e3944. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.D.; Sørensen, C.S. The caspase-activated DNase: Apoptosis and beyond. FEBS J. 2017, 284, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Gubina, N.; Leboeuf, D.; Piatkov, K.; Pyatkov, M. Novel Apoptotic Mediators Identified by Conservation of Vertebrate Caspase Targets. Biomolecules 2020, 10, 612. [Google Scholar] [CrossRef]
- Flütsch, A.; Ackermann, R.; Schroeder, T.; Lukarska, M.; Hausammann, G.J.; Weinert, C.; Briand, C.; Grütter, M.G. Combined inhibition of caspase 3 and caspase 7 by two highly selective DARPins slows down cellular demise. Biochem. J. 2014, 461, 279–290. [Google Scholar] [CrossRef]
- Rodríguez-Berriguete, G.; Torrealba, N.; Ortega, M.A.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; Guil-Cid, M.; Fraile, B.; Royuela, M. Prognostic value of inhibitors of apoptosis proteins (IAPs) and caspases in prostate cancer: Caspase-3 forms and XIAP predict biochemical progression after radical prostatectomy. BMC Cancer 2015, 15, 809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Y.; Liu, K.; Liu, B.; Xu, W.; Gao, J.; Ding, L.; Tao, L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019, 52, e12543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, D.; Luo, Q.; Li, J.; Liu, J. Pterostilbene Inhibits Human Renal Cell Carcinoma Cells Growth and Induces DNA Damage. Biol. Pharm. Bull. 2020, 43, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, K.; Gao, F.; Huang, J.; Guan, X. Clinical considerations of CDK4/6 inhibitors in triple-negative breast cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188590. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.; Brady, M.F. BAX Gene. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Park, S.Y.; Gurung, R.; Hwang, J.H.; Kang, J.H.; Jung, H.J.; Zeb, A.; Hwang, J.I.; Park, S.J.; Maeng, H.J.; Shin, D.; et al. Development of KEAP1-targeting PROTAC and its antioxidant properties: In vitro and in vivo. Redox Biol. 2023, 64, 102783. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Zhang, X.; Yu, S.; Li, X.; Wen, X.; Liu, S.; Zu, R.; Ren, H.; Li, T.; Yang, C.; Luo, H. Research progress on the interaction between oxidative stress and platelets: Another avenue for cancer? Pharmacol. Res. 2023, 191, 106777. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [PubMed]
- Tang, Z.; Huang, G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Ding, Y.; Cheng, J.; Yang, S.; Ding, Z.; Dai, Q.; Ding, Z. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2019, 124, 972–980. [Google Scholar] [CrossRef]
- Yiasmin, M.N.; Islam, M.S.; He, H.; Liu, Y.; Wang, M.; Yang, R.; Hua, X. Purification, isolation, and structure characterization of water soluble and insoluble polysaccharides from Maitake fruiting body. Int. J. Biol. Macromol. 2020, 164, 1879–1888. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Z.; Zou, Y.; Sun, X.; Huang, G. Extraction and deproteinization process of polysaccharide from purple sweet potato. Chem. Biol. Drug Des. 2022, 99, 111–117. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, L.; Wang, Y.; Wan, P.; Zhou, H. Basic characterization, antioxidant and immunomodulatory activities of polysaccharides from sea buckthorn leaves. Fitoterapia 2023, 169, 105592. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Jiao, C.; Tong, J.; Zhang, L.; Chang, Y.; Sun, W.; Jin, Q.; Cai, Y. Optimization of quercetin extraction method in Dendrobium officinale by response surface methodology. Heliyon 2019, 5, e02374. [Google Scholar] [CrossRef]
- Ma, L.; Bian, M.; Gao, H.; Zhou, Z.; Yi, W. A novel 3-acyl isoquinolin-1(2H)-one induces G2 phase arrest, apoptosis and GSDME-dependent pyroptosis in breast cancer. PLoS ONE 2022, 17, e0268060. [Google Scholar] [CrossRef]
- Guo, J.; Chen, W.; Bao, B.; Zhang, D.; Pan, J.; Zhang, M. Protective effect of berberine against LPS-induced endothelial cell injury via the JNK signaling pathway and autophagic mechanisms. Bioengineered 2021, 12, 1324–1337. [Google Scholar] [CrossRef]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The antioxidant activities of balsam pear polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef]
- Abot, A.; Fried, S.; Cani, P.D.; Knauf, C. Reactive Oxygen Species/Reactive Nitrogen Species as Messengers in the Gut: Impact on Physiology and Metabolic Disorders. Antioxid. Redox Signal. 2022, 37, 394–415. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Silva, D.C.; Freitas, A.L.P.; Barros, F.C.N.; Lins, K.; Alves, A.; Alencar, N.M.N.; de Figueiredo, I.S.T.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; et al. Polysaccharide isolated from Passiflora edulis: Characterization and antitumor properties. Carbohydr. Polym. 2012, 87, 139–145. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, B.; Yan, J.; Zhao, F.; Xiao, J.; Yao, L.; Zhao, B.; Huang, Y. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr. Polym. 2012, 87, 392–396. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, S.; Su, J.; Xu, Y.; Ordaz-Ortiz, J.J.; Li, N.; Wu, J.; Wang, H.; Wang, S. Structural characterization of a heteropolysaccharide from fruit of Chaenomelese speciosa (Sweet) Nakai and its antitumor activity. Carbohydr. Polym. 2020, 236, 116065. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Xie, H.; Li, X.; Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus mori. Carbohydr. Polym. 2018, 190, 232–239. [Google Scholar] [CrossRef]
- Zeng, C.; Luo, S.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Phenolic Composition, Antioxidant and Anticancer Potentials of Extracts from Rosa banksiae Ait. Flowers. Molecules 2020, 25, 3068. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef]
- Leng, X.; Li, J.; Miao, W.; Liu, Y.; Haider, M.S.; Song, M.; Fang, J.; Li, Q. Comparison of physicochemical characteristics, antioxidant and immunomodulatory activities of polysaccharides from wine grapes. Int. J. Biol. Macromol. 2023, 239, 124164. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and Anti-Diabetic Activities of Polysaccharides from Guava Leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Reis, M.B.E.; Maximo, A.I.; Magno, J.M.; de Lima Bellan, D.; Buzzo, J.L.A.; Simas, F.F.; Rocha, H.A.O.; da Silva Trindade, E.; Camargo de Oliveira, C. A Fucose-Containing Sulfated Polysaccharide from Spatoglossum schröederi Potentially Targets Tumor Growth Rather Than Cytotoxicity: Distinguishing Action on Human Melanoma Cell Lines. Mar. Biotechnol. 2024, 26, 181–198. [Google Scholar] [CrossRef]
- Yücel, N.T.; Asfour, A.A.R.; Evren, A.E.; Yazıcı, C.; Kandemir, Ü.; Özkay, Ü.D.; Can, Ö.D.; Yurttaş, L. Design and synthesis of novel dithiazole carboxylic acid Derivatives: In vivo and in silico investigation of their Anti-Inflammatory and analgesic effects. Bioorganic Chem. 2024, 144, 107120. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Zeyaullah, M.; Alsayegh, A.A.; Mahmood, S.E.; AlShahrani, A.M.; Khan, M.S.; Shama, E.; Hamouda, A.; Elbendary, E.Y.; et al. Ganoderma lucidum: Novel Insight into Hepatoprotective Potential with Mechanisms of Action. Nutrients 2023, 15, 1874. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Nagahawatta, D.P.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Jayawrdhana, H.; Kim, J.I.; Jeon, Y.J. Sulfated Polysaccharides from Seaweeds: A Promising Strategy for Combatting Viral Diseases-A Review. Mar. Drugs 2023, 21, 461. [Google Scholar] [CrossRef]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Algae Food Products as a Healthcare Solution. Mar. Drugs 2023, 21, 578. [Google Scholar] [CrossRef]
- Wang, L.; Wei, T.; Zheng, L.; Jiang, F.; Ma, W.; Lu, M.; Wu, X.; An, H. Recent Advances on Main Active Ingredients, Pharmacological Activities of Rosa roxbughii and Its Development and Utilization. Foods 2023, 12, 1051. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, Y.; Shi, Y.; Feng, D.; Zuo, Y.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods 2022, 11, 4108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Liu, X.; Li, J.; Zhang, J.; Liu, D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chin. Herb. Med. 2022, 14, 187–209. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, Y.; Zhang, S.; Bao, J.; Wang, H.; Dong, C.; Ohizumi, Y.; Xu, J.; Guo, Y. Structural analysis and biological effects of a neutral polysaccharide from the fruits of Rosa laevigata. Carbohydr. Polym. 2021, 265, 118080. [Google Scholar] [CrossRef]
- Zhai, B.W.; Zhao, H.; Zhu, H.L.; Huang, H.; Zhang, M.Y.; Fu, Y.J. Triterpene acids from Rosa roxburghii Tratt fruits exert anti-hepatocellular carcinoma activity via ROS/JNK signaling pathway-mediated cell cycle arrest and mitochondrial apoptosis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 119, 154960. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zhang, J.; Guo, J.P.; Niu, Y.P. Effects of Rosa roxburghii on insulin resistance in obese rats and its mechanisms. Chin. J. Appl. Physiol. 2022, 38, 670–675. [Google Scholar]
- Wu, P.H.; Han, S.C.; Wu, M.H. Beneficial Effects of Hydroalcoholic Extract from Rosa Roxburghii Tratt Fruit on Hyperlipidemia in High-Fat-Fed Rats. Acta Cardiol. Sin. 2020, 36, 148–159. [Google Scholar] [PubMed]
- Yuan, H.; Wang, Y.; Chen, H.; Cai, X. Protective effect of flavonoids from Rosa roxburghii Tratt on myocardial cells via autophagy. 3 Biotech 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, N.; Wu, Z.; Feng, Y.; Meng, X. Anti-tumor activity of a polysaccharide from blueberry. Molecules 2015, 20, 3841–3853. [Google Scholar] [CrossRef] [PubMed]




| Serial Numbers | A | B | C | D | Y/% (Extraction Yield) |
|---|---|---|---|---|---|
| 1 | −1 | 1 | 0 | 0 | 25.64 |
| 2 | 0 | 0 | 1 | 1 | 24.19 |
| 3 | 1 | 0 | 0 | −1 | 26.96 |
| 4 | 0 | 0 | −1 | 1 | 26.94 |
| 5 | −1 | 0 | 1 | 0 | 25.97 |
| 6 | 0 | 0 | 0 | 0 | 29.85 |
| 7 | −1 | 0 | 0 | 1 | 24.29 |
| 8 | 0 | −1 | 0 | −1 | 27.46 |
| 9 | 0 | −1 | −1 | 0 | 26.34 |
| 10 | 1 | 0 | −1 | 0 | 27.45 |
| 11 | 0 | 0 | 0 | 0 | 29.74 |
| 12 | 0 | 0 | −1 | −1 | 27.24 |
| 13 | 0 | −1 | 0 | 1 | 25.62 |
| 14 | 1 | −1 | 0 | 0 | 27.53 |
| 15 | 1 | 0 | 1 | 0 | 25.32 |
| 16 | 0 | 1 | 1 | 0 | 27.15 |
| 17 | 0 | 0 | 0 | 0 | 28.95 |
| 18 | 0 | 1 | 0 | 1 | 25.98 |
| 19 | 1 | 1 | 0 | 0 | 28.28 |
| 20 | 0 | 1 | 0 | −1 | 28.72 |
| 21 | 0 | 0 | 1 | −1 | 26.55 |
| 22 | 0 | 0 | 0 | 0 | 29.84 |
| 23 | 0 | 1 | −1 | 0 | 30.56 |
| 24 | −1 | 0 | 0 | −1 | 26.35 |
| 25 | −1 | −1 | 0 | 0 | 24.73 |
| 26 | 0 | −1 | 1 | 0 | 25.38 |
| 27 | 0 | 0 | 0 | 0 | 29.18 |
| 28 | −1 | 0 | −1 | 0 | 26.89 |
| 29 | 1 | 0 | 0 | 1 | 24.58 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 80.96 | 14 | 5.78 | 10.18 | <0.0001 | ** |
| A | 6.31 | 1 | 6.31 | 11.11 | 0.0049 | ** |
| B | 7.16 | 1 | 7.16 | 12.61 | 0.0032 | ** |
| C | 12.06 | 1 | 12.06 | 21.24 | 0.0004 | ** |
| D | 11.37 | 1 | 11.37 | 20.02 | 0.0005 | ** |
| AB | 0.0064 | 1 | 0.0064 | 0.011 | 0.9170 | |
| AC | 0.18 | 1 | 0.18 | 0.31 | 0.5864 | |
| AD | 0.026 | 1 | 0.026 | 0.045 | 0.8349 | |
| BC | 1.5 | 1 | 1.5 | 2.64 | 0.1263 | |
| BD | 0.2 | 1 | 0.2 | 0.36 | 0.5599 | |
| CD | 1.06 | 1 | 1.06 | 1.87 | 0.1932 | |
| A2 | 30.1 | 1 | 30.1 | 53.01 | <0.0001 | ** |
| B2 | 3.28 | 1 | 3.28 | 5.77 | 0.0307 | * |
| C2 | 11.81 | 1 | 11.81 | 20.79 | 0.0004 | ** |
| D2 | 20.56 | 1 | 20.56 | 36.21 | <0.0001 | ** |
| Residual | 7.95 | 14 | 0.57 | |||
| Lack of Fit | 7.25 | 10 | 0.72 | 4.14 | 0.0915 | non-significant |
| Pure Error | 0.7 | 4 | 0.17 | |||
| Cor Total | 88.91 | 28 |
| Item | Value | Item | Value |
|---|---|---|---|
| Std.Dev. | 0.7535 | R-Squared | 0.9106 |
| Mean | 27.02 | Adjusted R-Squared | 0.8212 |
| C.V./% | 2.79 | Pred R-Squared | 0.5058 |
| PRESS | 43.94 | Adequate Precision | 11.3 |
| Serial Number | A | B | C | Decolorizing Rate (%) | Retention Rate (%) | Comprehensive Score (S) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 21.45 | 84.54 | 57.00 |
| 2 | 1 | −1 | 0 | 71.55 | 66.48 | 61.82 |
| 3 | −1 | 1 | 0 | 44.49 | 83.11 | 63.8 |
| 4 | 1 | 1 | 0 | 77.2 | 49.36 | 63.28 |
| 5 | −1 | 0 | −1 | 12.34 | 89.55 | 57.95 |
| 6 | 1 | 0 | −1 | 57.98 | 67.27 | 62.63 |
| 7 | −1 | 0 | 1 | 64.44 | 63.67 | 64.06 |
| 8 | 1 | 0 | 1 | 87.38 | 27.58 | 61.58 |
| 9 | 0 | −1 | −1 | 23.29 | 80.07 | 51.68 |
| 10 | 0 | 1 | −1 | 31.74 | 75.22 | 58.48 |
| 11 | 0 | −1 | 1 | 62.66 | 57.35 | 60.01 |
| 12 | 0 | 1 | 1 | 80.55 | 30.71 | 55.63 |
| 13 | 0 | 0 | 0 | 76.56 | 67.23 | 73.35 |
| 14 | 0 | 0 | 0 | 76.89 | 68.59 | 72.74 |
| 15 | 0 | 0 | 0 | 73.91 | 71.45 | 72.68 |
| 16 | 0 | 0 | 0 | 71.58 | 70.46 | 71.02 |
| 17 | 0 | 0 | 0 | 74.64 | 69.85 | 72.25 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 711.71 | 9 | 79.08 | 70.43 | <0.0001 | ** |
| A | 5.28 | 1 | 5.28 | 4.7 | 0.0667 | |
| B | 14.26 | 1 | 14.26 | 12.7 | 0.0092 | ** |
| C | 13.89 | 1 | 13.89 | 12.37 | 0.0098 | ** |
| AB | 7.13 | 1 | 7.13 | 6.35 | 0.0398 | * |
| AC | 12.82 | 1 | 12.82 | 11.41 | 0.0118 | * |
| BC | 31.25 | 1 | 31.25 | 27.83 | 0.0012 | ** |
| A2 | 35.75 | 1 | 35.75 | 31.84 | 0.0008 | ** |
| B2 | 270.76 | 1 | 270.76 | 241.14 | <0.0001 | ** |
| C2 | 265.38 | 1 | 265.38 | 236.36 | <0.0001 | ** |
| Residual | 7.86 | 7 | 1.12 | |||
| Lack of fit | 4.84 | 3 | 1.61 | 2.13 | 0.2388 | non-significant |
| Pure error | 3.02 | 4 | 0.76 | |||
| Cor total | 719.57 | 16 |
| Item | Value | Item | Value |
|---|---|---|---|
| Std.Dev. | 1.06 | R-Squared | 0.9891 |
| Mean | 63.53 | Adjusted R-Squared | 0.9750 |
| C.V./% | 63.53 | Pred R-Squared | 0.8859 |
| PRESS | 82.11 | Adequate Precision | 26.339 |
| Levels | Factors | ||
|---|---|---|---|
| A Time (h) | B Temperature (°C) | C Resin Content (g) | |
| −1 | 2 | 40 | 2.0 |
| 0 | 3 | 50 | 3.0 |
| 1 | 4 | 60 | 4.0 |
| Level | A Extraction Time (h) | B Extraction Temperature (°C) | C Material–Liquid Ratio (g/mL) | D Extraction Frequency (Freq) |
|---|---|---|---|---|
| −1 | 2 | 50 | 1:20 | 2 |
| 0 | 3 | 60 | 1:30 | 3 |
| 1 | 4 | 70 | 1:40 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Chen, G. Inhibition of Proliferation and Induction of Apoptosis in Prostatic Carcinoma DU145 Cells by Polysaccharides from Yunnan Rosa roxburghii Tratt. Molecules 2024, 29, 1575. https://doi.org/10.3390/molecules29071575
Yang Z, Chen G. Inhibition of Proliferation and Induction of Apoptosis in Prostatic Carcinoma DU145 Cells by Polysaccharides from Yunnan Rosa roxburghii Tratt. Molecules. 2024; 29(7):1575. https://doi.org/10.3390/molecules29071575
Chicago/Turabian StyleYang, Ziyan, and Guiyuan Chen. 2024. "Inhibition of Proliferation and Induction of Apoptosis in Prostatic Carcinoma DU145 Cells by Polysaccharides from Yunnan Rosa roxburghii Tratt" Molecules 29, no. 7: 1575. https://doi.org/10.3390/molecules29071575
APA StyleYang, Z., & Chen, G. (2024). Inhibition of Proliferation and Induction of Apoptosis in Prostatic Carcinoma DU145 Cells by Polysaccharides from Yunnan Rosa roxburghii Tratt. Molecules, 29(7), 1575. https://doi.org/10.3390/molecules29071575







